Abstract
Depletion of selected regulatory CD4+ T cell subsets induces the spontaneous onset of various immune or autoimmune disorders. It is not clear, however, whether a given subset, notably CD4+CD25+ regulatory T cells, protects from a wide spectrum of immune disorders, or whether specialized subsets of regulatory T cells control each given disease or group of diseases. We report here, using diabetes prone nonobese diabetic (NOD) mice, that depending on the regulatory T cells that are depleted, i.e., CD25+, CD62L+, or CD45RBlow, distinct immune diseases appear after transfer into NOD severe combined immunodeficiency (SCID) recipients. Thus, reconstitution of NOD SCID mice with CD25- T cells induces major gastritis and late-onset diabetes, but no or mild colitis. Reconstitution with CD62L- T cells induces fulminant diabetes with no colitis or gastritis. Reconstitution with CD45RBhigh T cells induces major colitis with wasting disease and no or very moderate gastritis and diabetes. Major differences among the three regulatory T cell subsets are also seen in vitro. The bulk of suppressor cells inhibiting the proliferation of CD4+CD25- T cells in coculture is concentrated within the CD25+ but not the CD62L+ or CD45RBlow T cell subsets. Similarly, cytokine production patterns are significantly different for each regulatory T cell subset. Collectively, these data point to the diversity and organ selectivity of regulatory T cells controlling distinct autoimmune diseases whatever the underlying mechanisms.
Experimental evidence has been collected indicating the existence of naturally occurring regulatory CD4+ T cells controlling autoimmunity in various models of experimentally induced lymphopenia (1, 2). Depletion of CD25+ T cells, as obtained after thymectomy at 3 days of age (in BALB/c mice) or by restoring BALB/c nude mice with CD25- T cells (3) induces the onset of gastritis, thyroiditis, and oophoritis or prostatitis/orchitis, depending on the mouse gender. Reconstitution of BALB/c severe combined immunodeficiency (SCID) mice with CD45RBhigh T cells (obtained after depletion of CD45RBlow T cells) leads to the onset of colitis with severe wasting disease (4). Last, reconstitution of nonobese diabetic (NOD) SCID mice with CD62L- T cells, obtained after depletion of T cells expressing CD62L (l-selectin), induces rapid onset insulin-dependent diabetes mellitus (5-7). One interpretation of these differences is that, depending on the regulatory T cell subset depleted (CD25+, CD62L+, or CD45RBlow), different immune disorders are induced. Another interpretation, favored by several groups, is that differences in mouse strains (BALB/c vs. NOD), experimental conditions (thymectomy, nude, or SCID recipients), and the autoimmune or immune disease examined in each given study could explain the results observed. This latter hypothesis was in keeping with the major attention focused on CD25+ T cells as the central actors in all these models. In addition, as far as colitis is concerned, differences in gut bacterial colonization could have differed according to animal facilities, an important factor because of the well established role of mucosal bacteria in the pathogenesis of inflammatory bowel diseases (8, 9).
We report here that the differences in organ specificity outlined above still hold in well controlled experimental conditions using a single mouse strain (NOD, NOD SCID). These data raise the question of the mechanisms explaining the preferential effect of CD25+, CD62L+, and CD45RBlow T cells in the control of immune disorders affecting different organs.
In addition, results from our in vitro studies (suppression of CD3 antibody-induced proliferation of CD4+CD25- T cells and cytokine production) further support the existence of a distinct functional pattern of each of the three regulatory CD4+ T cell subsets.
Materials and Methods
Mice. NOD (Kd, I-Ag7,Db) and NOD SCID mice were bred in our animal facilities under specific pathogen-free conditions. Colorimetric strips were used to monitor glycosuria (Glukotest, Boehringer Mannheim) and fasting glycemia (Haemoglukotest and Reflolux F, Boehringer Mannheim). When needed, diabetic mice were treated with insulin (1-2 units/day).
Flow Cytometry. Biotin, phycoerythrin (PE), FITC, or allophycocyanin (APC)-labeled antibodies to CD62L (MEL-14), CD25 (7D4 or PC61), CD19 (1D3), CD45RB(16A), CD4, CD8, and PE- and APC-labeled streptavidin were from BD Pharmingen. Cells were stained in PBS plus 5% heat-inactivated FCS (Invitrogen) plus 0.01% sodium azide and fixed by using PBS plus 2.5% formaldehyde (Sigma-Aldrich). Control staining was performed by using isotype-matched biotin, FITC, or PE-labeled irrelevant antibodies. cellquest software (Beckton Dickinson) was used for the acquisition and the analysis.
Cell Recovery and Purification. Splenocytes were purified by using magnetic bead-activated cell sorting (MACS, Miltenyi Biotec, Bergisch-Gladbach, Germany), as reported (5, 10). In brief, spleen cells were incubated for 20 min at 4°C in ice with the appropriate concentration of the biotinylated CD4, CD25, CD62L, or CD45RB antibodies. The cells were washed and incubated with streptavidincoated paramagnetic beads (10 μl/10 × 106 cells) for 15 min at 4°C. After washing, the cells were passed through MS or LS columns within the MACS device. The buffer used throughout the whole procedure was PBS supplemented with 5% FCS. T cell purity was >97%, and recovery ranged from 50% to 70%. For some experiments, cells were purified by using fluorescence-activated cell sorting (FACS, FACSVantage, Becton Dickinson). CD4+ T cells, recovered by negative selection by using a CD4+ T cell isolation kit (MACS), were stained with FITC, phycoerythrin, and allophycocyanine-Sav-conjugated antibodies to CD25, CD62L, or biotinylated antibody to CD45RB. The cells were washed, resuspended in a 2% FCS-containing culture medium deprived of phenol red, and FACS-sorted (purity was ≈98%).
