Abstract
Syphilis is a frequent coinfection with human immunodeficiency virus (HIV). Whereas systemic syphilis infection increases plasma HIV RNA levels (viral load; VL), effects of syphilis on cerebrospinal fluid (CSF) VL are unknown. We hypothesized that intrathecal immune activation in neurosyphilis would selectively increase CSF VL in coinfected patients. In this study, HIV-infected research subjects (N = 225) were categorized into three groups based on serum rapid plasma reagin (RPR), microhemaglutination for Treponema pallidum (MHA-TP) MHA-TP, and CSF VDRL: 23 with neurosyphilis (NS +; reactive serum RPR and MHA-TP and positive CSF VDRL); 42 with systemic syphilis but not neurosyphilis (Syph+; reactive serum RPR and MHA-TP; negative CSF VDRL), and 160 without syphilis (Syph−; nonreactive serum RPR). Plasma and CSF HIV VL were quantified by reverse transcriptase–ploymerase chain reaction (RT-PCR) (Amplicor, Roche) in log10 copies/ml. To adjust for covariates previously shown to influence CSF HIV VL (i.e., plasma VL, CD4, pleocytosis, and highly active antiretroviral therapy [HAART]), multivariable linear regression was used. Lumbar punctures (LP) done for research purposes diagnosed 23 with neurosyphilis; most (83%) of these reported prior syphilis treatment. Among subjects with detectable plasma VL, CSF VL was highest in NS+, followed by Syph+ and Syph− (P =.006). This relationship was independent of the level of plasma VL or CSF pleocytosis. By contrast, among subjects with undetectable plasma HIV VL, CSF VLs were similar in the three syphilis subgroups (P = .50). Neurosyphilis may amplify intrathecal HIV replication, possibly through immune activation that persists even after syphilis treatment. Because elevated CSF VL is associated with subsequent neurocognitive decline, future studies should evaluate the impact of neurosyphilis on the course of central nervous system (CNS) HIV infection.
Keywords: neurosyphilis, CSF, HIV, RNA, immune activation, syphilis, intrathecal activation, viral load
Introduction
Individuals are often coinfected with human immunodeficiency virus (HIV) and Treponema pallidum because both of these infections are sexually transmissible (Timmermans and Carr, 2004). Both pathogens invade and replicate in the central nervous system (CNS) shortly after exposure; and may repeatedly invade the CNS during the course of prolonged latent infections. Coinfection is increasingly important because of the high global prevalence of both infections and a growing epidemic among men who have sex with men (MSM) in large cities in North America, Europe, and Latin America (Golden et al. 2003; Griemberg et al. 1997, 2006; Simms et al. 2005; Simon, 2004).
HIV infection has been shown to alter both the clinical manifestations and diagnosis of syphilis. For example, HIV-infected patients have a higher frequency of syphilitic ophthalmologic and neurological involvement (Chan, 2005; Lynn and Lightman, 2004) and false-negative syphilis serologic testing (Chan, 2005; Lynn and Lightman, 2004; Marra et al. 2004a, 2004b). However, little attention has been given to the impact of syphilis on HIV-induced CNS disease. Primary syphilis transiently increases systemic HIV replication as measured by plasma viral load (VL) (Buchacz et al. 2004), but the effect of syphilis and neurosyphilis on HIV in the cerebrospinal fluid (CSF) has not been evaluated. In this study, we evaluated the hypothesis that HIV and Treponema pallidum coinfection, even after antitreponemal therapy, results in long-term increases in HIV viral replication in the CNS.
