Abstract
Objectives. We investigated environmental lead pollution and its impact on children's blood lead levels (BLLs) in a rural area of China.
Methods. In 2007, we studied 379 children younger than 15 years living in 7 villages near lead mines and processing plants, along with a control group of 61 children from another village. We determined their BLLs and collected environmental samples, personal data, and information on other potential exposures. We followed approximately 86% of the children who had high BLLs (> 15 μg/dL) for 1 year. We determined factors influencing BLLs by multivariate linear regression.
Results. Lead concentrations in soil and household dust were much higher in polluted villages than in the control village, and more children in the polluted area than in the control village had elevated BLLs (87%, 16.4 μg/dL vs 20%, 7.1 μg/dL). Increased BLL was independently associated with environmental lead levels. We found a significant reduction of 5 micrograms per deciliter when we retested children after 1 year.
Conclusions. Our data show that the lead industry caused serious environmental pollution that led to high BLLs in children living nearby.
High concentration of lead in children's blood is among the leading public health issues worldwide.1 Elevated blood lead levels (BLLs) not only cause central nervous system disorders, but also affect the kidneys, blood, and cardiovascular system as children grow and develop.2 Some developed countries, such as the United States, have made great efforts to reduce lead exposure and eliminate elevated BLLs among children by 2010 (information on whether this goal was met was not yet available at the time of writing).3 However, health problems stemming from environmental lead pollution remain a serious public health issue in many developing countries, including China.4–6
China experienced dramatic industrial and economic growth over the past decades, but at a high cost to the environment and public health.7 Among the major environmental problems is lead pollution, which began in large industrialized cities8,9 but has spread into the vast rural areas, as a consequence of booming small- and medium-scale township–village enterprises. One survey in 19 cities showed that median BLL in children aged 3 to 5 years ranged from 3.5 to 13.4 micrograms per deciliter.6 Nationally, nearly 30% of urban children aged 3 to 5 years had a BLL higher than 10 micrograms per deciliter.6 Comparable data from rural areas are limited.10,11 Policymakers have paid little attention to this issue, and a routine national BLL survey system has not yet been established.
Fujian Province, located in southeast China, is a relatively developed coastal area with abundant mineral resources, including lead. More than 100 lead-related enterprises (mines and processing plants) were established during the past 2 decades, most of which operated in poorly regulated rural areas. The activities associated with mining, separating, smelting, and transportation resulted in serious environmental pollution and posed a health threat to local residents, particularly children. News media increasingly called attention to high BLLs in local children. However, no tangible effort was made to evaluate the extent of environmental lead pollution and health outcomes in the general population.
We conducted a study in a rural area of Fujian Province, where several town-owned lead-related enterprises operated between 1996 and 2007. The investigation was triggered by a news report about children living near lead industries who were found to have high BLLs after their parents demanded that they be tested. The report aroused concern among local residents, who successfully advocated for testing all children younger than 15 years living in the vicinity. Great public pressure led local authorities to require all lead enterprises to close by September 2007. We undertook an exposure assessment of environmental lead pollution during October and November of the same year. We aimed to elucidate the relationship between environmental lead pollution and children's BLLs.
METHODS
We studied a polluted region, estimated to contain more than 20 million tons of natural lead and zinc ore deposits, in a rural mountainous area in the center of Fujian Province. Three lead mines and 2 lead-smelting processing plants had been operating since 1996, producing up to 675 000 tons of raw lead and 55 000 tons of lead products annually.
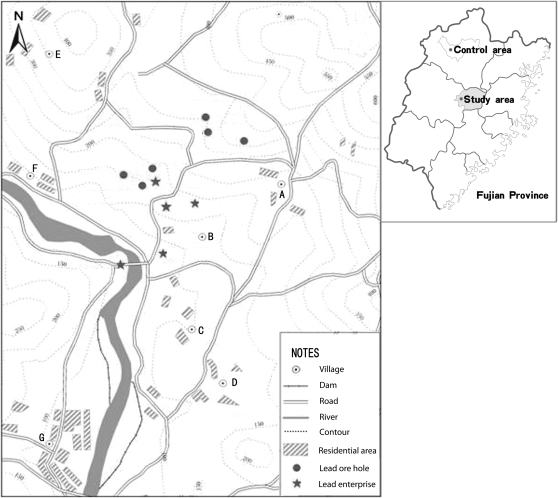
This area covered approximately 18 square kilometers, with 2450 people residing in 7 villages. Nearly half of the local families had at least 1 member working in a plant or mine. Several partially paved roads, approximately 2 to 3 meters wide, ran through this area and were mainly used to transport lead products. In addition, several explored ore holes dotted the area (Figure 1).
