Abstract
Type 1 immunity relies on the differentiation of two major subsets of T lymphocytes, the CD4+ T helper (Th) cell and the CD8+ cytotoxic T cell, that direct inflammatory and cytotoxic responses essential for the destruction of intracellular and extracellular pathogens. In contrast to CD4 cells, little is known about transcription factors that control the transition from the CD8 naïve to effector cell stage. Here, we report that the transcription factor T-bet, known to regulate Th cell differentiation, also controls the generation of the CD8+ cytotoxic effector cell. Antigen-driven generation of effector CD8+ cells was impaired in OT-I T cell receptor transgenic mice lacking T-bet, resulting in diminished cytotoxicity and a marked shift in cytokine secretion profiles. Furthermore, mice lacking T-bet responded poorly to infection with lymphocytic choriomeningitis virus. T-bet is a key player in the generation of type 1 immunity, in both Th and T cytotoxic cells.
The development, differentiation, and activation of the CD8+ cytotoxic T lymphocyte (CTL) are fundamental to the successful clearance of most pathogenic microorganisms. Upon stimulation, naïve CD8+ T cells undergo rapid antigen-specific selection, expansion, and differentiation, generating a concerted population of effector CTLs (1). Compared to the naïve precursor, the effector CD8+ T cell exhibits a distinct profile of surface markers and effector molecules required for pathogen eradication (2). Once an infection has been successfully cleared, the effector cell population diminishes rapidly through extensive cell death, while maintaining a pool of long-lived memory cells. Although the course of a CD8+ T cell response has been described for numerous pathogens, the factors that control the transition of a naïve CD8+ precursor to an activated, lytic effector cell are poorly understood (3). Stat4, for example, whose activation by IL-12 or IFN-α in CD8+ T cells does modulate IFN-γ production, has not been reported to affect CTL function (4, 5).
T-bet, a T helper 1 (Th1)-specific transcription factor previously isolated and characterized in this laboratory, has been shown to be required for the development of the CD4+ Th1 subset. T-bet directly activates transcription of the IFN-γ gene and has the remarkable property of redirecting committed Th2 populations to a Th1 phenotype (6). The importance of T-bet in Th1 immunity has been most clearly illustrated through the analysis of mice with a targeted disruption of the T-bet locus. CD4+ T cells lacking T-bet are severely impaired in their ability to produce IFN-γ, yet secrete elevated levels of the opposing Th2 subset cytokines, IL-4 and IL-5 (7). Furthermore, T-bet-deficient mice, on the otherwise resistant C57BL/6 background, are highly susceptible to Leishmania major infection, are protected from autoimmune inflammatory bowel disease, and spontaneously develop inflammation and airway remodeling resembling human asthma, indicating a marked in vivo shift of the Th1/Th2 balance toward the Th2 pathway (7–9).
Interestingly, in our initial analysis, whereas CD4+ T cell function was greatly impaired in the absence of T-bet, CD8+ T cells were unaffected by the loss of T-bet, as assessed by their production of IFN-γ and cytolytic activity in vitro (7). This finding was unexpected given that T-bet is rapidly induced to comparable levels in both cell types by signals emanating from the IFN-γ receptor and T cell receptor (TCR) (7, 10, 11). However, it has been pointed out that stimulation of T cells by antigen-nonspecific stimuli, most commonly through engagement of the TCR and costimulatory receptors with activating antibodies, may not mimic the more physiologic activation of these cells by a cognate antigen (11).
Here, using the OT-I TCR transgenic system, we demonstrate that under antigen-specific stimulation conditions T-bet is required for the differentiation of naïve CD8+ T cells into effector CTLs. T-bet-deficient CD8+ cells fail to acquire a bona fide effector surface marker profile after antigenic stimulation, and, likewise, are severely impaired in their killing capacity. Similar to previous findings in CD4+ T cells, T-bet-deficient OT-I, CD8+ T cells proliferate and expand normally, but secrete low levels of IFN-γ, whereas IL-4, IL-2, and most strikingly, IL-10, production is elevated. Furthermore, the diminished cytotoxicity observed appears to be independent of the altered cytokine secretion pattern. Finally, in the absence of T-bet, in vivo CD8+-mediated responses after lymphocytic choriomeningitis virus (LCMV) infection and DNA vaccination are severely compromised.
Materials and Methods
Mice and Cell Lines. C57BL/6 T-bet-deficient mice, backcrossed eight generations, were crossed onto the OT-I TCR transgenic background. IFN-γ-deficient OT-I mice were kindly provided by A. Lichtman (Harvard Medical School). The mouse thymoma, EL4, fibroblast line, MC57, and peritoneal exudate cells served as targets for CTL assays.
Cytokines and Antibodies. Recombinant mouse cytokines, IFN-γ, IL-4, IL-10, and IL-12, were purchased from PeproTech (Rocky Hill, NJ). Human recombinant IL-2 was a gift from Chiron. Capture and horseradish peroxidase-conjugated ELISA antibodies against IL-2, IL-4, and IFN-γ were purchased from Pharmingen, and antibodies against IL-10 were purchased from R & D Systems. All FITC-, phycoerythrin (PE)-, and CyC-conjugated antibodies were obtained from Pharmingen for fluorescence-activated cell sorting (FACS) analysis.
