Abstract
Successful goal pursuit involves repeatedly engaging self-control against temptations or distractions that arise along the way. Laboratory studies have identified the brain systems recruited during isolated instances of self-control, and ecological studies have linked self-control capacity to goal outcomes. However, no study has identified the neural systems of everyday self-control during long-term goal pursuit. The present study integrated neuroimaging and experience-sampling methods to investigate the brain systems of successful self-control among smokers attempting to quit. A sample of 27 cigarette smokers completed a go/no-go task during functional magnetic resonance imaging before they attempted to quit smoking and then reported everyday self-control using experience sampling eight times daily for 3 weeks while they attempted to quit. Increased activation in right inferior frontal gyrus, pre-supplementary motor area, and basal ganglia regions of interest during response inhibition at baseline was associated with an attenuated association between cravings and subsequent smoking. These findings support the ecological validity of neurocognitive tasks as indices of everyday response inhibition.
Keywords: self-control, smoking cessation, brain-as-predictor, right inferior frontal gyrus, response inhibition, text messaging
Ridding oneself of an unwanted habit or tendency is a war that consists of a series of momentary self-control skirmishes. A longtime smoker may decide to quit, but success in reaching that goal will depend on the individual outcomes of a series of battles with cigarette cravings. Understanding the neural processes involved in these brief repeated struggles, in smoking and in other domains, is essential to understanding how self-control works in the trenches of real-world goal pursuit. The investigation reported here focused on response inhibition as one key factor that influences the ultimate success or failure of goal pursuit, and overcoming addiction in particular. Behavioral studies have examined how response inhibition during lab-based tasks relates to general real-world success at overriding an unwanted habitual behavior in favor of a desired novel one (Wood & Neal, 2007). Similarly, cognitive neuroscience studies have examined the neural correlates of response inhibition in the lab. However, because of limitations inherent to these two methods, no study has identified the neural systems that support effective response inhibition during the brief and repeated self-control episodes in daily life that are integral to successful long-term goal pursuit. In the current study, we investigated this question using a novel integration of methods, combining within-scanner measures of response inhibition with assessment of daily, momentary self-control along the way to a larger habit-changing goal.
Behavioral performance on simple laboratory response-inhibition tasks (e.g., go/no-go) has been consistently linked to success in reaching a variety of real-world goals that involve self-regulation. For instance, the capacity to engage response inhibition has been linked to success at dieting (Rothman, Sheeran, & Wood, 2009), increased exercise (Achtziger, Gollwitzer, & Sheeran, 2008), and improved academic competency (Oaten & Cheng, 2006). Conversely, diminished response-inhibition capacity has been linked to alcoholism (Nigg et al., 2006), methamphetamine abuse (Monterosso, Aron, Cordova, Xu, & London, 2005), and even domestic violence (Finkel, DeWall, Slotter, Oaten, & Foshee, 2009). These studies have demonstrated a robust association between behavioral performance on simple behavioral tasks assessing response inhibition and important real-world outcomes, but have not focused specifically on the brief and repeated instances of self-control that occur as part of goal pursuit and collectively contribute to long-term success.
Neuroscience studies have converged in identifying a consistent network of brain regions that are active during brief, laboratory-based manipulations of response inhibition. A number of functional neuroimaging (Aron, Robbins, & Poldrack, 2004; Leung & Cai, 2007) and lesion (Aron, Fletcher, Bull-more, Sahakian, & Robbins, 2003; Chambers et al., 2006) studies have implicated the right inferior frontal gyrus (rIFG) as the primary brain region for response inhibition. Many studies have also found that the dorsal anterior cingulate cortex (dACC), the anterior insula, the pre-supplementary motor area (pre-SMA), and subcortical regions such as the basal ganglia are coactive with the rIFG during response inhibition (Aron et al., 2007; Wager et al., 2005). Though the precise role of each of these regions in the human response-inhibition network is unclear, recent studies have suggested that the pre-SMA and dACC are involved in detection of potential conflict between the pre-potent and desired response (Botvinick, Cohen, & Carter, 2004; Mostofsky & Simmonds, 2008; Nachev, Wydell, O'Neill, Husain, & Kennard, 2007), the rIFG plays a role in representing the mapping between the inhibition cue and stopping (Van Gaal, Ridderinkhof, Scholte, & Lamme, 2010), and the subcortical structures are important for directly inhibiting the motor response (Aron et al., 2007). These explanations fit well into the broader view that the prefrontal cortex executes top-down control via a neuroanatomical control loop including the basal ganglia and primary and supplementary motor areas (Fuster, 2008). These studies characterize the brain networks involved in response inhibition at a single point in time, but do not capture the repeated and motivationally relevant nature of response inhibition during real-world goal pursuit.
