Abstract
Parkinson’s disease (PD) brains show evidence of mitochondrial respiratory Complex I deficiency, oxidative stress, and neuronal death. Complex I-inhibiting neurotoxins, such as the pesticide rotenone, cause neuronal death and parkinsonism in animal models. We have previously shown that DJ-1 over-expression in astrocytes augments their capacity to protect neurons against rotenone, that DJ-1 knock-down impairs astrocyte-mediated neuroprotection against rotenone, and that each process involves astrocyte-released factors. To further investigate the mechanism behind these findings, we developed a high-throughput, plate-based bioassay that can be used to assess how genetic manipulations in astrocytes affect their ability to protect co-cultured neurons. We used this bioassay to show that DJ-1 deficiency-induced impairments in astrocyte-mediated neuroprotection occur solely in the presence of pesticides that inhibit Complex I (rotenone, pyridaben, fenazaquin, and fenpyroximate); not with agents that inhibit Complexes II-V, that primarily induce oxidative stress, or that inhibit the proteasome. This is a potentially PD-relevant finding because pesticide exposure is epidemiologically-linked with an increased risk for PD. Further investigations into our model suggested that astrocytic glutathione and heme oxygenase-1 anti-oxidant systems are not central to the neuroprotective mechanism.
Keywords: Parkinson’s disease, rotenone, pyridaben, fenazaquin, fenpyroximate, heme oxygenase-1
Parkinson’s disease (PD) brains show evidence of mitochondrial respiratory chain Complex I deficiency, oxidative stress, and neurodegeneration (Schapira et al. 1989, Schapira et al. 1990, Yoritaka et al. 1996, Braak et al. 2003). Pharmacologic inhibition of Complex I, which can be induced by MPTP and some environmental pesticides (e.g. rotenone), causes oxidative stress and neurodegenerative parkinsonism in animal models (Ballard et al. 1985, Langston et al. 1984, Nicklas et al. 1987, Betarbet et al. 2000, Cannon et al. 2009, Smith et al. 1994, Panov et al. 2005). Occupational exposure to environmental pesticides, many of which inhibit Complex I, is epidemiologically-associated with an increased risk for human PD (Sherer et al. 2007, Brown et al. 2006, Ascherio et al. 2006, Gash et al. 2008). Therefore, it is conceivable that environmental toxicant/pesticide-induced Complex I inhibition is a risk factor for sporadic PD.
DJ-1 was first linked to PD when deletional mutations in its gene (PARK7) were discovered to cause a genetic form of the disease (Bonifati et al. 2003). Our own work in PD brain tissues demonstrated that DJ-1 is abundantly-expressed in reactive astrocytes, but not in neurons (Rizzu et al. 2004). This was an interesting finding since both astrocytes and DJ-1 can promote neuronal survival. For example, astrocytes support and protect surrounding neurons in vitro, and often do so by supplying anti-oxidant and bioenergetic molecules (Damier et al. 1993, Dringen et al. 1999, Makar et al. 1994, Sagara et al. 1996, Sagara et al. 1993, Wang & Cynader 2000, Chen et al. 2009). Further, DJ-1 is a predominantly cytoprotective protein in vitro, and often acts to promote anti-oxidant and anti-apoptotic mechanisms (Liu et al. 2008a, Junn et al. 2005, Taira et al. 2004, Xu et al. 2005, Canet-Aviles et al. 2004, Blackinton et al. 2009, Zhou & Freed 2005, Clements et al. 2006, Aleyasin et al. 2007).
These collective observations prompted us to form two main hypotheses regarding the human brain. First, astrocytic DJ-1 over-expression in sporadic PD may represent an attempt by the brain to protect itself against disease progression. In this scenario, astrocytes may up-regulate DJ-1 to protect themselves and neighboring neurons against environmental Complex I-inhibiting toxicants. Second, the absence of astrocytic DJ-1 in genetic PARK7 PD may be more pathologically permissive than the absence of neuronal DJ-1. In this scenario, the inability of astrocytes to express DJ-1 may render the brain more sensitive to environmental toxicants.
As an initial in vitro test of these hypotheses, we recently reported that plasmid-mediated DJ-1 over-expression in astrocytes augmented their capacity to protect co-cultured neurons against the Complex I-inhibiting pesticide rotenone (Mullett & Hinkle 2009). This model of sporadic PD astrocytes supported the hypothesized protective role for astrocytic DJ-1 over-expression. We also demonstrated that small interfering RNA (siRNA)-mediated DJ-1 knock-down impaired astrocyte-mediated neuroprotection against rotenone (Mullett & Hinkle 2009). This model of genetic PARK7 PD astrocytes supported a pathologically permissive role for astrocytic DJ-1 deficiency. In each case, astrocyte-released soluble factors appeared to be central to the protective mechanism.
Our findings suggest that DJ-1 expression is critical for mechanisms of astrocyte-mediated neuroprotection. To investigate this further, we sought to determine whether the protective mechanism in our in vitro model was (i) selective for Complex I-mediated neurotoxicity and (ii) mediated by astrocyte anti-oxidant systems. To approach these questions, we first developed a 96-well plate-based bioassay in which the effects of astrocytic gene manipulation on neuronal survival could be studied. We used the new bioassay to demonstrate here that DJ-1-deficient astrocytes are, in fact, selectively impaired in their capacity to protect neurons against Complex I-inhibiting pesticides. This is a novel finding that may be highly PD-relevant. Investigating our model further, we found no evidence to support significant roles for glutathione (GSH), heme oxygenase-1 (HO-1), or other selected anti-oxidant systems in the mechanism of astrocyte-mediated neuroprotection.
Materials and methods
Cell cultures
Primary astrocyte-enriched and neuron-astrocyte contact co-cultures were produced as previously described (Mullett & Hinkle 2009). Briefly, astrocyte cultures were prepared from postnatal day 1 (P1) CD1 mouse cerebral cortex tissues by dissociation into Neurobasal media (Invitrogen, Carlsbad, CA) containing 10% fetal calf serum (FCS, Hyclone, Logan, UT) and 1X antibiotic-antimycotic (ABAM, Invitrogen). The plating density was 7.3 × 104 trypan blue-excluding cells/cm2. The cultures were fed with Dulbecco’s modified Eagle media (DMEM)/F12 (Sigma, St. Louis, MO)/FCS/ABAM for the first week, then maintained in DMEM/F12/ABAM containing 10% calf serum (CS, Hyclone). Monolayers prepared in this fashion contained ~97% glial fibrillary acidic protein immunoreactive (GFAP+) astrocytes and ~0.5% microglia (Iba1+).
