Abstract
Functional MRI (fMRI) is routinely used to non-invasively localize language areas. Magnetoencephalography (MEG) is being explored as an alternative technique. MEG tasks to localize receptive language are well established although there are no standardized tasks to localize expressive language areas. We developed two expressive language tasks for MEG and validated their localizations against fMRI data. Ten right-handed adolescents (μ=17.5 years) were tested with fMRI and MEG on two tasks: verb generation to pictures and verb generation to words. MEG and fMRI data were normalized and overlaid. The number of overlapping voxels activated in fMRI and MEG were counted for each subject, for each task, at different thresholding levels. For picture verb generation, there was 100% concordance between MEG and fMRI lateralization, and for word verb generation, there was 75% concordance. A count showed 79.6% overlap of voxels activated by both MEG and fMRI for picture verb generation and 50.2% overlap for word verb generation. The percentage overlap decreased with increasingly stringent activation thresholds. Our novel MEG expressive language tasks successfully identified neural regions involved in language production and showed high concordance with fMRI laterality. Percentage overlap of activated voxels was also high when validated against fMRI, but showed task-specific and threshold-related effects. The high concordance and high percentage overlap between fMRI and MEG activations confirm the validity of our new MEG task. Furthermore, the higher concordance from the picture verb generation task suggests that this is a promising task for use in the young clinical population.
Keywords: functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG), expressive language, adolescence, validation
INTRODUCTION
Advances in neuroimaging technology have increased our understanding of the complex neural networks underlying human language. While the networks involved in language are complicated, the classical model of language organization [1] still provides a helpful framework for designing language studies [2]. The Wernicke-Geschwind model suggests that expressive language is subsumed in Broca’s area [3] located at the pars triangularis and operculum of the left inferior frontal gyrus. Receptive language is located in Wernicke’s area [4] at the left temporo-parietal junction. These areas have complex, reciprocal connections between primary sensory, secondary sensory, and association areas [5]. Notwithstanding the limitations inherent in a simplified model, the application of this model to the study of language is of practical use.
Within the clinical setting, evaluation of language dominance is a critical step prior to surgery. Traditionally, this was accomplished using the intracarotid amobarbital test [6], considered to be the gold standard for language lateralization. More recently, fMRI has become the modality of choice for non-invasive localization [7]. In the pediatric environment, one drawback of fMRI is that the noise, possible claustrophobia, and intolerance of any head movement during scanning, can be challenging for children [8]. Magnetoencephalography (MEG) has been gaining acceptance as an adjunctive part of the pre-surgical functional mapping procedure [9]. It has been suggested that, in pediatric cases, the quiet environment of the MEG room and the head-motion-tracking feature available in newer MEG machines, increase the likelihood of success with testing young children. Furthermore, since the fMRI and MEG measure the hemodynamic and neurophysiological response, respectively, it was thought that the MEG might be a good complementary measure in situations where the fMRI results are equivocal.
The use of MEG to localize receptive language areas to Wernicke’s area has been well documented [10] and validated [11–14]. The development of MEG protocols to study expressive language has been more challenging for a number of reasons, the primary one being the computational limitations of the traditional dipole source localization method [14]. Advances in source localization methods have addressed this issue, and several groups have reported identifying Broca’s area in adults [15–19].
In the fMRI literature, a number of tasks, including word verb generation, have been used to successfully to activate Broca’s area [20]. Previously, we reported MEG localization of Broca’s area in healthy adults and children using a covert picture verb generation task [21]. In the current study, we directly compared fMRI and MEG voxel co-localization, in the same subjects, in a group of adolescents, on a verb generation task with both words and pictures. While we expected task differences between words and pictures, with words activating components of the reading network and pictures activating object recognition and memory, we presume that the language portions of both tasks would be subsumed in the language dominant hemisphere. Our goal was to assess the validity of our MEG task against fMRI for both word and picture stimuli in this adolescent control cohort. If high concordance is demonstrated between MEG and fMRI, then this would be evidence that we can use the MEG picture verb generation task in our young pre-literate clinical populations.