Adoptive Cell Transfer. Recipients were adult 6-wk-old NOD SCID mice. Mice were transferred i.v. with single purified T cell subsets. For each experiment, cell numbers were expressed in terms of CD4+ T cells, independently from the presence of B or CD8+ T cells, which were deliberately not removed from the cell inoculum.
For cotransfer experiments, mice were injected either with just diabetogenic cells or with mixtures of diabetogenic and purified putative regulatory T cells.
Autoantibody Measurements.Antithyroglobulin antibodies. Autoantibodies were detected by solid-phase ELISA (11). Flat-bottom microtiter plates (Nunc, Life Technologies, Paisley, U.K.) were coated overnight at 4°C with porcine thyroglobulin (Sigma) (100 μg/ml in PBS). The plates were incubated for 2 h at room temperature with PBS containing 5% FCS. Fifty microliters of each serum sample was diluted 1/10 in PBS plus 5% FCS and incubated in duplicate for 2 h at room temperature. The plates were then incubated with a sufficient concentration of a horseradish peroxidase-labeled goat anti-mouse IgG antiserum (P.A.R.I.S., Compiègne, France). The enzymatic activity was revealed by using O-phenylenediamine dihydrochloride (Sigma) as a substrate. The reaction was stopped by 50 μl of 3 M HCl, and the optical density was evaluated at 490/650 nm.
Gastric anti-H+/K+ATPase antibodies. Antibodies were detected by ELISA, as described (12). Porcine H+/K+ ATPase (α and β subunits) was purified by tomato-lectin affinity chromatography (13). Purity was assessed by SDS/PAGE and silver staining. Flat-bottom microtiter plates (Nunc) were coated overnight at 4°C with 100 μl of purified H+/K+ ATPase (0.5 μg/ml) in 50 mM carbonate-bicarbonate buffer, pH 9.6. Plates were then incubated for 2 h at 37°C with PBS plus FCS 5%. Fifty microliters of serum samples (diluted at 1/10 in PBS plus FCS 5%) was incubated for2hat room temperature. The plates were then incubated 1 h at room temperature with the adequate concentration of a streptavidin-biotinylated horseradish peroxidase-labeled goat anti-mouse IgG. The enzymatic activity was revealed as described.
Histopathology. Stomach, colon, pancreas, salivary glands, and thyroid were fixed in 4% formalin and processed according to standard methods. Five-micrometer-thick paraffin sections stained with hematoxylin/eosin-safran were examined. For each organ, mononuclear infiltration was scored as minimal, moderate, or severe.
For gastritis, inflammation was found around fundic glands, sometimes destroying the underlying glands leading to glandular atrophy. Neither epithelial dysplasia nor metaplasia was observed.
Colitis sometimes manifested as ulcerative colitis, with neutrophils infiltration, glandular atrophy, and necrosis.
In Vitro Proliferation Assays and Cytokine Production For proliferation assays, cells were cultured in RPMI medium 1640 supplemented with glutamax/10% FCS/0.05 mM 2-mercaptoethanol/penicillin/streptomycin. Purified subsets using MACS or FACS were seeded in round-bottom 96-well microplates (0.2 × 105 cells of each subset per well), in a total volume of 200 μl per microwell. Cells were stimulated with purified soluble CD3 antibody (145 2C11, provided by Jeffrey A. Bluestone, University of California, San Francisco) (500 ng per microwell) in the presence of antigen-presenting cells (APCs) (1.25 × 105 mitomycin-treated APCs per well). Cells were cultured at 37°C for 72 h in a humidified atmosphere containing 5% CO2 and pulsed with 1 μCi of [3H]thymidine (2 μCi/mmol) (Amersham Pharmacia) for the last 18 h of culture. Then cells were harvested, and thymidine incorporation was assessed by using a Beta Plate scintillation counter (Perkin-Elmer). Data from the cocultures were expressed as the percent inhibition deduced as follows:
% Inhibition = [1 - (cpm (CD4+CD25- + CD4+CD25+)/cpm CD4+CD25-)] × 100.
For cytokine production, lymphocytes were plated at 0.2 × 106 cells per well in a total volume of 200 μl per microwell and stimulated with immobilized CD3 antibody. Supernatants were recovered at 24, 48, and 72 h of culture and stored at -80°C until tested for IFNγ, IL-4, and IL-10.
il-4 elisa. Antibodies used were 11B11 (provided by W. Paul, National Institutes of Health, Bethesda) and biotinylated BVD6 (BD Pharmingen). Mouse recombinant IL-4, used as standard, was from R & D Systems. The sensitivity of the assay was 10 pg/ml.
il-10 elisa. Antibodies used were JES5-A5 (provided by A. O'Garra, DNAX) and biotinylated JES5-16E3 (BD Pharmingen). Mouse recombinant IL-10, used as standard, was from R & D Systems. The sensitivity of the assay was 10 pg/ml.
ifnγ elisa. Antibodies used were R46A2 and biotinylated AN18 (provided by A. O'Garra). Mouse recombinant IFNγ, used as standard, was from R & D Systems. The sensitivity of the assay was 10 pg/ml.
Statistical Analysis. Diabetes incidence was plotted by using the Kaplan-Meier method, i.e., nonparametric cumulative survival plot. The statistical comparison between the curves was performed by using the log-rank (Mantel-Cox) test that provided the corresponding χ2 values. Mean values were compared by using Student's t test.