Neuronal cultures were prepared from embryonic day 15 (E15) CD1 mouse cortex by plating the cell suspensions at 4.5 × 104 trypan blue-excluding cells/cm2 in Neurobasal/10% FCS/ABAM directly onto astrocyte monolayers that were previously transfected to alter DJ-1 levels. After overnight incubation, the media was replaced with serum-free Neurobasal/1X B27 (Invitrogen)/0.5 mM GlutaMAX (Invitrogen)/ABAM and the cultures maintained until treatments commenced (astrocyte day in vitro [DIV] 20/neuron DIV 6). This procedure consistently produced >97% microtubule associated protein 2 immunoreactive (MAP2+) neurons.
DJ-1 knock-down and over-expression in astrocytes
Transfections with siRNA were used to suppress DJ-1 protein levels (DJ-1 knock-down) in the astrocytes to ~5% of endogenous levels prior to neuronal plating (detailed methods, transfection efficiencies, and Western blot characterization data are available in Mullett & Hinkle 2009). Briefly, a 21 nucleotide double-stranded anti-mouse DJ-1 siRNA of sequence AGG CGC GGC TGC AGT CTT TAA (siDJ#2, Invitrogen) was used to effect DJ-1 knock-down. A non-silencing siRNA of sequence AAT TCT CCG AAC GTG TCA CGT (siNS, Invitrogen) was used as a transfection control. The siNS had no sequence matches on BLAST analysis and no effect on DJ-1, α-tubulin, or β-actin protein levels. The astrocyte cultures were exposed to siRNAs over DIV 10–13. The siRNAs were then removed and the cells washed and allowed to recover overnight prior to E15 neuronal plating on astrocyte DIV 14.
Full-length mouse wild-type DJ-1 cDNA (mDJwt, NCBI ID BC002187, American Type Culture Collection)-containing pCMV-SPORT6 mammalian expression plasmid transfections were used to over-express DJ-1 protein in the astrocytes by ~2-fold (detailed methods, transfection efficiencies, and Western blot characterization data are available in Mullett & Hinkle 2009). Vector plasmid was used as a transfection control and did not affect DJ-1 or β-actin protein levels. The astrocytes were exposed to the plasmids for 4 h on DIV 10. The plasmids were then removed and the cells washed and allowed to recover prior to E15 neuronal plating on astrocyte DIV 14. The astrocytes were co-transfected with siRNA and plasmid to produce wild-type (siNS + vector), DJ-1 knock-down (siDJ#2 + vector), or DJ-1 over-expressing (siNS + mDJwt) cells when all three conditions were to be directly compared.
Experimental treatments
Neuron/transfected-astrocyte contact co-cultures were exposed to neurotoxins for 72 h (all from Sigma). The following Complex I inhibitors were assessed: rotenone [0–80 nM], pyridaben [0–100 nM], fenazaquin [0–1 μM], and fenpyroximate [0–100 nM]. We also assessed the mitochondrial Complex II inhibitor 3-nitroproprionic acid [3-NPA, 0–1 mM], the Complex III inhibitor antimycin A [0–10 μM], the Complex IV inhibitor sodium azide [0–1 mM], and the Complex V/ATP synthase inhibitor oligomycin [0–1 mM]. Hydrogen peroxide [0–1 mM], paraquat [0–100 μM], and hemin [0–100 μM] were evaluated as primarily oxidative stress-inducing neurotoxins. Lactacystin [0–10 μM] was tested at as a proteasome inhibitor. Glutamic acid was tested as an excitotoxin [0–1 mM]. The toxins were diluted into Neurobasal/1X B27 antioxidant free (Invitrogen)/1X ABAM and compared to media-only controls. Rotenone, pyridaben, fenpyroximate, and fenazaquin required initial dilution into dimethyl sulfoxide (DMSO, Sigma). These toxins were compared to media + vehicle controls.
Several agents (all from Sigma) were supplemented to rotenone-containing media to assess their capacity to restore astrocyte-mediated neuroprotection. The following were assessed as anti-oxidant molecules: GSH [0–10 mM], membrane-permeable GSH ethyl ester [GSHee, 0–10 mM], N-acetyl-L-cysteine [NAC, 0–10 mM], and (+)-α-tocopherol/vitamin E [VitE, 0–10 mM]. Lactic acid [0–10 mM] was assessed as a potential bioenergetic molecule (e.g., the astrocyte-to-neuron “lactate shuttle”). Succinic acid [0–10 mM] and coenzyme Q10 [0–100 μM] were assessed as possible effectors of Complex I bypass.
Immunocytochemistry and neuronal survival bioassay
Assessments of neuronal survival were made by Odyssey infrared imaging (LiCor Biosciences, Lincoln, Nebraska) using a new, 96-well plate-based bioassay. Surviving neurons and astrocytes were identified by immunocytochemistry (ICC) for MAP2 and GFAP, respectively (Mullett & Hinkle 2009). Briefly, the cells were lightly fixed in 4% paraformaldehyde, blocked, and probed with primary antibody. MAP2 was identified using a monoclonal anti-rat MAP2 primary antibody (Sigma) at 1:1000. GFAP was identified using a polyclonal rabbit anti-GFAP primary antibody (Dako, Denmark) at 1:350. MAP2 signal detection/quantification was achieved using a secondary antibody tagged with LiCor IRdye-800 at 1:1000. GFAP ICC detection/quantification was simultaneously performed to confirm the presence of astrocytes in the co-cultures using an Alexa 680-conjugated secondary antibody (Invitrogen) at 1:5000. Infrared signal was quantified in each well of a 96-well plate using an Odyssey imager. Background signal was removed, and the average of 4–8 replicate wells per treatment was used to generate each data point. All assessments were performed within the linear range of the machine.
Western blots
Protein lysates were prepared from astrocytes using Laemmli-sodium dodecyl sulfate extraction and subjected to quantitative Western blot analysis using an Odyssey infrared scanner as previously described (Mullett & Hinkle 2009). The following primary antibodies were used: rabbit anti-glutathione peroxidase 1 polyclonal (GPx1, Abcam, Cambridge, MA) at 1:100, anti-HO-1 monoclonal (StressGen, Ann Arbor, MI) at 1:250, and anti-β-actin monoclonal (Sigma) at 1:2,000. The membranes were then incubated with secondary antibodies: IRDye 800-conjugated goat anti-mouse (LiCor, for β-actin) and Alexa 680-conjugated goat anti-rabbit (Invitrogen, for GPx1 and HO-1), each at 1:20,000. GPx1 and HO-1 band intensities were quantified and normalized to same-lane β-actin bands.
Glutathione measurements
GSH was quantified using a modification of a previously published protocol (Shvedova et al. 2000, Shvedova et al. 2002). Briefly, 10 μM ThioGlo-1 (Calbiochem, La Jolla, CA) in PBS was incubated with astrocyte lysate or 3-day astrocyte conditioned media for 5 minutes and the intensity of the 500 nm emission recorded in experimental samples relative those of simultaneously-assessed GSH standard curves. This method measured all immediately available low molecular weight thiols, and this population is composed almost entirely of GSH.
