Abstract
Objectives. We examined whether drinking untreated rainwater, a practice that is on the rise in developed countries because of water shortages, contributes to community gastroenteritis incidence.
Methods. We conducted a double-blinded, randomized controlled trial in Adelaide, Australia. Sham or active water treatment units were installed, and participants recorded incidences of illness in a health diary for 12 months. The primary outcome was highly credible gastroenteritis (HCG; characterized by a specified number of loose stools or vomiting alone or in combination with abdominal pain or nausea in a 24-hour period), and we used generalized estimating equations to account for correlations between numbers of HCG events for individuals in the same family.
Results. Participants reported 769 episodes during the study (0.77 episodes/person/year), with an HCG incidence rate ratio (active vs sham) of 1.05 (95% confidence interval [CI] = 0.82, 1.33). Blinding of the participants was effective (index = 0.65; 95% CI = 0.58, 0.72).
Conclusions. Our results suggest that consumption of untreated rainwater does not contribute appreciably to community gastroenteritis. However, our findings may not be generalizable to susceptible and immunocompromised persons because these groups were specifically excluded from the study.
Climate change and population growth have resulted in diminished potable water supplies globally.1 In developed countries such as Australia, parts of Europe, and the United States, water restrictions and the promotion of alternative water sources, including rainwater, have reduced demands on water mains. However, several studies have shown significant variability in the microbial quality of stored rainwater: 75% of tanks were found to contain fecal and total coliforms.2–6 Unlike conventional tap water, household rainwater supplies are usually untreated. Thus, the susceptibility of collected rainwater to contamination makes it a potential source of pathogenic microorganisms.
In Australia, household rainwater tank usage increased 40% between 2004 and 2007.7,8 Harvesting rainwater for drinking remains the choice of the consumer, but health authorities generally do not endorse consumption of untreated rainwater if a potable water system exists.9,10 Statistics nevertheless show that 2.5% of persons living in urban Australia drink rainwater; Adelaide, in South Australia, has the highest proportion at 10.6%.8
Increasing rainwater consumption, with its potential associated health risks from deliberate or inadvertent contamination, has created a need for research on rainwater quality and the health effects of rainwater usage. Some previous epidemiological studies have shown an association between rainwater consumption and illness,11 although 1 South Australian study showed no significant difference in highly credible gastroenteritis (HCG) among consumers of rainwater and consumers of mains water,12 and another suggested that rainwater consumption was protective.13 HCG is characterized by a specified number of loose stools or vomiting alone or in combination with abdominal pain or nausea in a 24-hour period.
Several randomized trials have been conducted to evaluate the effect of mains water on the incidence of gastroenteritis,14–16 but no trials have been conducted on the effect of rainwater consumption. We therefore conducted a double-blinded, randomized, controlled trial to determine whether consumption of untreated rainwater contributes to community gastroenteritis incidence.
METHODS
We conducted a double-blinded, randomized, controlled trial in South Australia between June 2007 and August 2008. We recruited 300 households that used untreated rainwater as their usual drinking water source.17 Participating households received either an active (intervention) or a sham (control) water treatment unit for filtering of all water intended for drinking or cooking.
At enrollment, written informed consent was obtained from all adult household members and from parents or guardians on behalf of children. One adult member of each household was designated as the reporting participant and was responsible for ensuring completion of a weekly health diary for each participant. This involved recording symptoms of diarrhea, vomiting, nausea, abdominal pains, and fever.
Variables and Study Area
We collected water consumption data on 3 occasions. Participants were asked to indicate the number of glasses (250 mL) of water consumed (combinations of hot or cold, filtered or unfiltered rainwater or other water source) for 1 weekday and 1 weekend day. At the end of data collection, participants were asked to indicate the filter type (active or sham) they thought had been installed during the study period to assess blinding.
