Abstract
Background
We previously reported 26 patients who underwent preoperative chemoradiotherapy (CXRT) for T3 rectal cancer and were subsequently offered full-thickness local excision (LE) as an alternative to total mesorectal excision (TME). At nearly 4 years’ follow-up, no difference in outcome was observed. This study compares outcomes in a larger cohort of patients and reevaluates the original 26 patients after longer follow-up.
Methods
Retrospective review was performed of patients who underwent preoperative CXRT (radiation doses of 45, 50.4, or 52.5 Gy with concurrent 5-fluorouracil-based chemotherapy) followed by surgery for T3 rectal cancer. Forty-seven patients underwent LE (Kraske [n = 6] or transanal excision [n = 41]). 473 patients underwent TME (abdominoperineal resection [n = 141] or low anterior resection [n = 332]). Local recurrence, disease-free survival (DFS), disease-specific survival, and overall survival (OS) rates were compared.
Results
Median follow-up was 63 months for the LE group and 59 months for the TME group. Twenty-three LE patients (49%) had a complete response to CXRT, 17 (36%) had microscopic residual disease, and 7 (15%) had gross residual disease, compared with 108 (23%), 89 (19%), and 276 (58%) TME patients, respectively. There was no significant difference between the 10-year actuarial local recurrence rate for the LE group versus the TME group (10.6% and 7.6%, respectively; P = .52), and no significant difference in DFS, disease-specific survival, or OS rates between groups.
Conclusions
In selected patients who demonstrate an excellent response to preoperative CXRT for T3 rectal cancer, full-thickness LE offers comparable local control, DFS, and OS to that achieved with proctectomy and TME.
A combination of chemoradiotherapy (CXRT) and surgery has become standard therapy for T3 rectal cancer because it results in lower rates of local recurrence (LR) and increased survival compared with surgery alone.1–5 CXRT can be administered either preoperatively or postoperatively. However, preoperative CXRT has become the standard of care in the United States because it is associated with reduced acute and chronic toxicity rates, improved local control, and increased sphincter-preservation rates.6,7
Surgery for rectal cancer includes proctectomy via low anterior resection (LAR) or abdominoperineal resection (APR). Critical to either procedure is total mesorectal excision (TME), in which the lymphatic drainage of the rectum is also removed. Proctectomy with TME has been demonstrated to result in a lower LR rate than proctectomy without TME.8–10 However, surgery for rectal cancer is associated with relatively high morbidity and mortality.9,11,12 Anastomotic leak, wound infection, sexual and urinary dysfunction, and frequent stooling are relatively common consequences of TME.12–14 In addition, APR involves a permanent stoma, and LAR often involves at least a temporary stoma.
The relatively high morbidity of rectal surgery can make these operations prohibitive for some patients with severe comorbidity. In addition, some patients refuse to consider a traditional surgical approach because of lifestyle and body image concerns related to a stoma. In the era of neoadjuvant CXRT, some groups have considered whether it is ever appropriate to manage patients with T3 rectal cancer with less than proctectomy and TME. Several small series of patients with T3 rectal cancer who underwent CXRT followed by full-thickness local excision (LE) have demonstrated LR rates similar to those achieved with CXRT followed by APR or LAR.15–18 Habr-Gama et al. have reported that neoadjuvant CXRT can lead to a complete response in up to 30% of cases, and have prospectively observed a cohort of patients with complete response with no initial surgical intervention. At almost 5 years of follow-up, they have reported LR, disease-free survival (DFS), and overall survival (OS) rates comparable to the patient cohort whose disease responded incompletely to CXRT and went on to immediate LAR or APR with TME.19–21
We previously reported a series of 26 patients with T3 rectal cancer who underwent CXRT and then full-thickness LE primarily because of medical comorbidity or patient refusal to undergo APR.22 At 46 months of follow-up, there was no difference between LR or OS rates between the LE group and a control group of patients with T3 rectal cancer who underwent CXRT followed by TME.
The purpose of the present study is to compare the outcome of LE versus TME in patients with T3 rectal cancer who underwent preoperative CXRT with a larger cohort of patients. In addition, we evaluated the outcome of the original group of 26 patients with longer follow-up.