Results
NOD and NOD SCID Mice Reconstituted with Syngeneic Total Spleen Cells Exhibit Overt Autoimmune Diabetes Concomitantly with Minor Gastritis and Colitis. In our colony, female NOD mice develop overt diabetes starting at 14-16 wk of age (95% incidence at 35 wk of age). At 18 wk of age, they also show, as already described, histological signs of thyroiditis and sialitis (14) (Table 1). Interestingly, they also exhibit histological manifestations typical of colitis (which, however, remain moderate and without wasting disease) but no gastritis (Table 1). No anti-H+/K+ ATPase or antithyroglobulin antibodies are detectable at this age.
Table 1. Histological analysis of pancreas, stomach, gut, thyroid, and salivary glands of NOD SCID mice reconstituted with distinct T cell subsets.
| T cell subset inoculum | Diabetes, % | Gastritis, % | Colitis, % | Thyroiditis, % | Sialitis, % |
|---|---|---|---|---|---|
| CD25- (n = 56) | 71 | 95 (22m, 26M, 5S) | 20 (4m, 7M) | 30 (3m, 1M, 3S) | 94 (29m, 19M) |
| CD62L- (n = 7) | 100 | 0 | 0 | ND | 57 (3m, 1S) |
| CD25-CD62L- (n = 15) | 100 | 30 (5m) | 0 | 0 | 67 (8m) |
| CD25-CD62L+ (n = 38) | 8 | 90 (3m, 17M, 14S) | 53 (15m, 5M) | 10 (2m, 2M) | 76 (19m, 10M) |
| CD4+CD45RBhigh (n = 8) | 0 | 12 (1m) | 100 (1M, 7S, 2 ulceratives) | 12 (1m) | 37 (2m, 1S) |
| Total splenocytes (n = 8) | 85 | 14 (1m) | 0 | 0 | 14 (1m) |
| NOD mice (18-week-old) (n = 30) | 80 | 0 | 66 (10m, 10M) | 3 (1S) | 66 (5m, 15M) |
| NOD SCID mice (10- to 18-week-old) (n = 32) | 0 | 0 | 0 | 0 | ND |
For each organ, mononuclear infiltration was scored as minimal (m), moderate (M), or severe (S). ND, not done. Values in parentheses are absolute numbers of mice exhibiting each pattern.
Immunoincompetent NOD SCID mice reconstituted with total syngeneic spleen cells from prediabetic (10-wk-old) NOD mice exhibited overt diabetes at 7-8 wk posttransfer with kinetics that correlated with the number of transferred cells (Fig. 1). These adoptively transferred recipients also showed moderate histological signs of colitis (here again without overt wasting disease), together with histological sialitis and mild gastritis but not thyroiditis.
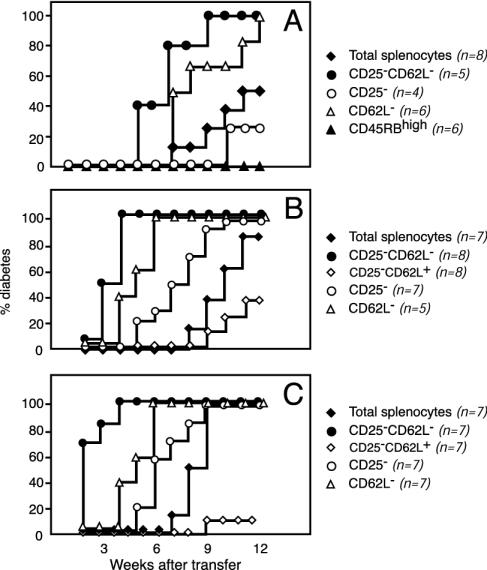
Fig. 1.
Diversity of regulatory CD4+ T cells controlling autoimmune diabetes. Results of a representative experiment show diabetes incidence in NOD SCID mice reconstituted with total spleen cells or spleen cells depleted in distinct regulatory T cell subsets from 10-wk-old NOD mice. Independent from the cell population analyzed, diabetes incidence correlated with the number of transferred cells, i.e., 1.25 × 106 (A), 4 × 106 (B), and 7 × 106 (C). The data highlight three main points. First, CD25-CD62L- T cells are significantly more effective in transferring diabetes than total spleen cells [P < 0.01 (A), P < 0.0002 (B), and P < 0.0001 (C)]. Second, purified CD25-CD62L- T cells triggered extremely severe diabetes at an incidence significantly higher than that observed in recipients of CD25-CD62L+ T cells [P < 0.0001 (B) and P < 0.0001 (C)]. Third, CD4+CD45RBhigh T cells that are very effective at transferring colitis were not diabetogenic at all.
Unreconstituted 5- to 7-wk-old NOD SCID mice showed as their only histopathological manifestation a minor mononuclear cell infiltration of the colon that spontaneously disappeared with aging (Table 1 and Fig. 2).
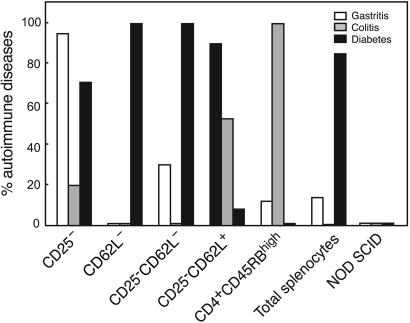
Fig. 2.
Gastritis, colitis, and diabetes in NOD SCID mice reconstituted with spleen cells depleted of distinct regulatory T cell subsets. Incidence of overt diabetes and histological gastritis and colitis in a total of 194 NOD SCID mice reconstituted with various T cell subsets is shown. The major finding was that CD25-CD62L+, CD4+CD45RBhigh, and CD4+CD62L- T cells were unique in their capacity to trigger almost exclusively one disease, namely gastritis, colitis, or diabetes, respectively. Intermediate patterns, both in terms of incidence and severity (see also Table 1), were observed for the other purified subsets.