Results
As described in Table 1, subjects were typically HIV+ men in their early 40s with a history of advanced HIV disease (CD4 nadir < 200 cells/µl), y of whom had experienced substantial immune reconstitution with antiretroviral treatment. In the NS+ group, 18 subjects reported a history of syphilis and prior treatment, 1 reported neurosyphilis, and 4 reported no history of syphilis. Most (68.8%) were taking antiretroviral therapy, yet plasma VL was detectable in about two thirds (65%). The Syph+ group was less likely than the other two groups to be taking antiretroviral therapy, probably because of higher CD4 nadirs (Table 1). Antiretroviral regimen types differed for the three groups, as shown in Table 2. The proportion of subjects on “other” regimens types was highest for the Syph+ group compared to the Syph− and NS+ groups (P =.0261, chi-square). However, CSF viral loads did not differ by regimen type (P = .70, ANOVA).
Table 1.
Demographic and clinical characteristics
| All | Syph− | Syph+ | NS+ | P | |
|---|---|---|---|---|---|
| N | 225 | 160 | 42 | 23 | |
| Age (years)1 | 40 (7.2) | 40.2 (6.1) | 39.7 (10.3) | 39.2 (7.1) | .65 |
| Male3 | 223 (99.1%) | 160 (100%) | 40 (95.2%) | 23 (100%) | .04 |
| Current CD4 (cells/µl)2 | 357 (194–543) | 345 (181–544) | 405 (245–533) | 388 (208–561) | .8 |
| CD4 nadir (cells/µl)2 | 191 (42–319) | 175 (29–300) | 274 (90–407) | 200 (97–296) | .04 |
| Log10 plasma VL (c/ml)2 | 3.2 (2.3–4.5) | 2.9 (2.3–4.5) | 3.7 (2.8–4.6) | 2.3 (2.7–4.7) | .19 |
| Plasma VL detectable3,5 | 145 (65%) | 99 (62%) | 33 (79%) | 13 (56%) | .08 |
| On ART3,4 | 154 (68.8%)4 | 113 (70.6%) | 24 (58.5%)4 | 17 (73.9%) | .28 |
| On HAART3,4 | 134 (59.8%)4 | 107 (66.9%) | 13 (31.7%)4 | 14 (60.9%) | <.0001 |
Mean (Standard deviation);
Median (Interquartile range);
N (%);
ARV information was available for 224/225 subjects (41/42 Syph+);
VLs were considered suppressed (limit of sensitivity of the assay) at 2.3 log10 copies/ml for plasma, and 1.7 log10 copies/ml for CSF.
Syph+: reactive serum RPR and MHA-TP; negative CSF VDRL.
NS+: positive CSF VDRL, reactive serum RPR and MHA-TP.
Syph−: nonreactive serum RPR.
Table 2.
Regimen types for subjects taking antiretroviral therapy (ART)
| All | Syph− | Syph+ | NS+ | P | |
|---|---|---|---|---|---|
| N | 154 | 113 | 24 | 17 | 0 |
| NNRTI-based regimen1 | 40 (26.0%) | 30 (18.8%) | 5 (20.8%) | 5 (21.7%) | 0.6 |
| PI-based regimen1 | 70 (45.5%) | 56 (35%) | 5 (20.8%) | 9 (39.1%) | 0.1 |
| Other regimen1 | 44 (28.6%) | 27 (16.9%) | 14 (58.3%) | 3 (13.0%) | 0 |
N (%).
Syph+: reactive serum RPR and MHA-TP; negative CSF VDRL.
NS+: positive CSF VDRL, reactive serum RPR and MHA-TP.
Syph−: nonreactive serum RPR.
We evaluated antiretroviral (ARV) CNS penetration using a validated ranking system (Letendre et al. 2008). The CNS penetration effectiveness (CPE) scores were not significantly associated with CSF VL in these subjects (Spearman’s rho −.068; P =.557). Further, subjects in the NS+ group did not have lower CPE scores than those in the Syph+ or Syph− group.