METHODS
After institutional review board approval of the project, the surgical records at the University of Texas M. D. Anderson Cancer Center were queried for patients who underwent transanal or transsacral procedures from January 1990 to July 2008. Patients were selected for inclusion into the LE group if they had rectal cancer, were clinically T3N0M0 or T3N1M0, underwent CXRT, and subsequently underwent LE via a Kraske procedure or transanal excision. A control group consisted of clinical T3N0M0 or T3N1M0 rectal cancer patients who underwent CXRT and subsequently underwent TME via LAR or APR between December 1989 and July 2004.
For all patients, records were reviewed and demographic variables were recorded. Patients were staged by means of clinical examination, digital rectal examination, chest X-ray, computed tomography (CT) of the abdomen and pelvis, and endoscopy. Endoscopic ultrasound (EUS) was routinely performed. Depth of invasion through the rectal wall and presence of abnormal lymph nodes was recorded for accurate T and N staging. In addition, tumor size and distance from the anal verge was recorded. Pathology slides from outside institutions were reviewed by University of Texas M. D. Anderson Cancer Center pathologists and confirmed to be rectal adenocarcinoma.
Several different CXRT regimens were used over the years as the standard of care and protocols changed. Before 2004, radiotherapy consisted of 45 Gy, administered to the posterior pelvis, in 25 fractions over 5 weeks (180 cGy per fraction, 5 days a week). After 2004, a regimen of 50.4 Gy was routinely used, in which patients received the initial 45 Gy and a sequential boost of 5.4 Gy during the sixth week of treatment. A small subset of patients received 52.5 Gy (45 Gy with an additional boost of 7.5 Gy to the tumor during the fifth week of treatment) on a specific protocol. Concurrent 5-fluorouracil-based chemotherapy was administered in all treatment regimens. Before 2002, infusional 5-fluorouracil was routinely used, but by 2004, it was gradually replaced by oral capecitabine.
Response to CXRT in LE patients was evaluated pre-operatively with proctoscopy and/or examination while under anesthesia. However, not all patients undergoing TME were evaluated preoperatively for response to CXRT in the same way. Therefore, response was based on final pathology after surgery. Patients were classified as having a final pathologic specimen with no residual disease (pathologic complete response: no residual mass and no malignant cells identified microscopically), microscopic residual disease (no residual mass; malignant cells identified microscopically), or gross residual disease (identifiable residual mass; malignant cells identified microscopically).
Surgery consisted of LAR or APR for all patients in the TME group, and either Kraske procedure or full-thickness transanal excision of the tumor site in the LE group. For both groups, intraoperative frozen section was used when necessary to maximize the chance of obtaining a negative margin, and additional margins were taken as needed. In three LE patients, a positive margin on final pathology was managed by reoperation and transanal reexcision; TME was recommended to all three patients but was refused.
Patients were scheduled for follow-up visits every 3 to 4 months for 2 years, then every 6 months up to 5 years, and yearly thereafter. Clinical examination, digital rectal examination, and measurement of the serum carcinoembryonic antigen level were performed on every visit. CT scan of the abdomen and pelvis and a chest X-ray were performed every 6 months for the first 2 years and yearly thereafter. Colonoscopy was performed at 1 year after surgery, and then every 1 to 3 years thereafter.
The statistical packages SAS version 9.1, SPLUS 8.0, and STATISTICA 6.1 were used for all statistical calculations. Comparisons of covariate distributions between the LE and TME groups were conducted by Wilcoxon rank sum tests and χ2 analysis. Kaplan-Meier product-limit survival probability estimates were calculated, and log rank tests were performed to compare the time to event outcome (LR, DFS, disease-specific survival [DSS], and OS) between groups.
RESULTS
Forty-seven patients underwent LE. Of these, 6 underwent a Kraske procedure and 41 underwent transanal excision. A total of 473 patients underwent TME. Of these, 141 underwent APR and 332 underwent LAR. Table 1 summarizes patient characteristics (age, tumor size, distance from the anal verge, and amount of radiation received) by group. As expected, there was a statistically significant difference between groups in age, tumor size, and distance from the anal verge: LE patients tended to be older and have smaller tumors that were closer to the anal verge.
TABLE 1.
