Abstract
Aniline exposure is associated with toxicity to the spleen leading to splenomegaly, hyperplasia, fibrosis and a variety of sarcomas of the spleen on chronic exposure. In earlier studies, we have shown that aniline exposure leads to iron overload, oxidative stress and activation of redox-sensitive transcription factors, which could regulate various genes leading to a tumorigenic response in the spleen. However, molecular mechanisms leading to aniline-induced cellular proliferation in the spleen remain largely unknown. This study was, therefore, undertaken on the regulation of G1 phase cell cycle proteins (cyclins), expression of cyclin-dependent kinases (CDKs), phosphorylation of retinoblastoma protein (pRB) and cell proliferation in the spleen, in an experimental condition preceding a tumorigenic response. Male SD rats were treated with aniline (0.5 mmol/kg/day via drinking water) for 30 days (controls received drinking water only), and splenocyte proliferation, protein expression of G1 phase cyclins, CDKs and pRB were measured. Aniline treatment resulted in significant increases in splenocyte proliferation, based on cell counts, cell proliferation markers including proliferating cell nuclear antigen (PCNA), nuclear Ki67 protein (Ki67) and minichromosome maintenance (MCM), MTT assay and flow cytometric analysis. Western blot analysis of splenocyte proteins from aniline-treated rats showed significantly increased expression of cyclins D1, D2, D3 and cyclin E, as compared to the controls. Similarly, real-time PCR analysis showed significantly increased mRNA expression for cyclins D1, D2, D3 and E in the spleens of aniline-treated rats. The overexpression of these cyclins was associated with increases in the expression of CDK4, CDK6, CDK2 as well as phosphorylation of pRB protein. Our data suggest that increased expression of cyclins, CDKs and phosphorylation of pRB protein could be critical in cell proliferation, and may contribute to aniline-induced tumorigenic response in the spleen.
Keywords: Aniline, splenic toxicity, cell proliferation, cyclins, CDKs, pRB
Introduction
Aniline, an aromatic amine, is a widely used industrial chemical with an annual production of over 1 billion pounds in the United States (Di Girolamo et al., 2009). Besides inducing hemolysis, and hemolytic anemia, aniline exposure is also associated with damage to the spleen leading to splenomegaly, hyperplasia, fibrosis, and the eventual formation of highly malignant soft tissue or mesenchymal tumors, most commonly fibrosarcomas on chronic exposure in rats (Goodman et al., 1984; Weinberger et al., 1985; Bus and Popp, 1987; Khan et al., 1993, 1999a). Splenomegaly is one of the earliest characteristic feature of aniline-induced splenotoxic responses preceding fibrosis and sarcomas (Weinberger et al., 1985; Bus and Popp, 1987; Khan et al., 1997, 1999a; Wang et al., 2005). As evidenced from previous studies, splenomegaly and splenotoxicity were associated with increased red pulp cellularity, increases in macrophages and fibroblasts, and such changes as iron overload, oxidative stress and activation of redox-sensitive transcription factors (Khan et al., 1997, 1999a, 1999b, 2003a, 2006; Wang et al., 2005, 2008). However, molecular mechanisms leading to aniline-induced cellular proliferation in the spleen remain largely unknown.
Cell proliferation, a complex and tightly controlled process, plays a fundamental role in chemical-induced cell injury, including the injury which leads to neoplasia (Swenberg et al., 1983; Williams and Iatropoulos, 2002; Park et al., 2009). Increases in cell proliferation and changes in cell cycle are essential ingredients of many stages of chemical carcinogenesis (Emmendoerffer et al., 2000; Williams and Iatropoulos, 2002; Park et al., 2009). The phases of the cell cycle include G1 (Gap 1), S (synthesis of DNA), G2 (Gap 2) and M (mitosis). G0 is a phase of resting cells outside the cell cycle. When cells are stimulated by exogenous stimuli, they enter to the first gap phase (G1) from G0. Two of the most important group of proteins involved in the cell cycle machinery are cyclins and cyclin-dependent kinases (CDKs) (Murray, 2004; Chulu and Liu 2009). Cell cycle progression is driven by changes in cyclin-CDK complexes, and if control of the cell cycle is disrupted, progress through the cycle might be stimulated by overexpressed cyclins, enhanced CDK activity or inactivated CDK inhibitors (Malumbres and Barbacid, 2001; Chulu and Liu 2009). Induction of cyclins leads to their binding and activation of the associated CDK4, CDK6 or CDK2 (Hunter and Pines, 1994; Keyomarsi et al., 1995; Sherr, 1995; Malumbres and Barbacid, 2001). In mammalian cells, cyclin D-CDK4/6 and cyclin E-CDK2 complexes are required to promote cell cycle entrance from quiescence, progression through the G1 phase and transition from G1 into S phase in response to mitogenic stimulation (Pardee, 1989; Malumbres and Barbacid, 2001; Chulu and Liu 2009). Deregulation of G1 cyclins is associated with tumorigenesis, and altered CDKs in G1 phase can also contribute to oncogenic process (Hunter and Pines, 1994; Keyomarsi et al., 1995; Malumbres and Barbacid, 2009).
The G1 phase of the cell cycle is a functional period during which cells prepare for S phase, and the control of post-embryonic cell proliferation occurs before S phase (Pardee, 1989; Lundberg and Weinberg, 1999). There is a check point in the G1-S transition, termed as ‘restriction point’ or ‘R point’ (Pardee, 1989), and R point is a central event in normal cellular proliferation control (Lundberg and Weinberg, 1999), because it determines if a normal proliferating cell in G1 will continue to cycle or will revert to quiescence. The retinoblastoma proteins (pRB) are best known for their roles in restraining the G1-S transition through the regulation of E2 transcription factor (E2F)-responsive genes. There are a variety of cyclin/CDK complexes formed during distinct phases and time windows of the cell cycle, however, they may both get co-overexpressed and modify pRB (Weinberg, 1995; Lundberg and Weinberg, 1998, 1999). Cyclin D-CDK4/6 and cyclin E-CDK2 complexes sequentially phosphorylate the pRB (Hatakeyama et al., 1994; Lundberg and Weinberg, 1998; Harbour et al., 1999), and the hyper-phosphorylated proteins release the E2F transcription factors that are required for the S phase entry (Sherr and Roberts, 1999; Sherr, 2000).
The precise molecular mechanisms in aniline-induced splenotoxicity, especially the molecular events in splenocyte proliferation and expression of the proteins which regulate cycling cells are unknown. This study was, therefore, focused on evaluating the cellular proliferation and expression of cell cycle proteins, especially G1 phase cyclins and CDKs in an experimental condition preceding a tumorigenic response, but known to induce iron overload and oxidative stress in the spleen (Khan et al., 1999a, 1999b; Wang et al., 2008) following aniline insult.