The primary endpoint of the study was HCG, defined as any of the following in a 24-hour period: 2 or more loose stools; 2 or more episodes of vomiting; 1 loose stool together with abdominal pain, nausea, or vomiting; or 1 episode of vomiting with abdominal pain or nausea.15 Cases of HCG were considered unique if the participant was symptom free for at least 6 days before new symptoms began. We also used a different definition of diarrheal illness (HCG-D), characterized by 3 or more loose stools in a 24-hour period regardless of other symptoms18; these episodes were also classified as unique if they followed 6 or more symptom-free days.
We consulted with the Department of Health, South Australia, to identify regions most likely to use rainwater for drinking.19 The study area comprised metropolitan Adelaide (including the Adelaide Hills) and Mount Barker. Metropolitan Adelaide, the capital city of South Australia, is a major urban area with a population of approximately 1.1 million, and Mount Barker, with a population of more than 11 500, is a fast-growing urban area located 35 kilometers southwest of the city.20
We collected data from 3 equal groups of participants (with both active and sham subgroups), with start dates 4 weeks apart. Data were collected for 52 weeks, with a 5-week suspension from December 17 to January 21 for the Christmas holiday period.
Water Treatment Units
Water treatment units were obtained from Freshwater Systems (Adelaide). The active units consisted of a water intake hose, an absolute 1-μm prefilter, a silver-impregnated ceramic filter cartridge (Sterasyl Doulton, Southfield, MI) to remove microorganisms, a pump to move water through the unit, and a delivery spout. The filter was rated to remove more than 99.9% of Escherichia coli, Salmonella typhi, Vibrio cholerae, Cryptosporidium, and Giardia cysts but was not capable of removing viruses.21 The filters and pump were enclosed in an external housing (335 mm [length] × 242 mm [width] × 330 mm [height]) with tamperproof seals.
The sham water treatment units were identical in appearance and power usage but contained no filtration cartridges. Initially, all households received bench-top sham water treatment units, which required participants to use a fill–filter–fill method in which they filled a bucket with rainwater and pumped the water through the unit into a 5-liter storage container. During the study, households with the rainwater tank piped into the kitchen were instead offered an under-sink unit consisting of the same basic unit without the external housing, to make filter use less cumbersome and therefore reduce loss to follow-up. Participants were advised to avoid filtering visibly dirty water.
Participants were advised to contact the study coordinator if problems arose with the unit. A routine service visit for all households was scheduled 4 to 5 months after the study began for replacement of the original filter with another of the same type (active or sham).
Participants
Households were eligible for participation if they were in the study area; used untreated rainwater from an aboveground tank as their normal drinking water source; had at least 4 eligible members, including at least 2 children living at home who were aged 1 to 15 years (as of March 2007); owned their home or had rented for 12 months or more, with no intention of moving in the next 12 months; and had household members with a reasonable command of English (to comprehensively understand the requirements of the study).
Households were excluded if tanks other than aboveground tanks were used; rainwater was always boiled before consumption or was routinely disinfected with chlorine, filtration devices, or other treatment methods capable of removing microorganisms; or the rainwater tank was routinely topped up with mains or carted water supplies. Individuals within households were excluded if the individuals were immunocompromised, had chronic diarrheal illness, or were on long-term antibiotic therapy. Persons who were pregnant or elderly were not specifically excluded.
A random number sequence generated by an independent researcher assigned households to receive the active or sham water treatment unit. Participants and researchers were blinded to the unit assigned; coded labels were used for communication between participants, researchers, and plumbers.
The study was designed to detect a 25% reduction in the overall rate of HCG episodes among the active group with 80% power at a 2-sided 5% significance level. We considered this reduction in the rate of gastrointestinal disease clinically relevant. We used the values of parameters estimated in our previous metropolitan study of water filters in Melbourne, Australia, to make the following assumptions: an average of 2 adults and 2.5 children per family; a dropout rate of 10%; a 12-month event rate for adults of 0.70 events per year and for children of 1.17 events per year; within-family correlations over a 12-month period for adult–adult, adult–child, and child–child of approximately 0.30; and a marginal Poisson overdispersion factor of 1.8.15 We calculated the required sample size to be 300 households (150 per group).