Patient characteristics stratified by group (TME vs. LE)
| Variable | n | Mean ± SD | Median (min, max) | P valuea |
|---|---|---|---|---|
| Age (y) | .02 | |||
| TME | 473 | 57.8 ± 12.5 | 58.3 (20.7, 87.8) | |
| LE | 47 | 62.5 ± 14.2 | 61.0 (21.3, 86.4) | |
| Tumor size (cm) | < .0001 | |||
| TME | 440 | 5.2 ± 1.9 | 5.0 (1.0, 15) | |
| LE | 38 | 3.9 ± 1.4 | 4.0 (0.5, 7) | |
| Distance from anal verge (cm) | .0001 | |||
| TME | 472 | 5.5 ± 3.0 | 5 (0,12.0) | |
| LE | 45 | 3.7 ± 1.6 | 4 (0, 8.0) | |
| Dose of radiation received (cGy) | .88 | |||
| TME | 472 | 4744 ± 371 | 4500 (1980, 5250) | |
| LE | 34 | 4687 ± 549 | 4500 (2400, 5250) |
min minimum value;
max maximum value;
TME total mesorectal excision;
LE local excision
Wilcoxon rank sum test
Patients underwent LE instead of TME for several reasons. Twelve patients (25%) were considered to have prohibitive comorbidity. Fifteen patients (32%) refused to undergo TME. Fifteen patients (32%) did not absolutely refuse TME, but were judged to have had a complete clinical response to CXRT and strongly preferred to undergo LE. In these patients, LE was offered to them as an alternative treatment after appropriate education and demonstration of their understanding that this treatment was not considered the standard of care. Five patients (11%) underwent LE for other or undocumented reasons.
Table 2 summarizes the clinical response to CXRT stratified by group. There was a statistically significant difference between LE and TME groups in the percentage of patients who were determined to have no residual disease, microscopic residual disease, or gross residual disease on final pathology. Overall, significantly more LE patients than TME patients had final pathology demonstrating no residual disease or microscopic residual disease. Far more TME patients than LE patients had final pathology demonstrating gross residual disease.
TABLE 2.
Response to chemoradiotherapy stratified by group (TME vs. LE)
| Final pathology | TME, n (%) (n = 473) | LE, n (%) (n = 47) | P valuea |
|---|---|---|---|
| No residual disease (path CR) | 108 (23%) | 23 (49%) | .0001 |
| Microscopic residual disease | 89 (19%) | 17 (36%) | .0049 |
| Gross residual disease | 276 (58%) | 7 (15%) | <.0001 |
TME total mesorectal excision; LE local excision; path CR complete response on final pathology
χ2 test
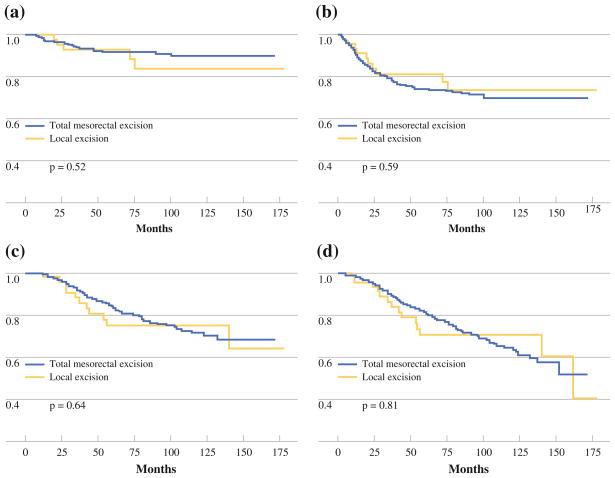
Median follow-up was 63 months (range, 9–178 months) for the LE group and 59 months (range, 4–172 months) for the TME group. Overall, 5 LE patients (10.6%) and 36 TME patients (7.6%) developed LR. There was no statistically significant difference in the LR rate between the LE group and the TME group (Fig. 1a).
FIG. 1.