FIGURE 1.
Locations in Fujian Province, China, of (A–G) 7 villages, 5 lead-related enterprises, several lead ore holes, and paved roads that were used mainly for transportation of lead products.
Source. Department of Environmental Protection in Fujian Province. Investigation report of children's elevated blood lead level in Youxi County in Fujian [internal report], January 2008.
Study Population and Blood Testing
According to official registry data, 401 children aged 2 months to 14 years were living in the 7 villages. We conducted interviews to collect relevant information and measured BLLs in all the children, except for 12 of them whose parents refused consent. We excluded 10 children because of incomplete information, leaving 379 children in our final analytical sample. We also selected 61 children aged younger than 15 years who lived in a village located 150 kilometers away in a mountainous area without lead-related industry. After 1 year, we retested the BLLs of 175 (85.8%) of the 209 children from the polluted area whose initial results were greater than 15 micrograms per deciliter.
Venous blood samples were drawn into metal-free heparin tubes from cleaned and sanitized skin at the children's elbow area by trained nurses at a local hospital. BLLs were determined by graphite furnace atomic absorption spectrometry in a certified laboratory. Blood samples were stored at −20°C or lower until analyzed. We applied both internal and external laboratory quality controls to ensure that the measurements were accurate. We monitored BLLs with quality control materials provided by the Chinese National Centers for Disease Control and Prevention. BLLs were expressed as micrograms per deciliter. We followed the same procedures in retesting the children's BLLs after 1 year.
Environmental Lead Pollution Assessment
Lead in soil.
We collected soil samples in each village with systematic grid sampling.12 Before sampling, we divided each village into square grids according to surface area and took a sample from the center of each grid. If a house stood in the center of the grid, we took at least 3 soil samples from the sides and back of the house, as well as from the children's play yards that were present in front of most houses. Then we combined these samples as composite example of the grid. Altogether, we collected 43 soil specimens in polluted villages and 22 in the control village. We used new plastic spoons to extract these specimens from the surface to 2.5 centimeters at each site. We measured soil lead with flame atomic absorption in a certified lab, following American Society for Testing and Materials standards.13 We placed soil samples in labeled polyethylene bags to be transported for laboratory drying, extraction, and acid digestion.
For quality control of specimen digestion, we performed identical treatments on blanks and specimens. We checked the precision of lead analyses by running lead content standards before and after each batch of specimens and intercalating a blank between every 10 specimens. The within-batch coefficient of variation did not exceed 2%, and the total coefficient of variation did not exceed 5% in control samples at low, midrange, and high concentrations in the analytical range. The lead concentration of soil was expressed as milligrams per kilogram. The detection limit with our method was 0.01 milligrams per kilogram.
Lead in household dust.
Our stratified sampling method drew data from a geographic information system about house location and resident density to select 60 of the 203 households in the polluted villages and 24 of the 53 households in the control village from which to take samples of dust to measure lead levels. One composite sample was taken from window sills or troughs in living rooms and children's play rooms, where lead-containing dust was known to accumulate. We collected a minimum of 10 grams of household dust from each selected home. We collected dust samples with moist wipes by a standard procedure.14 We followed the same methods to analyze lead concentration in household dust as to determine levels of lead in soil. Dust lead concentration was expressed as milligrams per kilogram.
Questionnaires and Interventions
We provided a structured questionnaire to be used by trained investigators to interview the children's parents and collect information on children's exposures to lead, including location, environment surrounding the house, condition of the house, and length of residence. The questionnaire probed for other potential sources of lead exposure, including frequency of consuming canned food or drinks; whether any family member worked in a lead industry and whether such workers often brought work suits or tools home; presence of peeling paint on windows, doors, or furniture; storage of other lead-containing materials, such as lead-containing pesticides or lead ore; and use of lead pottery at home. We also collected information on children's hygienic habits (hand washing, bathing frequency, and pica), milk consumption, smoking by family members at home, parental education level, and family income.
All of the lead mines and plants were forced to close at the time of our investigations. Lead mining, smelting, and transportation activities in the area stopped completely. A village-based health education program was launched as part of our study, delivering 1 class every 4 months. The classes focused on the adverse health effects of lead and how to protect children from lead exposure. Local residents were called on to help clean up the environment by, for example, washing yards, removing lead ore from their homes, and performing standard house cleaning. In cooperation with local professionals, we carried out a follow-up evaluation 1 year after these interventions in the polluted area to evaluate their effects on children's BLLs.