In Vitro OT-I Stimulation and Proliferation. CD8 T cells were isolated by magnetic bead purification (MACS, Miltenyi Biotec) from the lymph nodes of 6- to 8-week-old T-bet-/- and WT OT-I TCR transgenic mice. CD8+ cells (1 × 106) were stimulated for 72 h in the presence of irradiated (2,000 rads) syngeneic C57BL/6 splenocytes (15 × 106) and 1 μM ovalbumin peptide, SIINFEKL amino acids 257–264 (Sigma), or plate-bound anti-CD3 (2 μg/ml), anti-CD28 (2 μg/ml) with recombinant human IL-2 (100 units/ml) then expanded for 4 days. Cells were then restimulated as above for 24 h by either irradiated splenocytes and peptide or plate-bound anti-CD3, and supernatants were collected for cytokine ELISA.
Intracellular Cytokine Staining and ELISA. Primary OT-I cultures were restimulated with either irradiated splenocytes and peptide for 18 h or combined phorbol 12-myristate 13-acetate (PMA) (50 ng/ml) and ionomycin (1 μM) for 5 h. The protein secretion inhibitor, monensin (3 μM), was added to the cultures for the final 6 and 2 h, respectively. Cells were then washed, fixed in 4% paraformaldehyde, permeabilized with 0.1% saponin/1% FCS/PBS, and stained with FITC-Vα2 and the appropriate PE-labeled cytokine antibody. Culture supernatants were incubated overnight at 4°C in antibody-coated 96-well plates (Costar) for cytokine capturing. Plates were incubated sequentially with biotinylated secondary antibodies, avidin-horseradish peroxidase, and phosphatase substrate (Sigma) for detection.
In Vitro CTL Assay. OT-I, CD8+ T cells were stimulated with irradiated splenocytes and peptide. After 72 h, cells were harvested, then incubated for 6 h with 51Cr-labeled EL4 syngeneic targets (3,000 per reaction) pulsed with SIINFEKL peptide. Reactions were performed in triplicate at the indicated effector/target ratios. Nonspecific lysis was assessed at the 10:1 (effector/target) ratio without peptide loading, maximum lysis, by treating labeled targets with 1% SDS, and spontaneous lysis, in the absence of effectors. Specific lysis was calculated as described (12).
In Vivo CTL Assay. T-bet-/- and WT OT-I CD8+ T cells were enriched from lymph node preparations by combined CD4 and CD19 MACS negative selection. Purity was assessed by combined Vα2-PE and Vβ5.1-FITC staining, and cells (1 × 106 Vα2+, Vβ5.1+) were transferred i.v. to normal C57BL/6 hosts. Recipients were immunized 2 days later with PBS or 200 μg ovalbumin/PBS (Sigma) by s.c. injections at the base of the tail. After 6 days, carboxyfluorescein diacetate-succinimidyl ester (CFSE)-hi (5 μM), peptide pulsed targets and CFSE-lo (0.5 μM), unpulsed control C57BL/6 splenocytes (10 × 106 per population) were transferred i.v. to each host. The draining inguinal nodes were harvested 10 h later, stained briefly with propidium iodide, and analyzed by FACS (13).
In Vitro Proliferation and in Vivo Expansion. OT-I CD8 T cells (2 × 105) were stimulated with irradiated splenocytes (2 × 106) and peptide at the indicated concentrations for 72 h. Tritiated thymidine (1 μCi) was added for the final 24 h, and proliferation was quantified as [3H] incorporation. For in vivo expansion analysis, CFSE (5 μM)-labeled T-bet-/- and WT CD8 OT-I cells (5 × 106) were transferred i.v. to WT C75BL/6 hosts. Recipients were immunized with ovalbumin as described above for in vivo CTL assays. The draining nodes were harvested 2 days after immunization, stained with CD8-CyC and Vα2-PE, and analyzed by FACS.
LCMV Infection and Intracranial Protection. T-bet-/-, T-bet+/-, and T-bet+/+ mice were infected with 105 plaque-forming units of LCMV (Armstrong clone 53b) i.p. and killed 14 days later. Spleen cells were then cultured with either the LCMV MHC-I peptide, GP33, or plate-bound anti-CD3 and anti-CD28, and intracellular IFN-γ production was determined by FACS. Secondary CTL assays were performed 30 days after LCMV infection. Spleen cells from LCMV-infected mice were cultured for 6 days with irradiated, LCMV-infected peritoneal exudate cells. CTL activity was quantified as 51Cr release from LCMV-infected MC57 cells. T-bet-/- and T-bet+/- or T-bet+/+ littermates were immunized twice, i.m., with plasmid encoding for the LCMV nucleoprotein, pCMV-NP. A normally lethal intracranial challenge of LCMV was given 7 days after the second vaccination to assess protection.