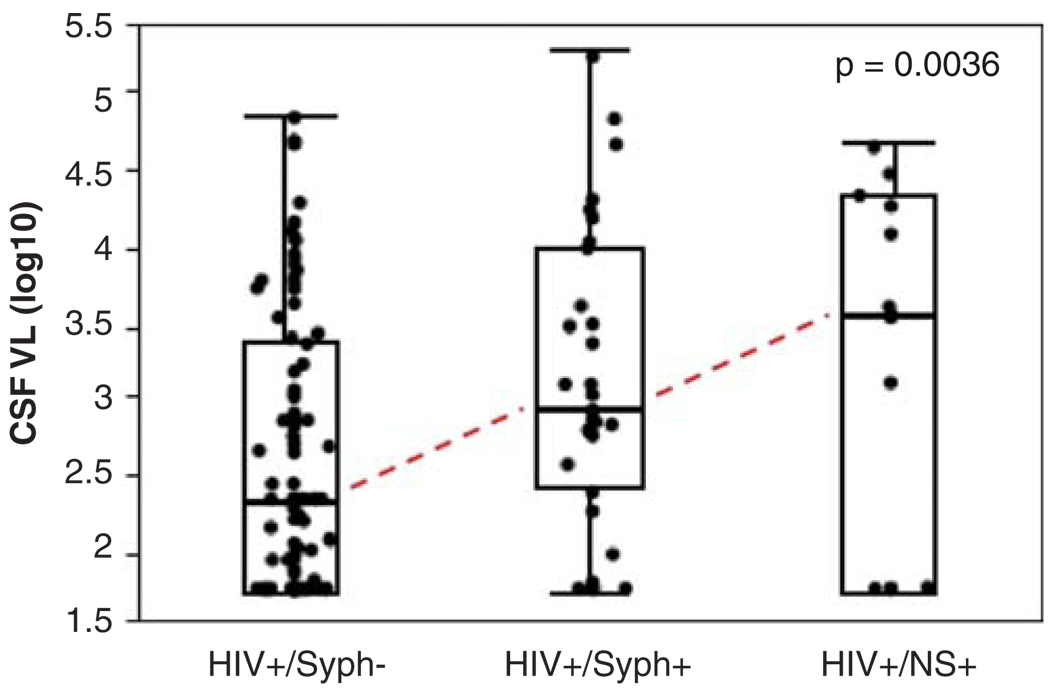
As shown in Figure 1, among patients with detectable plasma VL (n = 145), CSF VL was highest in the NS+ group (n = 13; median 3.5 [interquatile range {IQR} 1.7, 4.3]), followed by Syph+ (n = 31; median 2.9 [IQR 2.3, 4.0]), and Syph− (n = 99; median 2.3 [IQR 1.7, 3.4]) (P =.0036). These findings were not affected by excluding the two women in the Syph+ group (P=.0023). In a multivariate model, among subjects with detectable plasma VL, the effects of neurosyphilis and syphilis on CSF VL were independent of plasma VL (full model R2 = .176; plasma VL, t ratio = 4.39, P < .0001; syphilis, t ratio = −2.86, P =.0049).
Figure 1.
Among HIV+ patients with detectable plasma VL; CSF VL was highest in those with neurosyphilis, followed by those with serologic evidence of syphilis, but not neurosyphilis and those without syphilis coinfection. Among subjects with undetectable plasma VL (data not shown), all but two had undetectable CSF VL.
As shown in Table 3, CSF pleocytosis (WBC > 5 cells/µl) was more common in Syph+ (48%) as compared to NS+ (28%) and Syph− (16%) (P < .0001). After adjusting for CSF WBC in a multivariate regression model, CSF VL remained highest in NS+ as compared to Syph+ and Syph− (least squares means 3.2, 3.0, 2.6; P =.003). Increased pleocytosis in Syph+ subjects was likely explained by higher current and nadir CD4 counts in this group (Table 1); numerous previous studies have documented increased trafficking of lymphocytes from blood into CSF in HIV-infected persons with less immunosuppression (Andersson et al. 1988; Marra et al. 2007; Spudich et al. 2005).
Table 3.
