Abstract
Summary
The Agence Française de Securite Sanitaire des Produits de Santé (Afssaps; French Health Products Safety Agency) is responsible, through its hemovigilance unit, for the organization and the functioning of the national hemovigilance network. In accordance with the French law, it receives all data on adverse transfusion reactions regardless of their severity. With the aim of evaluating the tolerance of two kinds of labile blood products (LBP), pooled platelet concentrates (PP) and apheresis platelet concentrates (APC), we screened the French national database from January 1, 2000 to December 31, 2006. We observed that the number of transfusion incident reports is more than twice as high with APC (8.61:1,000 LBP) than with PP (4.21:1,000 LBP). The difference between these two ratios is statistically significant as shown by chi-square test (e = 21.00 with α = 5%). The risk to suffer adverse reactions of any type, except for alloimmunization, is higher with APC, and the major type of diagnosis related to APC is allergic reaction (1:200 APC issued) even if those allergic reactions are rarely serious. The new French National Hemovigilance Commission should impel a working group evaluating this topic and above all the impact of additive solutions which have been used since 2005 to put forward preventives measures.
Key Words: French hemovigilance, Apheresis platelet concentratece, Pooled platelet concentrate, Adverse reaction
Abstract
Zusammenfassung
Die Agence Française de Sécurité Sanitaire des Produits de Santé (Afssaps) ist über ihre Hämovigilanzabteilung verantwortlich für die Organisation und das Funktionieren des nationalen Hämovigilanznetzwerks. In Übereinstimmung mit dem französischen Gesetz erhält sie alle Daten über unerwartete Transfusionsnebenwirkungen unabhängig von deren Schwere. Mit dem Ziel, die Toleranz gegenüber zwei Typen von labilen Blutprodukten-Apheresethrombozytenkonzentrate (APC) und gepoolte Thrombozytenkonzentrate (PP) – zu evaluieren, überprüften wir den nationalen französischen Datenbestand für den Zeitraum 1. Januar 2000 bis 31. Dezember 2006. Dabei stellten wir fest, dass die Zahl der Berichte von Transfusionszwischenfällen bei APC mehr als doppelt so hoch war (8,61:1000) wie bei PP (4,21:1000). Dieser Unterschied war statistisch signifikant (Chi-Quadrat-Test; e = 21,00 mit α = 5%). Das Risiko für unerwartete Nebenwirkungen ist mit APC mit Ausnahme der Alloimmunisation höher. Die häufigsten auftretende Nebenwirkung bei APC sind allergische Reaktionen (1:200 verabreichte APC), wenngleich diese allergischen Reaktionen selten schwerwiegend sind. Die neue französische Hämovigilanzkommission soll eine Arbeitsgruppe initiieren, die diese Fragestellung, besonders den Einfluss von additiven Lösungen seit 2005, untersucht, um die Präventivmaβnahmen zu verbessern.
Introduction
For more than 14 years, hemovigilance has aimed to prevent the occurrence of adverse reactions (AR) related to the transfusion of labile blood products (LBP). In order to reduce the viral residual risk (lower donor exposure), apheresis platelet concentrates (APC) are more often applied in platelet transfusion (80.5 versus 19.5%) than pooled platelet concentrates (PP). From 1995 to 2006, the amount of APC used in France has increased regularly: from 130,000 APC to 190,000 APC. On the other hand the amount of PP has decreased from 72,000 to 24,000 in 2002, but increased again to 43,000 in 2006. The present study aimed at evaluating the tolerance of these two kinds of LBP in terms of AR observed in recipients. We therefore screened the database of the national hemovigilance network that gives an exhaustive view of the AR notifications in France in combination with a complete traceability of the transfused LBP for AR possibly, probably or definitely attributable to the transfusion of these two types of platelet concentrates (PC) between January 1, 2000 and December 31, 2006.
The French Hemovigilance System
The Agence Française de Sécurité Sanitaire des Produits de Santé (Afssaps; French Health Products Safety Agency) is responsible for the organization and the functioning of the national hemovigilance network. In accordance with the French laws, all AR, regardless of their severity, are notified to the Afssaps by hemovigilance correspondents (in public and private hospitals as well as in blood establishments) on standardized forms (online reporting system). In France, we use five levels of seriousness and evidence each.