Results
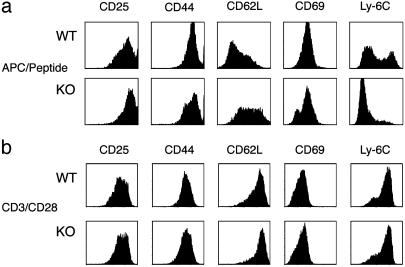
CD8+ Effector Development in the Absence of T-Bet. To elucidate a potential role for T-bet in antigen-driven differentiation and activation of CD8+ T cells, we generated T-bet-deficient C57BL/6 mice expressing the MHC class I restricted OT-I TCR transgene (T-bet-/-OT-I) specific for the ovalbumin peptide, SIINFEKL (14). CD8 cells were present at comparable numbers in WT and T-bet-/- OT-I transgenic thymus, spleen, and lymph node, indicating that T-bet is not required for the generation of the CD8 compartment. This finding is in contrast to mice lacking NFκB activity caused by the overexpression of a dominant negative IκB protein, which exhibit a block in the positive selection of CD8+ thymocytes (15, 16). We first examined the activation and effector differentiation status of stimulated T-bet-/-OT-I CD8+ T cells (Fig. 1). With proper activation, the surface of a CD8+ T cell undergoes significant remodeling, resulting in a signature effector profile (17). After stimulation with peptide in conjunction with antigen-presenting cells (APCs)/peptide, WT OT-I CD8+ T cells had elevated levels of CD25, CD44, CD69 and Ly-6C, while exhibiting considerable loss of CD62L expression. Although T-bet-/-OT-I CD8+ T cells expressed levels of CD25 similar to WT, they demonstrated a slight reduction in CD44, incomplete elevation of CD69, failure to down-regulate CD62L, and virtually complete failure to up-regulate Ly-6C expression (Fig. 1a). Of note, a fraction of the T-bet-/-OT-I CD8+ population down-regulated CD8 expression after stimulation (data not shown). In addition, the differences in the signature effector profile seen with APC/peptide stimulation were not reproduced with anti-CD3/CD28-induced activation. The expression profiles of each marker were nearly identical upon polyclonal stimulation; notably, neither WT nor T-bet-/- CD8 cells down-regulated CD62L efficiently, whereas Ly6-C expression was dramatically up-regulated by cells of both genotypes (Fig. 1b). These data demonstrate a substantial block in the antigen-driven generation of effector CTLs from naïve CD8+ T cells in the absence of T-bet. Furthermore, this block appears to be bypassed by polyclonal activation.
Fig. 1.
T-bet-deficient CD8+ T cells fail to acquire an effector phenotype after antigenic stimulation. OT-I CD8 T cells were isolated by magnetic bead purification from the lymph nodes of T-bet-/- and WT OT-I TCR transgenic mice. Cells were stimulated with irradiated C57BL/6 splenocytes and peptide (1 μM) (a) or plate-bound anti-CD3 and anti-CD28 with recombinant human IL-2 (b) for 72 h, then expanded. Populations were stained with PE (CD25, CD44, CD62L)- and FITC (CD69, Ly6C)-conjugated antibodies and analyzed by FACS. KO, knockout.
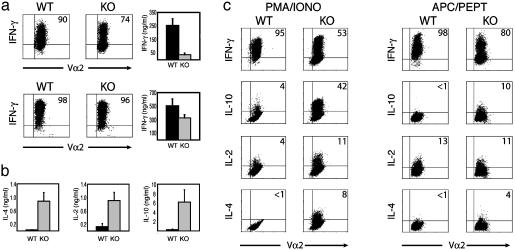
Altered Cytokine Production by T-Bet-Deficient OT-I+, CD8+ T Cells. The production of IFN-γ is another functional hallmark of the effector CD8+ T cell. Unlike naïve cells, which produce little IFN-γ, effector CD8+ T cells secrete large amounts of IFN-γ immediately after activation, a key mechanism in combating viral infections. IFN-γ is a direct inhibitor of viral replication and facilitates antigen processing of viral peptides by inducing expression of MHC class I molecules, TAP (transporter associated with antigen processing) transporter proteins, and components of the proteasome (18, 19). In contrast to what we observed previously using antigen-nonspecific stimuli, T-bet-/- OT-I CD8+ T cultures exhibited a profound reduction in both total IFN-γ secretion and the number of IFN-γ-producing cells after APC/peptide stimulation (Fig. 2a Upper). However, these same T-bet-/- OT-I CD8+ T cells produced only modestly diminished levels of IFN-γ when stimulated by antibodies against the TCR and CD28 receptors, consistent with the surface marker profiles above and data previously reported (Fig. 2a Lower). Similar to T-bet-/- CD4+ T cells, the defect in IFN-γ was accompanied by a substantial increase in the production of IL-4 (Fig. 2b) and IL-5 (data not shown), even in the absence of Tc2 (IL-4, anti-IFN-γ, and anti-IL-12) polarizing conditions. T-bet has been reported to repress the transcription of the IL-2 gene in CD4+ cells (6). Similarly, T-bet-/- OT-I cells produced significantly higher amounts of IL-2 than WT controls (Fig. 2b). Indeed the elevation in IL-2 production was far more pronounced in CD8+ than CD4+ T-bet-/- cells and was also observed with greater reproducibility after APC/peptide stimulation compared to other forms of activation. Of particular interest was the marked increase in levels of the Th2-associated immunosuppressive cytokine, IL-10, which is ordinarily secreted at very low levels in secondary stimulated CD8+ T cells (Fig. 2b). Intracellular cytokine staining confirmed the differing cytokine profiles observed. The T-bet-/-OT-I cultures exhibited a reduction in IFN-γ-producing cells and a concomitant increase in the number of cells producing IL-4 and IL-10 (Fig. 2c). Differences in IL-2 intracellular staining were variable, although the levels of secreted IL-2 were consistently elevated in the absence of T-bet (Fig. 2 b and c).