The Main Candidate Regulatory T Cell Subset Phenotypes (CD25+, CD62L+, and CD45RBlow) Only Partially Overlap. CD4+ T cells from 6- and 10-wk-old NOD mice were studied by flow cytometry for their expression of CD25, CD62L, and CD45RB. As shown in Table 2, there was only a modest correlation between these three phenotypic markers reported on CD4+ regulatory T cells.
Table 2. Analysis of CD25, CD45RB, and CD62L expression by spleen CD4+ T cells from 10-wk-old NOD mice.
| T cells | Percent CD62L+ T cells | Percent CD62L– T cells | Percent CD25+ T cells | Percent CD25– T cells |
|---|---|---|---|---|
| CD45RBhigh | 20–32 | 1–5 | 0–1 | 3–7 |
| CD45RBlow | 33–42 | 10–17 | 4–12 | 55–77 |
| CD45RB– | 3–8 | 14–22 | 2–6 | 14–23 |
| Total | 61–75 | 25–38 | 6–14 | 85–94 |
| Percent | Percent | |||
| CD62L+ T cells | CD62L– T cells | |||
| CD25+ | 2–6 | 0–2 | ||
| CD25– | 70–80 | 21–32 | ||
| Total | 68–82 | 21–33 |
Values shown for each given subset are expressed as the percentage of total CD4+ T cells. For each subset, the range of values obtained from two to four independent experiments is presented.
Thus, it was observed that both CD45RBlow and CD45RBhigh T cells included a minority of CD25+ T cells with a quasiabsence of CD25+ T cells among CD45RBhigh lymphocytes (Table 2). Similarly, CD62L+ T cells included very low proportions of CD25+ T cells (≈5%), only twice as many as the CD62L- T cell compartment (Table 2). Last, CD62L+ T cells included a similar proportion of CD45RBlow T cells as compared to CD62L- T cells (≈40%) (data not shown).
Taken together, these results show that the regulatory T cell subsets defined by the CD25, CD62L, and CD45RB markers overlap only partially, suggesting that either these subsets are functionally distinct or that the relevant regulatory subset(s) represents only a minority of the cells composing the three populations defined by these markers.
Gastritis, Diabetes, and Colitis Are Selectively Induced on Adoptive Transfer of T Cells Depleted of Distinct Regulatory CD4+ T Cell Subsets. Adoptive transfer experiments in NOD SCID recipients were performed by using CD4+ T cells from 10-wk-old NOD females depleted in CD25+, CD62L+, or CD45RBlow regulatory T cells. In these various experimental conditions, recipients were monitored for the occurrence of various immune and autoimmune diseases, namely diabetes, gastritis, thyroiditis, sialitis, and colitis. The purified CD4+ T cell subsets were supplemented with adequate proportions of purified B cells to allow the analysis of gastritis-specific anti-H+/K+ ATPase antibodies and of CD8+ T cells, known to be mandatory for diabetes induction (15). Given the different kinetics of the various diseases analyzed when diabetes occurred, mice were treated with exogenous insulin to allow survival until 12 wk posttransfer, the time point at which the stomach, thyroid, salivary glands, and gut were recovered for histological examination.
Cells were purified by either MACS or FACS. For CD25+- and CD62L+-depleted populations, similar results were obtained by using the two methods. In the case of CD45RBlow-depleted cells, induction of overt disease with weight loss and wasting was obtained only by using FACS purification. MACS-purified T cells induced only histological colitis.
Gastritis is predominantly induced on adoptive transfer of CD4+CD25+-depleted T cells. As expected, diabetes was observed within 7-8 wk after transfer of CD4+CD25+-depleted T cells. The kinetics were similar to those observed in mice reconstituted with unseparated spleen cells (Fig. 1).
The results shown in Fig. 2 and Table 1 demonstrate that CD25+ T cell depletion induced the onset of gastritis in 95% of recipients. This gastritis was associated with high serum levels of anti-H+/K+ ATPase autoantibodies, detectable as early as 6-7 wk after reconstitution (Fig. 3).
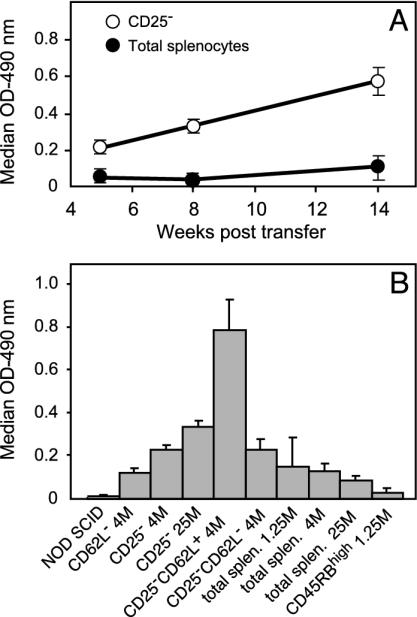
Fig. 3.
Anti-H+/K+ ATPase autoantibody serum levels. Specific autoantibody levels were determined by ELISA. (A) Kinetics of anti-H+/K+ ATPase autoantibody production in mice reconstituted with 4 × 106 of either total spleen cells (•) or purified CD25- T cells (○). (B) Antibody levels at 12 wk posttransfer in the various groups of recipients analyzed. The x axis details the phenotype and the number (M, millions of cells) for each transferred lymphocyte subset. Mean antibody levels detected in recipients injected with CD25-CD62L+ T cells were significantly higher than those observed in all other groups (P < 0.001).