Materials and methods
Animals and treatments
Male Sprague-Dawley rats (~200g), obtained from Harlan Sprague-Dawley (Indianapolis, IN), were housed in wire-bottom cages over adsorbent paper with free access to tap water and Purina lab chow and maintained in a controlled environment animal room (temperature, 22°C; relative humidity, 50%; photoperiod, 12-h light/dark cycle) for 7 days prior to the treatments. The experiments were performed in accordance with the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee at University of Texas Medical Branch. The animals were divided into two groups of six each. One group of animals received 0.5 mmol/kg/day aniline hydrochloride (~97%; Aldrich, Milwaukee, WI) via drinking water (pH of the solution adjusted to ~ 6.8), while the other group received water only and served as controls (Khan et al., 1993, 1999a, 1999b, 2003a, 2006; Wang et al., 2005; Ma et al., 2008). The drinking pattern of experimental and control rats was similar. Choice of dose and duration of exposure was based on earlier studies (Khan et al., 1993, 1999a, 1999b, 2006; Wang et al., 2005; Ma et al., 2008). After 30 days, the animals were euthanized under nembutal (sodium pentobarbital) anesthesia and the spleens were aseptically removed, weighed and divided into several portions for use in various analyses. Portions of the spleen were snap-frozen in liquid nitrogen and stored at −80°C for RNA isolation and protein extraction, whereas another portion of the spleen was processed to obtain splenocytes.
Isolation and culture of splenocytes
A portion of the spleen was passed through a cell strainer (BD Biosciences, Bedford, MA) in RPMI 1640 culture medium. The cell suspension was centrifuged at 1000 g for 5 min at 4°C. The cells were resuspended with Hanks’ balanced salt solution (HBSS) without calcium and magnesium. The splenocytes were isolated as described earlier (Khan et al., 2006; Wang et al., 2008). Briefly, the suspended cells were layered onto 6 ml of Histopaque 1083 (Sigma Chemical Co., St. Louis, MO) and centrifuged at 700 g for 30 min at 20°C. After centrifugation, the splenocyte layers were carefully removed, transferred to 50 ml Falcon plastic tubes, and washed twice with 20 ml HBSS. The cells were resuspended in RPMI 1640 culture medium supplemented with 2mM L-glutamine, 50 μg/ml gentamycin and 10% heat inactivated FBS (Sigma). Splenocytes were counted microscopically and the cell viability was determined by trypan blue exclusion method. The viability of splenocytes isolated from both control and aniline-treated rats was more than 95%.
MTT assay
MTT (3-[4, 5-Dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) was dissolved in phosphate buffered saline (PBS, 5 mg/ml) and filtered through 0.22 μm filter. The MTT assay was done as described earlier (Mosmann, 1983) with minor modifications. Briefly, the isolated splenocytes, suspended in complete RPMI 1640, were plated in 96-well flat bottom plates (1 × 105/100 μl/well) (Corning Inc., Corning, NY) and cultured in a humidified incubator with 5% CO2 at 37°C for 24, 48 and 72 h. After addition of 20 μl MTT solution in each well, the incubation was continued for another 4 h at 37°C. One hundred μl of stop solution (10% SDS-0.01 N HCl) was then added to each well, and the absorbance was read at 570 nm (test) and 630 nm (reference) 5 minutes later.
Flow cytometric analysis for splenocyte proliferation
Splenocytes isolated from control and aniline-treated rats were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) using the CellTrace CFSE Cell Proliferation kit (Molecular Probes, Eugene, OR) and then plated into 24-well flat-bottom plates at 1× 106/well in a total volume of 1ml. The splenocytes were incubated at 37°C with 5% CO2, and after 72h, harvested for flow cytometric analysis using a Becton-Dickinson FacsCanto flow cytometer (BD Biosciences, San Jose, CA).
Preparation of total protein extracts
Total spleen tissue lysates were prepared by using the lysis buffer essentially as described by the manufacturer (Cell Signaling, Beverly, MA). Protein concentration in the lysates was determined by Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA). The lysates were used for the detection of cyclin D1, cyclin D2, cyclin D3, cyclin E, CDK2, CDK4, CDK6, pRB and phospho-pRB proteins.
Preparation of nuclear protein extracts (NEs)
NEs were prepared essentially as described earlier (Ma et al., 2008). Spleen tissues (from control and aniline-treated rats) were cut into small pieces, homogenized briefly with a loose glass pestle in cold hypotonic buffer [10mM HEPES-KOH, 10 mM KCl, 100μM EDTA, 100 μM EGTA, 1 mM DTT, 0.5mM PMSF, 2 μg/ml pepstatin, and a complete protease inhibitor cocktail (Roche, Germany)], and incubated on ice for 20min. Tissues were then further homogenized using a tight pestle and centrifuged at 800g for 4 min to obtain nuclear pellets. Pellets were gently washed two times with homogenizing buffer. Nuclear proteins were extracted in a high salt buffer (20mM HEPES-KOH, 410 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1mM DTT, 1 mM PMSF, 2 μg/ml pepstatin, and protease inhibitor cocktail) by incubating for 45min on ice with reverse mixing at intervals of 10 min. The NEs were cleared by centrifugation (16,000g, 10 min) and adjusted to 15% with glycerol and stored at −80°C until further analysis. Protein concentration was determined by using Bio-Rad detergent compatible (DC) protein assay reagents (Bio-Rad). The nuclear protein extracts were used for the detection of proliferating cell nuclear antigen (PCNA), nuclear Ki67 protein (Ki67) and minichromosome maintenance (MCM) proteins (cell proliferation markers).
Western blot analysis for protein expression
Western blot analysis was performed following previous studies (Khan et al., 2006; Wang et al., 2008, 2010). Lysates containing 50 μg of total proteins from whole spleen or nuclear extracts were resolved by SDS-PAGE (10%) and transferred to PVDF membranes (Amersham, Arlington Heights, IL). After blocking with non-fat dry milk (5%, w/v), the membranes were incubated with antibodies specific for the above mentioned proteins (Santa Cruz Biotechnology, Santa Cruz, CA) for detecting their expression. To confirm even loading, membranes were stripped and probed with an actin antibody (Sigma). Blots were quantitated by densitometry and normalized using the actin signal to correct for differences in loading of the proteins from the control and experimental groups. All other procedures for immunoblotting were the same as described earlier (Khan et al., 2006; Wang et al., 2008, 2010).
Real-time PCR analysis for cyclins D1, D2, D3 and cyclin E gene expression in splenocytes
RNA isolation
Total RNA was isolated from spleen tissues using a RiboPure kit (Ambion, Austin, TX) as per the manufacturer’s instructions. To eliminate genomic DNA contamination, isolated RNA was treated with RNase-free DNase I (DNA-free kit; Ambion). The total RNA concentration was determined by measuring the absorbance at 260 nm. RNA integrity was verified electrophoretically by ethidium bromide staining and by measuring the A260/A280 ratio.
Real-time RT-PCR
Real-time RT-PCR was performed essentially as described earlier (Wang et al., 2005, 2008, 2010; Ma et al., 2008). First-strand cDNA was prepared from total RNA by using the SuperScript first-strand synthesis kit (Invitrogen, Carlsbad, CA) as per the manufacturer’s instructions. Real-time PCR employing a two-step cycling protocol (denaturation and annealing/extension) was carried out using a Mastercycler Realplex (Eppendorf, Westbury, NY) and the primer pairs used in the real-time PCR process are shown in Table 1. For each cDNA sample, parallel reactions were performed in triplicate for the detection of 18S and rat cyclins D1, D2, D3 and cyclin E. The reaction samples in a final volume of 25μl contained 2 μl cDNA templates, 2 μl primer pair, 12.5 μl iQ SYBR Green Supermix, and 8.5μl water. Amplification conditions were identical for all reactions: 95°C for 2min for template denaturation and hot-start before PCR cycling. A typical cycling protocol consisted of three stages, 15s at 95°C for denaturation, 30 s at 60°C for annealing, and 30s at 72°C for extension and an additional 20-s hold for fluorescent signal acquisition. To avoid nonspecific signal from primer dimers, the fluorescence signal was detected at 2°C below the melting temperature (Tm) of individual amplicons and above the Tm of the primer dimmers. A total of 40 cycles were performed.