Grade of Seriousness
-
–
Grade 4: death during or after transfusion.
-
–
Grade 3: life-threatening AR.
-
–
Grade 2: long-term morbidity (i.e. above all transfusion-transmitted viral infection and alloimmunization).
-
–
Grade 1: minor AR.
-
–
Grade 0: inappropriate transfusion of LBP consecutive to one or several dysfunctions, without any clinical or biological consequence for the recipient.
Evidence Levels
-
–
Level 4: certain = conclusive evidence for attributing the AR to the blood transfusion.
-
–
Level 3: likely = evidence in favor of attributing the AR to the blood transfusion, without any other obvious causes.
-
–
Level 2: possible = evidence is indeterminate for attributing the AR either to the blood transfusion or to alternative causes.
-
–
Level 1: doubtful = other possible causes but no evidence for excluding the role of the blood transfusion in the occurrence of the AR.
-
–
Level 0: excluded = conclusive evidence for attributing the AR to causes other than the blood transfusion.
Requirements of Hemovigilance
Requirements of hemovigilance are:
-
–
the notification of suspected AR observed in a recipient to the competent authority (Afssaps),
-
–
a high level of traceability, i.e. the ability to trace each individual unit of LBP from the donor to the recipient or disposal.
Source of Data
-
–
Afssaps
-
–
EFS (the French National Blood Service)
-
–
CTSA (the Army blood center)
-
–
Hemovigilance correspondents reporting AR.
Results
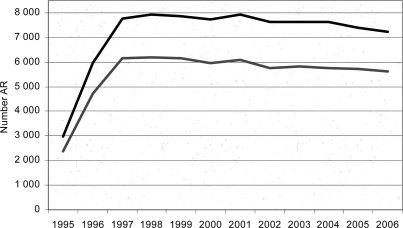
From 1995 to 2006, about 32,074,000 LBP were transfused, and 85,051 AR associated with transfusion were registered. Until 1999, the AR/LBP rate had increased, and then had remained stable between 2.8 and 3 per 1,000 LBP. Thus, our results included data from 2000 to 2006 (fig 1).
Fig. 1.
Time series AR 1995–2006.▃All notifications. ▃ Notifications with evidence level ≥ 2.
On average we have received 7,500 notifications of AR/year, more than 73.2% of the notifications were AR of minor gravity (grade 1).
From 2000 to 2006, there were 96 deaths, with an evidence level from possible to certain, under complete investigation. Transfusion-associated circulatory overload (TACO) is the most common cause of transfusion-related death. Before 2002, transfusion-related acute lung injury (TRALI) were not individualized on the reporting form. Approximately half of the deaths were of evidence level 2. Frequency of death with APC is twice as high as with PP (1 death in 54,410 APC versus 1 death in 105,310 PP issued (table 1)).
Table 1.
Frequency of transfusion related deaths, evidence level > 2 complete investigations (France 2000–2006)
| 2000–2006 | ACP | PP | 1 for xx LBP | 1 for xx APC | 1 for xx PP | |
|---|---|---|---|---|---|---|
| TACO | 27 | 1 | 1 | 656,160 | 1,251,390 | 210,630 |
| Immunologie imcompatibility | 16 | 4 | 0 | 1,107,440 | 312,870 | |
| Unknown | 16 | 4 | 0 | 1,107,440 | 312,870 | |
| TRALI | 12 | 6 | 0 | 1,476,580 | 208,570 | |
| TTBI | 9 | 6 | 1 | 198,770 | 208,570 | 210,630 |
| Allergy | 5 | 1 | 0 | 3,543,790 | 1,251,390 | |
| Other immediate TIR | 8 | 1 | 0 | 2,214,870 | 1,251,390 | |
| Hemosiderosis | 2 | 0 | 0 | 8,859,480 | ||
| Plasmodium falcipurum* | 1 | 0 | 0 | 17,718,960 | ||
| Total grade 4 | 96 | 23 | 2 | 184,570 | 54,410 | 105,310 |
TACO = Transfusion-associated circulatory; TRALI = transfusion-related acute lung injury; TTBI = transfusion-transmitted bacterial infections; TIR = transfusion incident reports.