Thus, on one hand, behavioral measures of response inhibition have been associated with a broad array of real-world outcomes, such as prevention of addiction relapse. On the other hand, the brain systems recruited for inhibiting responses during brief laboratory tasks are being mapped with increasing precision. Juxtaposing the behavioral and neuroscience literatures on response inhibition highlights why the neural processes underlying real-world instances of response inhibition have remained unexplored. There is almost no overlap between these literatures beyond similarity in the tasks used to assess response inhibition. Consequently, it is unknown whether the neural systems involved in laboratory assessments of response inhibition are the same ones recruited in the brief and repeated everyday battles between habit and self-control. For example, it is possible that the neural systems recruited during the stop-signal task are different from those associated with increasing exercise. Linking these disparate levels of analysis (i.e., neural and social/behavioral) and time scales (i.e., seconds/minutes and days/weeks) requires a paradigm for examining response inhibition during real-life situations and also during neuroim-aging tasks in the same sample of individuals.
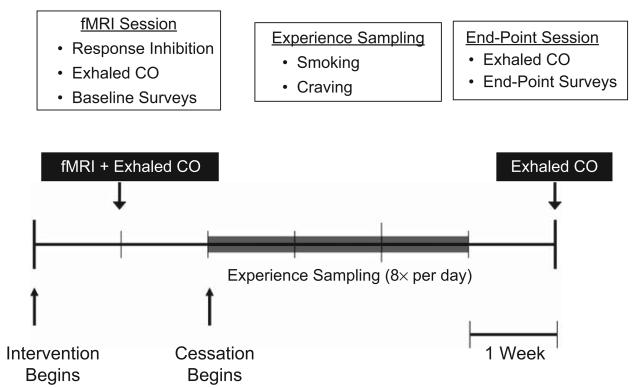
Accordingly, we made this link by measuring the neural mechanisms and everyday implementation of response inhibition within a single study. We recruited a sample of individuals just before they were to engage in the long-term, real-life response-inhibition task of quitting cigarette smoking and used functional MRI (fMRI) to examine their neural activation during a laboratory response-inhibition task. Next, we used experience sampling to track their progress throughout each day for the first 3 weeks of their smoking-cessation attempt (Fig. 1). Brain data in a priori regions of interest were then used to predict successful craving regulation on a daily basis during smoking cessation. This approach allowed us to test whether, and how, response-inhibition-related neural activation during the laboratory task related to response inhibition in the real world. We hypothesized that activation in the brain regions thought to be the most directly involved in inhibiting a motor response—the rIFG, pre-SMA, and basal ganglia—would predict successful regulation of daily craving.
Fig. 1.
Timeline of the experiment. The baseline (scanning) session occurred following registration in a smoking-cessation program but prior to smoking reduction. During this session, participants performed the functional MRI (fMRI) response-inhibition (go/no-go) task and completed baseline measures of self-reported smoking; exhaled carbon dioxide (CO) was also measured. The experience-sampling phase began the day prior to the targeted quit date and continued for 21 consecutive days. Participants reported smoking and cravings at eight time points that were evenly spaced between wake-up time and bedtime. During the endpoint session, which occurred approximately 4 weeks following the targeted quit date, additional surveys were administered, and exhaled CO was measured.
Method
Participants
Thirty-one participants (15 female, 16 male) were recruited from smoking-cessation programs in Los Angeles via in-person announcements at orientation sessions. All participants were heavy smokers (> 10 cigarettes/day, 7 days/week, for at least 1 year and urinary cotinine > 1,000 ng/mL) enrolled in a professionally led cessation program (e.g., Freedom From Smoking). To be included in the study, participants also were required to have a score of 9 or 10 (out of 10) on the Contemplation Ladder, a single-item measure of intentions to quit (Biener & Abrams, 1991), and a cumulative score of at least 18 (out of 20) on the Action subscale of the Readiness to Change Questionnaire (Rollnick, Heather, Gold, & Hall, 1992), a four-item measure of the action stage of change. Participants ranged in age from 28 to 69 years (M = 46, SD = 9.7) and had smoked for 11 to 53 years (M = 28.4, SD = 2.0). The sample was 52% Caucasian, 26% Hispanic, 19% African American, and 3% other ethnicities. Participants were excluded if they were left-handed, did not speak English, consumed more than 10 alcoholic drinks per week, or had any of the following conditions: dependence on substances other than nicotine at the time of study, dependence on substances within the previous year, neurological or psychiatric disorders, cardiovascular disease, pregnancy, claustrophobia, or any other condition contraindicated for MRI.