METHODS
Subjects
Ten teenagers (5 girls; 15–19 yrs; mean=17.5+/−1.08) participated. All were right-handed (Edinburgh Handedness Inventory; mean=85.7+/−11.63; range= + 60 to +100) [22], had English as their first and primary language, and in age-appropriate grades at school. None reported neurological, neuropsychological, or academic deficits. All subjects gave informed consent.
Expressive language tasks
Subjects completed both tasks in MEG and fMRI. Nine of ten subjects were tested on fMRI and MEG on the same day; due to scanner scheduling conflicts, one subject was tested on two consecutive days.
To minimize head movement, the language task was covert. Subjects received an overt practice session to confirm adequate performance. To maintain attention, a vigilance trial was inserted with 15% probability. The vigilance trial consisted of a picture of a hand pressing a computer mouse. Upon presentation of this picture, subjects also pressed a button. Responses were monitored and subjects demonstrated 98% accuracy.
Two language tasks were completed: picture verb generation and word verb generation. For the picture verb generation task, colour drawings of a set of common objects were presented, and subjects were instructed to rapidly and silently think of one action word that corresponded to the object. The baseline stimulus was a colour-frequency scrambled image created from the picture stimuli. Baseline and active pictures were of the same size, brightness, and contrast. For the word verb generation task, simple, single syllable words (3–5 letters long) were presented, and subjects were instructed to rapidly think of an action word that corresponded to the presented noun. The baseline task was a presentation of false-font stimuli, which are alphabet-like characters but not real characters, matched for word length and letter-character frequency to the real word stimuli.
MEG
Stimulus presentation & task parameters
For both tasks, the task stimuli (either pictures or words) were presented for 500 ms, immediately followed by the baseline stimuli for 2 sec. This continued for 92 trials with 12 embedded vigilance trials. The task stimuli subtended 5 degrees of arc visually. All subjects pressed the button response box with their right hands, but these trials were identified and removed prior to further processing.
Data acquisition
MEG data were acquired on a CTF Omega (VSM Medtech, Canada) system at The Hospital for Sick Children. Subjects were tested supine on the MEG bed in the darkened magnetically shielded room. Stimuli were controlled with Presentation (Neurobehavioral Systems, Inc., Albany, CA), sent to a video projector and back-projected via mirrors to a screen located 65 cm from the subject’s nose.
The subjects’ heads were localized before and after each test condition. Our criterion was that conditions with head movements greater than 5 mm would be repeated; no subjects required repeat due to head movement. MEG data were acquired continuously (625 Hz ADC; 0–100 Hz bandpass) and epoched from 500 ms pre-stimulus to 1000 ms post-stimulus.
Data analysis
Vigilance trials were removed from the MEG data prior to further analyses. Synthetic aperture magnetometry (SAM) analyses were used. This is a spatial filtering approach to source reconstruction which allows the localization of cortical oscillations without the need for time-averaging of the stimulus locked responses [23–25]. Differential estimates of power integrated over pre-defined time windows for both active and baseline activity are computed [26]; these use single-trial data over specified frequency bands and time windows throughout the brain and generate a distribution of pseudo-t values which can be displayed on individual MRIs resulting in co-registered 3-dimensional source images. Negative pseudo-t values indicate event-related desynchrony which has been shown to be related to cognitive function [27].
Systematic differential SAM analyses were performed for each subject at consecutive time windows and frequency bands. The baseline was defined as the 200 ms time window preceding stimulus onset (−200 to 0 ms) and the active states were defined as consecutive 200 ms windows starting 100 ms after stimulus onset (100–300, 200–400, 300–500, 400–600, 500–700, 600–800 ms). The region of interest was set to include the whole cerebral cortex with a 5-mm voxel resolution. Pseudo-t images were computed for three different frequency bands (5–15 Hz, 15–25 Hz, and 25–50 Hz). These ranges were selected on the basis of previous observations of spectral power changes during language related tasks [17,27]. The SAM results for each subject were transferred into AFNI (http://afni.nimh.nih.gov/) and normalized onto a Talaraich template brain [28]. Both individual and group averaged data were examined.
fMRI
Stimulus presentation & task parameters
fMRI tasks were block design format, employing the same stimuli as the MEG tasks. Each run consisted of fourteen 26-second blocks alternating task and baseline stimuli. Each block was composed of 12 images presented at 2-second intervals, with a randomly embedded vigilance trial.