One Plasmodium falciparum declared lately for 2006, i.e. 2 AR for the period 2000–2006 and an incidence ratio of 1 for 8 859 480 LBP.
All notifications of AR (evidence level 2, 3, 4) from 2000 to 2006 were reported to blood products issued. We observed that the number of transfusion incident reports (TIR) is more than twice as high with APC (8.61:1,000 LBP) than with PP (4.21:1,000 LBP). The difference between these two ratios is statistically significant as shown by chi-square test (e = 21.00 with α = 5%) (table 2).
Table 2.
Notification, evidence level ≥ 2 complete investigations, 2000–2006
| LBP | LBP issued | AR/1,000 LPB | AR grade 4/100,000 LBP |
|---|---|---|---|
| RBCC | 14,098,804 | 1.85 | 0.50 |
| APC | 1,251,391 | 8.61 | 1.84 |
| PP (MCP) | 210,628 | 4.21 | 0.95 |
| Plasma | 1,896,460 | 0.50 | 0.05 |
RBCC = Red blood cell concentrate.
The respective ratios for AR due to transfusion-transmitted bacterial infections (TTBI) are 0.0328:1000 APC and 0.0285:1000 PP. being not significantly different according to chi-square analysis (e = 0.320 with α = 5%).
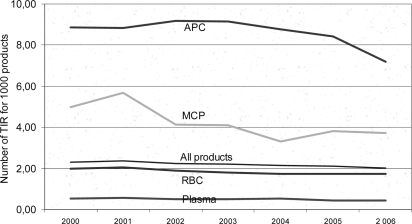
It could also be shown that the risk to suffer AR of any type, except for alloimmunization, is higher with APC than with PP, even though most of the AR (93%) are not serious (fig 2).
Fig. 2.
Notification of adverse events, evidence levell ≥ 2, 2000–2006.
The most frequently observed AR related to APC and PP transfusion were allergic reactions. 10,025 allergic reactions were reported 6,258 of which were associated with APC transfusion. The risk of allergic reactions subsequent to APC and PP transfusion is 1:200 and 1:630, respectively (table 3).
Table 3.
Notification of adverse events, evidence level > 2,2000–2006
| AR/1,000 APC | AR/1,000 PP | |
|---|---|---|
| FNHTR | 1,617 | 0,883 |
| Allergy | 5,001 | 1,595 |
| Alloimmunization | 0,292 | 0,864 |
| Unknown | 0,797 | 0,351 |
| Immunologie imcompatibility | 0,391 | 0,370 |
| Transfusion inefficiency | 0,244 | 0,062 |
| TACO | 0,062 | 0,024 |
| Virus infection | 0,002 | 0,000 |
| Other immediate AR | 0,121 | 0,009 |
| TTBI | 0,037 | 0,033 |
| TRALI | 0,033 | 0,005 |
| Other delayed AR | 0,015 |
0,000 |
| A11AR | 8,612 | 4,211 |
| Death | 0,018 | 0,009 |
FNHTR = Febrile nonhemolytic transfusion reaction; TACO = transfusion-associated circulatory; TRALI = transfusion-related acute lung injury; TTBI = transfusion-transmitted bacterial infections.
Conclusions and Discussion
The data of the French hemovigilance show that APC transfusion appears to be the greatest risk factor for AR of the ‘allergy’ type even if it should be remembered that allergic reactions are rarely serious.
Nevertheless, only APC permits the manufacturing of an HLA-compatible PC.
Since 2005, a slight decrease in the number of notified allergic reactions has been observed with APC. The impact of platelet additive solutions should be evaluated with the benefit of hindsight.
The new French national hemovigilance commission should impel a working group evaluating the topic of allergy and APC to put forward preventive measures.