Statistical Analysis
Independent cultures, transfections, and treatments were used for each experimental replicate (5–6 in most cases). Paired t-tests were used to assess the effect of a single DJ-1 manipulation relative to wild-type at a single toxin concentration when no time effect was measured. ANOVA with post-hoc Tukey’s range test comparisons were employed if multiple DJ-1 manipulations, treatments, and times were to be analyzed within an experiment. Data were reported as significant when p < 0.05.
Results
Validation of the plate-based neuronal survival bioassay
Several key points exist regarding the assessments of neuronal survival in the co-culture experiments. First, the astrocytes were always transfected then washed prior to neuronal seeding in fresh media. Thus, the neurons were never exposed to the transfection reagents. Second, we have previously established that siDJ#2-induced DJ-1 knock-down persists throughout the entire experimental period, and that it is fully reversible by co-transfection with a non siDJ#2-targeted mouse DJ-1 rescue cDNA (Mullett & Hinkle 2009). Third, we have also previously established that mDJwt-induced DJ-1 over-expression persists throughout the experimental period. Since these controls have already been published for this in vitro system, and since they establish DJ-1 levels prior to and regardless of toxin treatment, we did not repeat them here.
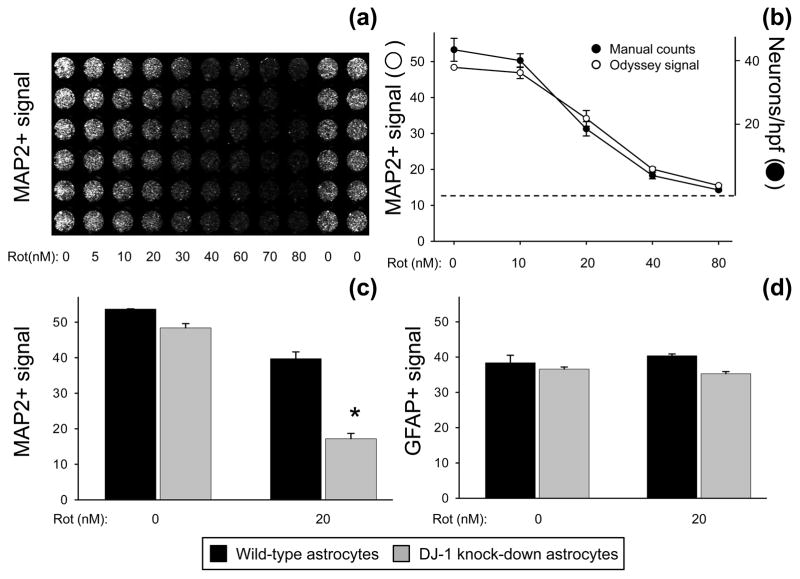
We originally performed the neuronal viability experiments by blinded manual counting of surviving neurons (Mullett & Hinkle 2009). Here we present and validate a new 96-well plate-based neuronal survival bioassay. This assay is capable of simultaneously measuring levels of MAP2 and GFAP signal that directly correlate with the number of living neurons and astrocytes, respectively, that remain attached to the plate after experimental treatments (Fig. 1). We initially established the optimum plating density and linear range of quantification for the Odyssey scanner. Using these parameters, rotenone dose-response curves were generated in neuron-astrocyte co-cultures to quantify the levels of MAP2 signal remaining after treatment (Fig. 1a–b). We were able to replicate the rotenone kill curve in neurons that we had previously published based on manual counts. In fact, the bioassay detected an identical linear range and 50% lethal dose (LD50) as the manual counts (Fig 1b). This indicated that the MAP2+ signal intensity detected by the scanner reflected the number of surviving neurons, not simply changes in MAP2+ ICC intensity.
Fig. 1.
Neuronal survival bioassay. (a) Portion of sample 96-well plate showing neuron-astrocyte co-cultures with Odyssey infrared scanner-visualized MAP2+ (neuronal) signal over a rotenone dose-response curve from 0–80 nM. (b) MAP2+ signal from the bioassay (open circles, Odyssey-generated intensity units) plotted with MAP2+ manual count data from the same wells (filled circles, neurons per high-power field [hpf]). The dotted line represents the background level for Odyssey detection and the zero cell level for manual counts. Mean ± SE shown, n = 4. (c) Pre-treatment neurons survived and developed equally well when co-cultured on wild-type (siNS-transfected, black bars) or DJ-1 knock-down (siDJ#2-transfected, gray bars) astrocytes. Neurons co-cultured with wild-type astrocytes survived significantly better than those co-cultured with DJ-1 knock-down astrocytes after 72 h treatment with 20 nM rotenone. The asterisk (*) represents p < 0.05 vs. wild-type by paired t-test. Mean ± SE shown, n = 5. (d) Neither DJ-1 manipulation nor 72 h treatment with 20 nM rotenone affected Odyssey-detected GFAP+ signal (astrocyte viability) in the co-cultures. Mean ± SE shown, n=5.
The bioassay also replicated our previous manual count findings that DJ-1 knock-down induces a deficiency in astrocyte-mediated neuroprotection against 72 h treatment with 10 nM and 20 nM rotenone (Fig. 1c, also Fig. 5b–e, Fig. 6, and Fig. 8). Further validation of this assay is provided in figures throughout the manuscript by detection of (i) consistent dose-response curves generated by 9 distinct neurotoxins, (ii) DJ-1-induced changes in astrocyte-mediated neuroprotection against multiple toxins, and (iii) vitamin E induced neuroprotection. Bioassay GFAP signal intensities (representing surviving astrocyte numbers) did not vary significantly with transfection conditions or toxin treatments unless specifically noted (Fig. 1d, data not shown for all toxins tested). Therefore, the changes in neuronal sensitivity to the toxins were not due to non-specific differences in astrocyte numbers or monolayer consistency.