Of the original 31 participants, all completed the scanning session, but 1 withdrew from participation in the experience-sampling phase, and 3 were excluded for insufficient data; thus, 27 participants were included in the analyses reported here. Participants were compensated $80 for completing the scanning session and an additional $1 for each experience-sampling response returned, for a possible total of $248. All participants provided written informed consent approved by the UCLA Institutional Review Board.
Procedure
Phone screening
Following recruitment, participants were contacted by telephone to assess their intentions to quit (with the Contemplation Ladder and Readiness to Change Questionnaire) and their targeted quit date (TQD), as well as whether they met any of the exclusion criteria. For qualifying participants, a baseline laboratory session was scheduled at least 1 day prior to the TQD.
Baseline (scanning) session
Participants came to the UCLA Ahmanson-Lovelace Brainmapping Center for a baseline session at least 1 day prior to their quit date (Fig. 1). After they provided written informed consent, their smoking status was confirmed with a urinary cotinine assay (Accutest NicAlert strips; JANT Pharmacal Corp., Encino, CA), and baseline exhaled carbon monoxide (CO) was measured (Micro-smoker-lyzer; Bedfont Scientific Ltd., Kent, United Kingdom). Participants were screened for amphetamines, cocaine, marijuana, opiates, and PCP via urine test (Syva RapidTest d.a.u. 5; Dade Behring Inc., Cupertino, CA).
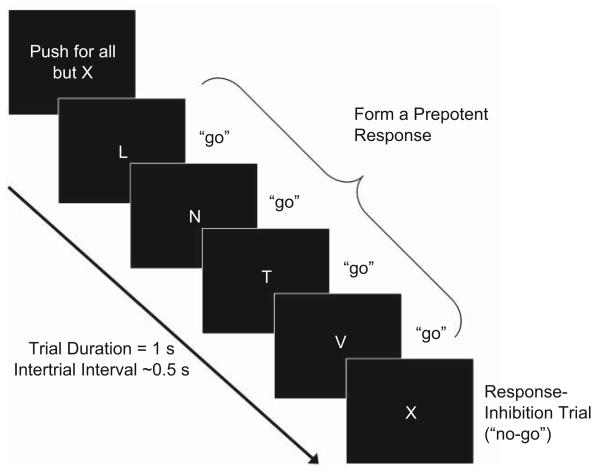
We used a go/no-go task to examine the neural activation associated with response inhibition (Fig. 2). The task consisted of 12 blocks containing a series of brief trials, each depicting a single letter centered in the screen. Each block began with the instruction to “push” or to “pull” the joystick lever. Then, depending on which instruction was given, participants pushed or pulled the lever whenever the letter L, N, T, or V appeared (go trials; ~82% frequency) and withheld a response when the letter X appeared (no-go trials; ~18% frequency). A neural measure of response inhibition was defined as the difference between brain activation during successful no-go trials (overriding the prepotent “go” response) and brain activation during go trials in an event-related analysis. Each block contained an average of 9 no-go trials and 41 go trials, and each trial lasted 1 s. The intertrial interval (ITI) was jittered according to a random gamma distribution (M = 0.5 s). Each block (50 trials and ITIs) lasted 75 s, and blocks were separated by 12-s rest periods. The blocks were divided across four fMRI runs.
Fig. 2.
The go/no-go task. Participants responded using the lever whenever the letter L, N, T, or V appeared (go trials) and withheld a response when the letter X appeared (no-go trials). In an event-related analysis, a neural measure of response inhibition was defined as the difference between brain activation during successful no-go trials (overriding the prepotent “go” response) and brain activation during go trials. Each of 12 blocks contained fifty 1-s trials (~41 go trials and ~9 no-go trials) separated by gamma-distributed jitter (M = 0.5 s).
After completing this task, participants were removed from the scanner and brought into a quiet testing room for the duration of the session. Participants completed measures of demographics, smoking history, waking hours, nicotine dependence (Fagerström Test of Nicotine Dependence; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), and smoking urges (Questionnaire on Smoking Urges; Tiffany & Drobes, 1991), in addition to several other questionnaires not relevant to the hypotheses tested here. Participants who did not have or preferred not to use their own cell phones were provided with and instructed to use a prepaid phone. Finally, participants were instructed in the use of text messages to receive and respond to experience-sampling prompts, and successfully completed a practice prompt.
Experience sampling
Following the scanning session, and beginning 1 day prior to their quit date, participants received prompts via text message eight times per day for 21 consecutive days. The first text prompt on each day was sent 15 min after morning rise, the last prompt was sent 15 min before bedtime, and the other six were evenly distributed throughout the day. Rise times and bedtimes were adjusted for each participant for weekdays and weekends. The interprompt interval varied across subjects between 1 hr 50 m and 2 hr 25 m.