Statistical Analysis
The data analysis centered on the association between environmental lead pollution and children's BLLs. We used environmental measurements in the different villages as surrogates of personal exposure levels, because no personal exposure data were available and not all soil samples corresponded exactly to the locations of children's homes. Soil lead corresponded well with the distances of the villages from lead mines or plants and prevailing wind direction. Therefore, we divided the villages (and the children residing in them) into 3 exposure categories according to the soil lead concentrations: exposure levels were high in villages A and B; medium in villages C, D, E, and F; and low in village G. As shown in Figure 1, villages A and B were nearest to and downwind from lead plants or mines (prevailing wind direction was west–northwest). Village G was located farthest away from the lead enterprises and separated by a river.
We applied multivariate linear regression analysis to determine the association between exposure levels and children's BLLs, with adjustment for gender, length of residence in the area, parental occupation and education, milk consumption, and possible sources of lead exposure at home. Actual BLLs were normally distributed among the children (skewness = 0.294; kurtosis = 0.022) and were used in the data analysis. We used a stepwise procedure with the criterion of entry (0.05) and removal (0.1) to select BLL-related variables; we coded categorical variables (including exposure level) as dummy variables. We then constructed a final model that incorporated the exposure category and all variables meeting the criteria. We included age and length of residence exclusively because of their colinearity. In determining a relationship between household dust lead and BLLs, we log-transformed both variables and applied Pearson correlation analysis.
To examine changes in BLLs 1 year after closure of the lead enterprises, we used paired t tests to compare the baseline and follow-up BLLs among retested children. We also used a multivariate linear model to compare changes in BLLs with exposure levels, with adjustment for potential confounding factors. All data analyses were carried out with the SPSS version 15.0 for Windows (SPSS Inc, Chicago, IL).
RESULTS
The geometric mean for soil lead in the low-exposure village was 119.93 milligrams per kilogram (range = 87.86–199.00 mg/kg; n [total soil samples] = 8); in the medium-exposure villages, 412.05 milligrams per kilogram (range = 104.49–2354.82 mg/kg; n = 24); and in the high-exposure villages, 1557.58 milligrams per kilogram (range = 541.65–4080.00 mg/kg; n = 11). All were higher than in the control village (32.11 mg/kg; range = 6.87–102.52 mg/kg; n = 22). The soil lead level in medium- and high-exposure villages far exceeded the maximum level of 250 milligrams per kilogram considered safe under Chinese regulations.
The geometric mean for household dust lead in the low-exposure village was 1088.81 milligrams per kilogram (range = 652.80–1321.90 mg/kg; n = 16); in the medium-exposure villages, 1283.18 milligrams per kilogram (range = 599.30–2577.70 mg/kg; n = 32); and in the high-exposure villages, 1326.30 milligrams per kilogram (range = 662.80–2240.20 mg/kg; n = 12). All were higher than in the control village (122.37 mg/kg; range = 62.90–321.00 mg/kg, n = 24).
Table 1 summarizes sociodemographic data and potential risk factors of children by exposure level. The average age of children in the sample was 7 to 8 years, with slightly more boys than girls. Parental education level was lower in the control group. In the entire polluted area, 41% of children lived in houses near a main road; 89% were similarly situated in the high-exposure villages. More than 40% of children in the polluted area but fewer in the control village had parents working in lead-related occupations. More than half of parents in the polluted area admitted bringing their work suits or tools home, but very few families used lead pottery at home. Daily milk consumption was more common and bathing was more frequent among children in the polluted area than it was among those in the control area. We identified 29 houses in the polluted area but none in the control village that were storing lead ore. The prevalence of other risk factors (canned food or drinks, lead pesticides, peeling paint, and pica) was too low to analyze (range = 3.4%–6.1%).
TABLE 1.