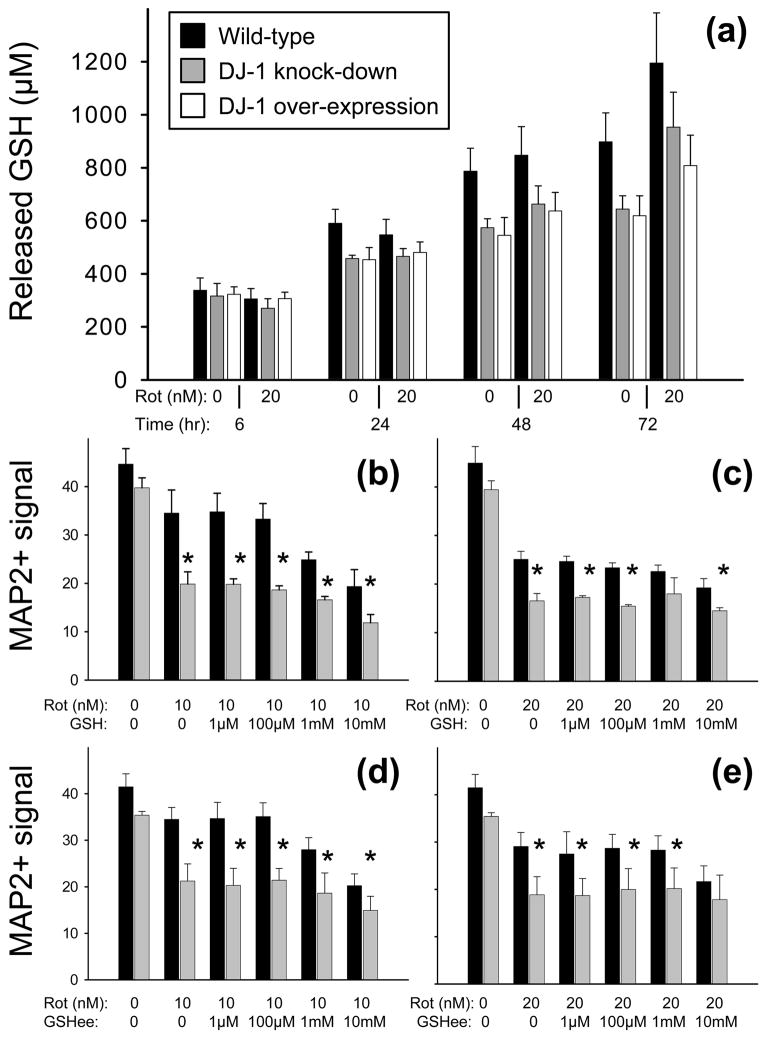
Fig. 5.
The astrocyte glutathione (GSH) system did not appear to be significantly active in DJ-1 modulated, astrocyte-mediated neuroprotection against rotenone. (a) Released GSH levels (μM) are shown in conditioned media collected from wild-type (siNS/vector-transfected, black bars), DJ-1 knock-down (siDJ#2/vector-transfected, gray bars), and DJ-1 over-expressing (siNS/mDJwt-transfected, white bars) astrocytes after rotenone treatment at the doses and durations shown. No significant differences were seen by ANOVA. Mean ± SE shown, n=6. (b-e) MAP2+ (neuronal) signal from bioassay is shown as mean ± SEM (n = 5) after 72 h of treatment with rotenone (Rot, 10 nM in b and d, 20 nM in c and e) ± GSH (b and c) or ± membrane permeable GSH ethyl ester (GSHee, d and e) at the doses shown. Asterisks (*) represent p < 0.05 vs. same-dose wild-type by paired t-tests.
Fig. 6.
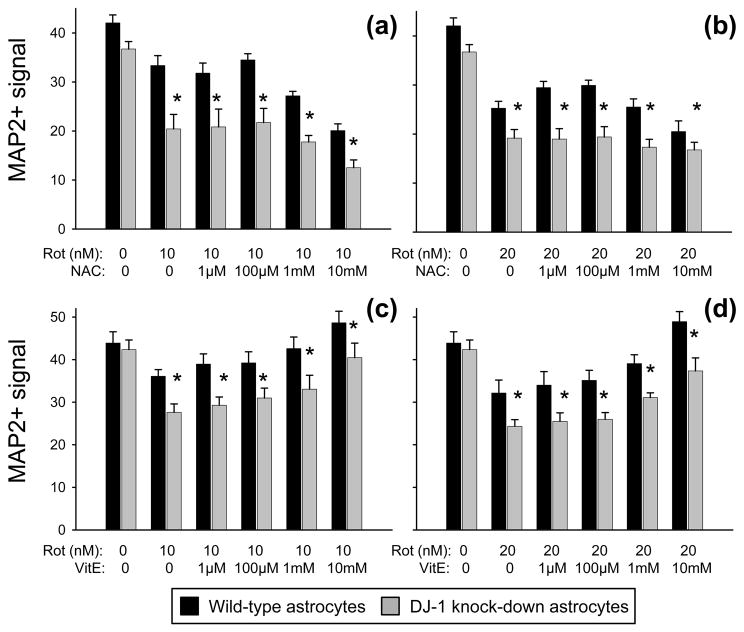
Neither N-acetyl-L-cysteine (NAC) nor vitamin E (VitE) replaced the DJ-1 knock-down-induced deficiency in neuroprotection against rotenone to wild-type levels. MAP2+ (neuronal) signal from bioassay is shown as mean ± SEM (n = 5) after 72 h of treatment with rotenone (Rot, 10 nM in a and c, 20 nM in b and d) ± NAC (a and b) or ± VitE (c and d) at the doses shown. Asterisks (*) represent p < 0.05 vs. same-dose wild-type by paired t-tests.
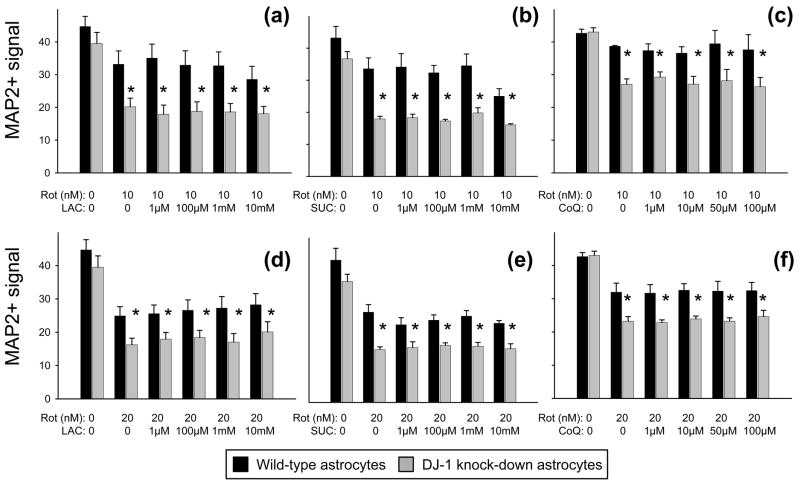
Fig. 8.
Neither lactic acid (LAC), succinic acid (SUC), nor coenzyme Q10 (CoQ) replaced the DJ-1 knock-down-induced deficiency in neuroprotection against rotenone to wild-type levels. (a-f) MAP2+ (neuronal) signal from bioassay is shown as mean ± SEM (n = 5) after 72 h of treatment with rotenone (Rot, 10 nM in a-c, 20 nM in d-f) ± LAC (a and d), ± SUC (b and e), or ± CoQ (c and f) at the doses shown. Asterisks (*) represent p < 0.05 vs. same-dose wild-type by paired t-tests.