Table 1.
Primer sequences of cyclins D1, D2, D3 and E for real-time PCR analysis
| Genes | Primer sequences (5′-3′) | Amplicon position (size) | Accession no. |
|---|---|---|---|
| Cyclin D1 | |||
| Forward | t g c t t g g g a a g t t g t g t t g g | 2131–2256 (126 ) | D14014 |
| Reverse | a a t g c c a t c a c g g t c c c t a c | ||
| Cyclin D2 | |||
| Forward | c t g a c t g c c g a a a a g c t g t g | 529–643 (115) | D16308 |
| Reverse | g g g t t a c t g c a g c c a g g t t c | ||
| Cyclin D3 | |||
| Forward | g g c c t t g g c t a g a a g a g c a a | 1649–1730 (82) | BC089819 |
| Reverse | g c a c c c t c a a g a c c c t c a a c | ||
| Cyclin E | |||
| Forward | g a a a a t c a g a c c g c c c a g a g | 474–596 (123) | D14015 |
| Reverse | c g c t g c a g a a a g t g c t c a t c | ||
| 18S | Universal 18S Internal Standard | 315 | |
Quantitation of PCR was done using the comparative CT method as described in User Bulletin No. 2 of Applied Biosystems (Foster City, CA) and reported as fold difference relative to the calibrator cDNA (QuantumRNA Universal 18S standards; Ambion). The fold changes in cyclins D1, D2, D3, cyclin E cDNA (target gene) relative to the 18S endogenous control were determined as fold change= 2 − ΔΔCT, where ΔΔCT= (CT aniline −CT 18S) − (CT control − CT 18S).
Statistical analyses
All data are expressed as means± SD. Comparison between the groups was made by p value determination using Student’s two-tailed t-test (GraphPad InStat 3 software, La Jolla, CA). A p value of <0.05 was considered to be statistically significant.
Results
Spleen weight and splenocyte population changes in aniline-treated rats
The body weight gain pattern of aniline-treated rats was similar to the controls (data not shown). However, spleens from aniline-treated rats appeared dark and remarkably enlarged (splenomegaly), and spleen weight in the aniline-treated rats (1.34 ± 0.09 g) increased by 79% in comparison to the controls (0.75 ± 0.04 g). Similarly, the spleen-to-body weight ratio also showed an increase of 84% in the aniline-treated rats. Also, the splenocyte population in the aniline-treated rats (304.0 ± 30.1 ×106) increased by 52% in comparison to control rats (200.0 ± 9.7 × 106), suggesting that the observed splenomegaly is due not only to the deposition of damaged erythrocytes but also to recruitment and/or proliferation of splenic cells.
Effects of aniline exposure on cell proliferation markers
Since our data clearly demonstrated an increased splenocyte population following aniline treatment, it was also deemed necessary to investigate the cell proliferation by measuring the expression of conventional proliferation markers, i.e., PCNA and Ki67 (Stuart-Harris et al., 2008). The results of PCNA and Ki67 expression are shown in Fig. 1. As evident from the figure, aniline exposure led to a 216% increase in splenic PCNA levels, whereas Ki67 levels increased by 247% in comparison to controls.
Fig. 1.
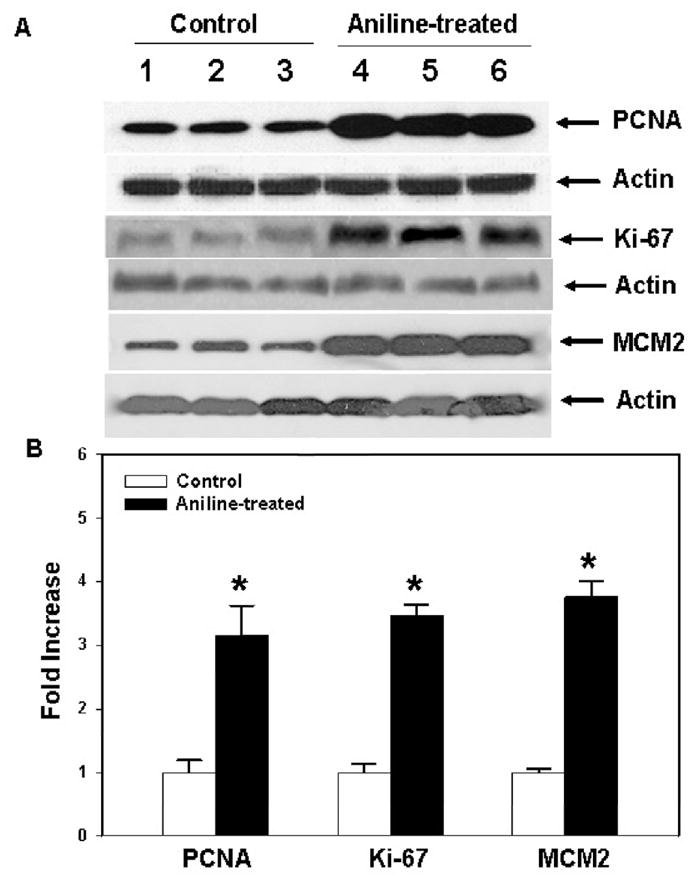
Protein expression of PCNA, Ki67 and MCM2 in the spleens from control and aniline-treated rats. (A) Western blot detection of PCNA, Ki67 and MCM2 proteins in the spleens of control and aniline-treated rats. (B) Densitometric analysis of protein bands. Values are mean ± SD (n=3); *p < 0.05.
MCM proteins play an essential role in DNA replication. They are not expressed in somatic cells that have been withdrawn from the cell cycle, but highly expressed in proliferating cells throughout the cycle. Thus, MCM proteins represent reliable markers for cell proliferation, cell cycle deregulation and probably tumor prognosis (Sington et al., 2004; Korkolopoulou et al., 2005). The protein expression of MCM2, considered the most reliable member of MCMs (Korkolopoulou et al., 2005), was quantified in the current study. Aniline treatment led to significantly increased (275%) expression of MCM2 in the spleens (Fig. 1). Taken together, our data showed that aniline exposure clearly induced overexpression of PCNA, Ki67 and MCM2, suggesting not only increased cell proliferation, but also the potential for leading to a tumorigenic response in the spleen.
Aniline exposure leads to enhanced cell proliferation ex vivo
To determine the capacity for proliferation of splenocytes ex vivo, MTT assay was carried out and the results are shown in Fig. 2. After incubation in complete RPMI 1640 for 24, 48 and 72 h, the proliferation rate of splenocytes from control rats did not change, while the proliferation rate of splenocytes from aniline-treated rats was significantly increased by 37%, 66% and 141% at 24, 48 and 72 h, respectively, in comparison to the respective controls.
Fig. 2.