References
- 1.Conway DJ. Molecular epidemiology of malaria. Clin Microbiol Rev. 2007;20:188–204. doi: 10.1128/CMR.00021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartl DL. The origin of malaria: mixed messages from genetic diversity. Nature Rev Microbiol. 2004;2:15–22. doi: 10.1038/nrmicro795. [DOI] [PubMed] [Google Scholar]
- 3.Fairhurst R, Wellems T. Plasmodium species (malaria) In: Mandell G, Bennett G, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 3121–3144. [Google Scholar]
- 4.Zoller T, Suttorp N. Malaria und Babesiose: Parasitäre Erkrankungen der Erythrozyten. In: Dietel M, Suttorp N, Zeitz M, editors. Harrisons Innere Medizin. Berlin: ABW Wissenschaftsverlag; 2005. pp. 1309–1323. [Google Scholar]
- 5.Kretschmer H, Bienzle U, Klauß V, Kremsner P, Leichsenring M. Malaria. In: Knobloch J, editor. Tropen- und Reisemedizin. Jena: Gustav Fischer; 1996. pp. 134–163. [Google Scholar]
- 6.WHO: World Malaria Report 2005. http://rbm.who.int./wmr2005
- 7.Robert Koch-Institut Reiseassoziierte infektionsbedingte Erkrankungen im Jahr 2004. Epidemiol Bull. 2005;35:317–324. [Google Scholar]
- 8.Lehky Hagen MR, Haley TJ, Christoph Hatz FR. Factors influencing the pattern of imported malaria. J Travel Med. 2005;12:72–79. doi: 10.2310/7060.2005.12203. [DOI] [PubMed] [Google Scholar]
- 9.Mouchet J. Airport malaria: a rare disease still poorly understood. Eurosurveillance. 2000;5:75–80. doi: 10.2807/esm.05.07.00016-en. [DOI] [PubMed] [Google Scholar]
- 10.Alweis RL, DiRosario K, Conidi G, Kain KC, Olans R, Tully JL. Serial nosocomial transmission of Plasmodium falciparum malaria from patient to nurse to patient. Infect Control Hosp Epidemiol; 2004;25:55–59. doi: 10.1086/502293. [DOI] [PubMed] [Google Scholar]
- 11.Moro ML, Romi R, Severini C, Casadio GP, Sarta G, Tampieri G, Scardovi A, Pozzetti C, Malaria Outbreak Group Patient-to-patient transmission of nosocomial malaria in Italy. Infect Control Hosp Epidemiol. 2002;23:338–341. doi: 10.1086/502062. [DOI] [PubMed] [Google Scholar]
- 12.Piro S, Sammud M, Badi S, Al Ssabi L. Hospital-acquired malaria transmitted by contaminated gloves. J Hosp Infect. 2001;47:156–158. doi: 10.1053/jhin.2000.0907. [DOI] [PubMed] [Google Scholar]
- 13.Chen KT, Chen CJ, Chang PY, Morse DL. A nosocomial outbreak of malaria associated with contaminated catheters and contrast medium of a computed tomographic scanner. Infect Control Hosp Epidemiol. 1999;20:22–25. doi: 10.1086/501557. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Local transmission of Plasmodium vivax malaria. – Palm Beach County, Florida, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(38):908–911. [PubMed] [Google Scholar]
- 15.Lucius R, Frank B. Parasitologie. Heidelberg: Spektrum Akademischer Verlag; 1997. [Google Scholar]
- 16.Fischer L. Einheimische Malaria und Anophelismus in der Nachkriegszeit. Dtsch Med Wochenschr. 1948;73:515–518. [Google Scholar]
- 17.Krüger A, Rech A, Xin-Zhuan S, Tannich E. Two cases of autochthonous Plasmodium falciparum malaria in Germany with evidence for local transmission by indigenous Anopheles plumbeus. Trop Med Intern Health. 2001;6:983–985. doi: 10.1046/j.1365-3156.2001.00816.x. [DOI] [PubMed] [Google Scholar]