DJ-1 deficient astrocytes were impaired in their capacity to protect neurons against multiple mitochondrial respiratory chain Complex I inhibitors
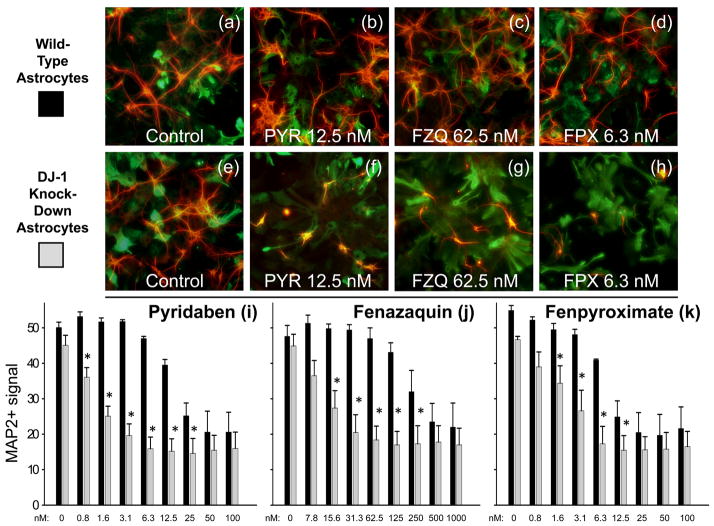
We sought to determine whether the results obtained in co-culture were specific to rotenone or more globally-related to mitochondrial Complex I inhibition. To do this, we compared the neuroprotective capacity of DJ-1 knock-down astrocytes with that of wild-type astrocytes against three other Complex I inhibitors: pyridaben, fenazaquin, and fenpyroximate (Fig. 2).
Fig. 2.
DJ-1 deficient astrocytes were impaired in their capacity to protect neurons against multiple Complex I inhibitors. (a-d) Photomicrographs of MAP2+ neurons (red) and co-cultured wild-type (siNS-transfected) GFAP+ astrocytes (green) remaining after 72 h treatment with no toxin (Control, a), pyridaben (PYR, b), fenazaquin (FZQ, c), or fenpyroximate (FPX, d). (e-h) Photomicrographs of MAP2+ neurons and co-cultured DJ-1 knock-down (siDJ#2-transfected) GFAP+ astrocytes remaining after treatment with no toxin (Control, e), pyridaben (PYR, f), fenazaquin (FZQ, g), or fenpyroximate (FPX, h). Bioassay-quantified dose-response of neuronal survival after 72 h treatment with pyridaben (i), fenazaquin (j), or fenpyroximate (k), at the doses shown, in co-culture with either wild-type (black bars) or DJ-1 knock-down (gray bars) astrocytes. Asterisks (*) represent p < 0.05 vs. same-dose wild-type by paired t-test. Mean ± SE shown, n = 5.
The neuron/transfected astrocyte co-cultures were exposed to each toxin for 72 h over a period in which astrocytic DJ-1 levels were maximally-suppressed. Visual assessments showed that DJ-1 knock-down and wild-type astrocytes were equally dense and morphologically healthy under all transfection and toxic conditions (Fig. 2a–h). The neurons were healthy and equally numerous when co-cultured with each type of astrocyte (Fig. 2a and e). However, DJ-1 knockdown astrocytes were significantly less protective of neuronal survival against all three Complex I inhibitors when compared to the wild-type astrocytes. This was true by both visual (Fig. 2f–h) and quantitative bioassay (Fig. 2i–k) assessments. For pyridaben, the LD50 for wild-type astrocyte co-cultured neurons was ~20 nM, whereas with DJ-1 knock-down astrocytes it shifted to ~1 nM (Fig. 2i). A significant deficiency in astrocyte-mediated neuroprotection was seen from 0.8 to 25 nM. For fenazaquin, the LD50 for wild-type astrocyte co-cultured neurons was ~200 nM, compared to ~12 nM with DJ-1 knock-down astrocytes (Fig. 2j). A significant deficiency of astrocyte-mediated neuroprotection was seen from 15.6 to 250 nM. For fenpyroximate, the LD50 for wild-type astrocyte co-cultured neurons was ~8 nM, whereas with DJ-1 knock-down astrocytes it was ~2 nM (Fig. 2k). A significant deficiency was seen from 1.6 to 12.5 nM.
DJ-1 over-expressing astrocytes were also compared with wild-type astrocytes against pyridaben and fenpyroximate using the bioassay. Although the effects were less robust, a significant neuroprotective effect was produced by astrocytic DJ-1 over-expression (Supplementary Fig. 1). This is similar to our previous results with rotenone.
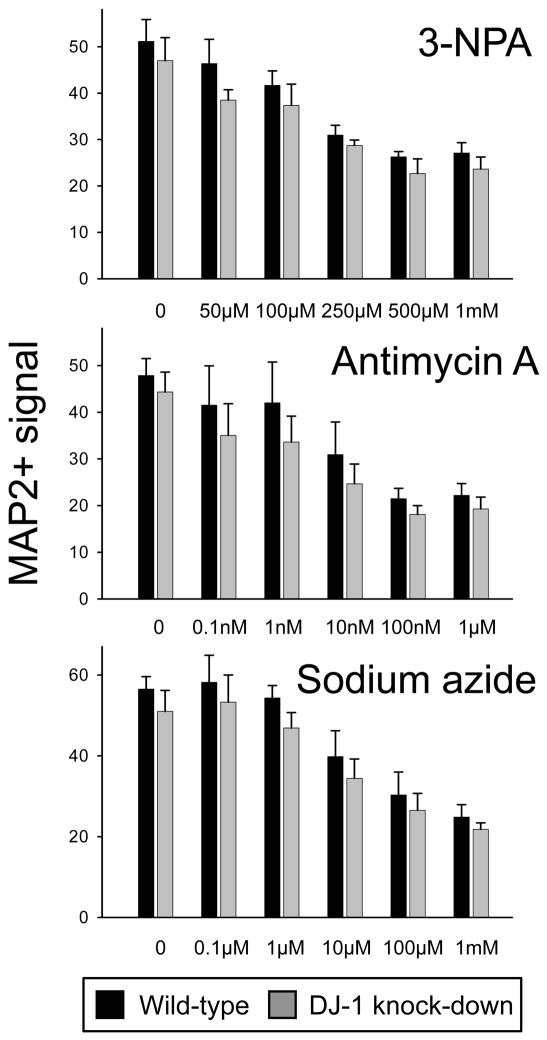
DJ-1 deficient astrocytes were not impaired in their capacity to protect neurons against mitochondrial respiratory chain Complex II, III, or IV inhibitors
The finding that four distinct Complex I inhibitors produced the same result in the co-culture experiments prompted us to consider whether these effects were specific to this Complex within the mitochondrial respiratory chain. To assess this we repeated the bioassay experiments using pharmacological inhibitors of Complex II (3-NPA), Complex III (antimycin A), Complex IV (sodium azide), and Complex V/ATP synthase (oligomycin). Each of these toxins produced dose-responsive neuronal kill curves after 72 h treatments. However, there was no difference in the neurotoxicity produced by Complex II, III, or IV inhibition when wild-type and DJ-1 knockdown astrocytes were compared (Fig. 3). Therefore, astrocytic DJ-1 deficiency did not render the neurons more susceptible to these agents under the same conditions in which they were more susceptible to all four Complex I inhibitors. Astrocyte-mediated neuroprotection could not be assessed for oligomycin because it was toxic to the astrocytes over the neurotoxic range.