It is important to note that, unlike diabetes, the prevalence of both gastritis and anti-H+/K+ ATPase autoantibody levels was higher than that observed in NOD SCID mice reconstituted with unseparated spleen cells (Table 1 and Fig. 3).
Last, there was also evidence in these recipients of severe sialitis, mild thyroiditis (without detectable antithyroglobulin autoantibodies), and only moderate colitis (without wasting disease) (Table 1 and Fig. 2).
Diabetes is selectively induced on adoptive transfer of CD4+CD62L+-depleted T cells. NOD SCID mice reconstituted with CD62L- T cells showed an extremely rapid appearance of fulminant diabetes, leading to death within 8 days in the absence of insulin treatment. This pattern of diabetes was significantly different from that observed in NOD SCID mice reconstituted with unseparated cells for whom the disease appeared later and in a much less severe form (Fig. 1). In contrast, as observed in NOD SCID mice reconstituted with unseparated cells, recipients of CD62L+-depleted T cells showed, on histological examination, sialitis, only moderate colitis (with no wasting disease), mild gastritis, and no thyroiditis (Table 1 and Fig. 2).
Similarly, in CD62L- T cell-reconstituted mice, antithyroglobulin and anti-H+/K+ ATPase antibodies were not found at higher frequency than in mice reconstituted with total spleen cells (Fig. 3). Colitis is selectively induced on adoptive transfer of CD4+CD45RBlow-depleted T cells. Injection of 0.5 × 106 of FACS-purified CD4+CD45RBhigh spleen cells into NOD SCID recipients induced severe colitis with wasting disease (Fig. 2). As described by others in BALB/c recipients, mice started to develop cachexia as early as 5-6 wk after reconstitution. They were culled at 8 wk after transfer, when they already showed major weight loss (≥15%). At this stage, histological examination revealed no or minor gastritis or insulitis (Table 1). This latter observation is at variance with our previous report showing that immunoincompetent NOD mice restored with total CD4+ T cells alone developed severe insulitis (16).
Experiments were performed in which CD4+CD45RBhigh spleen cells were complemented with CD8+ T cells and B cells. Despite the occurrence of colitis in these recipients, no diabetes was observed, although mice could be monitored up to 8 wk posttransfer, a time point when diabetes had already occurred in mice reconstituted with either CD62L- or CD25- T cells.
In addition, no anti-H+/K+ ATPase autoantibodies were observed in these same recipients, at a time point when NOD SCID mice reconstituted with CD25- T cells already showed significant autoantibody levels (Fig. 3).
Direct Evidence for the Selective Protection from Gastritis and Diabetes Afforded by CD25+CD62L- and CD25-CD62L+ T Cells. As previously mentioned, CD25- T cells (CD25+-depleted) were effective in transferring both severe gastritis and mild diabetes. Moreover, CD62L- T cells (CD62L+-depleted) triggered fulminant diabetes but no gastritis. We therefore wanted to explore whether combining the depletion of CD25+ and CD62L+ T cells would lead to the appearance of both severe gastritis and diabetes. Surprisingly, results showed that transfer of purified CD25-CD62L- T cells induced extremely severe diabetes but no gastritis, suggesting that CD25-CD62L+ T cells have a positive influence on gastritis induction. We then analyzed in greater detail the disease-protecting ability of CD25-CD62L+ T cells.
As shown in Table 1 and Fig. 2, CD25-CD62L+ T cells induced only severe gastritis, with levels of anti-H+/K+ATPase autoantibodies significantly higher (P < 0.001) than those scored in the other groups (Fig. 3) but not diabetes. These results argue for the selective gastritis-protective capacity of CD25+CD62L- T cells and the diabetes-protective capacity of CD25-CD62L+T cells. This latter conclusion was further supported by our cotransfer data.
Young NOD SCID recipients were injected i.v. with mixtures of two distinct populations, namely splenocytes from diabetic NOD mice (diabetogenic cells) and CD25+ or CD25-CD62L+ T cells from the spleen of 6-wk-old prediabetic NOD mice. As shown in Fig. 4, as few as 1 × 106 purified CD4+CD25+ spleen T cells from prediabetic NOD mice significantly protected from diabetes transfer [P < 0.0001 as assessed by the log-rank test (Mantel-Cox)]. Interestingly, the CD62L+ subset included within the CD4+CD25- compartment also provided very efficient protection from diabetes transfer (Fig. 4).
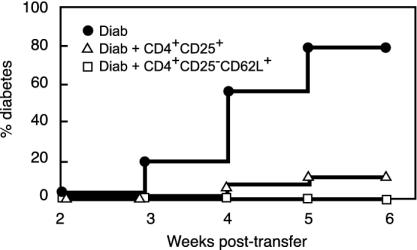
Fig. 4.
Spleen CD4+CD25+ and CD4+CD25-CD62L+ T cells from prediabetic NOD mice are protective in vivo. Diabetes was monitored in NOD SCID recipients injected with diabetogenic cells alone (Diab) or with mixtures of diabetogenic cells and 1 × 106 CD4+CD25+ or CD4+CD25-CD62L+ purified T cells. In the two latter groups, significant diabetes protection was observed (P < 0.0001 for CD4+CD25+ and CD4+CD25-CD62L+). Shown are combined data of six to seven independent experiments.