At each prompt, participants responded to three questions: “How many cigarettes have you smoked since the previous signal?” (numerical response), “How much are you craving a cigarette right now?” (0 = not at all, 1 = a little, 2 = somewhat, 3 = a lot, 4 = extremely), and “Overall, how is your mood right now?” (0 = extremely negative, 1 = somewhat negative, 2 = neutral, 3 = somewhat positive, 4 = extremely positive). Participants responded to all three questions with a single text message back to the experimenters. See the Supplemental Text (Supplementary Methods) in the Supplemental Material available online for further details.
End-point session
An end-point session was scheduled within 7 days of the end of the 21-day experience-sampling period. Exhaled CO was reassessed along with nicotine dependence (Fagerström Test of Nicotine Dependence) and smoking urges (Questionnaire on Smoking Urges). Participants were compensated $1 for each text-message response (M = $141, SD = $38).
fMRI data acquisition and analysis
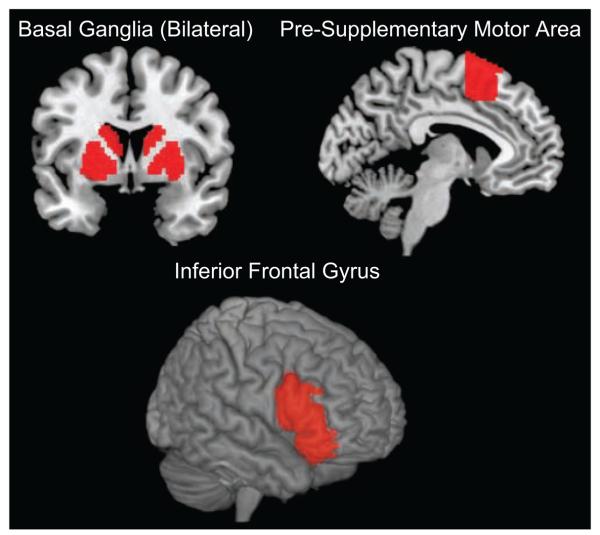
Brain-imaging data were acquired on a 3-T Siemens Trio scanner at the UCLA Ahmanson-Lovelace Brainmapping Center using standard data-acquisition and preprocessing steps (see the Supplemental Text in the Supplemental Material). The main effect of response inhibition was defined using a linear contrast for each participant (i.e., no-go > go). Contrast images were averaged across runs for each participant and then entered into a random-effects analysis at the group level. We constructed regions of interest (ROIs; Fig. 3) for the rIFG (pars triangularis, pars orbitalis, and pars opercularis; Aron et al., 2004), basal ganglia (encompassing caudate, putamen, and globus pallidus; Williams et al., 2006), and pre-SMA (y > 0; Aron & Poldrack, 2006) using the Automated Anatomical Labeling (AAL) toolbox (Tzourio-Mazoyer et al., 2002) within the Wake Forest University Pickatlas (Maldjian, Laurienti, Kraft, & Burdette, 2003). Also using AAL, we constructed control ROIs for discriminant validity in the bilateral precuneus and amygdala. These regions were chosen to represent one cortical and one subcortical region for which activation is often observed in cognitive neuroscience tasks but not typically during response inhibition. Analyses based on ROIs used a two-tailed significance threshold of .05.
Fig. 3.
The a priori anatomical target regions of interest from the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). The basal ganglia comprised the caudate, putamen, and globus pallidus; the pre-supplementary motor area was defined using the AAL and was restricted to be anterior to the anterior commissure (i.e., Montreal Neurological Institute y coordinates > 0); and the right inferior frontal gyrus was defined according to the AAL pars opercularis, triangularis, and orbitalis.
Experience-sampling data-analysis strategy
Multilevel linear modeling was used to address the nested nature of the experience-sampling data (HLM 6; Scientific Software International, Lincolnwood, IL; Raudenbush, Bryk, Cheong, & Congdon, 2004). A three-level model was constructed with time points (Level 1) nested within days (Level 2) nested within participants (Level 3). This model allowed us to examine the time-lagged relationship between craving and smoking within days while accounting for the nested structure of the data. The primary dependent measure of smoking was nonnormally distributed because it was reported as a count at each time point. Accordingly, we used a Poisson model with a log link function at the first level. Thus, all parameters are reported in log-expected likelihood units. Significance values were calculated using estimates of standard errors that are robust to violations of sphericity (see Supplemental Text in the Supplemental Material).