Completed health diaries were mailed to the study coordinator every 4 weeks. Diaries were scanned, and accuracy of data and completeness were verified with Teleform software, version 10.1 (Cardiff Software, Vista, CA), before being entered into a Microsoft Access database (Microsoft Corp, Bellingham, WA). Reporting participants were telephoned for clarification if information was missing or ambiguous.
Statistical Analysis
Data were extracted from the database into SPSS, version 15.0.1 (SPSS Inc, Chicago, IL); we queried to determine the number of valid HCG and diarrheal events. We compared HCG event rates between the active filter and sham filter groups according to the number of HCG events for each individual over the observation period. We used generalized estimating equations, assuming an overdispersed Poisson model for individuals with an exchangeable correlation structure within families and with individual observation time as an offset, to account for the correlation between the numbers of HCG events of individuals within the same family. In our principal analysis, active versus sham water treatment was the only covariate; we adjusted further analyses for age, gender, location, and starting time.
Analyses of duration of HCG episodes and water consumption comparing the active and sham filters used median regression; we computed confidence intervals with an application of the bootstrap method that used the family as the resampling unit and therefore respected the family clustering present in the study design. We assessed the success of blinding with the blinding index quantified by James et al.22 We obtained a smoothed plot of HCG rates with a weighted running mean smoother in which each point represented the weighted average of the 8 weeks of HCG rates surrounding it. We used Stata, version 10 (StataCorp LP, College Station, TX), and SPSS, version 16, for all analyses.
RESULTS
We initially screened 810 callers; 433 met the eligibility criteria and received further information. Of these households, 332 were willing to participate, 325 were enrolled, and 300 were randomized and assigned water treatment units. Full details about our recruitment and enrollment of participants are available elsewhere.17
Participants
The 300 households had 1352 residents, and the active and sham groups had similar demographics at baseline (Table 1). The mean number of participants per household was 4.59 in the active group and 4.42 in the sham group. The mean number of children (aged < 16 years) was 2.29 in the active group and 2.25 in the sham group.
TABLE 1.
Baseline Characteristics of Participating Households Consuming Untreated Rainwater: Adelaide and Mount Barker, Australia, 2007
| Households in Active Groupa (n = 152), No. Persons (%) | Households in Sham Groupb (n = 148), No. Persons (%) | |
| Participants | 698 (51.6) | 654 (48.4) |
| Male | 345 (49.4) | 341 (52.1) |
| Age, y | ||
| < 5 | 95 (13.6) | 90 (13.8) |
| 6–15 | 270 (38.7) | 258 (39.4) |
| 16–24 | 34 (4.9) | 15 (2.3) |
| 25–44 | 198 (28.4) | 197 (30.1) |
| 45–64 | 97 (13.9) | 93 (14.2) |
| ≥ 65 | 4 (0.6) | 1 (0.2) |
| Attendance at child care (children aged ≤ 5 y) | 44 (6.3) | 56 (8.6) |
| Educational attainment among adults | ||
| Primary school | 3 (1.0) | 4 (1.4) |
| Secondary school | 99 (31.7) | 97 (32.8) |
| Trade school | 38 (12.1) | 36 (12.2) |
| Tertiary school | 150 (47.9) | 138 (46.6) |
| Employed adultsc | 243 (77.6) | 251 (84.8) |
| Location | ||
| Metropolitan Adelaide | 117 (77.0) | 109 (73.6) |
| Mount Barker | 35 (23.0) | 39 (26.4) |
Note. Percentages may not total to 100% because of missing data or rounding.
The active group received water treatment units containing filters for their rainwater.
The sham group received water treatment units that were identical to the active units, except that they did not contain filters. Investigators and participants were blinded to the identity of the active and sham households.
Information on work status missing for 44 adults: 23 from the active group and 21 from the sham group.