CSF characteristics
| All | Syph− | Syph+ | NS+ | P | |
|---|---|---|---|---|---|
| N1 | 225 | 160 | 42 | 23 | |
| CSF total protein (mg/dl)2 | 39 (31–48) | 38 (31–47) | 41 (34–54) | 35 (30–52) | .2 |
| CSF glucose (mg/dl)2 | 62 (56–66) | 62 (57–67) | 62 (56–66) | 60 (54–66) | .56 |
| CSF WBC (cells/µl)2 | 2 (1–4) | 2 (1–3) | 2 (1–10) | 2 (1–7) | .0008 |
| % CSF WBC > 5/µl3 | 51 (23%) | 25 (16%) | 20 (48%) | 5 (28%) | <.0001 |
Mean (Standard deviation);
Median (Interquartile range);
N (%).
Syph+: reactive serum RPR and MHA-TP; negative CSF VDRL.
NS+: positive CSF VDRL, reactive serum RPR and MHA-TP.
Syph−: nonreactive serum RPR.
Discussion
Among HIV-infected individuals with detectable plasma VL, we found higher CSF VL in those with serologic evidence of neurosyphilis (NS+) than in subjects with either systemic syphilis alone or no syphilis coinfection. The pattern of results was the same after adjusting for important covariates such as plasma VL and CSF pleocytosis. Previous studies have demonstrated that vaccination and acute systemic infections including syphilis are associated with increases in plasma VL. Acute CNS opportunistic infections also increase CSF VL, but this study is the first to show that chronic neurosyphilis is associated with elevated CSF VL.
We cannot attribute the increase in CSF VL to active neurosyphilis for several reasons. First, most NS+ subjects reported a prior diagnosis of and appropriate treatment for syphilis. Second, these were ambulatory volunteers undergoing routine lumbar punctures for research purposes who reported no neurological symptoms suggestive of active neurosyphilis. Finally, indicators of disease activity such as pleocytosis and elevated protein levels were no more common in the NS+ group than in those without syphilis. Thus, the increases in CSF VL in subjects with positive CSF VDRL occurred in the absence of clinical or inflammatory indicators of active neurosyphilis.
As CSF viral loads did not differ by regimen type, regimen type could not account for the group differences in CSF viral load. We also considered the possibility that differential CNS penetration of the antiretroviral regimens prescribed to subjects in the three groups might explain higher CSF viral load in the neurosyphilis group. For example, if subjects in the NS+ group had more poorly penetrating regimens than those in the other groups, this could be reflected in higher CSF viral loads. However, CNS penetration was not significantly associated with CSF VL and subjects in the NS+ group did not have significantly lower penetration scores than those in the Syph+ or Syph− group.
Higher CSF HIV VL levels may be a consequence of intrathecal immune activation and subsequent amplification of HIV replication in CSF. Increased cellular activation by coinfection may enhance the surface expression of HIV receptors and coreceptors (CD4, CCR5, CXCR4), facilitating HIV cell entry. Furthermore, transcription of viral genes—even in the absence of replication—may be up-regulated by cytokines expressed in response to coinfection.
These immune effects may persist substantially beyond the period of active coinfection. The adverse effect of CNS neurological coinfections such as neurosyphilis on clinical outcomes of HIV CNS infection may be explained in part by persisting intrathecal immune disturbances that enhance CNS HIV replication. Such increased CNS replication might promote genotypic diversity (Buchacz et al. 2004), increasing the likelihood that transient CNS infection will evolve into autonomous infection (Staprans et al. 1999), with subsequent increased risk of developing an HIV-related neurocognitive disorder including dementia.
Treponema pallidum coinfection may be particularly likely to interact with HIV because the two pathogens share the same antigen-presenting cells. Thus, CNS tissue macrophages and microglia may present treponemal antigens to lymphocytes. These cells constitutively express major histocompatibility complex (MHC) class I, and after stimulation by coinfection or cytokine activation (e.g., interferon [IFN]-γ) may also express MHC class II (Kreutzberg, 1996; Sedgwick, 1995; Sedgwick et al. 1998). Additionally, endothelial cells of the blood-brain barrier (BBB) constitutively express MHC classes I and II, thus being able to act also as antigen-presenting cells (Sedgwick, 1995).