Integration of fMRI and experience-sampling data
To assess everyday response inhibition, we estimated the prospective relationship between craving for a cigarette at one time point and smoking at the subsequent time point. The magnitude of the relationship between these measures provided an ecological measure of response inhibition because cravings are among the primary impulses that must be regulated in successful smoking cessation (Allen, Bade, Hatsukami, & Center, 2008; Shiffman et al., 1997). To assess the relationship between laboratory neural and real-world behavioral measures of response inhibition, we imported neural activation parameters from the fMRI task into the HLM model as a person-level (Level 3) moderator of the slope between past craving and subsequent smoking. We entered three anatomically defined ROIs (rIFG, basal ganglia, and pre-SMA; Fig. 3) into separate analyses because they were multicollinear during the contrast of interest (nogo > go). The two control ROIs (amygdala and precuneus) were also entered separately for discriminant validity. Finally, we completed an exploratory whole-brain search for regions that predicted smoking reductions and subjected these results to a cross-validation analysis (see Supplemental Text in the Supplemental Material).
Results
Behavioral responses to the go/no-go task
Participants completed 108 no-go and 492 go trials across 12 go/no-go blocks. The error rate on no-go trials was 4.6%. Error trials were included in the model but not examined because of insufficient N. The mean response time on go trials was 547.9 ms (SD = 160.5).
Experience-sampling response rates
Participants responded to 84% of the prompts during the experience-sampling phase of the study (~6.7 responses out of 8 prompts daily). Most responses were sent within 23 min of the signal (SD = 44 min). For a given participant, a day was excluded if it contained fewer than four responses. In total, 90 days were excluded (M = 3.33 per participant). Robustness analyses suggest that the missing data did not affect the results (see Supplemental Text in the Supplemental Material). There were a total of 3,811 Level 1 observations (time points within days), 477 Level 2 observations (days within participants), and 27 Level 3 observations (participants) in our multilevel model.
Smoking and craving during experience sampling
Participants smoked 20.2 cigarettes per day (SD = 9.4) at baseline and 5.2 cigarettes per day (SD = 5.4) at the end point (mean change = 15.0), t(26) = 7.62, p < .01. Nicotine dependence and urges also decreased significantly (Table 1). Exhaled CO was marginally reduced, t(26) = 1.94, p = .06 (Table 1). The relatively high rate of lapse is common for smokers in the early weeks of a quitting attempt (Shiffman et al., 2007).
Table 1.
Mean Change on Smoking-Related Measures
| Measure | Baseline | End Point | Change |
|---|---|---|---|
| Global smoking self-report (no. cigarettes/day) | 20.24 (9.36) | 5.17 (5.45) | 15.07** (10.28) |
| Exhaled carbon monoxide | 18.93 (11.65) | 13.44 (10.89) | 5.49† (14.70) |
| FTND | 6.37 (2.04) | 2.63 (2.62) | 3.74** (2.49) |
| QSU: positive smoking urges | 4.82 (1.18) | 2.54 (1.57) | 2.28** (1.50) |
| QSU: negative smoking urges | 3.24 (1.21) | 2.00 (0.97) | 1.24** (1.35) |
Note: Standard deviations are given in parentheses. N = 27. FTND = Fagerström Test of Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991); QSU = Questionnaire on Smoking Urges (Tiffany & Drobes, 1991).
p = .06.
p < .01.
There was a positive within-day relationship between craving at one time point and smoking at the next when craving was entered alone into the model (i.e., without neural activations; log-expectation γ = .19, SE = .08), t(476) = 2.14, p < .05. Reductions in the number of cigarettes smoked per day were inversely related to the daily craving-smoking link (see Supplemental Text in the Supplemental Material for analysis of the relationship between the craving-smoking slope and smoking reductions).
Predicting everyday response inhibition from neuroimaging data
To examine the association between neural activation at baseline and longitudinal outcomes, we extracted activations from the no-go > go contrast for anatomically defined ROIs in the IFG, basal ganglia, and pre-SMA (Fig. 3), as well as the precuneus and amygdala control ROIs (all bilaterally). These activations were used as moderators of the within-day relationship between craving at time i and smoking at time i + 1. As in prior research (e.g., Wager et al., 2005), all target regions were significantly more active during no-go than during go trials (all ps < .01, corrected for multiple comparisons) and none of the control ROIs were differentially active (all ps > .2). Because of multicollinearity among the target ROIs (all rs > .6, all ps < .01), each ROI was entered into its own model with no other neural predictors. All models controlled for the linear decline in smoking across days, a quadratic pattern within days (increased smoking in the afternoon and evening compared with the morning), and baseline nicotine dependence. The results remained unchanged with age entered as a covariate.