During the first 3 months of the study, 21 households (12 from the active group and 9 from the sham group) withdrew prior to providing any data and were excluded from further analyses. For the entire data collection period, 93 (31%) households withdrew from the study, 45 (31%) from the active group and 48 (32%) from the sham group (P = .6). Reasons for withdrawal were failure to return 3 consecutive diaries (60.2%), inconvenience of using the filter (12.9%), relocation from the study area (7.6%), empty tank or switch to mains water (7.5%), house renovation or tank removal (5.4%), and change of mind (2.2%); 4.3% gave no reason for withdrawing. To reduce inconvenience, households with a piped-in rainwater supply were offered an under-sink unit partway through the study; 25.9% (50 households) of those eligible accepted this offer, with equal numbers in the active and sham groups.
Gastrointestinal Illness and Water Consumption
We collected 51 857 person-weeks of data (73.8%) out of a potential 70 304 person-weeks. We observed 769 HCG events, 411 in the active group and 358 in the sham group.
The overall HCG rate was 0.77 episodes per person per year (0.78 for the active group and 0.76 for the sham group, respectively). HCG episodes were experienced by 41.7% (266) of participants in the active group (multiple episodes in 88 participants) and 38.6% (235) of participants in the sham group (multiple episodes in 78 participants; Table 2).
TABLE 2.
Incidence of Highly Credible Gastroenteritis (HCG) in Active and Sham Water Treatment Groups: Adelaide and Mount Barker, Australia, 2007–2008
| All participants |
Children Aged ≤ 5 Years |
|||
| No. of HCG Events | Active Group (n = 638), No. Persons (%) | Sham Group (n = 609), No. Persons (%) | Active Group (n = 81), No. Persons (%) | Sham Group (n = 90), No. Persons (%) |
| 0 | 372 (58.3) | 374 (61.4) | 25 (30.9) | 31 (34.4) |
| 1–2 | 232 (36.4) | 206 (33.8) | 46 (56.8) | 48 (53.3) |
| 3–4 | 27 (4.2) | 26 (4.3) | 8 (9.9) | 8 (8.9) |
| 5–7 | 7 (1.1) | 3 (0.5) | 2 (2.5) | 3 (3.3) |
Note. HCG was defined as any of the following in a 24-hour period: 2 or more loose stools; 2 or more episodes of vomiting; 1 loose stool together with abdominal pain, nausea, or vomiting; 1 episode of vomiting with abdominal pain or nausea. The active group received water treatment units containing filters for their rainwater. The sham group received water treatment units that were identical to the active units, except that they did not contain filters. Investigators and participants were blinded to the identity of the active and sham households.
The ratio of HCG event rates in the active group versus the sham group was 1.05 (95% confidence interval [CI] = 0.82, 1.33; P = .72), with an intrafamily correlation of 0.26 and overdispersion factor of 1.57. After adjustment for age, gender, location, and starting group, the rate ratio was negligibly different at 1.03 (95% CI = 0.81, 1.30; P = .83). We calculated the HCG rate ratios for children aged 5 years and younger as 0.90 (95% CI = 0.66, 1.23; P = .52) and persons older than 5 years as 1.08 (95% CI = 0.84, 1.40; P = .56), but these were not significantly different (P = .47).
The overall median duration of HCG episodes among those with at least 1 episode was 2 days (interquartile range [IQR] = 1–3) for both groups. The range of episode duration was 1 to 71 days for the active group and 1 to 52 days for the sham group; the difference in median duration was statistically nonsignificant (difference = 0; 95% CI = −0.58, 0.58; P ≥ .99 ).
We observed 265 episodes of HCG-D (3 or more loose stools in a 24-hour period) among all participants. Four participants in the active group and 7 in the sham group experienced multiple episodes (Table 3). The overall event rate was 0.27 cases of diarrhea events per person per year (0.24 in the active and 0.29 in the sham group). The ratio of HCG-D event rates for the active group versus the sham group was 0.85 (95% CI = 0.57, 1.27; P = .44). For children aged 5 years and younger, the HCG-D rate ratio was 0.89 (95% CI = 0.42, 1.90; P = .77); for persons aged older than 5 years, the HCG-D rate ratio was 0.84 (95% CI = 0.59, 1.20; P = .77). This difference was not statistically significant (P = .88).