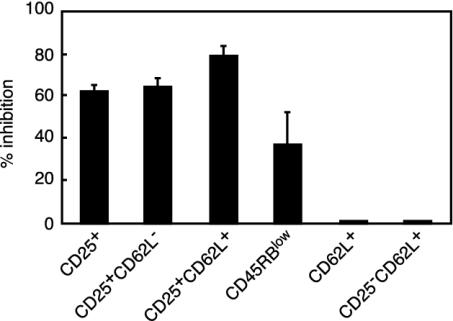
In Vitro Studies.In vitro suppression. The capacity of the various T cell subsets discussed above to inhibit the proliferation of CD25- and of CD25-CD62L- T cells was studied in a systematic fashion. As shown in Fig. 5, no inhibition was afforded by T cell subsets not expressing CD25. Thus, CD62L+CD25- T cells did not suppress. Conversely, total CD25+, CD25+CD62L+, and CD25+CD62L- T cells significantly inhibited the proliferation of the responder population.
Fig. 5.
In vitro suppression of CD4+CD25- T cell proliferation by distinct regulatory T cell subsets. Data collected from different experiments are expressed as percent inhibition of proliferation. Results show that cells expressing the CD25 marker are the most effective suppressors in vitro. In great distinction, no suppression was exhibited by T cell subsets expressing CD62L but no CD25.
At variance with CD25, CD62L did not prove to be a good marker for in vitro suppressor cells because both CD62L+ and CD62L- T cells proliferated and were not suppressive.
CD45RB expressed some capacity to discriminate between proliferative and suppressor cells because CD45RBhigh T cells proliferated more than CD45RBlow T cells and CD45RBlow T cells partially inhibited the proliferation of CD45RBhigh T cells (that included >99% CD25- T cells).
To ensure that total CD62L+ T cells and CD25-CD62L+ T cells did not exhibit in vitro regulatory capacities, it was verified that carboxyf luorescein diacetate succinimidyl ester-labeled CD25-CD62L- T cells underwent the same number of cell divisions when cultured alone or in the presence of CD62L+ T cells or CD25-CD62L+ T cells (data not shown).
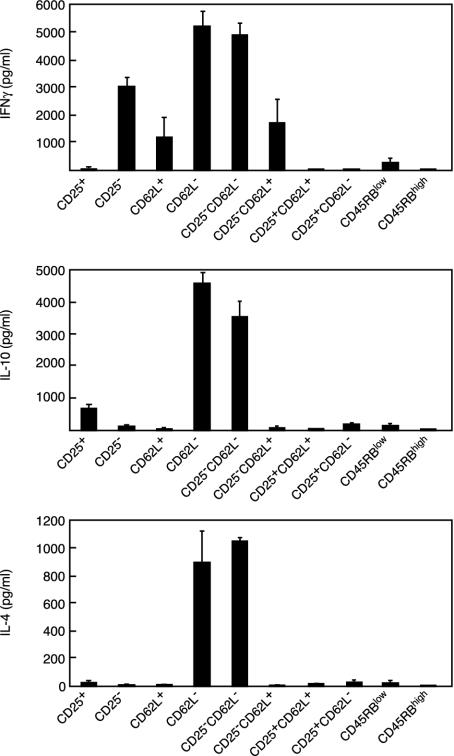
Cytokine production. The production of IFN-γ, IL-10, and IL-4 by the various cell subsets was analyzed after in vitro stimulation with CD3 antibody (Fig. 6). Although no absolute pattern was observed, clear-cut differences were noted in the cytokine pattern expressed by the various regulatory T cell subsets. Expression of CD25 was associated with low production of IFN-γ and IL-4 but with significant production of IL-10 whether CD25+CD62L+ or CD25+CD62L- T cells were taken into consideration. CD4+CD25- T cells produced significantly larger amounts of IFN-γ and significantly lower amounts of IL-10 than CD4+CD25+ T cells (P < 0.0001 and P < 0.015, respectively). IFN-γ was secreted in higher amounts by CD4+CD62L- T cells than by CD4+CD62L+ T cells. Production of IL-4 and IL-10 was quasiabsent in CD62L+ T cells but quite high in the CD62L- population. Removal of CD25+ T cells from the CD62L- population (CD25-CD62L-)did not modify IFN-γ and IL-4 production but slightly decreased that of IL-10. Conversely, removal of CD25+ T cells from the CD62L+ population (CD25-CD62L+) had no effect on the production of the three cytokines. Last, both CD45RBlow and CD45RBhigh T cells produced extremely low amounts of IFN-γ, IL-10, and IL-4.
Fig. 6.
Cytokine production by selected T cell subsets after CD3 antibody-induced stimulation. Results show that the cytokine-producing ability greatly differed among the distinct subsets analyzed. Mean values observed with CD62L- and CD25-CD62L- T cells were significantly higher for the three cytokines analyzed than those observed for all other T cell subsets tested (P < 0.0001).
Interestingly, when CD25-CD62L- T cells were injected into NOD SCID mice, the T cells recovered from the diabetic recipient mice showed a cytokine pattern different from that of the initial cell inoculum. In brief, IFN-γ production remained high, whereas IL-4 and IL-10 production was dramatically reduced (data not shown).
Discussion
There is compelling evidence to show that depletion of one or several candidate regulatory T cell subsets (CD45RBlow, CD25+, and CD62L+) induces distinct clinical manifestations such as colitis, autoimmune gastritis, or diabetes (1, 2). There is a questionable trend supporting the notion that a single population of regulatory T cells, predominantly CD25+, is involved in the control of these three immune diseases (3, 17, 18). Differences in results obtained after mouse reconstitution with CD25- or CD45RBhigh T cells have been explained by the variety of the experimental conditions used (thymectomy, SCID vs. nude mice). To address this issue, we studied a model in which the three immune diseases could be induced by using a single mouse strain (NOD), in a given lymphopenic condition (NOD SCID), thus avoiding biases due to variable experimental conditions. Importantly, the colony of mice used in the present study harbored intestinal bacteria necessary for the induction of colitis, as assessed by the presence of both histological signs of mild colitis in wild-type NOD mice and by the development of severe colitis and wasting disease after reconstitution of NOD SCID mice with purified CD45RBhigh T cells.