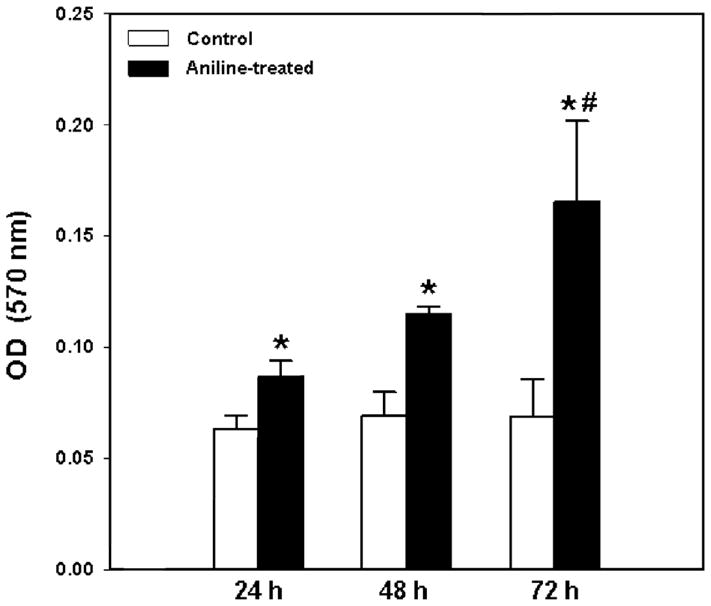
Proliferation patterns (MTT assay) of splenocytes from control and aniline-treated rats. Splenocytes were isolated and cultured for 24, 48 and 72 h and evaluated for their proliferation using MTT assay. Values are mean ± SD (n=3); *p < 0.05 vs. respective controls; #p<0.05 vs. 24h and 48h.
To further examine the impact of aniline exposure on splenocytes, the cell proliferation was also quantified using flow cytometric analysis. As evident from Fig. 3, splenocytes from aniline-treated rats showed 143% increase in cell proliferation compared to the controls after culturing for 72h. These results further support the findings of MTT assay, and suggest that the splenocytes from aniline-treated rats were primed and showed greater potential for proliferation in culture medium without further stimulation.
Fig. 3.
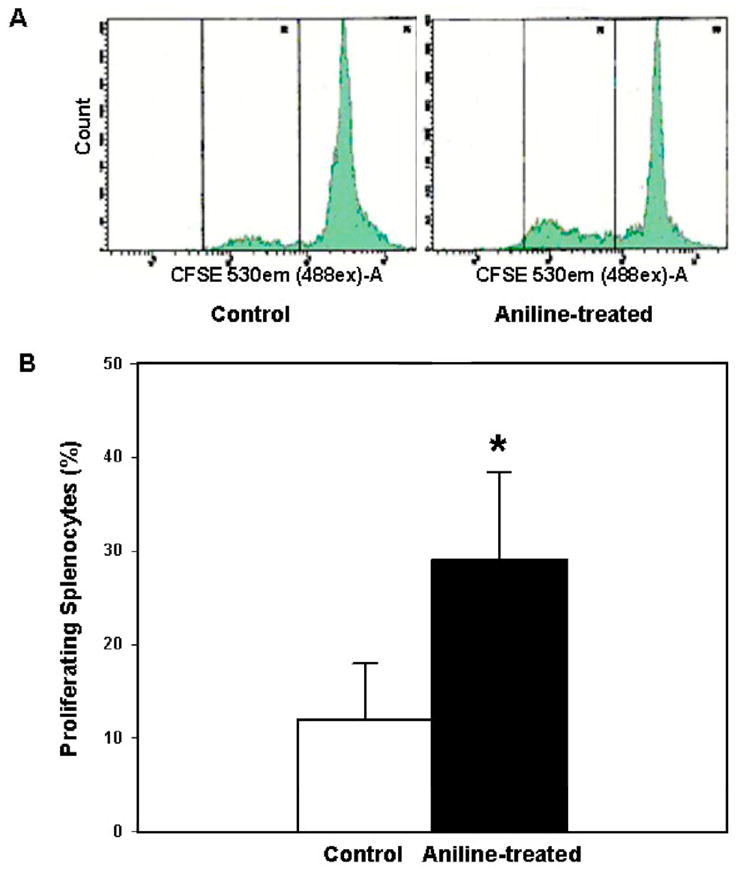
Flow cytometric analysis of splenocyte proliferation. Splenocytes isolated from control and aniline-treated rats were labeled with CFSE, cultured for 72 h, and analyzed by flow cytometry. Values are mean ± SD (n=3); *p < 0.05.
Effects of aniline exposure on protein expression of cyclins D1, D2, D3 and E
There is increasing evidence that cyclins play important roles in regulating cell cycle progression (Malumbres and Barbacid, 2001; Olashaw and Pledger, 2002; Murray, 2004; Chulu and Liu, 2009). Induction of cyclins leads to their binding and activation of the associated CDKs, such as CDK2, CDK4 and CDK6 (Hunter and Pines, 1994; Sherr, 1995; Malumbres and Barbacid, 2001). In mammalian cells, cyclin D-CDK4/6 and cyclin E-CDK2 complexes are required to promote cell cycle entrance from quiescence, progression through the G1 phase and transition from G1 into S phase in response to mitogenic stimulation (Sherr, 1995; Malumbres and Barbacid, 2001; Chulu and Liu, 2009). As shown in Fig. 4, the protein expression of cyclins D1, D2 and D3 from the spleens of aniline-treated rats increased by 129%, 99% and 284%, respectively, than the controls. Cyclin E level also increased and showed a remarkable increase of 938% than the controls.
Fig. 4.
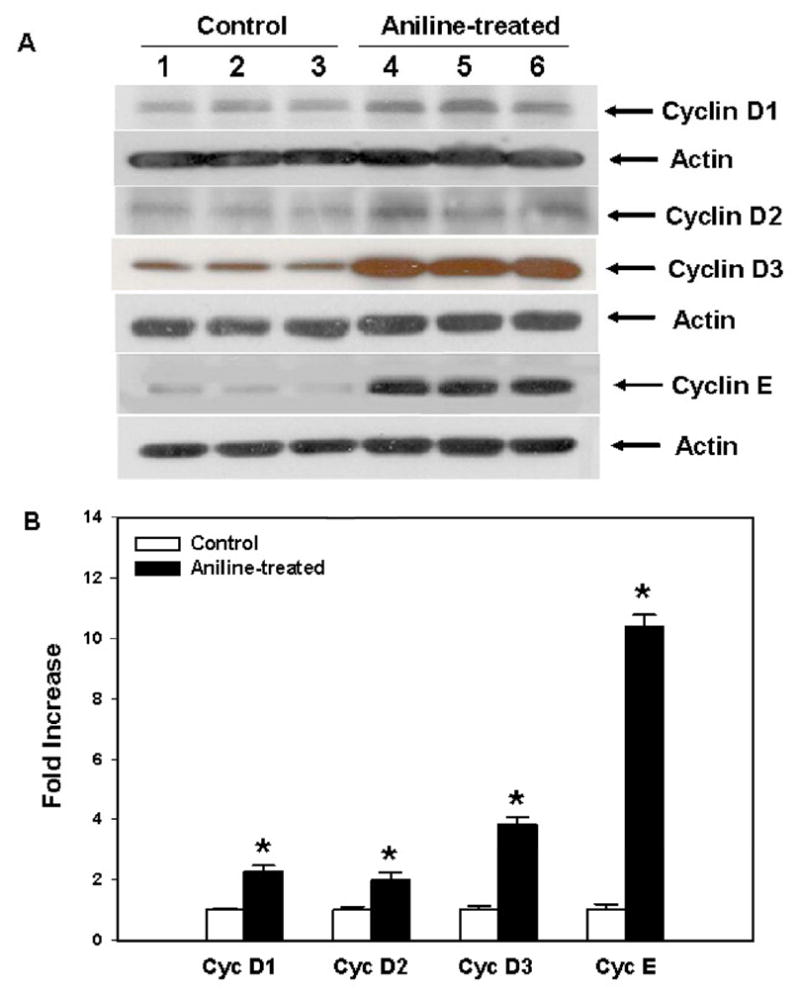
Cyclins D and cyclin E expression in the spleens of rats treated with aniline. (A) Western blot detection of cyclins D1, D2, D3 and E in the spleens of control and aniline-treated rats. (B) Densitometric analysis of cyclin protein bands. Values are mean ± SD (n=3); *p < 0.05.