TABLE 3.
Incidence of Highly Credible Gastroenteritis Defined by Diarrheal Events During Water Treatment Intervention: Adelaide and Mount Barker, Australia, 2007–2008
| All Participants |
Children Aged ≤ 5 Years |
|||
| No. of Diarrheal Events | Active Group (n = 638), No. Persons (%) | Sham Group (n = 609), No. Persons (%) | Active Group (n = 81), No. Persons (%) | Sham Group (n = 90), No. Persons (%) |
| 0 | 533 (83.5) | 502 (82.4) | 62 (76.5) | 68 (75.6) |
| 1–2 | 101 (15.8) | 100 (16.4) | 16 (19.8) | 20 (22.2) |
| 3–5 | 4 (0.63) | 7 (1.1) | 3 (3.7) | 2 (2.2) |
Note. Highly credible gastroenteritis defined by diarrheal events was characterized by 3 or more loose stools in a 24-hour period, regardless of other symptoms. The active group received water treatment units containing filters for their rainwater. The sham group received water treatment units that were identical to the active units, except that they did not contain filters. Investigators and participants were blinded to the identity of the active and sham households.
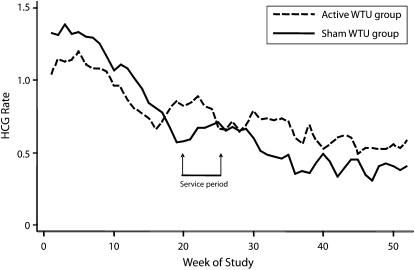
Overall, there was a decreasing rate of reported HCG events over time (Figure 1); however, the observed decline was not constant between the groups (for differing linear trends, P = .024). The relative risk of HCG among active versus sham water treatment users was 0.85 (95% CI = 0.64, 1.12) for the first quarter and increased to 1.41 (95% CI = 0.82, 2.42) for the last quarter. We observed a similar pattern for HCG-D (for linear trend in relative risks, P = .09). All water treatment units were serviced during the second quarter, and data were thus analyzed by comparing HCG events before and during servicing with events after servicing; 441 episodes were reported before and during servicing, and 338 episodes were reported after servicing. The ratio of HCG event rates before and during servicing to event rates after servicing was 0.83 (95% CI = 0.64, 1.08; P = .17; 0.96 in the active group and 1.11 in the sham group) and 1.29 (95% CI = 0.98, 1.70; P = .07; 0.66 in the active group and 0.49 in the sham group), respectively, and these ratios were significantly different from each other (P = .004).
FIGURE 1.
Rate of highly credible gastroenteritis (HCG; cases/person/year; 8-week moving average) during the study period (2007–2008) among participants with active and sham water treatment units (WTUs): Adelaide and Mount Barker, Australia, 2007.
Note. Data collection was suspended for 5 weeks during the Christmas holiday period (December 17—January 20). The Christmas holiday period occurred at week 20, 24, or 28 of the study, depending on the start dates of the 3 batches of participants.
Water consumption data were obtained from 894 individuals in 201 households; participants in the active group drank a daily average of 5.42 glasses of water (median = 4.98; IQR = 3.7–6.9) and in the sham group drank an average of 5.65 glasses (median = 5.92; IQR 3.5–7.3); the difference was not statistically significant (P = .31). The active group drank an average of 2.90 glasses of cold filtered rainwater per day (median = 2.6; IQR = 1.5–3.9), and the sham group drank 3.30 glasses per day (median = 3.0; IQR = 1.8–4.4); the difference was not statistically significant (P = .14).
Effectiveness of Blinding
Of the 207 households that completed the study, 173 (83.6%) returned the blinding questionnaire. The blinding index was 0.65 (95% CI = 0.58, 0.72) with a large proportion (111) of reporting participants either answering “don't know” or giving an incorrect answer. In the sham group, 76.5% of respondents were unaware of the unit type; 53.3% of respondents from the active group were unaware of the unit type. The difference in unawareness rate reflects evidence of blinding that could not be explained by guessing about filter assignment.