Demographic Data and Potential Risk Factors Among Children by Level of Lead Exposure: Fujian Province, China, 2007
| Polluted Area |
||||
| Characteristics | Low Exposure (n = 131), No. (%) or Mean (SD) | Medium Exposure (n = 193), No. (%) or Mean (SD) | High Exposure (n = 55), No. (%) or Mean (SD) | Control Village (n = 61), No. (%) or Mean (SD) |
| Boys | 76 (58.0) | 103 (53.4) | 31 (56.4) | 32 (55.5) |
| Age, y | 8.27 (3.83) | 8.39 (4.06) | 7.32 (4.18) | 7.78 (4.45) |
| Residence in polluted area, y | 7.55 (3.37) | 7.79 (3.50) | 6.83 (3.59) | 0 |
| Parents’ education, y | ||||
| 0 | 16 (12.2) | 36 (18.7) | 15 (27.3) | 26 (42.6) |
| < 7 | 71 (54.2) | 86 (44.6) | 26 (47.3) | 24 (39.3) |
| ≥ 7 | 44 (33.6) | 71 (36.7) | 14 (25.4) | 11 (18.1) |
| House near main road | 57 (44.5) | 79 (40.9) | 48 (88.9) | 10 (17.5) |
| Parental occupation related to lead | 53 (40.5) | 85 (44.0) | 36 (65.5) | 7 (11.5) |
| Work suits or tools brought home | 68 (51.9) | 130 (67.4) | 30 (54.5) | 10 (16.4) |
| Lead pottery used | 2 (1.5) | 11 (5.7) | 0 (0) | 1 (1.6) |
| Lead ore stored in house | 11 (8.4) | 6 (3.1) | 12 (21.8) | 0 (0) |
| Milk consumption | ||||
| Almost daily | 56 (42.8) | 92 (47.7) | 23 (41.8) | 17 (27.9) |
| 2–3 times/wk | 38 (29.0) | 56 (29.0) | 14 (25.5) | 26 (42.6) |
| Occasionally or never | 37 (28.2) | 45 (23.3) | 18 (32.7) | 18 (29.5) |
| Bathing | ||||
| Every 1–2 d | 110 (84.0) | 130 (67.9) | 52 (94.5) | 14 (23.0) |
| Every 3–5 d | 19 (14.5) | 47 (24.3) | 3 (5.5) | 42 (68.8) |
| Every 6–7 d or less | 2 (1.5) | 15 (7.8) | 0 (0.0) | 5 (8.2) |
Table 2 presents children's BLLs. The mean value for 379 children in the polluted area was 16.38 micrograms per deciliter (95% confidence interval [CI] = 15.79, 16.96), which was much higher than it was in the control group (7.12 μg/dL; 95% CI = 5.89, 8.34). We found BLLs of 10 micrograms per deciliter or higher in 86.8% (95% CI = 83.4%, 90.2%) of children in the polluted villages and in 19.7% (95% CI = 9.4%, 29.9%) of children in the control village. Among children in the polluted area, average BLLs increased with exposure level, suggesting an exposure–response gradient. We observed a higher prevalence of elevated BLLs in high-exposure villages, where 95% of the children had BLLs of 10 micrograms per deciliter or higher and 36.4% had levels of 20 micrograms per deciliter or higher.
TABLE 2.
Blood Lead Levels of Children by Level of Lead Exposure: Fujian Province, China, 2007
| Polluted Area |
|||||
| BLL, μg/dL | Low Exposure (n = 131) | Medium Exposure (n = 193) | High Exposure (n = 55) | Total (n = 379) | Control Village (n = 61) |
| Mean (SD) | 15.15 (5.41) | 16.48 (5.66) | 18.92 (6.23) | 16.38 (5.78) | 7.12 (4.78) |
| Range | 4.90–33.52 | 5.24–36.75 | 6.91–34.86 | 4.90–36.75 | 0.19–23.01 |
| No. (%) | |||||
| < 10 | 21 (16.0) | 26 (13.5) | 3 (5.5) | 50 (13.2) | 49 (80.3) |
| 10–19 | 89 (67.9) | 115 (59.6) | 32 (58.2) | 236 (62.3) | 10 (16.4) |
| 20–24 | 15 (11.5) | 41 (21.2) | 12 (21.8) | 68 (17.9) | 2 (3.3) |
| ≥ 25 | 6 (4.6) | 11 (5.7) | 8 (14.5) | 25 (6.6) | 0 (0) |
Note. BLL = blood lead level.
The local health department sent 25 children with BLLs greater than 25 micrograms per deciliter to the hospital. Of these children, 7 were reported to have behavior problems, such as hyperkinetic syndromes or attention deficit disorder; 6 had digestive disorders, such as stomachache, abdominal distension, or partiality for a particular kind of food; and 4 had anemia (hemoglobin = 95–108 g/L). These children received supportive and chelation therapy.