Fig. 3.
DJ-1 deficient astrocytes were not impaired in their capacity to protect neurons against mitochondrial Complex II, III, or IV inhibitors. Dose-response of neuronal survival after 72 h treatment with 3-nitroproprionic acid (3-NPA, a Complex II inhibitor), antimycin A (a Complex III inhibitor), or sodium azide (a Complex IV inhibitor), at the doses shown, in co-culture with either wild-type (siNS-transfected, black bars) or DJ-1 knock-down (siDJ#2-transfected, gray bars) astrocytes. Mean ± SEM bioassay data is shown, n = 4 for each toxin. No significant differences were detected by paired t-tests comparing wild-type with DJ-1 knock-down.
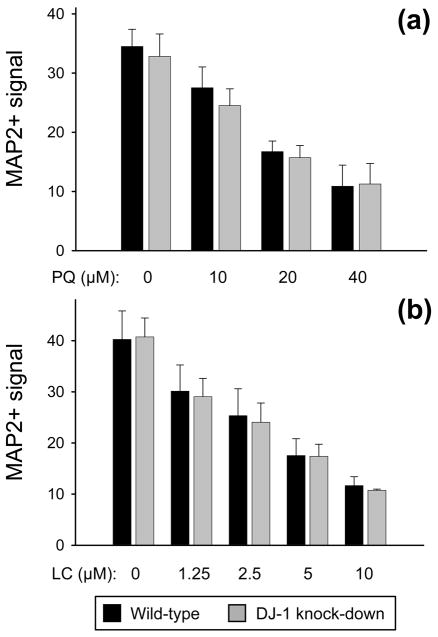
Astrocytic DJ-1-deficiency did not augment the neurotoxicity of primarily oxidative stress-inducing or proteasome-inhibiting neurotoxins
We next assessed our co-culture bioassay system for the capacity of more directly oxidative stress-inducing neurotoxins to produce the same results as the Complex I inhibitors. To do this, we repeated the experiments using paraquat (a strong inducer of oxidative stress, but relatively weak inhibitor of Complex I) and hydrogen peroxide. Paraquat produced a dose-responsive neuronal kill curve, but no differences were seen in neurotoxicity between the wild-type and DJ-1 knock-down astrocyte co-cultures (Fig. 4a). Hydrogen peroxide was toxic to astrocytes over the neurotoxic concentration range, therefore, astrocyte-mediated neuroprotection could not be assessed. The same was true for glutamic acid, an excitotoxin. We also evaluated lactacystin as a model of proteasome dysfunction, which occurs with oxidative stress, but again found no difference in the level of neuroprotection provided by the two types of astrocytes (Fig. 4b).
Fig. 4.
DJ-1 deficient astrocytes were not impaired in their capacity to protect neurons against paraquat or lactacystin. MAP2+ (neuronal) bioassay data is shown as mean ± SEM (n = 5) after 72 h of treatment with paraquat (PQ, a) or lactacystin (LC, b), at the doses shown, in co-culture with either wild-type (siNS-transfected, black bars) or DJ-1 knock-down (siDJ#2-transfected, gray bars) astrocytes. No significant differences were detected by paired t-tests comparing wild-type with DJ-1 knock-down.
The glutathione anti-oxidant system did not appear to be central to the mechanism of DJ-1-deficiency induced impairments in astrocyte-mediated neuroprotection
Astrocytic DJ-1 may protect surrounding neurons against Complex I inhibitor-induced oxidative toxicity via stabilization of astrocytic nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (Clements et al. 2006). In non-astrocytic cells, this stabilization can result in up-regulation of γ-glutamyl cysteine ligase (the rate-limiting enzyme for GSH production), GPx1 (oxidizes GSH to GSSG, promoting removal of hydrogen peroxide), and/or HO-1 (Malhotra et al. 2008). We therefore hypothesized that DJ-1 knock-down would reduce astrocytic GSH-related enzyme levels, GSH release, and HO-1 levels. We employed several methods to test these possibilities.
First, we used quantitative Western blots to measure astrocytic Nrf2 levels. Although Nrf2 was easily detectable in our cultured neurons, it was undetectable in our astrocytes. We similarly assessed for γ-glutamyl cysteine ligase. Although this enzyme was detectable in our astrocytes, the levels were too low to permit quantification. GPx1 was detectable and quantifiable in our astrocytes. However, we identified no effect of rotenone treatment, DJ-1 knock-down, or DJ-1 over-expression on its levels after 6 h or 24 h (Supplementary Fig. 2).
We also found no significant effect of rotenone treatment, DJ-1 knock-down, or DJ-1 over-expression on the levels of GSH released into astrocyte conditioned media after 6, 24, 48, or 72 h of treatment (Fig. 5a). Maximum released GSH levels reached ~1 mM after 72 h accumulation into the conditioned media, and trended lower for both the DJ-1 knock-down and the DJ-1 over-expressing astrocytes compared to wild-type. Intracellular GSH levels were not found to be different between the wild-type (68 ng GSH/μg total protein), DJ-1 knock-down (69 ng/μg), and DJ-1 over-expressing (71 ng/μg) astrocytes.
We also repeated the bioassay co-culture experiments with 10 or 20 nM rotenone in the additional presence of up to 10 mM GSH or GSHee (a membrane-permeable version of GSH). Neither of these anti-oxidants replaced the DJ-1 knock-down induced deficiency in astrocyte-mediated neuroprotection, and neither was generally neuroprotective (Fig. 5b-e). Similar bioassay experiments were performed using up to 10 mM of supplemental NAC or vitamin E. Again, neither of these anti-oxidants replaced the DJ-1 knock-down induced deficiency in astrocyte-mediated neuroprotection against rotenone (Fig. 6). However, unlike the other anti-oxidants tested, vitamin E was generally neuroprotective at higher levels. Despite this protective effect, the neuroprotective gap between wild-type and DJ-1 knock-down astrocytes was maintained (Fig. 6c–d).