Kaplan-Meier estimates for a freedom from local recurrence, b disease-free survival, c disease-specific survival, and d overall survival
The five LRs recurrences among LE patients occurred at 20, 21, 27, 72, and 76 months after surgery. Two of these patients underwent salvage surgery and currently have no evidence of disease at 6 and 7 years after surgery. A third patient underwent salvage surgery but developed pulmonary metastatic disease 7 months later. A fourth patient was offered salvage surgery but was unwilling to consider APR. Instead, she underwent CXRT (39 Gy with concurrent capecitabine) and is currently undergoing chemotherapy with capecitabine and oxaliplatin. Her most recent staging studies demonstrate a decrease in the size of the recurrence, and no evidence of disease elsewhere. A final patient was initially lost to follow-up, but when she sought care again 72 months later, she was discovered to have developed simultaneous LR and metastatic disease. None of the LE patients who developed LR have died of rectal cancer. Of the five LE patients who developed LR, final pathology revealed microscopic residual disease in four patients (80%) and gross residual disease in one patient (20%).
Overall, 10 LE patients (21.3%) and 118 TME patients (24.9%) developed disease recurrence (LR or distant metastatic disease). There was no statistically significant difference in the DFS rate between the LE group and the TME group (Fig. 1b). Disease recurrence in the LE group included the five aforementioned patients who developed LR, and five patients who developed metastatic disease with no evidence of LR at 4, 6, 12, 13, and 24 months after surgery. Four of the five LE patients who developed metastatic disease with no evidence of LR have died from rectal cancer; the fifth patient died of other causes. Final pathology in these five patients revealed microscopic residual disease in four patients (80%) and no residual disease in one patient (20%).
Because the LE group was older than the TME group, and because we presumed there may be higher baseline comorbidity in the LE group (25% were offered LE because of prohibitive comorbidity), we evaluated DSS as well as OS. DSS events were defined as deaths confirmed to be related to rectal cancer, as well as deaths of unknown cause. Only deaths confirmed to be unrelated to rectal cancer were excluded. There was no difference in DSS (Fig. 1c) or OS (Fig. 1d) between the LE and TME groups.
To control for response to CXRT, patients were subdivided into those with no residual disease, those with microscopic residual disease, and those with gross residual disease on final pathology. There was no difference in LR, DFS, DSS, or OS rates between the LE and TME groups for each category of response to CXRT, although small sample sizes in these analyses resulted in limited power (data not shown).
The presence of N1 disease was detected by preoperative EUS or CT in 13 LE patients (27.7%) and in 254 TME patients (53.7%). N1 disease was not confirmed by biopsy. In all but three LE cases, N1 disease seen on EUS was not appreciated as abnormal lymphadenopathy on CT. In the three cases of CT-visualized N1 disease, lymph nodes were normal on post-CXRT CT imaging performed before LE. Of the 13 LE patients with N1 disease, 3 underwent LE because of prohibitive medical comorbidity, 4 because of patient refusal of TME, 4 because of strong patient preference for LE, and 2 for undocumented reasons. LR developed in 3 (23.1%) of the 13 LE patients with N1 disease versus 2 (5.9%) of the 34 LE patients with N0 disease, a difference that was not statistically significant (Fisher exact test, P = .1213).
There were nine complications (19%) overall in the LE group. Five complications (10.6%) occurred in the first 30 days after surgery, and included readmission for pain control 1 week postoperatively (two transanal excision patients), urinary retention requiring Foley catheter placement for 6 days postoperatively in one transanal excision patient, rectal bleeding 2 weeks postoperatively in a transanal excision patient on enoxaparin for a deep venous thrombosis, and one episode of small bowel obstruction 2 weeks after a Kraske procedure with diverting loop ileostomy in one patient. Four complications (8.5%) occurred after the first 30 days postoperatively. Two patients developed rectocutaneous fistula after Kraske procedure, one of which healed spontaneously, and one of which required diverting colostomy and eventual APR. Two patients developed rectal stricture after transanal excision, which required endoscopic dilation twice between 6 and 18 months postoperatively. Overall, 3 (50%) of 6 patients who underwent Kraske procedure, and 6 (14.6%) of 41 patients who underwent transanal excision developed complications, a difference that showed a trend toward statistical significance (Fisher’s exact test, P = .0747).
The subset of 26 patients in our original, previously published LE group was analyzed separately.22 Median follow-up for this group was 89 months (range, 23–174 months). LR occurred in three patients (11.5%). Again, there was no difference in LR, DFS, DSS, or OS rates when the original LE group was compared to the current TME control group (P = .72, P = .57, P = .94, and P = .62 respectively).