Fig. 2.
Diminished IFN-γ, but increased IL-2 and Tc2 cytokine production by antigen-stimulated T-bet-deficient CD8 T cells. T-bet-/- and WT OT-I CD8 cells were stimulated with APC/peptide (a Upper, b, and c) or anti-CD3, anti-CD28, and recombinant human IL-2 (a Lower), as in Fig. 1. (a) Defective IFN-γ production by T-bet-/- OT-I CD8 cells only under antigen-specific stimulation conditions. Cells were restimulated for 24 h by either APC/peptide (Upper Right) or plate-bound anti-CD3 (Lower Right), and secreted IFN-γ was measured by ELISA. Intracellular IFN-γ staining was performed after stimulation with either APC/peptide (Upper) for 18 h or PMA/ionomycin (Lower)for 5 h. (b) Elevated levels of IL-2 and Tc2 cytokines in T-bet-deficient CD8 cells. IL2, IL-4, and IL10 production was measured by ELISA of supernatants taken after a 24-h secondary APC/peptide stimulation. (c) The altered cytokine profile of antigen-stimulated T-bet-/- OT-I CD8 cells is independent of secondary stimulation conditions. After an initial 72-h APC/peptide stimulation and expansion, cells were restimulated for intracellular cytokine analysis by either PMA/ionomycin (Left) or APC/peptide (Right). Dot plot values represent the percentage of Vα2+ cells that stained positive for the indicated cytokine. KO, knockout.
In CD4 cells, T-bet acts at an early stage of Th differentiation to mold genetic programs in the naïve Th progenitor cell (6). Interestingly, T-bet also acted at an early stage in the naïve CD8 cell to determine effector cell fate, as evidenced by the finding that the cytokine profiles of both WT and T-bet-/-OT-I CD8+ T cells were contingent on the nature of the primary stimulus. Thus, WT and T-bet-/- CD8 cells, stimulated with APC/peptide in primary cultures, still showed pronounced differences in cytokine expression profiles after secondary stimulation either by APC/peptide (Fig. 2c Right) or the polyclonal activity of PMA/ionomycin (Fig. 2c Left). Although CD8+ Tc2 cells can be generated in vitro under Tc2-polarizing conditions, their frequency is much lower than CD4+ Th2 populations, and their actual existence in vivo has been controversial. Our data suggest that one function of T-bet in CD8 cells in vivo may be to act as a potent repressor of the Tc2 phenotype.
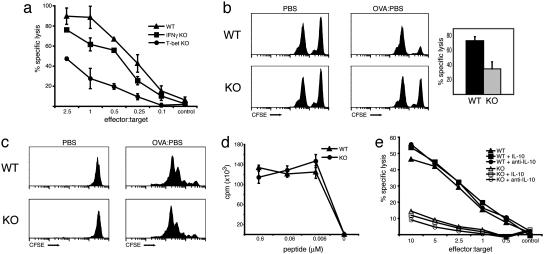
Antigen-Specific CD8+ Cytotoxicity Requires T-Bet. Having observed a marked deficiency of a central effector molecule, IFN-γ, and the noticeably impaired profile of effector cell markers, we next compared the cytolytic activity of T-bet-/- OT-I CD8+ and WT OT-I CD8+ cells in two separate antigen-specific assays. First, after in vitro priming with APC/peptide for 72 h, CTL activity was quantified by chromium release from the thymoma cell line, EL4, pulsed with SIINFEKL peptide (12). Although T-bet-deficient CTLs displayed detectable cytolytic activity, the amount of cytolysis was greatly reduced across all effector/target ratios when compared with WT CTLs (Fig. 3a), a defect that could not be attributed to the impaired production of IFN-γ (see below).
Fig. 3.