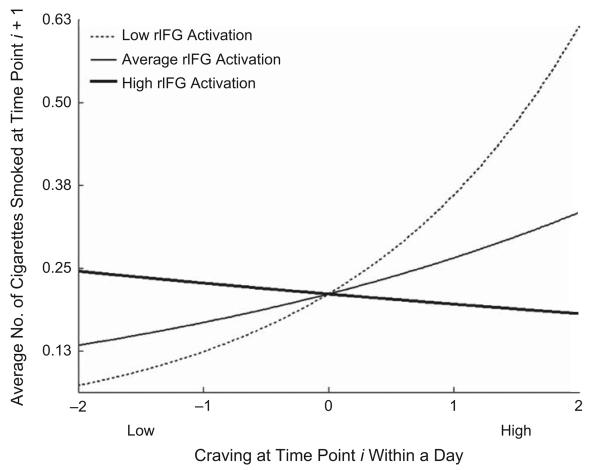
There was an overall positive relationship between craving at time i and smoking at time i + 1. The IFG, basal ganglia, and pre-SMA ROIs each significantly and negatively moderated that slope (Table 2); greater activity in these regions during the laboratory inhibition task related to attenuation of the link between craving and subsequent smoking in the real world. Though cravings were followed by increased smoking on average, participants who showed more inhibition-related activation in the target ROIs at baseline showed less coupling between cravings and later smoking. The moderating effect for the rIFG ROI is depicted in Figure 4, which indicates that individuals with low activation in rIFG (1 SD below the mean) in the no-go > go contrast showed a strong positive relationship between cravings and subsequent smoking, simple slope (log units) = 0.53, t(25) = 2.79, p < .01 (calculation following Bauer & Curran, 2005). Individuals at the mean showed a modest positive (though non-significant) relationship, simple slope (log units) = 0.25, t(25) = 1.20, n.s., and individuals with high activation (1 SD above the mean) showed no relationship between craving and smoking, simple slope (log units) = −0.04, t(25) = 0.21, n.s. In other words, an individual with average cravings on an average day would be expected to decrease his or her smoking by 33.6% for each standard deviation increase in rIFG activation during response inhibition at baseline; this translates to an increase of 4.48 cigarettes per day for the average subject. Within the basal ganglia ROI, activation in bilateral putamen and left caudate significantly moderated the craving-smoking link (see Supplemental Text in the Supplemental Material for details). Activity in right amygdala (a control ROI) positively moderated the relationship, such that individuals with higher right amygdala activation during response inhibition at baseline were more likely to smoke given high prior cravings (Table 2). None of the other control ROIs was a significant moderator of the craving-smoking link.
Table 2.
Regression Parameters From the Hierarchical Linear Model Predicting Expected Smoking at Each Time Point
| Region | Overall intercept |
Slope of prior cravings |
Moderation of craving slope by brain activation |
|---|---|---|---|
| Target regions | |||
| Right IFG | 0.51 (5.35) | 0.24 (0.20) | −0.29* (0.12) |
| Left IFG | 0.51 (4.64) | 0.18 (0.15) | −0.23** (0.05) |
| Right pre-SMA | 0.50 (5.60) | 0.19 (0.18) | −0.20* (0.09) |
| Left pre-SMA | 0.50 (5.45) | 0.26* (0.12) | −0.23* (0.11) |
| Right basal ganglia | 0.54 (4.96) | 0.31* (0.14) | −0.27* (0.12) |
| Left basal ganglia | 0.52 (4.99) | 0.25* (0.12) | −0.32** (0.12) |
| Control regions | |||
| Right amygdala | 0.51 (5.22) | 0.24* (0.11) | 0.22* (0.09) |
| Left amygdala | 0.50 (5.89) | 0.16 (0.17) | 0.03 (0.07) |
| Right precuneus | 0.49 (5.82) | 0.19 (0.20) | 0.11 (0.08) |
| Left precuneus | 0.50 (6.06) | 0.19 (0.20) | 0.11 (0.08) |
Note: All parameters are reported in natural log units. Standard errors are given in parentheses. All models controlled for the linear decline in smoking across days, the negative quadratic pattern within days, and baseline nicotine dependence. IFG = inferior frontal gyrus; pre-SMA = pre-supplementary motor area.
p < .05.
p < .01.
Fig. 4.
Activation in right inferior frontal gyrus (rIFG) in the no-go > go contrast as a moderator of the relationship between cravings and subsequent smoking. The average number of cigarettes smoked at time i + 1 as a function of craving at time i is shown for participants with high (1 SD above the mean), average, and low (1 SD below the mean) rIFG activation in the contrast.