DISCUSSION
In the United States, standard therapy for locally advanced rectal adenocarcinoma is CXRT followed by TME (LAR or APR). However, these operations carry a high risk of morbidity and mortality and can also greatly alter a patient’s quality of life. We and others have previously reported small series evaluating outcomes after full-thickness LE after CXRT, in most cases because of prohibitive medical comorbidity or patient refusal to undergo an operation that carried the risk of an ostomy.15–18,22 Habr-Gama et al. have taken this concept to the next level and have observed a cohort of patients with locally advanced rectal cancer who had a complete response to CXRT, with no intervention other than close follow-up.19–21 The encouraging results from these studies have led us to question whether there exists a subset of patients who could be spared the morbidity of TME and for whom a local operation might be sufficient. It is an important question because patient preferences and advancing technology have driven every medical field toward less invasive approaches, and certainly the field of rectal surgery is no exception. However, because of the unpredictable nature of the disease process and the bias in selecting the patients, it is not possible to specify exactly which patients are candidates for LE; the decision must be made on a case-by-case basis, taking individual circumstances into account.
Although LE for T3 rectal cancer is controversial, we have shown that in a group of patients who were too sick to undergo TME, refused to undergo TME, or (in recent years) had an apparent complete response to CXRT and strongly preferred to avoid TME, LE offers comparable local control and survival to TME. We again emphasize that offering LE as an alternative therapy to TME for eligible patients with an apparent complete response to CXRT must be done in the context of extensive patient education; patients must understand that LE is not the standard of care for locally advanced rectal cancer.
Our LE patients were older, had smaller tumors, and had tumors closer to the anal verge than our TME group. The difference in age is expected because 25% of our LE patients underwent LE instead of TME simply because they had medical comorbidities that prohibited the more radical operation, and increasing comorbidity often correlates with older age. The difference in the size and location of the tumors is also expected. We would expect patients with smaller tumors to have a better response to CXRT, and in recent years, we have offered LE to patients with an apparent clinical complete response to CXRT who did not absolutely refuse TME but who strongly preferred LE and clearly understood that it was not the standard of care. Also, patients with very distal tumors were more likely to have been recommended APR, which requires a permanent ostomy, and these patients were probably more likely to refuse TME and insist on LE. However, although these differences between groups were statistically significant, they were small and unlikely to be clinically meaningful.
The difference in final pathology between the LE and the TME groups—with no residual disease and microscopic residual disease being more common in the LE group, and gross residual disease being more common in the TME group—is also expected. One third of the patients in the LE group were offered LE on the basis of a perceived complete response to preoperative CXRT, and therefore, the increased incidence of LE patients with no or microscopic residual disease in their final pathologic specimen probably reflects that the correct group was selected to undergo LE instead of TME. Nonetheless, it is important to recognize that clinical impression is not perfect. In a previous report from our institution, 41% of patients who had either no visible mucosal abnormality or only a residual scar on preoperative evaluation after CXRT had residual disease on pathologic examination of their proctectomy specimen.23 However, the number of these patients in this group with only microscopic residual disease (and thus potential candidates for LE) was not identified.
There was no statistically significant difference in recurrence rate between the LE and the TME groups. LR typically occurred during the first 2 years after surgery, but two LRs occurred late, at 72 and 76 months after surgery. Thus, we thought it was important to look at very long-term follow-up in the group of original patients on whom we previously reported, who underwent CXRT followed by LE.22 Even after considerably longer follow-up in this group (89 months), we did not detect a higher rate of LR.
There were also no statistically significant differences between the LE and TME groups in any of the other outcome measures (DFS, DSS, or OS). Several patients in the LE group developed metastatic disease early—less than a year after surgery. This is consistent with the aggressive nature of the tumors in those particular patients. One of those patients had no residual disease on final pathology after LE. Again, this suggests aggressive tumor biology rather than failure of surgical control.
Several studies have reported that the response to CXRT is an independent predictor of survival.24,25 A statistically significantly higher incidence of patients in the LE group had either no residual disease or microscopic residual disease compared with the TME group. However, a comparison of LE and TME patients within the same category of response to CXRT on the basis of final pathology (no residual disease, microscopic residual disease, or gross residual disease) still revealed no difference in any of the outcome measures. The numbers of patients, particularly for the patients with gross residual disease (n = 7), were very small, so the significance of this finding is unclear.