Defective CTL effector function in T-bet-deficient OT-I CD8 cells. (a and e) WT, T-bet-/-, and IFN-γ-/- OT-I CD8 cells were stimulated with APC/peptide for 72 h, harvested, and then incubated for 6 h with 51Cr-labeled, peptide-pulsed EL4 targets. Data presented represent triplicate reactions at each effector/target ratio from one experiment (n = 5). (b) Lymph node T-bet-/- and WT OT-I CD8 cells were enriched by combined CD4 and CD19 negative selection and transferred to C57BL/6 hosts i.v. Recipients were immunized by s.c. injection with either PBS (control) or ovalbumin (experimental). After 6 days, equal numbers of peptide-pulsed CFSE-hi targets and unpulsed CFSE-lo control splenocytes were injected i.v. to each host. The draining inguinal nodes were processed 10 h later, and specific lysis was assessed by FACS. Histograms represent propidium iodide-negative, CFSE-positive acquisitions from one mouse per group and are representative of two independent experiments. Specific lysis was also quantified and graphed as the mean ± SEM of both experiments, totaling six recipient mice per group. (c and d) T-bet-/- OT-I CD8 cells proliferate comparably to WT controls. (c) CFSE-labeled T-bet-/- and WT OT-I, CD8 cells were labeled and transferred to WT C75BL/6 hosts. Recipients were immunized as described above (b). The draining nodes were harvested 2 days after ovalbumin immunization, stained with CD8-CyC and Vα2-PE antibodies, and analyzed by FACS. Histograms represent CFSE intensity of CD8+, Vα2+ gated populations. (d) OT-I, CD8 cells were stimulated with splenocytes and peptide at the indicated concentrations for 72 h. Reactions were performed in triplicate and pulsed with [3H]thymidine for the final 24 h of stimulation. (e) Diminished CTL capacity cannot be attributed to the imbalance in cytokine production. Purified OT-I, CD8 cells were stimulated as described in the presence of recombinant murine IL-10 (10 μg/ml), recombinant murine IFN-γ (10 ng/ml), anti-IL10 (10 μg/ml), or anti-IFN-γ (75 μg/ml) for 72 h, followed by CTL analysis. KO, knockout.
Cytolytic potential was also assessed by using an in vivo cross priming assay, as described by Robinson and colleagues (13). WT and T-bet-/- OT-I CD8+ T cells were enriched from lymph node preparations by combined anti-CD19 and anti-CD4 magnetic bead depletion, and then transferred i.v. to WT C57BL/6 hosts. Recipients were then immunized s.c. with ovalbumin protein or mock PBS. After 6 days, CFSE-labeled, peptide-pulsed target (bright) and unpulsed control (dim) splenocytes were coinjected i.v., and their survival was assessed by flow cytometry 10 h later. Consistent with the in vitro CTL assays, the number of peptide-pulsed splenocytes recovered was markedly reduced in hosts that received WT OT-I CD8+ T cells as compared to T-bet-/- OT-I CD8+ recipients (Fig. 3b). The impaired CTL activity could not be explained by defective proliferation or antigenic response of T-bet-/- CD8+ T cells, as observed in mice lacking the transcription factor Runx3 (20). CFSE-labeled T-bet-/-OT-I and WT OT-I CD8+ T cells, transferred to C57BL/6 hosts and immunized as above, progressed through a comparable number of cell divisions in response to antigen (Fig. 3c). Furthermore we observed normal proliferation of T-bet-/- OT-I CD8+ T cells after in vitro APC/peptide stimulation (Fig. 3d). Finally, TCR signaling in effector T cells results in the activation and serine phosphorylation of downstream mitogen-activated protein kinases, ERK1 and ERK2 (21). Using an antibody specific for phosphorylated ERK1/2, we observed comparable activation of ERK1/2 in T-bet-/- and WT OT-I CD8+ T cells after 30 min of APC/peptide secondary stimulation (data not shown).
We considered that the diminished CTL activity observed in the absence of T-bet could be explained by the profoundly altered patterns of cytokine expression. IL-10 and IFN-γ cross-regulate each other in inflammatory responses and have been reported to play a central, although controversial, role in CD8+ effector mechanisms (2, 22, 23). Although in vitro-generated Tc2 cells have been reported to retain cytolytic activity, others have described conditions where CD8 T cells lose CTL function while producing elevated amounts of IL-4, IL-5, and IL-10 (24, 25). Also, a deficiency in c-Rel expression that resulted in diminished CTL activity was overcome with the addition of exogenous cytokine (26). To address this possibility, T-bet-/- OT-I and WT OT-I CD8+ T cells were cultured in the presence of exogenous recombinant cytokines (IFN-γ, IL-10), cytokine blocking antibodies and Tc1 (recombinant IL-12 and anti-IL-4), or Tc2 (IL-4, anti-IFN-γ and anti-IL-12) skewing conditions. We observed no significant effects on CTL function in either WT or T-bet-/- OT-I CD8+ T cells under any of the conditions tested (Fig. 3e and data not shown). To further address the role of IFN-γ in CTL development, we examined CTL activity of IFN-γ-deficient OT-I transgenic CD8+ T cells. Previous studies using such mutant mice have generated conflicting data on the role of IFN-γ in viral infections (3, 27, 28). Again, we observed only a modest reduction in the generation of SIINFEKL-specific CTL activity in the absence of IFN-γ (Fig. 3a). In addition, the cytolytic capacity of T-bet-/-, IFN-γ-/- and WT OT-I CTLs was not altered by stimulation with IFN-γ-deficient splenocytes, ruling out the possibility that APC-derived IFN-γ could overcome deficiencies in the generation of IFN-γ-/- CTLs (data not shown). We conclude from these studies that T-bet controls the generation of effector CTLs by mechanisms beyond the regulation of IFN-γ, IL-4, and IL-10. Dissociation of IFN-γ production from cytolytic activity has also been observed in mice lacking NFκB activity caused by overexpression of a dominant negative IκB protein that secrete normal levels of IFN-γ yet do exhibit modest cytolytic defects (M. Boothby, personal communication) (16).