- 18.Warhurst DC, Williams JE. ACP Broadsheet no 148, July 1996. Laboratory diagnosis of malaria. J Clin Pathol. 1996;49:533–538. doi: 10.1136/jcp.49.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snounou GS, Viriyakosol S, Zhu XP, Jara W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 20.Johnston SP, Pieniazek NJ, Xayavong MV, Slemenda SB, Wilkins PP, da Silva AJ. PCR as a confirmatory technique for laboratory diagnosis of malaria. J Clin Microbiol. 2006;44:1087–1089. doi: 10.1128/JCM.44.3.1087-1089.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhotra I, Dent A, Mungai P, Muchiri E, King CL. Real-time quantitative PCR for determining the burden of Plasmodium falciparum parasites during pregnancy and infancy. J Clin Microbiol. 2005;43(8):3630–3635. doi: 10.1128/JCM.43.8.3630-3635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsayed S, Plewes K, Church D, Chow B, Zhang K. Use of molecular beacon probes for real-time PCR detection of Plasmodium falciparum and other Plasmodium species in peripheral blood specimens. J Clin Microbiol. 2006;44:622–624. doi: 10.1128/JCM.44.2.622-624.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangold KA, Manson RU, Koay ESC, Stephens L, Regner MA, Thomson RB, Peterson LR, Kaul KL. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–2440. doi: 10.1128/JCM.43.5.2435-2440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perandin F, Manca N, Calderaro A, Piccolo G, Galati L, Ricci L, Medici MC, Arcangeletti MC, Snounou G, Dettori G, Chezzi C. Development of a real-time PCR assay for detection of Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale for routine clinical diagnosis. J Clin Microbiol. 2004;42:1214–1219. doi: 10.1128/JCM.42.3.1214-1219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grobusch MP, Alpermann U, Schwenke S, Jelinek T, Warhurst DC. False-positive rapid tests for malaria in patients with rheumatoid factor. Lancet. 1999;353:297. doi: 10.1016/s0140-6736(05)74930-8. [DOI] [PubMed] [Google Scholar]
- 26.Hänscheid T, Valadas E. Poor accuracy of rapid diagnostic tests and misdiagnosis of imported malaria: are PCR-based reference laboratories the answer? J Clin Microbiol. 2002;40:736–737. doi: 10.1128/JCM.40.2.736-737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mankhambo L, Kanjala M, Rudman S, Lema VM, Rogerson SJ. Evaluation of the OptiMal rapid antigen test and species-specific PCR to detect placental Plasmodium falciparum infection at delivery. J Clin Microbiol. 2002;40:155–158. doi: 10.1128/JCM.40.1.155-158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Playford EG, Walker J. Evaluation of the ICT malaria P.f/P.v and the OptiMal rapid diagnostic tests for malaria in febrile returned travellers. J Clin Microbiol. 2002;40:4166–4171. doi: 10.1128/JCM.40.11.4166-4171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson DC, Ciach M, Zhong KJY, Crandall I, Kain KC. Evaluation of the Makromed dipstick assay versus PCR for diagnosis of Plasmodium falciparum malaria in returned travelers. J Clin Microbiol. 2002;40:4528–4530. doi: 10.1128/JCM.40.12.4528-4530.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jelinek T, Grobusch MP, Harms G. Evaluation of a dipstick test for the rapid diagnosis of imported malaria among patients presenting within the network TropNetEurop. Scand J Infect Dis. 2001;33:752–754. doi: 10.1080/003655401317074563. [DOI] [PubMed] [Google Scholar]