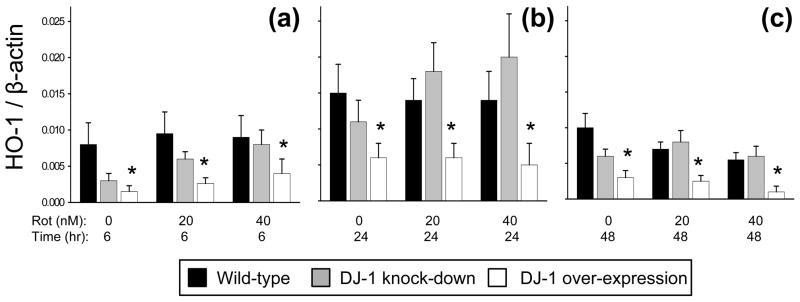
The HO-1 anti-oxidant system did not appear to be central to the mechanism of DJ-1-deficiency induced impairments in astrocyte-mediated neuroprotection
We employed quantitative Western blots to assess the potential role of HO-1 in our system, but found no significant effects of rotenone treatment or DJ-1 knock-down on astrocytic HO-1 levels (Fig. 7). However, we were surprised to discover that DJ-1 over-expression significantly reduced astrocytic HO-1 levels. To verify this result, we treated the astrocytes with hemin, a strong inducer of HO-1 (Supplementary Fig. 3). We replicated the predicted effect of hemin-induced HO-1 stimulation in the wild-type astrocytes. We also found strong stimulation in the DJ-1 knock-down cells. However, as was seen with rotenone, hemin exposure was not able to overcome the strong suppression of HO-1 induced by DJ-1 over-expression.
Fig. 7.
Astrocyte heme oxygenase-1 (HO-1) was not significantly affected by rotenone treatment or DJ-1 knock-down, but was suppressed by DJ-1 over-expression. (a-c) Dose- and time-response for quantitative Western blots showing HO-1 signal normalized to same-lane β-actin signal, in arbitrary units, after treatment of wild-type (siNS/vector-transfected, black bars), DJ-1 knock-down (siDJ#2/vector-transfected, gray bars), and DJ-1 over-expressing (siNS/mDJwt-transfected, white bars) astrocytes with rotenone (Rot) at the doses shown for 6 (a), 24 (b), or 48 (c) hours. Asterisks (*) represent p < 0.05 versus same-treatment wild-type by ANOVA. Mean ± SE shown, n=5.
Bioenergetic/Complex I bypass molecule supplementations did not replace the DJ-1-deficiency induced impairments in astrocyte-mediated neuroprotection
Our data suggest a mechanistically-important class effect of the Complex I-inhibiting neurotoxins that is not rescued by anti-oxidants. Thus, we attempted further rescue experiments in neuron/DJ-1 knock-down astrocyte co-cultures by supplementing the 10 nM and 20 nM rotenone-containing media with agents that may bypass the effects of inhibited Complex I. We tested lactic acid as an energy supplement (via the astrocyte-to-neuron “lactate shuttle”), succinic acid as a direct Complex II substrate, and coenzyme Q10 as a facilitator of downstream electron transport. Each agent was assessed over a wide concentration range, but none acted to replace the DJ-1 knock-down induced deficiency in astrocyte-mediated neuroprotection (Fig. 8). In addition, none were generally neuroprotective.
Discussion
We have here introduced a new, high-throughput bioassay that can be used to assess how genetic manipulations in astrocytes affect their capacity to protect co-cultured neurons against toxic insults. Using this bioassay we have demonstrated that DJ-1deficiency-induced impairments in astrocyte-mediated neuroprotection apply selectively to neurotoxicity induced by Complex I inhibiting pesticides. This may be a highly relevant finding to human disease because (i) genetic DJ-1 deficiency causes familial PD, (ii) sporadic PD reactive astrocytes over-express DJ-1, (iii) pesticide exposures are linked to elevated PD risk, and (iv) PD brain tissues exhibit reduced Complex I activity (Schapira et al. 1990, Rizzu et al. 2004, Bonifati et al. 2003, Ascherio et al. 2006, Brown et al. 2006, Gatto et al. 2009, Richardson et al. 2009, Wilk et al. 2006). Thus, it is conceivable that DJ-1 deficient PARK7 PD astrocytes are not able to protect surrounding neurons against Complex I deficiency induced by environmental toxicants. Conversely, DJ-1 over-expression in sporadic PD astrocytes may represent an attempt by the brain to protect itself against the same process.
The new data presented here support our previous rotenone findings (Mullett & Hinkle 2009) in several important manners. First, a novel plate-based bioassay was employed to replicate the original manual count data in our neuron/transfected astrocyte co-culture system. Second, independent bioassay assessments of multiple Complex I-inhibiting pesticide neurotoxins (rotenone, pyridaben, fenazaquin, and fenpyroximate) each produced the same result: DJ-1 deficient astrocytes were less neuroprotective than wild-type astrocytes. Third, bioassay experiments with pyridaben and fenpyroximate detected modest, but significant, augmentation of astrocyte-mediated neuroprotection induced by astrocytic DJ-1 over-expression. This pattern of more robust effects with DJ-1 knock-down than DJ-1 over-expression is similar to our findings with rotenone. It likely reflects the high endogenous DJ-1 levels in the cultured astrocytes, where a significant loss of DJ-1 expression (to ~5%) appears to be more physiologically active than a moderate gain (to ~2-fold).
The most important finding from our studies is that astrocytic DJ-1 knock-down induced a selective deficiency in astrocyte-mediated neuroprotection against Complex I inhibition. Specifically, DJ-1 deficient astrocytes were significantly less neuroprotective than wild-type astrocytes against four distinct Complex I inhibitors. This disparity in astrocyte-mediated neuroprotection did not exist against Complex II-IV inhibitors, direct inducers of oxidative stress, or proteasome inhibitors. Astrocyte viability was stable under all of these conditions. However, the Complex V inhibitor oligomycin, the oxidative toxin hydrogen peroxide, and the excitotoxin glutamic acid were all toxic to the astrocytes over the neurotoxic range. Therefore, astrocyte-mediated neuroprotection could not be assessed for these agents.