Coinfections such as syphilis and other chronic bacterial, parasitic, and helminthic infections tend to be more prevalent in developing countries. The majority of HIV-1–infected individuals live in these developing countries, where viral replication and disease may be accelerated by chronic immune activation caused by coinfecting pathogens (Lawn, 2004; Pennycook et al. 2000). Efforts should be geared to target treatments for both HIV itself and coinfections in subpopulations of HIV-1–infected individuals with coinfections.
Previous studies suggest that HIV infection is associated with higher rates of syphilis, more frequent recurrence of syphilis after treatment, and greater rates of recurrence of syphilis after treatment (Funnye and Akhtar, 2003; Handsfield, 2000; Johns et al. 1987; Lynn and Lightman, 2004; Nnoruka and Ezeoke, 2005; Rolfs et al. 1997). Conversely, syphilis also impacts HIV disease and treatment. Thus, for example, primary syphilitic ulcers increase HIV transmission (Sheffield et al. 2007). Recurrent syphilis can increase viral replication and worsen CD4 lymphocyte loss.
Similar potentiation of HIV replication in the CNS by neurosyphilis could predispose to a higher frequency of neurocognitive impairment (Brew et al. 1997; Ellis et al. 1997, 2002; McArthur et al. 1997). Although neuro-cognitive evaluation was not the objective of this study, a previous study by Wallace et al found that HIV-infected subjects with a history of either syphilis or gonorrhea had poorer neurocognitive performance testing than those with no such coinfection (Wallace et al. 1997). This difference was not explained by education, age, race, or CD4 count and was not observed in the HIV-uninfected control subjects. The study by Wallace et al did not address CSF VL. Our finding that CSF VL is higher in systemic syphilis than in controls is consistent with the findings of Wallace et al and suggests that systemic inflammation acting on the CNS could be the mechanism. Future studies should evaluate whether syphilis coinfection is associated with higher rates of neurocognitive impairment.
Materials and methods
Study design
We performed a retrospective comparison of three groups of participants selected from among participants in longitudinal HIV research studies undergoing routine, protocol-mandated phlebotomy and lumbar puncture (LP) performed at the National Institutes of Health (NIH)-funded HIV Neurobehavioral Research Center (HNRC) at the University of California, San Diego. Subjects included 225 HIV-infected volunteers divided into the following three groups: (a) systemic syphilis, (b) neurosyphilis, and (c) no serological evidence of syphilis. All participant visits occurred over a 14-year period between 1990 and 2004. Subjects were excluded for evidence of current CNS opportunistic infections or infectious disease other than syphilis, based on detailed neurological examination and CSF analysis and clinical follow-up. HNRC protocols are approved by the University of California San Diego (UCSD) Human Subjects Protections Committee.
All participants were HIV seropositive by both screening and confirmatory antibody tests. For subjects taking antiretrovirals (ARVs), highly active antiretroviral therapy (HAART) comprised regimens containing at least three agents. Some subjects took only one or two antiretroviral medications; these were classified as ART (non-HAART) regimens. For some analyses, regimens were further subgrouped into three categories: protease inhibitor (PI) based, non-nucleoside reverse transcriptase inhibitor (NNRTI) based, and other (e.g., three-class regimens, dual therapy).
Routine serologic screening of blood and CSF samples for syphilis began with a rapid plasma reagin (RPR) test to detect reaginic antibodies in the blood. All RPR-positive sera were confirmed by an antitreponemal antibody test, the microhemaglutination for Treponema pallidum (MHA-TP), and then screened for neurosyphilis by performing the reaginic Venereal Disease Research Laboratory (VDRL) test on CSF. If de novo evidence of syphilis was detected by laboratory testing, permission to contact the patients’ primary care provider was sought in order to coordinate appropriate medical management. Based on serologic testing, subjects were divided into three groups: (1) the neurosyphilis group (NS+) with positive serum RPR and MHA-TP and positive CSF VDRL; (2) the systemic syphilis group (Syph+) with positive serum RPR and MHA-TP and negative CSF VDRL; and the control group (Syph−) with negative serum and CSF VDRL. Most patients in the neurosyphilis group reported having been previously treated for syphilis.