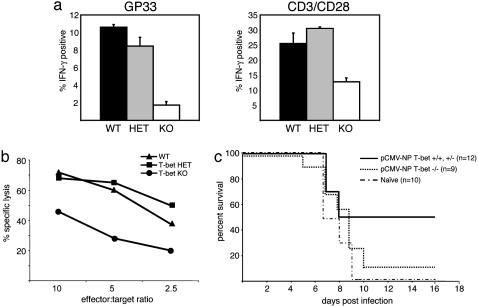
T-Bet Directs CD8+-Mediated Responses in Vivo. Given the striking differences in T-bet function revealed by varying the mode of stimulation (antigen-specific vs. nonspecific polyclonal), it was critical to evaluate the function of T-bet in an in vivo immune response against a pathogen. Because T-bet is critical in the generation of CD4+ Th1 cells, we examined an antiviral immune response that depends on the CD8+ T cell compartment. The response to LCMV primarily requires CD8+ but not CD4+ cells as demonstrated by the uncompromised CTL response against LCMV observed in mice lacking CD4 or MHC class II (29, 30). T-bet-/-and control littermates were infected i.p. with a single dose of LCMV, 1 × 105 plaque-forming units, and analyzed 14 days after infection. Spleen cells were cultured with LCMV MHC class I peptide (gp33) or anti-CD3/CD28, and the number of LCMV-specific, IFN-γ producing cells were measured by double staining for intracellular IFN-γ and the relevant TCR, using GP33–41 and NP396–404 peptide-loaded H-2Db tetramers, the dominant Db-restricted LCMV epitope (31, 32). A substantial reduction in numbers of IFN-γ producer cells in the absence of T-bet was observed under both antigen-specific and polyclonal activation protocols, but was more pronounced in the former (Fig. 4a). Cytolytic activity was examined 30 days after LCMV infection in secondary CTL assays after 6 days of culture with syngeneic LCMV-infected peritoneal exudate cells. Consistent with our observations using the OT-I TCR transgenic system, T-bet-/-LCMV-specific CD8 cells exhibited a marked decrease in cytotoxic effector function compared to WT and heterozygous controls (Fig. 4b).
Fig. 4.
T-bet-deficient mice fail to generate substantial viral-specific CTL activity and exhibit inadequate protection against LCMV infection. (a) Spleen cells of LCMV-infected mice were cultured with peptide, GP33, or plate-bound anti-CD3/CD28. IFN-γ production was assessed by intracellular staining and presented as percent IFN-γ+ of gated CD8+ populations. Data represent the mean ± SEM of two to three mice per group. (b) LCMV-infected T-bet-/-, T-bet+/-, and T-bet+/+ spleen cells were cultured for 6 days with LCMV-infected peritoneal exudate cells. CTL activity was quantified as 51Cr release from LCMV-infected MC57 cells at the indicated effector/target ratios. (c) T-bet-/- and T-bet+/- or T-bet+/+ mice were vaccinated with pCMV-NP, 7 and 14 days before intracranial LCMV challenge. naïve, unimmunized mice served as controls for lack of protection against infection. KO, knockout; HET, heterozygote.
To further address CD8 effector function in vivo, we assessed the protective capacity of T-bet-/- CTLs after DNA vaccination (33). It has been demonstrated that CTL clones can clear infection and that a vaccine that induced only CD8 cells was protective (34, 35). T-bet-/-, T-bet+/-, and WT mice received two injections of a DNA vector encoding for the LCMV nucleoprotein (pCMV-NP). Immunized and naïve, unimmunized control mice were then challenged with a normally lethal dose of LCMV by intracerebral inoculation. Within 10 days, nearly all T-bet-/- mice (8/9) succumbed to infection, paralleling the course of naïve controls (10/10). In contrast, a significant proportion of T-bet+/- and T-bet+/+ (6/12) were completely protected (Fig. 4c).
Discussion
Here, we have demonstrated that the generation and function of the effector CD8+ T cell compartment relies on the transcription factor T-bet. Discovering the function of T-bet in CD8 effector cell generation relied solely on assays involving antigen-specific stimuli. Other studies, centered on Th cell differentiation, have revealed discrepancies when comparing varied activation protocols (11). Indeed, although profound defects in T-bet-/-CD4 cells were observed under both antigen-specific and polyclonal stimulation, they were more marked in the former (7). Although numerous reports have described common signaling pathways shared by CD4 and CD8 T cells, differing thresholds and requirements for activation of these subsets have been documented (36, 37). For example, the exquisite sensitivity with which CD8 T cells can respond to minimal antigenic stimulations has been noted (38, 39). Such considerations may have contributed to the initial masking and subsequent elucidation of a T-bet dependent phenotype in CD8 T cells. Like T-bet, other factors central to Th1 development could prove to be potent mediators of CTL differentiation when examined in an appropriate, antigen-specific setting.