A higher percentage of TME patients had N1 disease on preoperative staging workup compared with the LE group. This could certainly represent a source of bias in favor of the LE group; however, this finding was expected, and underscores the idea that the appropriate patients are specifically selected as good candidates for LE instead of TME in the setting of T3 disease.
Although a higher percentage of patients with N1 disease experienced LR compared with patients with N0 disease, this difference was not statistically significant. The fact that N1 disease was visualized by CT in only 3 of 13 cases is consistent with EUS as a more sensitive imaging modality for nodal staging. However, EUS may in fact have overstaged some patients by identifying tiny sub-centimeter lymph nodes of uncertain significance; in the German trial, Sauer et al. reported that 18% of patients were overstaged by EUS.6 Patients are not routinely restaged by EUS after CXRT; however, any patient with visible nodal disease on CT after CXRT is strongly discouraged from undergoing LE.
Although the LE group experienced a 19% overall complication rate, most of the complications were relatively minor. In our series, the most significant adverse events (rectocutaneous fistula and small bowel obstruction) occurred in patients who underwent a Kraske procedure, whereas patients who underwent transanal excision experienced minimal morbidity. Because of the very low risk of morbidity, transanal excision is our preferred technique for LE, and we try to avoid a Kraske procedure unless it is absolutely necessary.
In conclusion, to our knowledge, our data represent the largest series of patients described in the literature who have been followed after LE for T3 rectal cancer treated with preoperative CXRT. Our results suggest that there exists a subset of patients with T3 rectal cancer who demonstrate an excellent response to CXRT and for whom LE might be appropriate therapy. These patients could thus be spared the morbidity of TME and could experience acceptable local control and survival. There is clearly a need for further study in this area; specifically, a randomized prospective trial would be extremely valuable in answering this question. However, it is unlikely that such a trial will be forthcoming because of ethical issues involved with randomizing patients to TME versus LE, as well as strong patient preferences.
The ongoing nonrandomized phase II ACOSOG Z 6041 trial is currently evaluating preoperative CXRT followed by LE in patients with clinical T2 rectal cancer. Patients whose pathology is T0 to T2 with negative margins are observed. Patients with T3 tumors or positive margins on pathology are managed at the discretion of their treating physician. Depending on the results of that trial, the next potential step could be a phase II nonrandomized trial in patients with clinical T3 rectal cancer. After preoperative CXRT followed by LE, patients with either no residual disease or microscopic residual disease and negative margins could be observed. Patients with more than microscopic residual disease could be managed at the discretion of their treating physician.
Until there is further definitive data, patient selection must continue to be made on a highly individualized basis. It is critical that patients receive appropriate education regarding TME as the current standard of care for locally advanced rectal cancer and that they agree to very close follow-up if they should elect to undergo LE.
Acknowledgments
We thank E. Lin and Wei Qiao for their assistance with statistical analysis.