Decreased flow through the unit was reported by 24% (73) of households; 86% (63) of these households were participants who had active water treatment units. However, 53 of these households responded to the blinding questionnaire, and only 28 (52.8%) correctly identified their unit type. In the sham group, 7 of 10 households that reported flow problems returned the questionnaire, and only 2 of these correctly identified their filter type.
DISCUSSION
Ours was the first randomized trial to assess the effect of rainwater consumption on health. Our results showed that the rates of HCG and HCG-D were not significantly different between the active and sham water treatment households.
Of the epidemiological studies conducted on rainwater to date, 2 have compared rates of gastroenteritis between populations of children (presumably a more susceptible population), and these unblinded studies showed no increased risk of illness associated with rainwater consumption. Our blinded study supports this conclusion and further indicates that adults have no increased risk of illness. Our results therefore suggest that consumption of untreated rainwater does not significantly contribute to community gastroenteritis incidence. This has important implications: at a minimum, inadvertent or accidental ingestion of small quantities of rainwater during showering and other water usage activities is highly unlikely to cause adverse health effects. Therefore, our results may be of significant interest to health authorities and may inform advice regarding safe uses of rainwater and potential conservation of conventional water sources.
The overall rate of gastrointestinal events reported decreased as the study progressed. Although data collection was staggered, this decrease was observed in all groups and suggests that as the novelty of the study wore off, recording of illness decreased. Several studies have reported similar decreases and have attributed this to fatigue in data recording.14–16
During the first 13 weeks of the study, the use of the active water treatment units appeared to provide a protective effect that was not observed in subsequent periods. Because our blinding was successful, we have no explanation for this observation. If the protective effect was attributable to a temporary improvement in water quality from active water treatment units, we would have expected a similar effect after the service visit, when units were cleaned and new cartridges were installed, but this did not occur. We checked unit code numbers and confirmed that the correct replacement scheme was conducted (i.e., active cartridge replaced with active, sham cartridge with sham). It is possible that there was a preferential drop-off in reporting of gastrointestinal events in the sham filter arm, although there is no obvious reason why this would have occurred.
We conducted limited water quality testing on rainwater tank samples. The prevalence of E. coli in 974 samples tested was 30.1%; levels ranged from 0 to 2400 cfu per 100 mL (data not shown). The protective effect of acquired immunity is presumed to vary for different pathogens but is likely to range from months to years,23 and although we could not relate water quality to health outcomes, it is possible that acquired immunity develops among regular rainwater drinkers from repeated exposure to residual microbial contaminants. Partial immunity may necessitate exposure to larger doses of contaminating microorganisms for infection to occur or symptoms to develop.23 Young children presumably have less acquired immunity, so more illness may be expected in this group; indeed, this was observed: children aged 2 years and younger experienced twice as many episodes (HCG rate ratio = 2.1; P < .001) as did persons older than 2 years (analysis pooled over filter groups; data not shown). However, because of the small numbers of infants in our sample (n = 28), these results should be interpreted with caution.
Blinding was successful: 64.2% of participants were unaware of the filter type installed. This result is comparable to that obtained by Colford et al. in a conventional drinking water supply study of similar design.16 In our study, only 39 (35.8%) households correctly identified the type of unit installed. Surprisingly, blinding was more effective among participants with a sham device, despite the fact that they might have been expected to deduce the type of unit supplied if solid particles passed through. If households assigned an active water treatment unit experienced clogging of the filter unit, this might also have caused unblinding. The overall success of blinding reduced chances of reporting bias.
Limitations
Our findings may not be generalizable to households who have recently begun using rainwater as a drinking water supply. Shorter-term users may not have acquired the partial immunity that long-term exposure is hypothesized to confer. In addition, we focused on gastroenteritis from microbial contamination and did not assess potential health effects from chemical exposures.
We found no statistically significant difference in the amount of total rainwater or cold rainwater consumed by the active and sham groups. However, the data should be interpreted with caution because consumption was estimated at only 3 time points, not continuously. In addition, self-reported data may over- or underestimate volumes consumed. Nevertheless, most water consumed was rainwater, suggesting good compliance with use of the unit.