This study establishes a vital role for T-bet, a transcription factor that controls Th1 development and activation, in CD8+ effector function. T-bet therefore directs lineage commitment and effector function of naïve progenitor cells of both major T cell subsets, making it an overall regulator of type 1 immunity. This finding is in contrast to recent studies, which have described factors that play specific roles in CD8+ but not CD4 effector function. For example, a deficiency in the runt domain transcription factor, Runx3, results in impaired CD8+ T cell proliferation, despite normal expression of effector molecules like perforin, while having no effect on the proliferation of CD4+ T cells (20). Although deficiencies in other transcription factors (Ets1, GATA-3, TCF/LEF1) result in profound developmental defects in both CD4+ and CD8+ T cells (40), the specificity with which T-bet modulates the type 1 immune response within both cell types is unique. In Th cells, T-bet controls the production of cytokines, and hence effector function because this is the primary means by which CD4 cells mediate delayed type hypersensitivity and inflammatory responses. In CD8 cells, T-bet also controls cytokine production and effector function, but in T-bet-deficient CD8 cells, these two functions are not linked.
Although roles in both CD4 and CD8 T cell function have been described, the mechanism by which T-bet directs effector differentiation in these two cell types is not fully understood. Recent studies have described T-bet-dependent gene expression in CD4 T cells (6, 11). However, the altered cytokine production observed in T-bet-deficient CD8 T cells does not account for the loss of CD8 effector function. Therefore, a novel T-bet-dependent mechanism, either shared by both CD4 and CD8 T cells or one unique to the CD8 subset, must exist. Preliminary gene profiling experiments in T-bet-/- CTLs (unpublished observations) have almost exclusively revealed impaired expression of genes (e.g., perforin, granzyme B, and Fas ligand) known to be fundamental to effector CD8s (41). Whether T-bet directly or indirectly regulates the expression of such genes remains to be established. T-bet should prove an attractive target in designing strategies to augment type 1 immunity against known and novel microorganisms.
Acknowledgments
We thank N. Iwakoshi, A. Erlebacher, M. Grusby, M. Oukka, and K. Mowen for thoughtful review of the manuscript. This work was supported by National Institutes of Health Grant AI48126 (to L.H.G.) and a grant from the Ellison Medical Foundation (to L.H.G.). S.J.S. is a recipient of the Burroughs Wellcome Foundation Career Development Award.
Abbreviations: CTL, cytotoxic T lymphocyte; Th, T helper; TCR, T cell receptor; LCMV, lymphocytic choriomeningitis virus; PE, phycoerythrin; FACS, fluorescence-activated cell sorting; PMA, phorbol 12-myristate 13-acetate; CFSE, carboxyfluorescein diacetatesuccinimidyl ester; APC, antigen-presenting cell.
References
- 1.Kaech, S. M., Wherry, E. J. & Ahmed, R. (2002) Nat. Rev. Immunol. 2, 251-262. [DOI] [PubMed] [Google Scholar]
- 2.Harty, J. T. & Badovinac, V. P. (2002) Curr. Opin. Immunol. 14, 360-365. [DOI] [PubMed] [Google Scholar]
- 3.Wong, P. & Pamer, E. G. (2003) Annu. Rev. Immunol. 21, 29-70. [DOI] [PubMed] [Google Scholar]
- 4.Carter, L. L. & Murphy, K. M. (1999) J. Exp. Med. 189, 1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen, K. B., Watford, W. T., Salomon, R., Hofmann, S. R., Pien, G. C., Morinobu, A., Gadina, M., O'Shea, J. J. & Biron, C. A. (2002) Science 297, 2063-2066. [DOI] [PubMed] [Google Scholar]
- 6.Szabo, S. J., Kim, S. T., Costa, G. L., Zhang, X., Fathman, G. C. & Glimcher, L. H. (2000) Cell 100, 655-669. [DOI] [PubMed] [Google Scholar]
- 7.Szabo, S. J., Sullivan, B. M., Stemmann, C., Satoskar, A. R., Sleckman, B. P. & Glimcher, L. H. (2002) Science 295, 338-342. [DOI] [PubMed] [Google Scholar]
- 8.Neurath, M. F., Weigmann, B., Finotto, S., Hildner, K., Glickman, J., Nieuwenhuis, E., Iijima, H., Mizoguchi, A., Mizoguchi, E., Autschbach, F., et al. (2002) J. Exp. Med. 195, 1129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finotto, S., Neurath, M. F., Glickman, J., Qin, S., Lehr, H. A., Green, F., Ackerman, K., Haley, K., Galle, P. R., Szabo, S., et al. (2002) Science 295, 336-338. [DOI] [PubMed] [Google Scholar]