Data acquisition
All MRI imaging was performed at the Hospital for Sick Children on an Excite HD 1.5 T MRI system (GE Medical Systems, Waukesha, WI). Prior to entering the scanner, the MEG fiducial sites were marked with vitamin E capsules to allow co-registration between MEG and fMRI. A 3-dimensional SPGR T1 anatomical MRI was acquired. Functional MRI acquisition used a T2-weighted echoplanar imaging (EPI) sequence with the following parameters: TE = 40 ms, TR = 2000 ms, flip angle = 90 degrees, FOV = 240 mm, matrix = 64 × 64, and slice thickness = 5 mm × 25 slices (no gap).
Data analysis
Functional MRI data were analyzed using AFNI. Raw functional data was motion-corrected and functional images were normalized into Talairach space using algorithms provided by the AFNI software. Cross-correlational analyses were performed using a binary-model of the hemodynamic response. Both individual and group averaged data were examined.
Cross-modality analysis
Using fMRI data as the standard to which we would compare our MEG results, examination of the fMRI data showed that despite variable patterns of activation, all subjects demonstrated a consistent activation in the left frontal lobe. This is consistent with the literature suggesting that in healthy right-handed adult individuals, there is a 95% probability that both expressive and receptive language reside in the left cerebral hemisphere [28]. For this reason, the region of comparison for MEG to fMRI was limited to the left frontal lobe quadrant, defined in Talairach space by x > 0 mm, y < 14 mm, z > 14 mm, and referred to as our region of interest (ROI).
For fMRI data, a t-test was performed on the functional volumes based on percent-change in BOLD signal. For each individual, the set of voxels within the ROI that reached significant t-values (p < 0.05, corrected) was identified and counted; a group average for each task was calculated. For picture verb generation, the average total number of voxels reaching significance in the ROI was 801±3. For word verb generation, 501±2 voxels were significant in the ROI on fMRI.
Instead of re-counting the number of activated voxels at more stringent p-values, we chose to compute a new “pseudo-threshold” by sorting our functional volumes by percent signal changed, and then selecting the highest voxels only. Specifically, for picture verb generation, we know that at p<0.05, 800 vowels were activated. We looked at the pattern of activation for the highest half of these voxels (i.e., the top 400 voxels) and the highest quartile of these voxels (i.e., the top 200 voxels). For word verb generation, we know that 500 voxels were significant at p<0.05, so we looked also at the top half (i.e., 250 voxels) and top quarter (i.e., 125 voxels).
For the MEG data, we applied the new “pseudo-threshold” to each individual subject’s data. The voxels activated by MEG SAM results were sorted by magnitude of desynchrony, or magnitude of power change. Following the same process as for fMRI, for the picture verb generation task on MEG, we selected the highest 800, 400, and 200 voxels. For word verb generation, we selected the highest 500, 250, and 125 voxels. We then compared the fMRI and MEG data for overlapping voxels.
Conjunction Analysis
Conjunction analysis consists of superimposing two functional volumes over each other and examining the areas of overlap. Since the grid of the two volumes (i.e., voxel size, FOV, etc) need to be identical, functional volumes with finer grids were re-sampled to match the coarser grid, and this was used as the master grid. Once the datasets were matched in AFNI, the number of voxels that were activated in both fMRI and MEG were counted, and a percent overlap computed.