Untreated rainwater containing fecal matter from birds may pose a special risk of Campylobacter and Salmonella infections. For healthy adults, exposure to such microbial pathogens may result in subclinical or short-term, self-limiting illness, but among the susceptible and immunocompromised, this could represent a more serious risk of morbidity and mortality. Thus, although our findings may be extrapolated to healthy adults, they may not be generalizable to susceptible or immunocompromised persons, young children, and the elderly.
The moderately high dropout rate also affects interpretation of results and may have led to an underestimation of the true incidence of gastroenteritis among this cohort. The dropout rate of 31% was greater than the 10% rate we expected,15 but 279 of the 300 households provided at least some data, enabling collection of 74% of the total possible information. However, the dropout rate was similar between the groups (30% of the active group, 32% of the sham group) so this would not have affected overall study findings, and the study retained 80% power to detect a rate reduction of 28% (by contrast with an anticipated 25% difference).
Contributing to the high dropout rate was the fill–filter–fill requirement of the water treatment unit protocol, which was particularly inconvenient for households with a piped-in rainwater supply. Consequently, these households were offered an under-sink unit partway through the study. It is also possible that a healthy participant bias existed: those in both groups who were generally healthy and enthusiastic were more likely to remain in the study. Because these are characteristics of individuals, however, such a bias likely did not differ across treatment arms.
Installation of rainwater tanks is an expensive undertaking, and government rebates in Australia have led to an increase in tank uptake. Although the costs of installation might skew recruitment toward households with higher socioeconomic status, the contribution of other factors, such as the poor aesthetic quality of mains water in the past, has meant that a wide range of households in South Australia use rainwater. Our results are therefore generalizable to urban areas of the United States, Germany, and the United Kingdom, all countries where rainwater use has also been increasing. However, bias resulting from voluntary recruitment of participants may have led to inclusion of persons particularly concerned about the quality of their water supply or the health risks of drinking untreated rainwater, thereby affecting the external generalizability of the study results.
Conclusions
Our study provides valuable insights into gastrointestinal morbidity among people primarily drinking rainwater. Although the possibility of some health effects below the levels of detection we were able to achieve cannot be ruled out, our results suggest that consumption of untreated rainwater does not appreciably contribute to community gastroenteritis incidence.
The success of blinding in our study shows that interventions can be conducted to provide valuable insights into the risks of waterborne diseases at the community level. Future work should be considered in households with new tank installations and to correlate rainwater quality with health outcomes.
Acknowledgments
This work was supported by the National Health Medical Research Council (grant 384226) and the Cooperative Research Centre for Water Quality and Treatment.
We are grateful to Kathryn Drew, Michelle Cruse, and Freya Goodhew, who conducted the recruitment and enrollment of participants into the study, to all householders who participated in the study, and to Julia Bell, who worked tirelessly to scan the health diaries.
Human Participant Protection
This study received approval from the Monash University standing committee on ethics in research involving humans and from the South Australia Department of Health human research ethics committee.