Procedures
Lumbar punctures were performed under aseptic conditions using a nontraumatic spinal needle. Freshly collected, unprocessed CSF was then delivered to the clinical laboratory for hematology (cell counts and differential) and chemistry (glucose and total protein). From each subject, 9 to 15 ml of CSF was centrifuged at low speed to precipitate cells, and cell-free supernatants were separated into 1-ml aliquots, frozen, and stored at −80°C. Blood was collected by phlebotomy. Plasma and CSF were assayed for HIV RNA by a reverse transcriptase–polymerase chain reaction (RT-PCR) method (Roche Amplicor HIV-1 Monitor test).
Statistical analysis
All viral loads were log10-transformed before further analysis. Because we hypothesized that intrathecal presence of syphilis would stimulate HIV replication as compared to extrathecal syphilis or absence of syphilis, we used a nonparametric Jonckhere test for ordered categories (Syph−, Syph+, NS +) rather than a simple analysis of variance (ANOVA) to assess differences in CSF HIV VLs. Multivariable linear regression examined the predictive value of groups, plasma and CSF white blood cells (WBCs).
Another analysis was conducted to investigate a possible influence of the CNS penetration effectiveness (CPE) of the subjects’ antiretroviral regimens. CPE was estimated using a validated ranking system (Letendre et al. 2008). These CPE scores were compared to CSF VL using a rank correlation statistic (Spearman's rho). To evaluate possible confounding due to differences between the groups in antiretroviral regimen types (PI-based, NNRTI-based, or other), we performed secondary analyses using ANOVA and nonparametric Kruskal-Wallis tests.
Acknowledgments
The San Diego HIV Neurobehavioral Research Center (HNRC) group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes Director: Igor Grant, MD; Co-Directors: J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, and J. Allen McCutchan, MD; Center Manager: Thomas D. Marcotte, PhD; Heather Bentley, CCRA; Melanie Sherman; Naval Hospital San Diego: Braden R. Hale, MD, MPH (P.I.); Neuromedical Component: Ronald J. Ellis, MD, PhD (P.I.), J. Allen McCutchan, MD, Scott Letendre, MD, Edmund Capparelli, PharmD, Rachel Schrier, PhD; Jennifer Marquie-Beck; Terry Alexander, RN; Neurobehavioral Component: Robert K. Heaton, PhD (P.I.), Mariana Cherner, PhD, Steven Paul Woods, PsyD, David J. Moore, PhD; Matthew Dawson; Neuroimaging Component: Terry Jernigan, PhD (P.I.), Christine Fennema-Notestine, PhD, Sarah L. Archibald, MA, John Hesselink, MD, Jacopo Annese, PhD, Michael J. Taylor, PhD, Brian Schweinsburg, PhD; Neurobiology Component: Eliezer Masliah, MD (P.I.), Ian Everall, FRCPsych, FRCPath, PhD, Cristian Achim, MD, PhD; Neurovirology Component: Douglas Richman, MD, (P.I.), David M. Smith, MD; International Component: J. Allen McCutchan, MD, (P.I.); Developmental Component: Ian Everall, FRCPsych, FRCPath, PhD (P.I.), Stuart Lipton, MD, PhD; Clinical Trials Component: J. Allen McCutchan, MD, J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, Scott Letendre, MD; Participant Accrual and Retention Unit: J. Hampton Atkinson, MD (P.I.), Rodney von Jaeger, MPH; Data Management Unit: Anthony C. Gamst, PhD (P.I.), Clint Cushman (Data Systems Manager), Daniel R. Masys, MD (Senior Consultant); Statistics Unit: Ian Abramson, PhD (P.I.), Florin Vaida, PhD, Christopher Ake, PhD.