We searched for possible determinants of BLL through linear regression analysis (Table 3). All 3 exposure levels were significantly associated with elevated BLLs compared with the control group. We detected a gradient of BLLs with exposure level: relative to the control village, high-exposure villages contributed to a BLL increase of 10 micrograms per deciliter; the medium- and low-exposure villages contributed to increases of 8 and 7 micrograms per deciliter, respectively. Other significant risk factors were male gender, longer residence in the polluted area, parental lead-related occupations, and parents often bringing work suits or tools home. Milk consumption and higher parental education were inversely associated with BLL. We found colinearity between age and years of residence in the polluted area (r = 0.725; 95% CI = 0.662, 0.791). Age was a significant predictor (b = 1.135; 95% CI = 0.060, 2.586) when we excluded years of residence from the model. We used years of residence in the polluted area in the final model.
TABLE 3.
Multiple Linear Regression Analysis for Determinants of Children's Blood Lead Levels: Fujian Province, China, 2007
| Characteristics | b (95% CI) |
| Exposure level | |
| Low | 7.18 (4.98, 9.37) |
| Medium | 8.24 (6.11, 10.38) |
| High | 10.27 (7.83, 12.71) |
| Control village (Ref) | 1.00 |
| Boys | 1.30 (0.31, 2.30) |
| Length of residence in polluted area | 0.17 (0.02, 0.32) |
| Parental occupation related to lead | 1.36 (0.28, 2.45) |
| Work suits or tools brought home | 1.13 (0.01, 2.25) |
| Milk consumption | |
| Almost daily | −2.53 (−3.84, −1.22) |
| 2–3 times/wk | −2.55 (−3.78, −1.31) |
| Occasionally or never (Ref) | 1.00 |
| Bathing | |
| Every 1–2 d | −1.33 (−3.75, 1.09) |
| Every 3–5 d | −1.24 (−3.59, 1.11) |
| Every 6–7 d or less (Ref) | 1.00 |
| Parents’ education, y | |
| 0 (Ref) | 1.00 |
| < 7 | −1.87 (−3.18, −0.55) |
| ≥ 7 | −3.26 (−4.69, −1.83) |
Note. We adjusted for use of lead pottery, house near main road, and lead ore stored in house. R2 = 0.38 for the model explanation; sample size = 440.
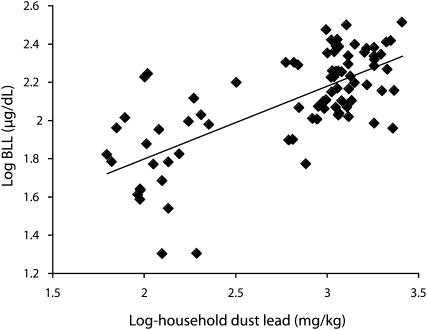
Figure 2 is a scatter plot of household dust lead (log) against BLLs (log) in 84 children whose household dust was sampled. Children's BLLs were moderately correlated with dust lead level (r = 0.699; 95% CI = 0.540, 0.855). A significant correlation remained even after excluding the control group.
FIGURE 2.
Scatter plot with fit line between log-household dust lead against log-BLL among 84 children: Fujian Province, China, 2007.
Note. BLL = blood lead level. Children's BLLs rose with increasing household dust lead, with a coefficient of 0.699.
Table 4 shows the changes in BLLs 1 year after the intervention. Among the entire group, the mean BLL decreased from 18.94 to 14.02 micrograms per deciliter, or 26%. Paired t tests showed that BLLs at each exposure level were significantly reduced at the 1-year follow-up (P < .001). The reduction tended to be greater among children in lower-exposure villages. In a further multiple linear regression, in which the difference between baseline and follow-up BLLs was the outcome variable, we found smaller reductions among children with medium (1.74 μg/dL; 95% CI = 0.29, 3.19) and high (2.77μg/dL; 95% CI = 0.93, 4.62) exposure than we did among children with low exposure, after adjusting for baseline BLL, age, and milk consumption.
TABLE 4.
Blood Lead Levels at 1-Year Follow-Up of Children With Elevated Baseline Levels by Level of Exposure: Fujian Province, China, 2008
| Exposure |
||||
| BLL, μg/dL | Low (n = 44), Mean (SD) or % | Medium (n = 99), Mean (SD) or % | High (n = 32), Mean (SD) or % | Total (n = 175), Mean (SD) or % |
| Baseline | 18.32 (3.75) | 18.70 (3.25) | 20.53 (4.30) | 18.94 (3.60) |
| 1-year follow-up | 12.38 (3.30) | 14.12 (4.69) | 15.97 (4.20) | 14.02 (4.61) |
| Follow-up − baseline | −5.94* (3.44) | −4.59* (3.48) | −4.55* (3.33) | −4.92* (3.44) |
| (Follow-up − baseline)/baseline | −32.4 | −24.4 | −22.2 | −26.1 |
Note. BLL = blood lead level.