- 10.Lighvani, A. A., Frucht, D. M., Jankovic, D., Yamane, H., Aliberti, J., Hissong, B. D., Nguyen, B. V., Gadina, M., Sher, A., Paul, W. E. & O'Shea, J. J. (2001) Proc. Natl. Acad. Sci. USA 98, 15137-15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afkarian, M., Sedy, J. R., Yang, J., Jacobson, N. G., Cereb, N., Yang, S. Y., Murphy, T. L. & Murphy, K. M. (2002) Nat. Immunol. 3, 549-557. [DOI] [PubMed] [Google Scholar]
- 12.Wunderlich, J., Shearer, G. & Livingstone, A. (1994) Curr. Protocols Immunol. 1, 3.11.1-3.11.20. [Google Scholar]
- 13.Nelson, D., Bundell, C. & Robinson, B. (2000) J. Immunol. 165, 6123-6132. [DOI] [PubMed] [Google Scholar]
- 14.Hogquist, K. A., Jameson, S. C., Heath, W. R., Howard, J. L., Bevan, M. J. & Carbone, F. R. (1994) Cell 76, 17-27. [DOI] [PubMed] [Google Scholar]
- 15.Hettmann, T. & Leiden, J. M. (2000) J. Immunol. 165, 5004-5010. [DOI] [PubMed] [Google Scholar]
- 16.Mora, A. L., Chen, D., Boothby, M. & Rubin, D. H. (1999) Eur. J. Immunol. 29, 2968-2980. [DOI] [PubMed] [Google Scholar]
- 17.Kreuwel, H. T., Aung, S., Silao, C. & Sherman, L. A. (2002) Immunity 17, 73-81. [DOI] [PubMed] [Google Scholar]
- 18.Farrar, M. A. & Schreiber, R. D. (1993) Annu. Rev. Immunol. 11, 571-611. [DOI] [PubMed] [Google Scholar]
- 19.Fruh, K. & Yang, Y. (1999) Curr. Opin. Immunol. 11, 76-81. [DOI] [PubMed] [Google Scholar]
- 20.Taniuchi, I., Osato, M., Egawa, T., Sunshine, M. J., Bae, S. C., Komori, T., Ito, Y. & Littman, D. R. (2002) Cell 111, 621-633. [DOI] [PubMed] [Google Scholar]
- 21.Dong, C., Davis, R. J. & Flavell, R. A. (2002) Annu. Rev. Immunol. 20, 55-72. [DOI] [PubMed] [Google Scholar]
- 22.Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O'Garra, A. (2001) Annu. Rev. Immunol. 19, 683-765. [DOI] [PubMed] [Google Scholar]
- 23.Akdis, C. A. & Blaser, K. (2001) Immunology 103, 131-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sad, S., Marcotte, R. & Mosmann, T. R. (1995) Immunity 2, 271-279. [DOI] [PubMed] [Google Scholar]
- 25.Erard, F., Wild, M.-T., Garcia-Sanz, J. A. & LeGros, G. (1993) Science 260, 1802-1805. [DOI] [PubMed] [Google Scholar]
- 26.Liou, H. C., Jin, Z., Tumang, J., Andjelic, S., Smith, K. A. & Liou, M. L. (1999) Int. Immunol. 11, 361-371. [DOI] [PubMed] [Google Scholar]
- 27.Graham, M. B., Dalton, D. K., Giltinan, D., Braciale, V. L., Stewart, T. A. & Braciale, T. J. (1993) J. Exp. Med. 178, 1725-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bot, A., Bot, S. & Bona, C. A. (1998) J. Virol. 72, 6637-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laufer, T., von Herrath, M., Grusby, M., Oldstone, M. & Glimcher, L. H. (1993) J. Exp. Med. 178, 589-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldstone, M. B. (2002) Curr. Top. Microbiol. Immunol. 263, 83-117. [DOI] [PubMed] [Google Scholar]
- 31.Oldstone, M. B. A., Nerenberg, M., Southern, P., Price, J. & Lewicki, H. (1991) Cell 65, 319-331. [DOI] [PubMed] [Google Scholar]
- 32.Ohashi, P. S., Oehen, S., Buerki, K., Pircher, H., Ohashi, C. T., Odermatt, B., Malissen, B., Zinkernagel, R. M. & Hengartner, H. (1991) Cell 65, 305-317. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama, M., Zhang, J. & Whitton, J. L. (1995) J. Virol. 69, 2684-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byrne, J. A. & Oldstone, M. B. (1984) J. Virol. 51, 682-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klavinskis, L. S., Whitton, J. L. & Oldstone, M. B. (1989) J. Virol. 63, 4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piccotti, J. R., Chan, S. Y., Li, K., Eichwald, E. J. & Bishop, D. K. (1997) J. Immunol. 158, 643-648. [PubMed] [Google Scholar]
- 37.Elloso, M. M. & Scott, P. (2001) Eur. J. Immunol. 31, 384-395. [DOI] [PubMed] [Google Scholar]
- 38.Kaech, S. M. & Ahmed, R. (2001) Nat. Immunol. 2, 415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Stipdonk, M. J., Lemmens, E. E. & Schoenberger, S. P. (2001) Nat. Immunol. 2, 423-429. [DOI] [PubMed] [Google Scholar]
- 40.Kuo, C. T. & Leiden, J. M. (1999) Annu. Rev. Immunol. 17, 149-187. [DOI] [PubMed] [Google Scholar]
- 41.Kaech, S. M., Hemby, S., Kersh, E. & Ahmed, R. (2002) Cell 111, 837-851. [DOI] [PubMed] [Google Scholar]