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award MH 62512 from NIMH.
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Andersson MA, Bergstrom TB, Blomstrand C, Hermodsson SH, Hakansson C, Lowhagen GB. Increasing intrathecal lymphocytosis and immunoglobulin G production in neurologically asymptomatic HIV-1 infection. J Neuroimmunol. 1988;19:291–304. doi: 10.1016/0165-5728(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Pemberton L, Cunningham P, Law MG. Levels of human immunodeficiency virus type 1 RNA in cerebrospinal fluid correlate with AIDS dementia stage. J Infect Dis. 1997;175:963–966. doi: 10.1086/514001. [DOI] [PubMed] [Google Scholar]
- Buchacz K, Patel P, Taylor M, Kerndt PR, Byers RH, Holmberg SD, Klausner JD. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004;18:2075–2079. doi: 10.1097/00002030-200410210-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DJ. Syphilis and HIV co-infection: when is lumbar puncture indicated? Curr HIV Res. 2005;3:95–98. doi: 10.2174/1570162052773031. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Hsia K, Spector SA, Nelson JA, Heaton RK, Wallace MR, Abramson I, Atkinson JH, Grant I, McCutchan JA. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997;42:679–688. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Moore DJ, Childers ME, Letendre S, McCutchan JA, Wolfson T, Spector SA, Hsia K, Heaton RK, Grant I. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59:923–928. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- Funnye AS, Akhtar AJ. Syphilis and human immunodeficiency virus co-infection. J Natl Med Assoc. 2003;95:363–382. [PMC free article] [PubMed] [Google Scholar]
- Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290:1510–1514. doi: 10.1001/jama.290.11.1510. [DOI] [PubMed] [Google Scholar]
- Griemberg G, Bautista CT, Pizzimenti MC, Orfus G, Alonso B, Fernandez T, Cando O, Martinez Peralta L. High prevalence of syphilis-HIV co-infection at four hospitals of the city of Buenos Aires, Argentina. Rev Argent Microbiol. 2006;38:134–136. [PubMed] [Google Scholar]
- Griemberg G, Pizzimenti MC, Famiglietti AM, Belli L, Vay C, Garcia S, Cardinalli A, Costa MA, Marcenac F, Casco RH. The impact of HIV infection on the incidence of syphilis and gonorrhea at a university hospital (1985–1994) Medicina (B Aires) 1997;57:1–6. [PubMed] [Google Scholar]
- Handsfield HH. Resurgent STD in gay and bisexual men: a public health crisis. Presented at the 28th Annual Meeting of the Infectious Diseases Society of America; Sept. 7–10, 2000; New Orleans, Louisiana. 2000. [Google Scholar]
- Johns DR, Tierney M, Felsenstein D. Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N Engl J Med. 1987;316:1569–1572. doi: 10.1056/NEJM198706183162503. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lawn SD. AIDS in Africa: the impact of coinfections on the pathogenesis of HIV-1 infection. J Infect. 2004;48:1–12. doi: 10.1016/j.jinf.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn WA, Lightman S. Syphilis and HIV: a dangerous combination. Lancet Infect Dis. 2004;4:456–466. doi: 10.1016/S1473-3099(04)01061-8. [DOI] [PubMed] [Google Scholar]
- Marra CM, Maxwell CL, Collier AC, Robertson KR, Imrie A. Interpreting cerebrospinal fluid pleocytosis in HIV in the era of potent antiretroviral therapy. BMC Infect Dis. 2007;7:37–41. doi: 10.1186/1471-2334-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra CM, Maxwell CL, Smith SL, Lukehart SA, Rompalo AM, Eaton M, Stoner BP, Augenbraun M, Barker DE, Corbett JJ, Zajackowski M, Raines C, Nerad J, Kee R, Barnett SH. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis. 2004a;189:369–376. doi: 10.1086/381227. [DOI] [PubMed] [Google Scholar]