Impact of aniline exposure on mRNA expression of cyclins
The D-type cyclins and cyclin E play key role in promoting pRB hyperphosphorylation and resulting cell cycle progression via assembling with CDKs to form cyclin-CDK complexes (Hatakeyama et al., 1994; Weinberg, 1995; Menon and Goswami, 2007; Chulu and Liu, 2009). Western blot analysis data in this study demonstrated that aniline treatment induced significant increases in protein expression of cyclins D and cyclin E in spleen tissues. To investigate whether aniline exposure also affects expression of cyclins at gene levels, cyclins D1, D2, D3 and cyclin E mRNA levels were analyzed by real-time PCR and the findings are shown in Fig. 5. Aniline exposure resulted in 1.84, 2.08, 4.82 and 12.82 fold increases, respectively, in cyclins D1, D2, D3 and cyclin E mRNA expression as compared to their respective controls, further supporting the observed increases in protein levels following aniline exposure.
Fig. 5.
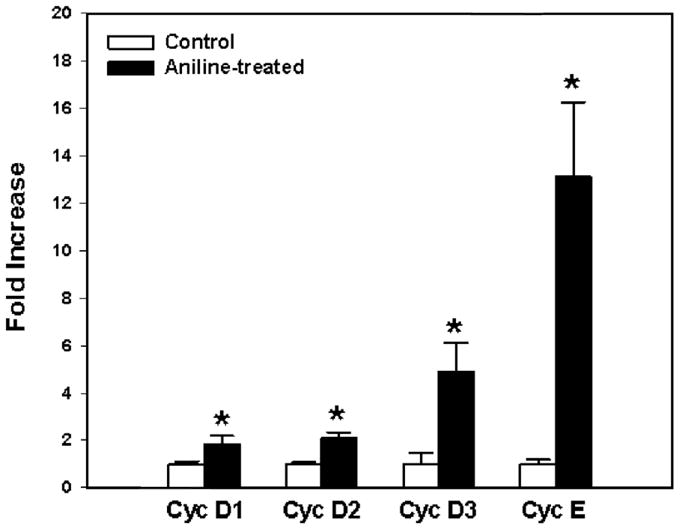
Real-time PCR analysis of cyclins D1, D2, D3 and E gene expression in the spleens of control and aniline-treated rats. Total RNA was extracted from spleens, real-time PCR was performed, and the fold changes in mRNA expression (2−ΔΔCT) were determined. Values are means ± SD ( n = 3). p < 0.05 vs. respective controls.
Aniline exposure increases the expression of CDKs
CDKs are core components of the cell cycle machinery and the vital regulators driving cell cycle progression (Malumbres and Barbacid, 2001; Olashaw and Pledger, 2002; Chulu and Liu, 2009; Malumbres and Barbacid, 2009). CDKs are enzymatically active when associated with cyclines, and three sets of cyclin-CDK complexes including cyclin D-CDK4, cyclin D/CDK6 and cyclin E-CDK2 complexes are sequentially assembled and activated during the G1 phase (Malumbres and Barbacid, 2001; Chulu and Liu, 2009). In order to evaluate the roles of CDKs in the cell cycle regulation of splenocytes, expression of CDK2, CDK4 and CDK6 was analyzed by using Western blot as presented in Fig. 6. CDK2, CDK4 and CDK6 protein expression in aniline-treated rats showed 338%, 343% and 556% increases, respectively, in comparison to the controls. These findings further suggest that aniline exposure has a stimulatory effect on the expression of cell cycle regulatory proteins.
Fig. 6.

Protein expression of cyclin-dependent kinases (CDKs) in rat spleens after aniline exposure. (A) Western blot detection of CDK2, CDK4 and CDK6 in the spleens of control and aniline-treated rats. (B) Densitometric analysis of CDKs bands. Values are mean ± SD (n=3); *p < 0.05.
Aniline exposure promotes pRB phosphorylation
The retinoblastoma protein (pRB) and pRB-related p107 and p130 comprise the “pocket protein” family, and are the primary substrates for CDK4/6 and CDK2 in G1 progression (Lundberg and Weinberg, 1998; Harbour et al., 1999; Malumbres and Barbacid, 2001; Cobrinik, 2005). Phosphorylation of pRB causes the inactivation of the growth inhibitory function of pRB via releasing E2F from an inhibitory complex, enabling it to promote the transcription necessary for progression into late G1 and S phase (Weinberg, 1995; Lundberg and Weinberg, 1998; Cobrinik, 2005). As evident from Fig 7, aniline treatment resulted in about ~8 fold increase in phospho-pRb protein expression in the spleens, whereas no changes were observed in total pRB, suggesting the importance of pRB phosphorylation in cell cycle progression following aniline exposure.
Fig. 7.
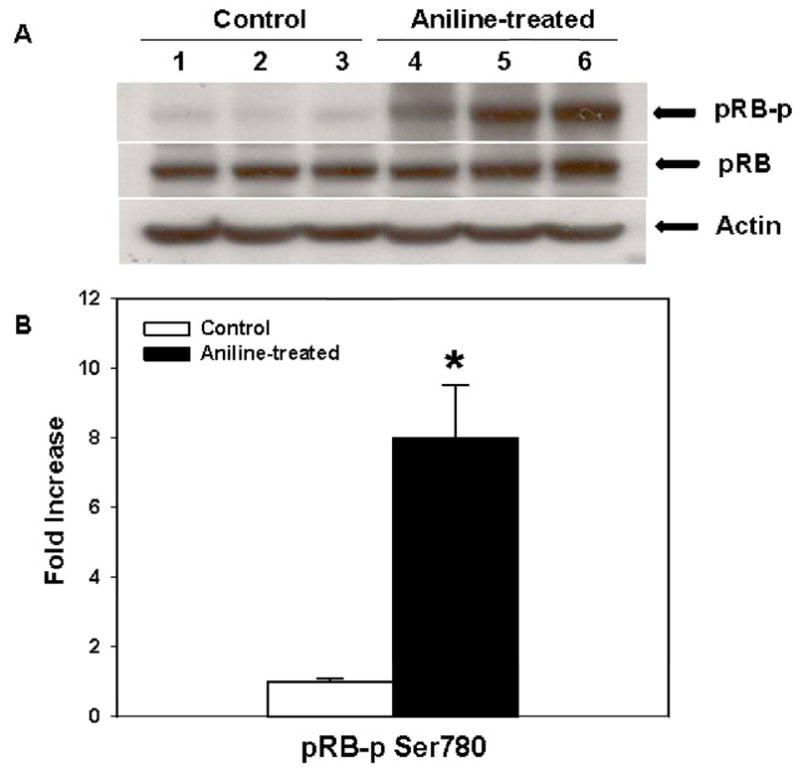
Phosphorylated pRB and pRB protein expression in rat spleens after aniline exposure. (A) Western blot detection of phosphorylated pRB and pRB protein expression in the spleens of control and aniline-treated rats. (B) Densitometric analysis of protein bands. Values are mean ± SD (n=3); *p < 0.05.