- 31.Joshi HH. Monoclonal antibody based ELISA: an effective diagnostic tool for the diagnosis of falciparum malaria. J Nep Med Assoc. 2005;44:79–83. [PubMed] [Google Scholar]
- 32.Trenholme KR, Boutlis CS, Kuns R, Lagog M, Bockarie MJ, Gatton M, Kemp DJ, Good MF, Anstey NM, Gardiner DL. Antibody reactivity to linear epitopes of Plasmodium falciparum cytoadherence linked asexual gene 9 in asymptomatic children and adults from Papua New Guinea. Am J Trop Med Hyg. 2005;72:708–713. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Crouch L, Richie TL, Nhan DH, Coppel RL. Naturally acquired antibody responses to the components of the Plasmodium falciparum merozoite surface protein 1 complex. Parasite Immunol. 2003;25:403–412. doi: 10.1111/j.1365-3024.2003.00647.x. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen MA, Vestergaard LS, Lusingu J, Kurtzhals JAL, Giha HA, Grevstad B, Goka BQ, Lemnge MM, Jensen JB, Akanmori BD, Theander TG, Staalsoe, Hviid L. Geographical and temporal conservation of antibody recognition of Plasmodium falciparum variant surface antigens. Infect Immun. 2004;72(6):3531–3535. doi: 10.1128/IAI.72.6.3531-3535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orlandi-Pradines E, Penhoat K, Durand C, Pons C, Bay C, Pradines B, Fusai T, Josse R, Dubrous P. Meynard JP, Durand JP, Migliani R, Boutin JP, Druilhe P, Rogier C: Antibody responses to several malaria pre-erythrocytic antigens as a marker of malaria exposure among travelers. Am J Trop Med Hyg. 2006;74:979–985. [PubMed] [Google Scholar]
- 36.Pierrot C, Wilson S, Lallet H, Lafitte S, Jones FM, Daher W, Capron M, Dunne DW, Khalife J. Identification of a novel antigen of Schistosoma mansoni shared with Plasmodium falciparum and evaluation of different cross-reactive antibody subclasses induced by human schistosmiasis and malaria. Infect Immun. 2006;74:3347–3354. doi: 10.1128/IAI.01724-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okocha EC, Ibeh CC, Ele PU, Ibeh NC. The prevalence of malaria parasitaemia in blood donors in a Nigerian teaching hospital. J Vector Borne Dis. 2005;42:21–24. [PubMed] [Google Scholar]
- 38.Nunez L, Linares J, Perez AH. Seroprevalence of antibodies against Plasmodium falciparum in volunteer donors from various cities in Venezuela. Sangre (Barc) 1992;37(2):141–143. [PubMed] [Google Scholar]
- 39.Bundesärztekammer Richtlinien zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten (Hämotherapie) gemäß §§12 und 18 des Transfusionsgesetzes (Novelle 2005) Bundesanzeiger. 2005;57 (Nr.209a). [Google Scholar]
- 40.Joint UKBTS/NIBSC Professional Advisory Committee: Whole Blood & Components Donor Selection Guidelines. http://www.transfusionguidelines.org.uk/index.asp?Publication-WB
- 41.Woolsey G. Transfusion for pernicious anemia: two cases. Ann Surg. 1911;53:132–134. [Google Scholar]
- 42.Bruce-Chwatt LJ. Blood transfusion and tropical disease. Trop Dis Bull. 1972;69:825–862. [PubMed] [Google Scholar]
- 43.Bruce-Chwatt LJ. Transfusion malaria revisited. Trop Dis Bull. 1982;79:827–840. [PubMed] [Google Scholar]