P < .001, paired t test.
DISCUSSION
We examined environmental lead pollution resulting from industrial sources and its impact on the BLLs of children in a rural area of China. The lead concentrations in soils were well above the already lax national standards. We did not report lead concentrations in the air because air samples were taken after the closure of lead industries and did not reflect environmental exposure levels. However, 43% of air samples (29 of 67) in the polluted area and none in the control village exceeded the national standard. The poorly regulated activities of mining, separating, transportation, and smelting of lead were responsible for the serious lead pollution in the area. After ruling out other potential exposure sources, we found that lead exposure levels resulting from industrial pollution were strongly associated with elevated BLLs in children. Our results revealed a huge impact of environmental lead pollution on children's health.
Environmental quality in Chinese rural areas was generally thought to be much better than in industrial urban cities, before the era of booming township–village enterprises.15 However, with the acceleration of industrialization, township–village enterprises mushroomed in rural areas throughout the country. Most of these alliances lacked the technology and facilities to treat hazardous industrial wastes. Although concrete data on the resultant health effects are scant, no doubt remains that the township–village enterprises are major sources of environmental pollutants in rural areas, and our study provides supportive evidence for this concern. Previous studies on children's BLLs in China8–11,16–20 focused mostly on urban areas. Among the limited studies conducted in rural areas, 1 revealed that a small battery-recycling plant caused serious environmental lead pollution and severe adverse affects on children's BLLs.16 Another study examined electronic waste recycling in a rural town and also reported elevated BLLs in children.10 None of these studies provided concurrent data on environmental lead levels, however.
We were able to measure lead in soil and household dust, thus documenting serious environmental pollution. We observed a clear gradient of BLLs in children with increasing exposure levels. We found a moderate correlation between household dust lead levels and BLLs in a subgroup of children whose house dust was sampled. All results pointed toward a strong relationship between environmental lead pollution and BLLs in children, and the association was independent of other potential sources of exposure and confounding factors.
The children could be exposed to lead from several major routes. Polluted air was a source, especially fumes from lead smelting, whose molecules are small enough to be absorbed through the bronchiole tree. Lead deposited in soil and dust could also be ingested by children directly or through the food chain. Lead may exist in the environment in different chemical forms. According to data provided by the Environmental Protection Department in Fujian, the major forms used in the lead industries (varying in different factories) were residual lead (30%–59%), carbonate lead (17%–42%), iron–manganese oxidation lead (18%–31%), and humic acid lead (11%–18%). In household dust in the various villages and households we studied, residual lead accounted for 15% to 47% of the lead content, carbonate lead for 15% to 25%, iron–manganese oxidation lead for 10% to 36%, and humic acid lead for 15% to 24%. A recent study analyzed the associations of exposure to different forms of lead with BLLs in Chinese children and found that carbonate lead and humic acid lead were significantly related to elevated BLLs.21 The available samples in our study area showed that the component ratio of these 2 forms of lead were not much different across the villages.
Parental occupations related to lead might result in indirect exposure. In the polluted study area, 46% of the families had at least 1 member working in such an industry; 60% of these parents admitted often bringing their work clothes and tools home. We found these 2 variables to be significant risk factors for elevated BLLs, in agreement with a previous study.22 High parental education, female gender, and daily milk consumption were protective factors, as previously reported.23–26
One notable finding was that 20% of the children in the control area had BLLs greater than 10 micrograms per deciliter, even though no lead industries were near their homes. A partial explanation could be that some of the children (12%) had parents whose occupations exposed them to lead, such as motor repair or welding. These children had a higher average BLL than did others in their village (11.0 μg/dL vs 6.6 μg/dL). Other sources of lead exposure might be lead-based paint, lead pesticides, or lead-containing toys and tableware, but not leaded gasoline, which was banned by China in 1999. This high background risk is typical for vast rural areas of the country. A recent article reviewed 49 studies (mostly published in Chinese journals) on BLLs in rural children from 1998 to 2006 and found levels ranging from 4.4 to 53.2 micrograms per deciliter.27
A notable finding in our follow-up to the remediation intervention was that BLLs significantly decreased among children at all exposure levels, although the average value was still higher than 10 micrograms per deciliter. Similar BLL reductions have been observed in children throughout the United States28 and in New York State29 and have been attributed to public awareness, lead paint remediation, replacement of older homes, and phasing out of lead-containing gasoline. On the other hand, lead in soil and other parts of the environment does not dissipate quickly, and these lead sources may exert lasting adverse effects on children.30 This factor might explain our finding that reductions in BLLs tended to be smaller among children from higher-exposure villages.