References
- 1.Thomas PR, Lindblad AS. Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: a review of the Gastrointestinal Tumor Study Group experience. Radiother Oncol. 1988;13(4):245–52. doi: 10.1016/0167-8140(88)90219-8. [DOI] [PubMed] [Google Scholar]
- 2.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324(11):709–15. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 3.Tveit KM, Guldvog I, Hagen S, et al. Randomized controlled trial of postoperative radiotherapy and short-term time-scheduled 5-fluorouracil against surgery alone in the treatment of Dukes B and C rectal cancer. Norwegian Adjuvant Rectal Cancer Project Group. Br J Surg. 1997;84(8):1130–5. [PubMed] [Google Scholar]
- 4.Bosset JF, Collette L, Calais G, et al. Chemotherapy with pre-operative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 5.Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radio-therapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24(28):4620–5. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 6.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 7.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on down-staging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17(8):2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 8.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341(8843):457–60. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 9.Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995;181(4):335–46. [PubMed] [Google Scholar]
- 10.Aitken RJ. Mesorectal excision for rectal cancer. Br J Surg. 1996;83(2):214–6. doi: 10.1046/j.1365-2168.1996.02057.x. [DOI] [PubMed] [Google Scholar]
- 11.Rothenberger DA, Wong WD. Abdominoperineal resection for adenocarcinoma of the low rectum. World J Surg. 1992;16(3):478–85. doi: 10.1007/BF02104451. [DOI] [PubMed] [Google Scholar]
- 12.Chessin DB, Enker W, Cohen AM, et al. Complications after preoperative combined modality therapy and radical resection of locally advanced rectal cancer: a 14-year experience from a specialty service. J Am Coll Surg. 2005;200(6):876–82. doi: 10.1016/j.jamcollsurg.2005.02.027. discussion 882–874. [DOI] [PubMed] [Google Scholar]
- 13.Neal DE, Williams NS, Johnston D. A prospective study of bladder function before and after sphincter-saving resections for low carcinoma of the rectum. Br J Urol. 1981;53(6):558–64. doi: 10.1111/j.1464-410x.1981.tb03260.x. [DOI] [PubMed] [Google Scholar]
- 14.Havenga K, Enker WE, McDermott K, et al. Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg. 1996;182(6):495–502. [PubMed] [Google Scholar]
- 15.Mohiuddin M, Marks G, Bannon J. High-dose preoperative radiation and full thickness local excision: a new option for selected T3 distal rectal cancers. Int J Radiat Oncol Biol Phys. 1994;30(4):845–9. doi: 10.1016/0360-3016(94)90359-x. [DOI] [PubMed] [Google Scholar]
- 16.Kim CJ, Yeatman TJ, Coppola D, et al. Local excision of T2 and T3 rectal cancers after downstaging chemoradiation. Ann Surg. 2001;234(3):352–8. doi: 10.1097/00000658-200109000-00009. discussion 358–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruo L, Guillem JG, Minsky BD, et al. Preoperative radiation with or without chemotherapy and full-thickness transanal excision for selected T2 and T3 distal rectal cancers. Int J Colorectal Dis. 2002;17(1):54–8. doi: 10.1007/s003840100327. [DOI] [PubMed] [Google Scholar]
- 18.Schell SR, Zlotecki RA, Mendenhall WM, et al. Transanal excision of locally advanced rectal cancers downstaged using neoadjuvant chemoradiotherapy. J Am Coll Surg. 2002;194(5):584–90. doi: 10.1016/s1072-7515(02)01128-6. discussion 590–581. [DOI] [PubMed] [Google Scholar]
- 19.Habr-Gama A, de Souza PM, Ribeiro U, Jr, et al. Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum. 1998;41(9):1087–96. doi: 10.1007/BF02239429. [DOI] [PubMed] [Google Scholar]
- 20.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–7. doi: 10.1097/01.sla.0000141194.27992.32. discussion 717–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habr-Gama A, Perez RO, Proscurshim I, et al. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006;10(10):1319–28. doi: 10.1016/j.gassur.2006.09.005. discussion 1328–1319. [DOI] [PubMed] [Google Scholar]
- 22.Bonnen M, Crane C, Vauthey JN, et al. Long-term results using local excision after preoperative chemoradiation among selected T3 rectal cancer patients. Int J Radiat Oncol Biol Phys. 2004;60(4):1098–105. doi: 10.1016/j.ijrobp.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 23.Bedrosian I, Rodriguez-Bigas MA, Feig B, et al. Predicting the node-negative mesorectum after preoperative chemoradiation for locally advanced rectal carcinoma. J Gastrointest Surg. 2004;8(1):56–62. doi: 10.1016/j.gassur.2003.09.019. discussion 62–53. [DOI] [PubMed] [Google Scholar]
- 24.Berger C, de Muret A, Garaud P, et al. Preoperative radiotherapy (RT) for rectal cancer: predictive factors of tumor downstaging and residual tumor cell density (RTCD): prognostic implications. Int J Radiat Oncol Biol Phys. 1997;37(3):619–27. doi: 10.1016/s0360-3016(96)00577-9. [DOI] [PubMed] [Google Scholar]
- 25.Janjan NA, Crane C, Feig BW, et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol. 2001;24(2):107–12. doi: 10.1097/00000421-200104000-00001. [DOI] [PubMed] [Google Scholar]