- 44.Chiodini PL, Hartley S, Hewitt PE, Barbara JA, Lalloo K, Bligh J, Voller A. Evaluation of a malaria antibody ELISA and its value in reducing potential wastage of red cell donations from blood donors exposed to malaria, with a note on a case of transfusion-transmitted malaria. Vox Sang. 1997;73:143–148. doi: 10.1046/j.1423-0410.1997.7330143.x. [DOI] [PubMed] [Google Scholar]
- 45.Mungai M, Tegtmeier G, Chamberland M, Parise M. Transfusion-transmitted malaria in the United States from 1963 through 1999. N Engl J Med. 2001;344:1973–1978. doi: 10.1056/NEJM200106283442603. [DOI] [PubMed] [Google Scholar]
- 46.Slinger R, Giulivi A, Bodie-Collins M, Hindieh F, John RS, Sher G, Goldman M, Ricketts M, Kain KC. Transfusion-transmitted malaria in Canada. CMAJ. 2001;164:377–379. [PMC free article] [PubMed] [Google Scholar]
- 47.Oehlecker F. Übertragung latenter Malaria bei direkter Bluttransfusion. Dtsch Med Wochenschr. 1920;46:1025–1026. [Google Scholar]
- 48.Schnitzler H. Übertragung von latenter Malaria tertiana durch Bluttransfusion. Zentralbl Chir. 1929;56:1438. [Google Scholar]
- 49.Majanz A. Beiträge zur Frage der Bluttransfusion. Dtsch Z Chir. 1930;224:171–185. [Google Scholar]
- 50.Stohlmann H. Malariaübertragung auf Blutspende. Münch Med Wochenschr. 1943;90:84–85. [Google Scholar]
- 51.Menk W. Bluttransfusion und Malaria. Münch Med Wochenschr. 1944;91:349–350. [Google Scholar]
- 52.Lampen H. Induzierte Malaria nach Bluttransfusion. Med Klin. 1947;42:371–372. [Google Scholar]
- 53.Mohr W. Malariaübertragung durch Bluttransfusionsgerät. Med Klin. 1950;45:94. [Google Scholar]
- 54.Nolte K, Stibbe W, Kuhlencord A, Bommer W, Gallasch E. Transfusionsbedingte Malaria tropica. Beitr Infusionsther Klin Ernähr. 1987;18:42–46. [PubMed] [Google Scholar]
- 55.Schunkert H, Handt S, de Wit M, Gladziwa U, Glöckner W, Sieberth H. Transfusionsmalaria bei Promyelozytenleukämie. Dtsch Med Wochenschr. 1988;113:1841–1843. doi: 10.1055/s-2008-1067898. [DOI] [PubMed] [Google Scholar]
- 56.Witt O, Iglauer A RJ, Bommer W, Eber S. Transfusionsmalaria als Ursache von unklarem postoperativen Fieber. Monatsschr Kinderheilkd. 1998;146:1054–1056. [Google Scholar]
- 57.Frey-Wettstein M, Maier A, Markwalder K, Munch U. A case of transfusion transmitted malaria in Switzerland. Swiss Med Wkly. 2001;131:320. doi: 10.4414/smw.2001.09720. [DOI] [PubMed] [Google Scholar]
- 58.Knobloch, Jürgen, editors. Malaria – Grundlagen und klinische Praxis. Bremen: Unimed; 2003. [Google Scholar]
- 59.Kitchen AD, Barbara JA, Hewitt PE. Documented cases of post-transfusion malaria occurring in England: a review in relation to current and proposed donor-selection guidelines. Vox Sang. 2005;89:77–80. doi: 10.1111/j.1423-0410.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- 60.Guerrero IC, Weniger BG, Schultz MG. Transfusion malaria in the United States, 1972–1981. Ann Intern Med. 1983;99:221–226. doi: 10.7326/0003-4819-99-2-221. [DOI] [PubMed] [Google Scholar]
- 61.Graves P, Gelband H. Vaccines for preventing malaria. Cochrane Database Syst Rev. 2006;(2 und 4):CD000129. doi: 10.1002/14651858.CD000129. [DOI] [PubMed] [Google Scholar]
- 62.Deutsche Gesellschaft für Tropenmedizin und Internationale Gesundheit (DTG): Empfehlungen zur Malariavorbeugung, 2006. www.dtg.org/Malaria.html
- 63.Leitlinie der AWMF (Association of the Medical Societies in Germany): Diagnostik und Therapie der Malaria. Leitlinienregister Nr. 042/001.