RESULTS
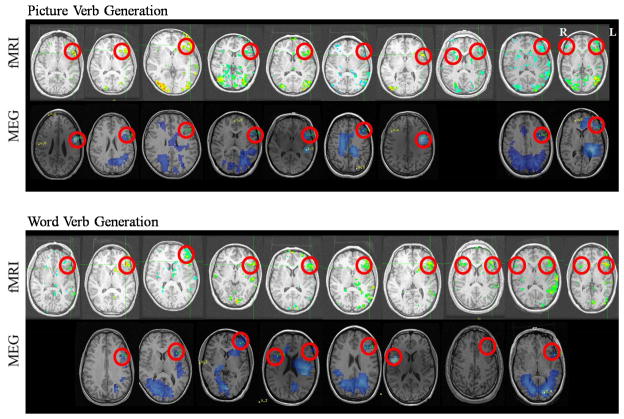
Figure 1 shows each subject’s fMRI and MEG data for both picture verb generation and word verb generation tasks. Table 1 summarizes these data and demonstrates that in both tasks, fMRI identified a significant activation in all 10 subjects while significant activations were only identified in 90% and 80% on MEG for the picture verb generation and word verb generation tasks, respectively. Table 1 also demonstrates that on the picture verb generation task, all 9 subjects (100%) with MEG activations reported concordance with fMRI, while on the word verb generation task, only 6 of 8 MEG subjects (75%) showed concordance.
Figure 1.
fMRI and MEG analyzed data for the picture verb generation and word verb generation tasks for each individual subject. MEG data are from 600–800 ms, 15–25 Hz for picture verb generation and 400–600 ms, 20–35 Hz for word verb generation.
Table 1.
Summary of frontal activation.
| Picture Verb Generation | Word Verb Generation | |||
|---|---|---|---|---|
| Subject # | fMRI | MEG | fMRI | MEG |
| 1 | left | left | left | |
| 2 | left | left | left | left |
| 3 | left | left | left | left |
| 4 | left | left | left | left |
| 5 | left | left | left | R>L |
| 6 | left | left | left | left |
| 7 | left | left | left | right |
| 8 | L>R | L>R | left | |
| 9 | left | left | L>R | left |
| 10 | L>R | left | L>R | |
MEG activations for picture verb generation were seen in the frontal areas of most subjects in the 600–800 ms time window and 15–25 Hz frequency band. For word verb generation, MEG activations in frontal areas were seen in the 400–600 ms time window and the 20–35 Hz frequency band. The activations from these windows and frequency bands are shown in Figure 1 and overlaid in Figure 2.
Figure 2.
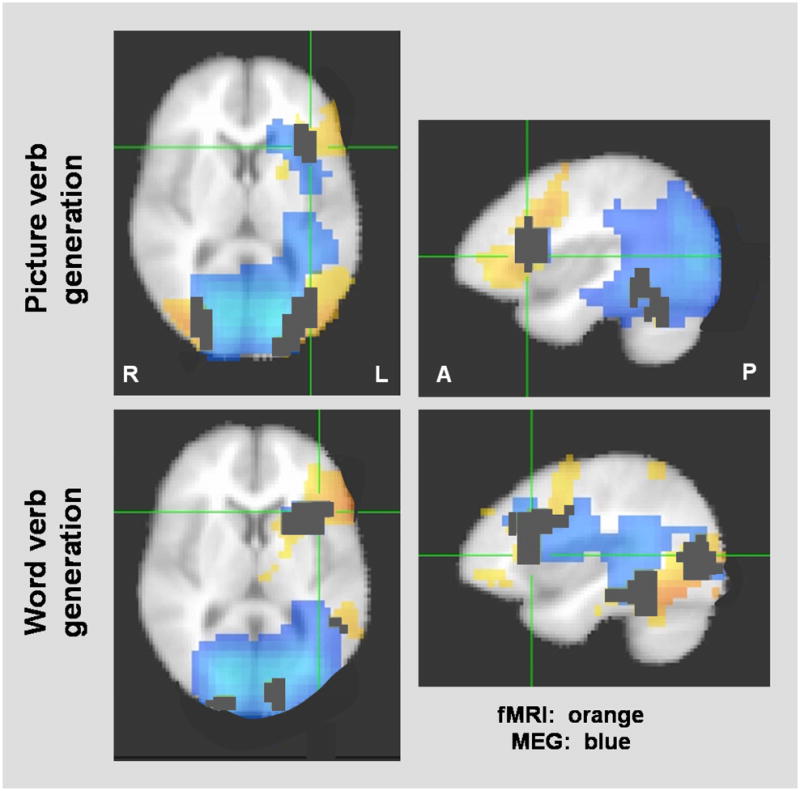
Group averaged data for the two tasks with fMRI (orange) and MEG (blue) activations overlaid. fMRI threshold is set at p<0.05 and overlapping voxels are marked in black.