To the best of our knowledge, ours is one of the very few studies reporting a positive association between environmental lead pollution and children's BLLs in a rural area of China. The association was unlikely to be a chance finding because of the adequate sample size and consistent results. Strengths of our study included a participation rate over 90% and inclusion of other possible lead exposure sources and potential confounding factors in the multivariate analysis. Environmental sources of lead and the outcome of BLL were objective parameters and were determined by standard laboratory protocols carried out independently of one another. In addition, we followed children with high BLLs to determine the change in their levels after 1 year, as a possible indicator for the effect of closure of the lead industries.
Limitations
We measured environmental lead levels by collecting samples at a village level only once, which might not have reflected the actual exposure of individual children. The lack of individual exposure data prevented us from documenting a more precise exposure–response relationship, although we observed a clear increasing trend of the children's BLLs with increasing environmental lead levels. We sampled household dust from only 60 families, largely because of limited resources. However, the lead levels in household dust were consistent with the soil lead levels in different exposed villages, indicating the samples were representative to a great extent.
We retested BLLs only in children with high initial levels, so we do not know what happened to the BLLs of the other children after 1 year. Furthermore, we did not remeasure environmental lead after 1 year, so we could not assess the degree of persistence of lead contamination in the environment and link it to the retested BLLs.
Recall bias and interviewer bias were unlikely, because the BLLs of the children were not known at the time of the interviews. Inaccuracies could have occurred during collection of information on other possible exposures or confounding factors and resulted in misclassifications, but such misclassifications were unlikely differential and thus did not significantly distort the association between environmental lead pollution and children's BLLs.
Conclusions
We assessed environmental lead pollution caused by rural industries and its impact on BLLs among children living nearby. We measured average BLLs as high as 16.4 micrograms per deciliter in some villages. Of the children in the study, 87% had an elevated BLL (> 10 μg/dL), and 25% had a level greater than 25 micrograms per deciliter. Our results could be a wake-up call for the revamping of governmental policies that supported rural industries without tightly regulating their hazardous discharges and environmental pollution. Efficient strategies and public health policies are urgently needed to control and prevent environmental lead pollution in both rural and urban areas.
The environmental lead pollution we report represents just the tip of an iceberg, and more studies are needed to evaluate environmental pollution from other harmful chemicals and its health effects on children in cities and in the vast rural areas of China. BLLs in children at high risk should be screened and monitored regularly. Children with high BLLs should receive medical attention and appropriate treatments, with financial support from the responsible industries. In addition, extensive education programs should be launched to enhance public awareness of lead exposure and its adverse health effects throughout the country.
Acknowledgments
We thank the staff of the Department of Environmental Protection of Fujian Province and the Monitor Center of the Environment, the local Center for Diseases Control and Prevention in the study area, and Ning Chen, Yiting Zhuang and Guiguo Bian for their participation and valuable help throughout the investigation.
Human Participant Protection
This study was approved by the Centre for Prevention and Control of Occupational Diseases and Chemical Poisoning, and the Fujian institutional review board. Oral informed consent was obtained from all study participants and their parents.
References
- 1.Tong S, Von Schirnding YE, Prapamontol T. Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ. 2000;78(9):1068–1077 [PMC free article] [PubMed] [Google Scholar]
- 2.Pocock SJ, Shaper AG, Ashby D, Delves T, Whitehead TP. Blood lead concentration, blood pressure, and renal function. Br Med J (Clin Res Ed). 1984;289(6449):872–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin R, Brown MJ, Kashtock ME, et al. Lead exposures in U.S. children, 2008: implications for prevention. Environ Health Perspect. 2008;116(10):1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falk H. International environmental health for the pediatrician: case study of lead poisoning. Pediatrics. 2003;112(1 Pt 2):259–264 [PubMed] [Google Scholar]