Discussion
Exposure to aniline is associated with toxicity to the spleen, which is characterized by splenomegaly, hyperplasia, fibrosis, and a variety of sarcomas on chronic exposure in rats (Goodman et al., 1984; Weinberger et al., 1985; Bus and Popp, 1987; Khan et al., 1993, 1999a, 2006; Pauluhn, 2004). Splenomegaly is one of the earliest characteristic features of aniline insult (Goodman et al., 1984; Weinberger et al., 1985; Bus and Popp, 1987; Khan et al., 1993, 1997, 2006). Despite the well-documented splenotoxic effects and tumorigenic responses resulting from aniline exposure (Bus and Popp, 1987; Khan et al., 1999a, 2006), precise cellular and molecular events and consequences of such events to eventual formation of fibrosarcomas are not known. In the present study, we report deregulation of G1 phase cyclins, enhanced expression of CDKs and phosphorylation of pRB in aniline-induced cellular proliferation in the spleen.
Consistent with earlier studies, we not only found that the spleens from the rats treated with aniline appeared dark and remarkably enlarged (Khan et al., 1999a, 1999b, 2003a, 2003b, 2006), but also observed substantial increases in the splenocyte population, suggesting that the splenomegaly is due not only to the deposition of damaged erythrocytes, but also to increased recruitment and/or proliferation of splenocytes. To further verify if aniline exposure indeed induces cellular proliferation and contributes to splenomegaly, molecular biomarkers of cell proliferation were evaluated. The most widely used conventional cell proliferation markers, PCNA and Ki67, identify proliferating cells because their expression coincides with DNA synthesis (Scholzen and Gerdes, 2000; Stuart-Harris et al., 2008). The observed increases in protein expression of both PCNA and Ki67 in this study suggest that aniline exposure promotes cell proliferation in the spleen. Since the cell proliferation marker PCNA might not be expressed until late G1, an extended stage in many proliferating cells, one more marker MCM2 was also evaluated. The MCM family of proteins (MCMs 2–7) are expressed at high levels in proliferating cells but down-regulated following cell cycle exit, making them very useful in distinguishing cycling cells from quiescent cells (Blow and Hodgson, 2002; Sington et al., 2004; Korkolopoulou et al., 2005). MCMs, therefore, serve as useful biomarkers of cell cycle progression, and deregulation of MCM function may contribute to tumorigenesis (Sington et al., 2004; Korkolopoulou et al., 2005; Lei, 2005). Aniline treatment in this study also led to greater MCM2 expression in the spleens. Taken together, the results from above three biomarkers, especially MCM2, as well as the increases in the spleen weight and splenocyte population, are not only indicative of cellular proliferation, but also suggest a potential progression to a tumorigenic response in the spleen.
To further evaluate the proliferation of splenocytes, MTT assay and flow cytometric analysis for splenocyte proliferation was conducted ex vivo. Both MTT and flow cytometric analyses data showed significantly increased proliferation rate for splenocytes from aniline-treated rats. The MTT data generally represents the final account of cell proliferation, and this assay can not exhibit the complexity of the cell proliferative processes in vitro (Mosmann, 1983; Muul et al., 2008). However, this limitation was overcome by flow cytometric assessment which measures the proliferation of individual cells and the cell cycle progression via visualizing up to 10 discrete cycles of cell division (Lyons, 2000; Muul et al., 2008). Thus, it is evident from the data on cell proliferation markers, MTT assay and flow cytometry that aniline exposure not only induced a proliferative response in vivo, but the splenocytes were also “primed” or “activated” and entered cell cycle progression in culture (ex vivo). Even though the mechanism(s) by which aniline induces a proliferative response for splenocytes is not known, one possibility appears to be iron overload and generation of reactive oxygen species (oxidative stress) which may serve as mitogenic stimulators, initiating the cell cycle progression from G0 and through the G1-S restriction point (Emmendoerffer et al., 2000; Pietrangelo, 2003; Brown et al., 2006; Olguín-Martínez et al., 2006; Kinoshita et al., 2007; Menon and Goswami, 2007; Sarsour et al., 2009).
Even though our data provided a compelling evidence that aniline exposure induces greater splenocyte proliferation, molecular mechanisms how aniline leads to such response is not known. Cell cycle progression plays pivotal role in cell proliferation, and the mechanisms of cell-cycle regulation in aniline-induced splenic toxicity remain unexplored. To overcome this, we especially focused on unraveling how aniline treatment influences the transition from quiescence to growth, the step considered as critical in cell cycle progression. This interval is particularly important since in adult tissues, most cells are withdrawn from the cell cycle and maintained in a quiescent state (G0), and cells in adult spleens would presumably re-enter the cell cycle and resume proliferation only in response to injury activation, or to replace cells lost during normal function (Pardee, 1989). G1 phase is regulated by cyclin D-CDK4, cyclin D-CDK6 and cyclin E-CDK2 complexes (Hunter and Pines, 1994; Sherr, 1995). Cell cycle progression is driven by changes in cyclin-CDK complexes, and if control of the cell cycle is disrupted, progress through the cycle might be stimulated by overexpressed cyclins, enhanced CDK activity or inactivated CDK inhibitors (Hunter and Pines, 1994; Sherr, 1995; Malumbres and Barbacid, 2001; Chulu and Liu, 2009).