Figure 2 shows the group averaged data for fMRI with MEG data overlaid. Clear areas of overlap can be seen, particularly left inferior frontal and bilateral occipital areas for both tasks, and left temporal-parietal areas for word verb generation. Also, of note, the MEG activations are more wide-spread and extend deeper compared to the fMRI activations.
In terms of voxel count comparisons for the two tasks, at p<0.05 (corrected), for picture verb generation, 80±15 of the same voxels were identified by both modalities resulting in a 79.6% voxel overlap, and for word verb generation, this overlap was 50.2%. At increasingly stringent thresholds on fMRI, for picture verb generation, the percent overlap decreased to 46±7.3% and 26± 8.0%, respectively, while for word verb generation, the percent overlap decreased to 28±6.4% and 8±3.5%, respectively.
DISCUSSION
Consistent with reports in the literature for children and adult subjects [30–32], fMRI expressive language tasks produced reliable activation of left frontal language areas in this cohort of healthy teenagers. Our finding that fMRI results were observable for every subject while MEG activations were less pronounced is consistent with another study comparing fMRI and MEG in adults during a naming task [33]. This pattern of strong fMRI and less pronounced MEG has also been seen in other cognitive tasks (for reviews, see [9,34]). This is most likely due to the hemodynamic versus neurophysiological properties of fMRI versus MEG. The fMRI response reflects cerebral blood flow changes involved in multiple, long-duration processes while the MEG response reflects discrete changes in local field potentials in a population of highly time- and/or phase-synchronized neurons [35–36]. Despite these qualitative differences, our new MEG expressive language tasks lateralized language very well. In fact, for subjects who showed activation on MEG, 100% showed concordance with fMRI in identifying language dominance on picture verb generation and 75% showed concordance on word verb generation.
With regards to the actual voxel overlap between fMRI and MEG, we discovered that this is task-specific and threshold-related. Specifically, with the picture verb generation task, we found a 79.6% overlap between voxels activated by both MEG and fMRI, while word verb generation showed a 50.2% overlap. Regardless of the threshold level selected, picture verb generation showed higher percentage of voxel overlap than word verb generation. Examination of Figure 2 suggests one explanation for this difference. Picture verb generation produced a more focal activation on MEG in the frontal lobes, whereas word verb generation produced a wider activation, encompassing the dorsolateral frontal language network and extending posteriorly to include parietal, temporal and occipital lobes. The more widespread activation seen with word verb generation probably reflects the recruiting of neural networks involved in reading [37]. This additional finding that picture verb generation shows greater overlap with fMRI results is promising as we designed this task for use in our pediatric clinical population who may be too young to read, or unable to read due to developmental delays.
The effect of threshold selection is consistent with reports in the literature that choice of thresholds is not straight-forward and needs to be carefully considered [33,38]. We found that at increasingly stringent thresholds, the percent overlap between MEG and fMRI decreased substantially. In general, increasing thresholds tightened the cluster and emphasized the discrepancy between the fMRI data, which was more superficial, and the MEG activations, which started deeper and seemed to move deeper. This finding again underlines the fact that the basic mechanisms underlying the fMRI and MEG responses are different [35].
Our observation, in the MEG data, of specific time windows and frequency bands when the response in the frontal area is maximal is of interest. Maximal frontal activation to picture verb generation was between 600–800 ms in the beta band (15–25 Hz) and earlier (400–600 ms) and in the low gamma band (20–35 Hz) for word verb generation. It is not surprising that different frequency bands desynchronized as there is evidence that the performance of different cognitive tasks is reflected in different bands; however, the latency difference is surprising. Since one presumes the word verb generation is a more difficult task because it involves reading, one presumes that this latency should be later; in fact, these findings suggest that reading, in highly fluent populations, is automated and probably very fast, so the time savings seen with word verb generation is probably a reflection of the reading abilities of this healthy cohort of adolescents.