We also examined the relationship between activity in each ROI and long-term cessation success (i.e., across 4 weeks). Only activity in basal ganglia, and not in the other two ROIs, predicted long-term reductions in smoking as measured by change in exhaled CO (Montreal Neurological Institute coordinates: x = 30, y = 5, z = 4; 86-voxel extent, t = 5.02, false-detection-rate-corrected p < .05; see Supplemental Text, Table S1, and Fig. S1 in the Supplemental Material). This result was further supported by a predictive cross-validation analysis (see Fig. S2 in the Supplemental Material).
Discussion
This study investigated the neural underpinnings of the brief, recurring episodes of everyday self-control that are integral to successful goal pursuit. We employed a joint fMRI/experience-sampling approach to link neuroimaging and ecological methods (cf. Eisenberger, Gable, & Lieberman, 2007). The results support the notion that laboratory neurocognitive measures of response inhibition relate meaningfully to real-world instances of self-control. Activation in three regions that have been consistently associated with response inhibition in laboratory go/no-go tasks—rIFG, pre-SMA, and basal ganglia—was related to attenuating the link between cigarette craving and subsequent smoking. More generally, we demonstrated that neural activations moderated the relationship between two momentary measures acquired in the real world.
These results add to emerging evidence supporting the predictive power of brain-imaging data. In contrast to traditional approaches, in which brain activation is modeled as a dependent measure regressed on time-course variables, the brain-as-predictor approach employed here models brain activation as an independent measure that may account for unexplained variance in other outcomes (Bandettini, 2009). Variants of this approach have been used to classify participants' visual-system activation into two categories (Haxby et al., 2001) and to predict decision outcomes of individual participants given a set of four choices (Soon, Brass, Heinze, & Haynes, 2008). More recently, we built upon these findings by showing that neural activation during exposure to a persuasive message predicted health-behavior change a week later (Falk, Berkman, Mann, Harrison, & Lieberman, 2010). The present study extended the brain-as-predictor approach further still by demonstrating that brain-imaging data had between-subjects predictive validity regarding an important health behavior over a span of 4 weeks and within-subjects discriminant validity in predicting a fine-grained self-regulatory process.
The present study also has several substantive implications for understanding the neural systems involved in smoking cessation. Though the rIFG, pre-SMA, and basal ganglia had been implicated in previous laboratory studies of response inhibition (e.g., Wager et al., 2005), and response-inhibition performance in the laboratory had been associated with addiction outcomes (e.g., Monterosso et al., 2005), it remained unclear whether brief response inhibition during the laboratory task relies on the same neural systems as do more prolonged forms of response inhibition, such as regulation of cravings for cigarettes across a period of weeks. Here, we found that the extent of neural activation in stopping a prepo-tent motor response (i.e., no-go trials) was related to success at repeatedly preventing a habitual response (i.e., smoking). This suggests that some of the interventions that have been shown to improve response inhibition in the laboratory (e.g., Muraven, 2010) may also improve real-world forms of response inhibition (Berkman, Burklund, & Lieberman, 2009). Further, the fact that baseline activation in these regions was specifically related to the regulation of cravings on a daily basis hints at the diagnostic utility of neuroimaging data in smoking cessation. For example, it may be possible to develop tailored smoking-cessation programs targeting craving regulation in individuals with relatively low baseline response-inhibition capacity. It is important to note that response-inhibition capacity is only one of many neurocognitive skills that are likely to be critical to effective smoking cessation (see, e.g., Hare, Camerer, & Rangel, 2009, on the modulation of the ventromedial prefrontal cortex valuation system during self-control).
Among all three regions that were related to an attenuated link between craving and smoking, only the basal ganglia also predicted overall reductions in smoking across the 1st month of cessation. It may be that a broad network including bilateral IFG, pre-SMA, and basal ganglia is involved in discrete instances of response inhibition, such as regulation of momentary cravings, whereas a subset of this network or a distinct network (including basal ganglia and other regions) is involved in overall smoking change (see Supplemental Text in the Supplemental Material). To the extent that overall change in smoking involves not only response inhibition but also many other processes, it makes sense that a contrast that isolates only response-inhibition-related activity to the exclusion of other processes would not relate to global smoking change. It is possible that the basal ganglia are active across a more general set of processes because of their direct anatomical involvement in coordinating motor actions. This would be consistent with the finding that both the caudate and the putamen contributed to the attenuated craving-smoking link. In this view, it makes sense that the basal ganglia predict overall smoking change better than the other members of the response-inhibition network do, as activation of the latter regions may be more specific to response inhibition and less sensitive to other processes.