References
- 1.Intergovernmental Panel on Climate Change Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2007 [Google Scholar]
- 2.Chapman H, Cartwright T, Huston R, O'Toole J. Water Quality and Health Risks From Urban Rainwater Tanks. Adelaide, Australia: Cooperative Research Centre for Water Quality and Treatment; 2008. Report 42 [Google Scholar]
- 3.Coombes PJ, Kuczera G, Kalma JD. Rainwater quality from roofs, tanks and hot water systems at Figtree Place. : 3rd International Hydrology and Water Resource Symposium. Perth, Australia: Water and Rivers Commission; 2000:1042–1047 [Google Scholar]
- 4.Simmons G, Hope V, Lewis G, Whitmore J, Gao W. Contamination of potable roof-collected rainwater in Auckland, New Zealand. Water Res. 2001;35(6):1518–1524 [DOI] [PubMed] [Google Scholar]
- 5.Thomas PR, Greene GR. Rainwater quality from different roof catchments. Water Sci Technol. 1993;28(3–5):291–299 [Google Scholar]
- 6.Yaziz MI, Gunting H, Sapari N, Ghazali AW. Variations in rainwater quality from roof catchments. Water Res. 1989;23(6):761–765 [Google Scholar]
- 7.Environmental Issues. People's Views and Practices. Canberra: Australian Bureau of Statistics; 2004:1–89 [Google Scholar]
- 8.Environmental Issues. People's Views and Practices. Canberra: Australian Bureau of Statistics; 2007:1–88 [Google Scholar]
- 9.Department of Sustainability and Environment, Department of Human Services. Rainwater use in and around the home. Available at: http://www.pic.vic.gov.au/resources/documents/DSE0603.pdf. Accessed June 20, 2009
- 10.New South Wales Department of Health Rainwater tanks brochure. Available at: http://www.health.nsw.gov.au/pubs/2007/rainwater_tanks.html. Accessed 20 June, 2009
- 11.Sinclair MI, Leder K, Chapman H. Public Health Aspects of Rainwater Tanks in Urban Australia. Adelaide, Australia: Cooperative Research Centre for Water Quality and Treatment; 2005 [Google Scholar]
- 12.Heyworth JS, Glonek G, Maynard E. The prevalence of gastroenteritis amongst young children and the potential role of drinking water. In: Proceedings of 18th Federal Convention of the Australian Water and Wastewater Association. Artarmon, Australia: Australian Water and Wastewater Association; 1999 [Google Scholar]
- 13.Heyworth J, McCaul K. Prevalence of non-specific health symptoms in South Australia. Int J Environ Health Res. 2001;11(4):291–298 [DOI] [PubMed] [Google Scholar]
- 14.Payment P, Siemiatycki J, Richardson L, Renaud G, Franco E, Prevost M. A prospective epidemiological study of gastrointestinal health effects due to the consumption of drinking water. Int J Environ Health Res. 1997;7(1):5–31 [Google Scholar]
- 15.Hellard ME, Sinclair MI, Forbes AB, Fairley CK. A randomized, blinded, controlled trial investigating the gastrointestinal health effects of drinking water quality. Environ Health Perspect. 2001;109(8):773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colford JM, Jr, Wade TJ, Sandhu SK, et al. A randomized, controlled trial of in-home drinking water intervention to reduce gastrointestinal illness. Am J Epidemiol. 2005;161(5):472–482 [DOI] [PubMed] [Google Scholar]
- 17.Rodrigo S, Sinclair M, Cunliffe D, Leder K. Effectiveness and cost of recruitment strategies for a community-based randomised controlled trial among rainwater drinkers. BMC Med Res Methodol. 2009;9:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall GV, Kirk MD, Ashbolt R, Stafford R, Lalor K. Frequency of infectious gastrointestinal illness in Australia, 2002: regional, seasonal and demographic variation. Epidemiol Infect. 2006;134(1):111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heyworth J, Maynard E, Cunliffe D. Who drinks what? Potable water use in South Australia. Water. 1998;25(1):9–13 [Google Scholar]
- 20.Adelaide—A Social Atlas: 2006 Census of Population and Housing. Canberra: Australian Bureau of Statistics; 2006 [Google Scholar]
- 21.Doulton USA. Sterasyl ceramic filter cartridges. Available at: http://doultonusa.com/sterasyl_specs.pdf. Accessed August 20, 2009
- 22.James KE, Bloch DA, Lee KK, Kraemer HC, Fuller RK. An index for assessing blindness in a multi-centre clinical trial: disulfiram for alcohol cessation—a VA cooperative study. Stat Med. 1996;15(13):1421–1434 [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg JN, Bartram J, Hunter PR. A public health perspective for establishing water-related guidelines and standards. : Fewtrell L, Bartram J, Water Quality: Guidelines, Standards and Health: Assessment of Risk and Risk Management for Water-Related Infectious Disease. London, UK: IWA Publishing; 2001:229–256 [Google Scholar]