In this study, we not only observed significantly increased protein expression of cyclins (D1, D2, D3, and E), and CDKs (CDK2, CDK4, CDK6), but also the mRNA expression of cyclins D1, D2, D3 and cyclin E in the spleens of aniline-treated rats. Their overexpression could contribute to cellular proliferation and/or lead to uncontrolled cellular proliferation in the spleen. The D-types cyclins (D1, D2, and D3) are components of the core cell cycle machinery, the most prominent regulators in the phosphorylation of pRB, and the ultimate recipients of mitogenic and oncogenic signals in mammalian cells (Weinberg, 1995; Bartkova et al., 1998; Sicinska et al., 2003; Menon and Goswami, 2007). Based on cyclin D expression, mammalian cells can be subdivided into five broad categories: (i) those expressing all three D-type cyclins; (ii) cells with cyclin D1 and D3; (iii) cells with cyclin D2 and D3; (iv) cells expressing cyclin D3 only; and (v) cells apparently lacking any of the cyclin D proteins (Bartkova et al., 1998). Our findings suggest that the splenocytes from aniline-treated rats fall in the first category, since all three D-type cyclins (D1, D2, D3) were co-expressed in these cells. However, both protein and mRNA expression of cyclin D3 were highest in those cells. Even through cyclin D3 is the most widely expressed cyclin, and is essential for specific oncogenic pathways in mammalian cells, the three D-type cyclins may work in concert to provide the cyclin D-dependent kinase activity required to promote G1/S progression, a potential step in the tumorigenesis (Weinberg, 1995; Bartkova et al., 1998; Sicinska et al., 2003). CDKs play a key role in cell cycle regulation, and CDK4 and CDK2 appear to be the most prominent partners of cyclins in macrophages and fibroblasts (Matsushime et al., 1992; Olashaw and Pledger, 2002), while cyclin D-CDK6 complexes predominate in peripheral blood T cells (Meyerson and Harlow, 1994). Interestingly, our data showed increased expression of CDK6 (556%), CDK4 (343%) and CDK2 (338%) in the spleens of aniline-treated rats. The highest increases in CDK6 would rather be expected as rat spleen has more T lymphocytes than macrophages and fibroblasts.
pRB family plays an important role in restraining the G1-S transition as a guardian by shutting the R point gate (Weinberg, 1995; Lundberg and Weinberg, 1998, 1999). Cyclin D-CDK4/6 and cyclin E-CDK2 complexes can sequentially phosphorylate the pRBs (Weinberg, 1995; Lundberg and Weinberg, 1998; Harbour et al., 1999), and this hyper-phosphorylated proteins release the E2F transcription factors that are required for the S phase entry (Sherr and Roberts, 1999; Sherr, 2000). It is generally accepted that hyperphosphorylation of pRB by multiple cyclin/CDK complexes in late G1-phase is required for its inactivation (phosphorylation) and cell cycle progression (Hatakeyama et al., 1994; Weinberg, 1995; Lundberg and Weinberg, 1998; Harbour et al., 1999). Cyclin D-CDK4/6 becomes active in mid to late G1-phase, whereas cyclin E-CDK2 in late G1-phase. Also, cyclin D-CDK4/6 induces a conformational change that permits phosphorylation by cyclin E-CDK2 (Lundberg and Weinberg, 1998; Harbour et al., 1999). Overexpression of G1 and G1-S phase cyclins and CDK proteins including cyclins D1, D2, D3, cyclin E and CDK2, CDK4 and CDK6 in the spleens from aniline-treated rats, as observed in this study, would thus be expected to contribute to increased phospho-pRB. Since pRB phosphorylation is a key molecular event leading to the S-phase commitment at the G1 restriction point in the cell cycle, the increased pPB phosphorylation may provide an explanation why spleen cells could proliferate ex vivo without further stimulation.
In conclusion, our data clearly suggest that increased cellular proliferation contributes to splenomegaly in rats treated with aniline, which is evidenced by increased splenocyte population, overexpressed cell proliferation marker proteins, PCNA, Ki67 and MCM2, and greater splenocyte proliferation rate ex vivo. These findings suggest that the splenocytes have gone through G1-S transition and could potentially lead to a tumorigenic response on chronic exposure (Goodman et al., 1984; Weinberger et al., 1985). Greater release and presence of iron in the spleen after aniline exposure, resulting in increased oxidative stress (Khan et al., 1997, 2003a, 2003b; Wang et al., 2005, 2010; Ma et al., 2008), may serve as mitogenic stimulator in initiating cell cycle progression, upregulation of cell cycle regulatory proteins, including cyclins D1, D2, D3, E and CDKs as observed in this study (Pietrangelo, 2003; Brown et al., 2006; Olguín-Martínez et al., 2006; Kinoshita et al., 2007; Menon and Goswami, 2007). These overexpressed proteins may subsequentially phosphorylate pRB, allowing the cells to go through R point, leading to S-phase commitment. To our knowledge, this is the first study to investigate the expression of proteins regulating the cell cycle machinery, including cyclins, CDKs and pRB in G1 phase and their contributions to aniline-induced splenic toxicity. The findings on splenocyte proliferation and deregulation of cell cycle proteins in G1 phase not only provide a better understanding of the mechanisms of splenic toxicity of aniline, but also open a new avenue for investigating the role of iron overload/oxidative stress in diseases involving cell cycle regulation. However, more studies are needed to explore the precise molecular mechanisms of splenic toxicity, including the initiation of cell cycle progression, the interaction of cell cycle regulatory genes/proteins, and involvement of proteins in other phases (S, G2 and M) of the cell cycle.
Acknowledgments
This work was supported by Grant ES06476 from the National Institute of Environmental Health Sciences (NIEHS), NIH, and it contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Footnotes
Conflict of interest statement
The authors declare that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartkova J, Lukas J, Strauss M, Bartek J. Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene. 1998;17:1027–1037. doi: 10.1038/sj.onc.1202016. [DOI] [PubMed] [Google Scholar]
- Blow JJ, Hodgson B. Replication licensing-defining the proliferative state? Trends Cell Biol. 2002;12:72–78. doi: 10.1016/s0962-8924(01)02203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KE, Mathahs MM, Broadhurst KA, Weydert J. Chronic iron overload stimulates hepatocyte proliferation and cyclin D1 expression in rodent liver. Transl Res. 2006;148:55–62. doi: 10.1016/j.trsl.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Bus JS, Popp JA. Perspectives on the mechanism of action of the splenic toxicity of aniline and structurally-related compounds. Food Chem Toxicol. 1987;25:619–626. doi: 10.1016/0278-6915(87)90024-x. [DOI] [PubMed] [Google Scholar]
- Chulu JL, Liu HJ. Recent patents on cell cycle regulatory proteins. Recent Pat Biotechnol. 2009;3:1–9. doi: 10.2174/187220809787172614. [DOI] [PubMed] [Google Scholar]
- Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- Di Girolamo F, Campanella L, Samperi R, Bachi A. Mass spectrometric identification of hemoglobin modifications induced by nitrosobenzene. Ecotoxicol Environ Saf. 2009;72:1601–1608. doi: 10.1016/j.ecoenv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Emmendoerffer A, Hecht M, Boeker T, Mueller M, Heinrich U. Toxicol Lett. 112–113. 2000. Role of inflammation in chemical-induced lung cancer; pp. 185–191. [DOI] [PubMed] [Google Scholar]