We demonstrate here that CD25+ T cells are essential in controlling the onset of gastritis, whereas CD45RBlow are indispensable for the control of colitis. Moreover, CD62L+ T cells play the key role in controlling diabetes with CD25+ T cells also participating, albeit to a lesser extent.
It was important to ensure that, in the adoptive transfer experiments, all lymphocyte subsets necessary for the induction of the immune disorders in question (B cells, CD4+, and CD8+ T cells) were present in the cell inoculum. Gastritis can be induced with purified CD4+CD25- T cells and does not require B cells if the read-out is limited to the infiltration of the stomach, but production of antigastric autoantibodies (anti-H+/K+ ATPase) requires the presence of B cells (data not shown). Colitis leading to wasting disease is observed solely through the transfer of purified CD4+CD45RBhigh T cells (4). Last, transfer of autoimmune diabetes, a T cell-mediated disease, requires the concomitant presence of CD4+ and CD8+ T cells when polyclonal diabetogenic T cells are used (15), even though, when islet-specific monoclonal T cells are used, disease transfer can be obtained solely by CD4+ or CD8+ lymphocytes (19, 20). In the present study, to allow a fair comparison of the three diseases (gastritis, colitis, and diabetes), the cell inocula were adapted to include the relevant cell populations (B cells for antigastric antibodies and CD8+ T cells for diabetes). The age of the cell donors was also carefully selected (10 wk) to ensure the presence of pathogenic cells for each model (21). Finally, the recipients were monitored for a sufficient time to allow each disease to develop. Thus, when diabetes occurred, mice were treated with insulin to allow survival for sufficient time to monitor for gastritis and colitis.
Another approach for dissecting regulatory T cells controlling autoimmunity consists of studying their capacity to inhibit CD3-specific antibody-induced T cell proliferation. For this purpose, we studied the capacity of each of the regulatory subsets just mentioned to suppress in coculture the proliferation of CD4+CD25- T cells (22, 23). In addition, we examined the cytokine pattern of each of the T cell subsets in question.
Taken together, the in vivo and in vitro data obtained in this study indicated that CD25+ T cells exhibit the unique capacity to protect from gastritis in vivo and to inhibit the CD3 antibody-induced proliferation of CD25- T cells. CD62L+ T cells have a very selective ability to protect from diabetes in vivo, and CD45RBlow T cells possess the unique ability to protect from colitis in vivo.
Among the possible mechanisms explaining these differences, one may first suggest variable homing capacities of the relevant regulatory T cells possibly relying on a differential expression of chemokine receptors. This is in keeping with the recent data showing that CD25+CD62L+ T cells that protect from diabetes in NOD mice selectively express CCR7 (18). In the same vein, one might consider differences in chemokine receptor expression on effector T cells. Other homing receptors could be involved, such as l-selectin, discussed in this paper. However, one should mention that CD62L-/- NOD mice do not exhibit accelerated diabetes onset (24).
Another hypothesis relates to the differential cytokine dependency of each autoimmune disorder. CD45RBlow T cell control of colitis is IL-10- and TGF-β-dependent (25, 26). Diabetes protection in NOD mice is TGF-β-dependent (L.C., unpublished results) but IL-4- and IL-10-independent (7). Gastritis protection by CD25+ T cells has not been shown to depend on any cytokine, suggesting the possible requirement of direct cell-cell contact (1). One may postulate that CD25+, CD62L+, and CD45RBlow T cells produce different cytokine patterns, either systemically or locally in the target organ, that in turn influence the organ specificity of the immune disorder they control. Our data are in keeping with such interpretation. CD25+ T cells exhibit a high IL-10:IFN-γ ratio compared to CD25- T cells. CD62L+ T cells produce hardly detectable levels of IL-4 and IL-10 but significant amounts of IFN-γ. In contrast, CD62L- T cells produce large amounts of the three cytokines. Although these data support the notion of a differential pattern of cytokine expression by individual regulatory T cell subsets, they do not fully correlate with the in vivo cytokine dependency just mentioned. The data may be reconciled by the fact that cytokine patterns may change after in vivo transfer, as exemplified by our observation with CD25-CD62L- T cells, which before transfer produced high amounts of IL-4 and IL-10 in vitro that significantly decreased after in vivo parking.
Last, we mention the possibility of different mechanisms for triggering immunoregulation according to the cellular subset. The autoantigen-specific triggering of CD25+ regulatory T cells has been suggested (1, 2), whereas autoantigen specificity has not been demonstrated for colitis, which involves antibacterial responses (8, 9). Moreover, in colitis, a role for homeostatic competition has been suggested. Thus, increasing the number of CD45RBhigh T cells did not provoke a more severe colitis but rather a significant decrease of its incidence (27), at variance with what was observed for transferred CD25- T cells, where the higher the number, the worse the disease (28). One may hypothesize that CD45RBlow T cells are particularly good competitors for homeostatic signals (such as IL-7 and MHC peptide recognition), whereas CD25+ T cells act essentially through antigen-specific mechanisms.
In conclusion, these data indicate that three of the main candidate regulatory T cell subsets show major differences in their functional activities both in vivo and in vitro. Each population appears to control a different immune disorder in a predominant fashion. In vitro suppression is essentially a property of CD25+ T cells, with no obvious role for CD62L+ T cells and a dubious one for CD45RBlow T cells. These results militate against a unitary view of regulatory T cells. They also pose the problem of the general significance of in vitro suppression in coculture and of the differences in the mechanisms involved in controlling the various immune disorders under study.