Within the clinical setting, localization of language areas prior to surgery is an essential aspect of the pre-surgical work-up. We have developed a new expressive language localization test for the MEG, which we hope is more child-friendly. In discussing our new task, we acknowledge a few caveats, namely, the need for MEG users to be cognizant of the effects of task choice, threshold selection, MEG latency window and frequency band selections for data analyses. However, we conclude that by testing the same subjects on the same tasks in both MEG and fMRI, we have demonstrated the validity of our MEG results and the potential utility of MEG as an alternative method for non-invasive language localization.
Acknowledgments
This study was supported by Canadian Institutes of Health Research (CIHR) MOP-81161 to Elizabeth Pang and Elizabeth Donner and CIHR MOP-89961 to Elizabeth Pang.
References
- 1.Geschwind N. The organization of language and the brain. Science. 1970;170(961):940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- 2.Price CJ. The anatomy of language: Contributions from functional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broca P. Perte de la parole, ramolissement chronique et destruction partielle du lobe anteriéur gauche du cerveau. Bulletin de la Société Anthropologique de Paris. 1861;2:235–238. [Google Scholar]
- 4.Wernicke C. Der Aphasiche symptomencomplex. Breslau: Cohn & Weigert; 1874. [Google Scholar]
- 5.Démonet J-F, Thierry G, Cardebat D. Renewal of the neurophysiology of language: functional neuroimaging. Physiological Review. 2005;85:49–95. doi: 10.1152/physrev.00049.2003. [DOI] [PubMed] [Google Scholar]
- 6.Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. Experimental and clinical observations. J Neurosurg. 1960;17:266–282. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Khalil B. An update on determination of language dominance in screening for epilepsy surgery: The Wada Test and newer non-invasive alternatives. Epilepsia. 2007;48:442–455. doi: 10.1111/j.1528-1167.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 8.Byars AW, Holland SK, Strawsburg RH, et al. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol. 2002;17:885–889. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salmelin R. Clinical neurophysiology of language: The MEG approach. Clin Neurophysiol. 2007;118:237–254. doi: 10.1016/j.clinph.2006.07.316. [DOI] [PubMed] [Google Scholar]
- 10.Papanicolaou AC, Pazo-Alvarez P, Castillo EM, et al. Functional neuroimaging with MEG: normative language profiles. NeuroImage. 2006;33:326–342. doi: 10.1016/j.neuroimage.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Breier JI, Simos PG, Zouridakis G, et al. Language dominance determined by magnetic source imaging: a comparison with the Wada test. Neurology. 1999;53:938–945. doi: 10.1212/wnl.53.5.938. [DOI] [PubMed] [Google Scholar]
- 12.Kamada K, Sawamura Y, Takeuchi F, et al. Expressive and receptive language areas determined by a non-invasive reliable method using functional magnetic resonance imaging and magnetoencephalography. Neurosurgery. 2007;60:296–306. doi: 10.1227/01.NEU.0000249262.03451.0E. [DOI] [PubMed] [Google Scholar]
- 13.Simos PG, Breier JI, Maggio WW, et al. Atypical language representation: MEG and intraoperative stimulation mapping correlations. Neuroreport. 1999;10:139–142. doi: 10.1097/00001756-199901180-00026. [DOI] [PubMed] [Google Scholar]
- 14.Billingsley-Marshall RL, Clear T, Mencl WE, et al. A comparison of functional MRI and Magnetoencephalography for receptive language mapping. J Neurosci Meth. 2007;161:306–313. doi: 10.1016/j.jneumeth.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Bowyer SM, Moran JE, Mason KM, et al. MEG localization of language-specific cortex utilizing MR-FOCUSS. Neurology. 2004;62:2247–2255. doi: 10.1212/01.wnl.0000130385.21160.7a. [DOI] [PubMed] [Google Scholar]