- Goodman DG, Ward JM, Reichardt WD. Splenic fibrosis and sarcomas in F344 rats fed diets containing aniline hydrochloride, p-chloroaniline, azobenzene, o-toluidine hydrochloride, 4,4′-sulfonyldianiline, or D & C red No. 9. J Natl Cancer Inst. 1984;73:265–273. [PubMed] [Google Scholar]
- Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M, Brill JA, Fink GR, Weinberg RA. Collaboration of G1 cyclins in the functional inactivation of the retinoblastoma protein. Genes Dev. 1994;8:1759–1771. doi: 10.1101/gad.8.15.1759. [DOI] [PubMed] [Google Scholar]
- Hunter T, Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Keyomarsi K, Conte D, Jr, Toyofuku W, Fox MP. Deregulation of cyclin E in breast cancer. Oncogene. 1995;11:941–950. [PubMed] [Google Scholar]
- Khan MF, Kaphalia BS, Boor PJ, Ansari GAS. Subchronic toxicity of aniline hydrochloride in rats. Arch Environ Contam Toxicol. 1993;24:368–374. doi: 10.1007/BF01128736. [DOI] [PubMed] [Google Scholar]
- Khan MF, Boor PJ, Gu Y, Alcock NW, Ansari GAS. Oxidative stress in the splenotoxicity of aniline. Fundam Appl Toxicol. 1997;35:22–30. doi: 10.1006/faat.1996.2259. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Boor PJ, Ansari GAS. Oxidative modification of lipids and proteins in aniline-induced splenic toxicity. Toxicol Sci. 1999a;48:134–140. doi: 10.1093/toxsci/48.1.134. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Alcock NW, Boor PJ, Ansari GAS. Iron exacerbates aniline-associated splenic toxicity. J Toxicol Environ Health, Part A. 1999b;57:173–184. doi: 10.1080/009841099157746. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Ansari GAS, Boor PJ. Malondialdehyde-protein adducts in the spleens of aniline-treated rats: immunochemical detection and localization. J Toxicol Environ Health, Part A. 2003a;66:93–102. doi: 10.1080/15287390306464. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Kaphalia BS, Boor PJ, Ansari GAS. Nitrotyrosine formation in splenic toxicity of aniline. Toxicology. 2003b;194:95–102. doi: 10.1016/j.tox.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Khan MF, Kannan S, Wang J. Activation of transcription factor AP-1 and mitogen-activated protein kinases in aniline-induced splenic toxicity. Toxicol Appl Pharmacol. 2006;210:86–93. doi: 10.1016/j.taap.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Wanibuchi H, Wei M, Yunoki T, Fukushima S. Elevation of 8-hydroxydeoxyguanosine and cell proliferation via generation of oxidative stress by organic arsenicals contributes to their carcinogenicity in the rat liver and bladder. Toxicol Appl Pharmacol. 2007;221:295–305. doi: 10.1016/j.taap.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Korkolopoulou P, Givalos N, Saetta A, Goudopoulou A, Gakiopoulou H, Thymara I, Thomas-Tsagli E, Patsouris E. Minichromosome maintenance proteins 2 and 5 expression in muscle-invasive urothelial cancer: a multivariate survival study including proliferation markers and cell cycle regulators. Hum Pathol. 2005;36:899–907. doi: 10.1016/j.humpath.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Lei M. The MCM complex: its role in DNA replication and implications for cancer therapy. Curr Cancer Drug Targets. 2005;5:365–380. doi: 10.2174/1568009054629654. [DOI] [PubMed] [Google Scholar]
- Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg AS, Weinberg RA. Control of the cell cycle and apoptosis. Eur J Cancer. 1999;35:531–539. [PubMed] [Google Scholar]
- Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Meth. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- Ma H, Wang J, Abdel-Rahman SZ, Boor PJ, Khan MF. Oxidative DNA damage and its repair in rat spleen following subchronic exposure to aniline. Toxicol Appl Pharmacol. 2008;233:247–253. doi: 10.1016/j.taap.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1:222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- Matsushime H, Ewen ME, Strom DK, Kato JY, Hanks SK, Roussel MF, Sherr CJ. Identification and properties of an atypical catalytic subunit (p34PSKJ3/CDK4) for mammalian D-type GI cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26:1101–1109. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- Muul LM, Silvin C, James SP, Candotti F. Measurement of proliferative responses of cultured lymphocytes. Curr Protoc Immunol. 2008;Chapter 7(Unit 7.10.1—7.10.24) doi: 10.1002/0471142735.im0710s82. [DOI] [PubMed] [Google Scholar]
- Olashaw N, Pledger WJ. Paradigms of growth control: relation to Cdk activation. Sci STKE. 2002;2002:1–14. doi: 10.1126/stke.2002.134.re7. [DOI] [PubMed] [Google Scholar]
- Olguín-Martínez M, Mendieta-Condado E, Contreras-Zentella M, Escamilla JE, Aranda-Fraustro A, El-Hafidi M, Hernández-Muñoz R. Rate of oxidant stress regulates balance between rat gastric mucosa proliferation and apoptosis. Free Radic Biol Med. 2006;41:1325–1337. doi: 10.1016/j.freeradbiomed.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Park DH, Shin JW, Park SK, Seo JN, Li L, Jang JJ, Lee MJ. Diethylnitrosamine (DEN) induces irreversible hepatocellular carcinogenesis through overexpression of G1/S-phase regulatory proteins in rat. Toxicol Lett. 2009;191:321–326. doi: 10.1016/j.toxlet.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Subacute inhalation toxicity of aniline in rats: analysis of time-dependence and concentration-dependence of hematotoxic and splenic effects. Toxicol Sci. 2004;81:198–215. doi: 10.1093/toxsci/kfh187. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A. Iron-induced oxidant stress in alcoholic liver fibrogenesis. Alcohol. 2003;30:121–129. doi: 10.1016/s0741-8329(03)00126-5. [DOI] [PubMed] [Google Scholar]
- Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- Sicinska E, Aifantis I, Le Cam L, Swat W, Borowski C, Yu Q, Ferrando AA, Levin SD, Geng Y, von Boehmer H, Sicinski P. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4:451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Sington JD, Freeman A, Morris LS, Vowler SL, Arch BN, Fisher C, Coleman N. Minichromosome maintenance protein in myxofibrosarcoma. Mod Pathol. 2004;17:235–240. doi: 10.1038/modpathol.3800044. [DOI] [PubMed] [Google Scholar]
- Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17:323–334. doi: 10.1016/j.breast.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Swenberg JA, Barrow CS, Boreiko CJ, Heck HD, Levine RJ, Morgan KT, Starr TB. Non-linear biological responses to formaldehyde and their implications for carcinogenic risk assessment. Carcinogenesis. 1983;4:945–952. doi: 10.1093/carcin/4.8.945. [DOI] [PubMed] [Google Scholar]
- Wang J, Kannan S, Li H, Khan MF. Cytokine gene expression and activation of NF-kappa B in aniline-induced splenic toxicity. Toxicol Appl Pharmacol. 2005;203:36–44. doi: 10.1016/j.taap.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang G, Ansari GAS, Khan MF. Activation of oxidative stress-responsive signaling pathways in early splenotoxic response of aniline. Toxicol Appl Pharmacol. 2008;230:227–234. doi: 10.1016/j.taap.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ma H, Boor PJ, Ramanujam VM, Ansari GAS, Khan MF. Up-regulation of heme oxygenase-1 in rat spleen after aniline exposure. Free Radic Biol Med. 2010;48:513–518. doi: 10.1016/j.freeradbiomed.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger MA, Albert RH, Montgomery SB. Splenotoxicity associated with splenic sarcomas in rats fed high doses of D & C Red No. 9 or aniline hydrochloride. J Natl Cancer Inst. 1985;75:681–690. [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Williams GM, Iatropoulos MJ. Alteration of liver cell function and proliferation: differentiation between adaptation and toxicity. Toxicol Pathol. 2002;30:41–53. doi: 10.1080/01926230252824699. [DOI] [PubMed] [Google Scholar]