- 64.Garfield MD, Ershler WB, Maki DG. Malaria transmission by platelet concentrate transfusion. JAMA. 1978;240:2285–2286. [PubMed] [Google Scholar]
- 65.Dover AS, Guinee VF. Malaria transmission by leukocyte component therapy. JAMA. 1971;217:1701–1702. [PubMed] [Google Scholar]
- 66.Lazner E, Newhouser E. Studies on the transmission of malaria by blood transfusions. Am J Med Sci. 1943;204:141–146. [Google Scholar]
- 67.Al-Saigul AM, Fontaine RE, Haddad Q. Nosocomial malaria from contamination of a multidose heparin container with blood. Infect Control Hosp Epidemiol. 2000;21:329–330. doi: 10.1086/501765. [DOI] [PubMed] [Google Scholar]
- 68.Winterberg DH, Wever PC, Rheenen-Veerberg CV, Kempers O, Durand R, Bos AP, Teeuw AH, Spanjaard L, Dunkert J. A boy with nosocoial malaria tropica contracted in a Dutch hospital. Ped Infect Dis J. 2005;24:89–91. doi: 10.1097/01.inf.0000148881.92329.f6. [DOI] [PubMed] [Google Scholar]
- 69.Besson P, Robert JF, Reviron J, Richard-Lenoble D, Gentilini M. 2 cases of transfusional malaria. Attempted prevention combining an indirect immunofluorescence test with clinical selection critera. Rev Fr Transfus Immunohematol. 1976;19:369–373. doi: 10.1016/s0338-4535(76)80076-1. [DOI] [PubMed] [Google Scholar]
- 70.Nahlen BL, Lobel HO, Cannon SE, Campbell CC. Reassessment of blood donor selection criteria for United States travelers to malarious areas. Transfusion. 1991;31:798–804. doi: 10.1046/j.1537-2995.1991.31992094665.x. [DOI] [PubMed] [Google Scholar]
- 71.Bruce-Chwatt LJ. Transfusion malaria. Bull World Health Organ. 1974;50:337–346. [PMC free article] [PubMed] [Google Scholar]
- 72.Seed CR, Kitchen A, Davis TM. The current status and potential role of laboratory testing to prevent transfusion-transmitted malaria. Transfus Med Rev. 2005;19:229–240. doi: 10.1016/j.tmrv.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vu TT, Tran VB, Phan NT, Le TT, Luong VH, O'Brien E, Morris GE. Screening donor blood for malaria by polymerase chain reaction. Trans R Soc Trop Med Hyg. 1995;89:44–47. doi: 10.1016/0035-9203(95)90652-5. [DOI] [PubMed] [Google Scholar]
- 75.Benito A, Rubio JM. Usefulness of seminested polymerase chain reaction for screening blood donors at risk for malaria in Spain. Emerg Infect Dis. 2001;7:1068. doi: 10.3201/eid0706.010632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silvie O, Rubinstein E, Franetich JF, Prenant M, Belnoue E, Rénia L, Hannoun L, Eling W, Levy S, Boucheix C, Mazier D. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 77.Smith TG, Kain KC. Inactivation of Plasmodium falciparum by photodynamic excitation of hemecycle intermediates derived from delta-aminolevulinic acid. J Infect Dis. 2004;190:184–191. doi: 10.1086/421503. [DOI] [PubMed] [Google Scholar]
- 78.Zavizion B, Serebryanik D, Chapman J, Alford B, Purmal A. Inactivation of Gram-negative and Gram-positive bacteria in red cell concentrates using INACTINE PEN110 chemistry. Vox Sang. 2004;87:143–149. doi: 10.1111/j.1423-0410.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- 79.Ferreira-da-Cruz MF, Teva A, Espindola-Mendes EC, dos Santos LG, Daniel-Ribeiro CT. Inactivation of Plasmodium falciparum parasites using gamma-irradiation. Mem Inst Oswaldo Cruz. 1997;92:137–138. doi: 10.1590/s0074-02761997000100029. [DOI] [PubMed] [Google Scholar]
- 80.Grellier P, Santus R, Mouray E, Agmon V, Maziere JC, Rigomier D, Dagan A, Gatt S, Schrével J. Photosensitized inactivation of Plasmodium falciparum and Babesia divergens- infected erythrocytes in whole blood by lipophilic pheophorbide derivatives. Vox Sang. 1997;72:211–220. doi: 10.1046/j.1423-0410.1997.7240211.x. [DOI] [PubMed] [Google Scholar]
- 81.Lustigman S, Ben Hur E. Photosensitized inactivation of Plasmodium falciparum in human red cells by phthalocyanines. Transfusion. 1996;36:543–546. doi: 10.1046/j.1537-2995.1996.36696269514.x. [DOI] [PubMed] [Google Scholar]
- 82.Singh Y, Sawyer LS, Pinkoski LS, Dupuis KW, Hsu JC, Lin L, Corash L. Photochemical treatment of plasma with amotosalen and long-wavelength ultraviolet light inactivates pathogens while retaining coagulation function. Transfusion. 2006;46:1168–1177. doi: 10.1111/j.1537-2995.2006.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]