The present study represents a step toward increasing the integration of functional neuroimaging methods, such as fMRI, with ecological methods, such as experience sampling. We linked across neurocognitive and behavioral measures of response inhibition in the domain of smoking cessation; we investigated merely one process within one health-relevant domain. This research yielded valuable insights about the mechanisms of response inhibition that would otherwise have been difficult or impossible to obtain. Yet within the domain of smoking cessation, there are several other central processes (e.g., goal maintenance, attention regulation) whose investigation using these methods might yield equally valuable insights (Berkman & Lieberman, 2009). The present investigation highlights the benefits of this approach, including the ability to connect otherwise-decontextualized neuroimaging data to the real world and to probe the temporal extent of processes of interest, and paves the way for future research to capitalize on the potential for this approach to forge links across measurement modalities.
Supplementary Material
Acknowledgments
We are grateful to Naomi Eisenberger, Edythe London, Jane Mendle, and Steven Reise, as well as two anonymous reviewers, for helpful feedback on earlier drafts of this manuscript.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Supplemental Material
Additional supporting information may be found at http://pss.sagepub.com/content/by/supplemental-data
References
- Achtziger A, Gollwitzer PM, Sheeran P. Implementation intentions and shielding goal striving from unwanted thoughts and feelings. Personality and Social Psychology Bulletin. 2008;34:381–393. doi: 10.1177/0146167207311201. [DOI] [PubMed] [Google Scholar]
- Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine & Tobacco Research. 2008;10:35–45. doi: 10.1080/14622200701705076. [DOI] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. The Journal of Neuroscience. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: Role of the subthalamic nucleus. The Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bandettini PA. What's new in neuroimaging methods? Annals of the New York Academy of Sciences. 2009;1156:260–293. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research. 2005;40:373–400. doi: 10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Burklund L, Lieberman MD. Inhibitory spillover: Intentional motor inhibition produces incidental limbic inhibition via right inferior frontal cortex. NeuroImage. 2009;47:705–712. doi: 10.1016/j.neuroimage.2009.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. The neuroscience of goal pursuit: Bridging gaps between theory and data. In: Moskowitz G, Grant H, editors. The psychology of goals. Guilford Press; New York, NY: 2009. pp. 98–126. [Google Scholar]
- Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, et al. Executive “brake failure” following deactivation of human frontal lobe. Journal of Cognitive Neuroscience. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Gable SL, Lieberman MD. Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion. 2007;7:745–754. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Mann T, Harrison B, Lieberman MD. Predicting persuasion-induced behavior change from the brain. The Journal of Neuroscience. 2010;30:8421–8424. doi: 10.1523/JNEUROSCI.0063-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel E, DeWall C, Slotter E, Oaten M, Foshee V. Self-regulatory failure and intimate partner violence perpetration. Journal of Personality and Social Psychology. 2009;97:483–499. doi: 10.1037/a0015433. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 4th ed. Academic Press/Elsevier; Boston, MA: 2008. [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Leung H-C, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. The Journal of Neuroscience. 2007;27:9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Muraven M. Building self-control strength: Practicing self-control leads to improved self-control performance. Journal of Experimental Social Psychology. 2010;46:465–468. doi: 10.1016/j.jesp.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. NeuroImage. 2007;36:T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Oaten M, Cheng K. Improved self-control: The benefits of a regular program of academic study. Basic and Applied Social Psychology. 2006;28:1–16. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon R. HLM6: Hierarchical linear and nonlinear modeling. Scientific Software International; Lincoln-wood, IL: 2004. [Google Scholar]
- Rollnick S, Heather N, Gold R, Hall W. Development of a short ‘readiness to change’ questionnaire for use in brief, opportunistic interventions among excessive drinkers. British Journal of Addiction. 1992;87:743–754. doi: 10.1111/j.1360-0443.1992.tb02720.x. [DOI] [PubMed] [Google Scholar]
- Rothman AJ, Sheeran P, Wood W. Reflective and automatic processes in the initiation and maintenance of dietary change. Annals of Behavioral Medicine. 2009;38:S4–S17. doi: 10.1007/s12160-009-9118-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, et al. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug and Alcohol Dependence. 2007;91:159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: Predicting smoking lapse from daily urge. Journal of Abnormal Psychology. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Soon CS, Brass M, Heinze H-J, Haynes J-D. Unconscious determinants of free decisions in the human brain. Nature Neuroscience. 2008;11:543–545. doi: 10.1038/nn.2112. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer MJ. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Gaal S, Ridderinkhof KR, Scholte HS, Lamme VAF. Unconscious activation of the prefrontal no-go network. The Journal of Neuroscience. 2010;30:4143–4150. doi: 10.1523/JNEUROSCI.2992-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Sylvester C-YC, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. NeuroImage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, et al. The mellow years?: Neural basis of improving emotional stability over age. The Journal of Neuroscience. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W, Neal DT. A new look at habits and the habit-goal interface. Psychological Review. 2007;114:843–863. doi: 10.1037/0033-295X.114.4.843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.