Acknowledgments
We are indebted to Fabrice Valette and Michael Garcia for managing the animal facility, to Meriam Belghith for assistance with cotransfer experiments, to Berta Segovia for important technical help, to Martine Netter for iconography, and to Jérôme Mégret for assistance with FACS. We also thank J. A. Bluestone, W. Paul, and A. O'Garra for providing the monoclonal antibodies used in the study. This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Association Claude Bernard, and the Juvenile Diabetes Research Foundation.
Abbreviations: NOD, nonobese diabetic; SCID, severe combined immunodeficiency; MACS, magnetic bead-activated cell sorting; FACS, fluorescence-activated cell sorting.
References
- 1.Shevach, E. M. (2002) Nat. Rev. Immunol. 2, 389-400. [DOI] [PubMed] [Google Scholar]
- 2.Bach, J. F. (2003) Nat. Rev. Immunol. 3, 189-198. [DOI] [PubMed] [Google Scholar]
- 3.Asano, M., Toda, M., Sakaguchi, N. & Sakaguchi, S. (1996) J. Exp. Med. 184, 387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powrie, F., Leach, M. W., Mauze, S., Caddle, L. B. & Coffman, R. L. (1993) Int. Immunol. 5, 1461-1471. [DOI] [PubMed] [Google Scholar]
- 5.Herbelin, A., Gombert, J. M., Lepault, F., Bach, J. F. & Chatenoud, L. (1998) J. Immunol. 161, 2620-2628. [PubMed] [Google Scholar]
- 6.Lepault, F., Gagnerault, M. C., Faveeuw, C., Bazin, H. & Boitard, C. (1995) Eur. J. Immunol. 25, 1502-1507. [DOI] [PubMed] [Google Scholar]
- 7.Lepault, F. & Gagnerault, M. C. (2000) J. Immunol. 164, 240-247. [DOI] [PubMed] [Google Scholar]
- 8.Hudcovic, T., Stepankova, R., Cebra, J. & Tlaskalova-Hogenova, H. (2001) Folia Microbiol. (Praha) 46, 565-572. [DOI] [PubMed] [Google Scholar]
- 9.Rath, H. C., Schultz, M., Freitag, R., Dieleman, L. A., Li, F., Linde, H. J., Scholmerich, J. & Sartor, R. B. (2001) Infect. Immun. 69, 2277-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gombert, J. M., Herbelin, A., Tancrede-Bohin, E., Dy, M., Carnaud, C. & Bach, J. F. (1996) Eur. J. Immunol. 26, 2989-2998. [DOI] [PubMed] [Google Scholar]
- 11.Tang, H., Mignon-Godefroy, K., Meroni, P. L., Garotta, G., Charreire, J. & Nicoletti, F. (1993) Eur. J. Immunol. 23, 275-278. [DOI] [PubMed] [Google Scholar]
- 12.Chuang, J. S., Callaghan, J. M., Gleeson, P. A. & Toh, B. H. (1992) Autoimmunity 12, 1-7. [DOI] [PubMed] [Google Scholar]
- 13.Callaghan, J. M., Toh, B. H., Simpson, R. J., Baldwin, G. S. & Gleeson, P. A. (1992) Biochem. J. 283, 63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bach, J. F. (1994) Endocr. Rev. 15, 516-542. [DOI] [PubMed] [Google Scholar]
- 15.Bendelac, A., Carnaud, C., Boitard, C. & Bach, J. F. (1987) J. Exp. Med. 166, 823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thivolet, C., Bendelac, A., Bedossa, P., Bach, J. F. & Carnaud, C. (1991) J. Immunol. 146, 85-88. [PubMed] [Google Scholar]
- 17.Read, S., Malmstrom, V. & Powrie, F. (2000) J. Exp. Med. 192, 295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szanya, V., Ermann, J., Taylor, C., Holness, C. & Fathman, C. G. (2002) J. Immunol. 169, 2461-2465. [DOI] [PubMed] [Google Scholar]
- 19.Haskins, K., Portas, M., Bergman, B., Lafferty, K. & Bradley, B. (1989) Proc. Natl. Acad. Sci. USA 86, 8000-8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong, F. S., Visintin, I., Wen, L., Flavell, R. A. & Janeway, C. A., Jr. (1996) J. Exp. Med. 183, 67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuniyasu, Y., Takahashi, T., Itoh, M., Shimizu, J., Toda, G. & Sakaguchi, S. (2000) Int. Immunol. 12, 1145-1155. [DOI] [PubMed] [Google Scholar]
- 22.Thornton, A. M. & Shevach, E. M. (1998) J. Exp. Med. 188, 287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi, T., Kuniyasu, Y., Toda, M., Sakaguchi, N., Itoh, M., Iwata, M., Shimizu, J. & Sakaguchi, S. (1998) Int. Immunol. 10, 1969-1980. [DOI] [PubMed] [Google Scholar]
- 24.Friedline, R. H., Wong, C. P., Steeber, D. A., Tedder, T. F. & Tisch, R. (2002) J. Immunol. 168, 2659-2666. [DOI] [PubMed] [Google Scholar]
- 25.Asseman, C., Mauze, S., Leach, M. W., Coffman, R. L. & Powrie, F. (1999) J. Exp. Med. 190, 995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powrie, F., Carlino, J., Leach, M. W., Mauze, S. & Coffman, R. L. (1996) J. Exp. Med. 183, 2669-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barthlott, T., Kassiotis, G. & Stockinger, B. (2003) J. Exp. Med. 197, 451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155, 1151-1164. [PubMed] [Google Scholar]