- Marra CM, Maxwell CL, Tantalo L, Eaton M, Rompalo AM, Raines C, Stoner BP, Corbett JJ, Augenbraun M, Zajackowski M, Kee R, Lukehart SA. Normalization of cerebrospinal fluid abnormalities after neurosyphilis therapy: does HIV status matter? Clin Infect Dis. 2004b;38:1001–1006. doi: 10.1086/382532. [DOI] [PubMed] [Google Scholar]
- McArthur JC, McClernon DR, Cronin MF, Nance-Sproson TE, Saah AJ, St Clair M, Lanier ER. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- Nnoruka EN, Ezeoke AC. Evaluation of syphilis in patients with HIV infection in Nigeria. Trop Med Int Health. 2005;10:58–64. doi: 10.1111/j.1365-3156.2004.01344.x. [DOI] [PubMed] [Google Scholar]
- Pennycook A, Openshaw P, Hussell T. Partners in crime: co-infections in the developing world. Clin Exp Immunol. 2000;122:296–299. doi: 10.1046/j.1365-2249.2000.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs RT, Joesoef MR, Hendershot EF, Rompalo AM, Augenbraun MH, Chiu M, Bolan G, Johnson SC, French P, Steen E, Radolf JD, Larsen S. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group. N Engl J Med. 1997;337:307–314. doi: 10.1056/NEJM199707313370504. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD. Immune surveillance and autoantigen recognition in the central nervous system. Aust N Z J Med. 1995;25:784–792. doi: 10.1111/j.1445-5994.1995.tb02882.x. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Ford AL, Foulcher E, Airriess R. Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J Immunol. 1998;160:5320–5330. [PubMed] [Google Scholar]
- Sheffield JS, Wendel GD, Jr, McIntire DD, Norgard MV. Effect of genital ulcer disease on HIV-1 coreceptor expression in the female genital tract. J Infect Dis. 2007;196:1509–1516. doi: 10.1086/522518. [DOI] [PubMed] [Google Scholar]
- Simms I, Fenton KA, Ashton M, Turner KM, Crawley-Boevey EE, Gorton R, Thomas DR, Lynch A, Winter A, Fisher MJ, Lighton L, Maguire HC, Solomou M. The re-emergence of syphilis in the United Kingdom: the new epidemic phases. Sex Transm Dis. 2005;32:220–226. doi: 10.1097/01.olq.0000149848.03733.c1. [DOI] [PubMed] [Google Scholar]
- Simon R. The great pox. Clin Infect Dis. 2004;38:1007–1008. doi: 10.1086/382539. [DOI] [PubMed] [Google Scholar]
- Spudich SS, Nilsson AC, Lollo ND, Liegler TJ, Petropoulos CJ, Deeks SG, Paxinos EE, Price RW. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis. 2005;5:98–118. doi: 10.1186/1471-2334-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans S, Marlowe N, Glidden D, Novakovic-Agopian T, Grant RM, Heyes M, Aweeka F, Deeks S, Price RW. Time course of cerebrospinal fluid responses to antiretroviral therapy: evidence for variable compartmentalization of infection. AIDS. 1999;13:1051–1061. doi: 10.1097/00002030-199906180-00008. [DOI] [PubMed] [Google Scholar]
- Timmermans M, Carr J. Neurosyphilis in the modernera. J Neurol Neurosurg Psychiatry. 2004;75:1727–1730. doi: 10.1136/jnnp.2004.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MR, Heaton RK, McCutchan JA, Malone JL, Velin R, Nelson J, Miller LK, Weiss PJ, Oldfield EC, 3rd, Grant I. Neurocognitive impairment in human immunodeficiency virus infection is correlated with sexually transmitted disease history. Sex Transm Dis. 1997;24:398–401. doi: 10.1097/00007435-199708000-00003. [DOI] [PubMed] [Google Scholar]