- 16.Breier JI, Papanicolaou AC. Spatiotemporal patterns of brain activation during an action naming task using Magnetoencephalography. J Clin Neurophysiol. 2008;25:7–12. doi: 10.1097/WNP.0b013e318163ccd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata M, Kato A, Taniguchi M, et al. Determination of language dominance with synthetic aperture magnetometry: comparison with the Wada test. NeuroImage. 2004;23:46–53. doi: 10.1016/j.neuroimage.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Kober H, Möller M, Nimsky C, Vieth J, Fahlbusch R, Ganslandt O. New approach to localize speech relevant brain areas and hemispheric dominance using spatially filtered Magnetoencephalography. Hum Brain Mapp. 2001;14:236–250. doi: 10.1002/hbm.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald CR, Thesen T, Hagler DJ, Jr, et al. Distributed source modeling of language with Magnetoencephalography: application to patients with intractable epilepsy. Epilepsia. 2009;50:2256–2266. doi: 10.1111/j.1528-1167.2009.02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema J-M, Karunanayaka PR, Shmithorst VJ, Yuan W, Plante E, Byars AW. Functional MRI of language lateralization during development in children. International Journal of Audiology. 2007;46:533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadis DS, Smith ML, Mills T, Pang EW. Expressive language mapping in children using MEG. Down Syn Quarterly. 2008;10:5–12. [Google Scholar]
- 22.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 23.Fawcett IP, Barnes GR, Hillebrand A, Singh KD. The temporal frequency tuning of human visual cortex investigated using synthetic aperture magnetometry. Neuroimage. 2004;21:1542–1553. doi: 10.1016/j.neuroimage.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 24.Cheyne D, Gaetz W, Garnero L, et al. Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Cognitive Brain Res. 2003;17:599–611. doi: 10.1016/s0926-6410(03)00173-3. [DOI] [PubMed] [Google Scholar]
- 25.Herdman AT, Wollbrink A, Chau W, Ishii R, Ross B, Pantev C. Determination of activation areas in the human auditory cortex by means of synthetic aperture magnetometry. NeuroImage. 2003;20:995–1005. doi: 10.1016/S1053-8119(03)00403-8. [DOI] [PubMed] [Google Scholar]
- 26.Robinson SE, Vrba J. Functional neuroimaging by synthetic aperture magnetometry (SAM) In: Yoshimoto T, Kotani M, Kuriki S, Karibe H, Nakasato N, editors. Recent advances in biomagnetism. Sendai: Tohoku; 1999. pp. 302–305. [Google Scholar]
- 27.Singh KD, Barnes GR, Hillebrand A, Forde EME, Williams AL. Task-related changes in cortical synchronization are spatially coincident with the hemodynamic response. NeuroImage. 2002;16:103–114. doi: 10.1006/nimg.2001.1050. [DOI] [PubMed] [Google Scholar]
- 28.Talairach J, Tournoux P. Co-Planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 29.Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein E-B, Hennngsen H. Handedness and hemispheric language dominance in healthy adults. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- 30.Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- 31.Holland SK, Plante E, Byars AW, Strawsburg RH, Schmithorst VJ, Ball WS. Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- 32.Szarflarski JP, Schmithorst VJ, Altaye M, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liljeström M, Hultén A, Parkkonen L, Salmelin R. Comparing MEG and fMRI views to naming actions and objects. Hum Brain Mapp. 2009;30:1845–1856. doi: 10.1002/hbm.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theoryof reading: a metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- 35.Shibasaki H. Human brain mapping: Hemodynamic response and electrophysiology. Clin Neurophysiol. 2008;119:731–743. doi: 10.1016/j.clinph.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 36.Winterer G, Carver FW, Musso F, Mattay V, Weinberger DR, Coppola R. Complex relationship between BOLD signal and synchronization/desynchronization of human brain MEG oscillations. Hum Brain Mapp. 2007;28:805–816. doi: 10.1002/hbm.20322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaillard WD, Pugliese M, Grandin CB, et al. Cortical localization of reading in normal children: An fMRI language study. Neurology. 2001;57:47–54. doi: 10.1212/wnl.57.1.47. [DOI] [PubMed] [Google Scholar]
- 38.Suarez RO, Whalen S, Nelson AP, et al. Threshold-independent functional MRI determination of language dominance: a validation study against clinical gold standards. Epilepsy Behav. 2009;16:288–297. doi: 10.1016/j.yebeh.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]