- 5.Shen X, Rosen JF, Guo D, Wu S. Childhood lead poisoning in China. Sci Total Environ. 1996;181(2):101–109 [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Zhang J. Blood lead levels in children, China. Environ Res. 2006;101(3):412–418 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization and United Nations Development Programme Environment and people's health in China. 2001. Available at: http://www.wpro.who.int/NR/rdonlyres/CDB68D58-C3C6-4EC0-AC09-F2F9DAE7890E/0/CHNEnvironmentalHealth.pdf. Accessed February 7, 2011
- 8.Wang C, Ouyang H, Wang J, Liu J, Zhang X, Wang Y. Impact of lead pollution in environment on children's health in Shenyang City [in Chinese]. Huan Jing Ke Xue. 2003;24(5):17–22 [PubMed] [Google Scholar]
- 9.Duzgoren-Aydin NS. Sources and characteristics of lead pollution in the urban environment of Guanzhou. Sci Total Environ. 2007;385(1–3):182–195 [DOI] [PubMed] [Google Scholar]
- 10.Huo X, Peng L, Xu X, et al. Elevated blood lead levels of children in Guiyu, an electronic waste recycling town in China. Environ Health Perspect. 2007;115(7):1113–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li HX, Song YL, Li HG, et al. An epidemiological survey on saturnism among children due to lead pollution released from township enterprise [in Chinese]. Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42(3):156–159 [PubMed] [Google Scholar]
- 12.Markus J, McBratney AB. A review of the contamination of soil with lead II. Spatial distribution and risk assessment of soil lead. Environ Int. 2001;27(5):399–411 [DOI] [PubMed] [Google Scholar]
- 13.Standard Practice for Field Collection of Soil Samples for Subsequent Lead Determination. West Conshohocken, PA: American Society for Testing and Materials; 2005. ASTM E 1727-05 [Google Scholar]
- 14.Standard Practice for Collection of Settled Dust Samples Using Wipe Sampling Methods for Subsequent Lead Determination. West Conshohocken, PA: American Society for Testing and Materials; 2003. ASTM E 1728-03 [Google Scholar]
- 15.Dumreicher H. Chinese villages and their sustainable future: the European Union–China–Research Project “SUCCESS.” J Environ Manage. 2008;87(2):204–215 [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Huang Q, Zhou X, et al. Study on the effects of lead from small industry of battery recycling on environment and children's health [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23(3):167–171 [PubMed] [Google Scholar]
- 17.Chen XX, Teng HH, Wang FZ, et al. Blood lead level and related risk factors among children aged 0–6 years in Beijing [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24(10):868–871 [PubMed] [Google Scholar]
- 18.Zhang SM, Dai YH, Xie XH, Fan ZY, Tan ZW. Study on blood lead level and related risk factors among children aged 0–6 years in 15 cities in China [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26(9):651–654 [PubMed] [Google Scholar]
- 19.Yan CH, Shen XM. Study review and prospect for saturnism prevention and control among Chinese children [in Chinese]. Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42(3):147–150 [PubMed] [Google Scholar]
- 20.He K, Wang S, Zhang J. Blood lead levels of children and its trend in China. Sci Total Environ. 2009;407(13):3986–3993 [DOI] [PubMed] [Google Scholar]
- 21.Bian GG. Association of child blood lead and its chemical form in the room dust [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30(4):356–359 [PubMed] [Google Scholar]
- 22.Whelan EA, Piacitelli GM, Gerwel B, et al. Elevated blood lead levels in children of construction workers. Am J Public Health. 1997;87(8):1352–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen XM, Yan CH, Wu SH, Shi R. Parental education to reduce blood lead levels in children with mild and moderate lead poisoning: a randomized controlled study [in Chinese]. Zhonghua Er Ke Za Zhi. 2004;42(12):892–897 [PubMed] [Google Scholar]
- 24.Wang L. Lead poisoning in children (Part A) [in Chinese]. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9(5):514–516 [PubMed] [Google Scholar]
- 25.Markowitz ME, Sinnett M, Rosen JF. A randomized trial of calcium supplementation for childhood lead poisoning. Pediatrics. 2004;113(1 Pt 1):e34–e39 [DOI] [PubMed] [Google Scholar]
- 26.Chuang HY, Tsai SY, Chao KY, et al. The influence of milk intake on the lead toxicity to the sensory nervous system in lead workers. Neurotoxicology. 2004;25(6):941–949 [DOI] [PubMed] [Google Scholar]
- 27.Bian Gui-guo Lead level in blood for Chinese countryside children [in Chinese]. Huangjing Kexue yu Jishu. 2008;l31:101–106 [Google Scholar]
- 28.Meyer PA, Pivetz T, Dignam TA, et al. Surveillance for elevated blood lead levels among children—United States, 1997–2001. MMWR Surveill Summ. 2003;52(10):1–21 [PubMed] [Google Scholar]
- 29.Haley VB, Talbot TO. Seasonality and trend in blood lead levels of New York state children. BMC Pediatr. 2004;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glorennec P. Analysis and reduction of the uncertainty of the assessment of children's lead exposure around an old mine. Environ Res. 2006;100(2):150–158 [DOI] [PubMed] [Google Scholar]