These results suggest that DJ-1-deficient astrocytes are specifically impaired in their capacity to support neurons against death induced by the PD-relevant process of Complex I inhibition. Several potential mechanisms exist for this finding, and we approached our investigations with the assumption that astrocyte-released, soluble factors were involved. This is based on our previous discovery that both DJ-1 modulated augmentation and impairment of astrocyte-mediated neuroprotection persist when astrocyte conditioned media is used on separate neuron-enriched cultures (Mullett & Hinkle 2009). Thus, it is conceivable that DJ-1 deficient astrocytes lose their capacity to release soluble neuroprotective molecules that are required by surrounding neurons to stabilize themselves against Complex I inhibition. Soluble factor candidates based on known astrocyte physiology include anti-oxidant molecules, bioenergetic molecules, cytokines, and peptide neurotrophic factors (Benvenisti-Zarom & Regan 2007, Calabrese et al. 2005, Chen et al. 2009, Damier et al. 1993, Dringen et al. 1999, Makar et al. 1994, Matz et al. 1996, Nakaso et al. 2000, Sagara et al. 1996, Sagara et al. 1993, Wang & Cynader 2000). Soluble factor candidates based on known DJ-1 mechanisms in non-astrocytic cells include DJ-1 itself and anti-oxidant molecules (Tsuboi et al. 2008, Andres-Mateos et al. 2007, Clements et al. 2006, Kim et al. 2005, Liu et al. 2008a, Meulener et al. 2005, Taira et al. 2004, Zhou & Freed 2005). Soluble factor candidates based on known/suspected mechanisms of rotenone/Complex I inhibitor induced neurotoxicity include those that protect against oxidative stress, energy failure, altered mitochondrial function, altered proteasome activity, excitotoxicity, apoptosis, cytoskeletal instability, and neuroinflammation (Betarbet et al. 2006, Feng 2006, Klintworth et al. 2007, Panov et al. 2005, Ramachandiran et al. 2007, Sanchez et al. 2008, Shamoto-Nagai et al. 2003, Sherer et al. 2003, Sherer et al. 2007, Wang et al. 2006).
Our approach was to initially focus on the mechanism that appeared to be most relevant to DJ-1, astrocytes, and rotenone: astrocytic production/release of anti-oxidant molecules. We first hypothesized that DJ-1 itself might be released into astrocyte conditioned media. This did not turn out to be true (Mullett & Hinkle 2009). We next hypothesized that primarily oxidative stress-inducing neurotoxins should reproduce the Complex I inhibitor results, particularly if oxidative stress was a critical mechanism in our system. The agents that we tested were each neurotoxic in a dose-dependent manner, but either did not reproduce the Complex I inhibitor results (paraquat) or were toxic to both cell types (hydrogen peroxide). The results for paraquat were particularly interesting because this toxin is a powerful inducer of oxidative stress that, relative to rotenone, only weakly inhibits Complex I and often acts through distinct mechanisms (Ved et al. 2005, Richardson et al. 2005, Ramachandiran et al. 2007, Klintworth et al. 2007, Wang et al. 2006). Thus, the inability of paraquat to reproduce the Complex I inhibitor co-culture findings strengthened the centrality of this mechanism to our system, and weakened the apparent importance of general oxidative stress.
We also investigated whether the DJ-1 modulated protective response involved astrocytic Nrf2-related anti-oxidant mechanisms. The rationale was that (i) DJ-1 can stimulate Nrf2-dependent GSH and HO-1 production in non-astrocytic cells and (ii) Nrf2, GSH, and HO-1 can respond to, and promote astrocyte-mediated neuroprotection against, oxidative stress in other models (Zhou & Freed 2005, Clements et al. 2006, Liu et al. 2008b, Wang & Cynader 2000, Chen et al. 2009, Dringen et al. 1999, Makar et al. 1994, Sagara et al. 1993, Nakaso et al. 2000, Matz et al. 1996, Benvenisti-Zarom & Regan 2007, Calabrese et al. 2005). Our hypothesis was therefore that astrocytic DJ-1 knock-down would reduce the protective influence of these anti-oxidant systems. We were not able to detect the “gateway” Nrf2 protein in our astrocytes, despite being able to do so in cultured neurons. This did not eliminate the possibility that GSH and/or HO-1 were active in our system, however.
Regarding the astrocyte GSH anti-oxidant system, γ-glutamyl cysteine ligase levels were too low to quantify and GPx1 was quantifiable but did not change significantly with rotenone treatments or DJ-1 manipulation. We further assayed for intracellular and released GSH, but again found no evidence for regulation by rotenone or DJ-1 in our astrocytes. Attempts to replace the DJ-1 knock-down induced deficiency in astrocyte-mediated neuroprotection by supplementing physiological levels of GSH and GSHee were unsuccessful. Experiments using supplemental NAC and vitamin E produced similar results, except that vitamin E had a global neuroprotective effect. Interestingly, the neuroprotective disparity between DJ-1 knock-down and wild-type astrocytes remained. This further strengthened our developing conclusion that astrocyte anti-oxidant systems were not significantly involved in the neuroprotective mechanism.
Regarding the HO-1 anti-oxidant system, neither rotenone treatment nor DJ-1 knock-down significantly affected HO-1 levels in the astrocytes. However, DJ-1 over-expression strongly, and consistently, suppressed HO-1 levels. This was true even in the presence of the powerful HO-1 inducer hemin. This finding ran counter to our hypothesis, and was difficult to interpret mechanistically, but supported the conclusion that DJ-1 over-expression does not augment astrocyte-mediated neuroprotection by inducing HO-1.
We next hypothesized that astrocyte-released, bioenergetic/Complex I-bypassing molecules might be involved in the mechanism. To test this possibility we supplemented rotenone-treated neuron/DJ-1 knock-down astrocyte co-culture media with lactic acid, succinic acid, or coenzyme Q10. None of these agents was able to replace the deficiency in astrocyte-mediated neuroprotective induced by DJ-1 knock-down, and none was generally neuroprotective. Therefore, we could draw no firm conclusions regarding their importance as astrocyte-released neuroprotective factors.
Thus, the investigated anti-oxidant, bioenergetic, and Complex I-bypassing mechanisms are not likely to be critical to astrocyte-mediated neuroprotection in our model system. We are therefore left with evidence that astrocytes release unknown, DJ-1 modulated factors that are protective against Complex I-selective mechanisms of neurodegeneration. We found no evidence that proteasome inhibition replicated the pesticide effects, and experiments with excitotoxins were inconclusive due to astrocytic toxicity. Neuronal mitochondrial functioning, particularly as it relates to apoptotic pathways, may be targeted by the DJ-1 modulated, astrocyte-released factors. Neuroinflammation is another potential avenue of inquiry. For example, microglia can contribute to rotenone neurotoxicity and DJ-1 knock-out astrocytes are impaired in their neuroprotective capacity against lipopolysaccharide (Gao et al. 2002, Gao et al. 2003, Waak et al. 2009). Neuronal cytoskeletal stability may also be targeted by both Complex I inhibition and the remediating factors (Feng 2006). These remaining possibilities are wide in scope and have not yet been tested formally.
In summary, we have shown that the deficient expression of a PD-relevant gene (DJ-1) in cells that robustly express it in PD (astrocytes) causes a selective impairment in neuroprotection against a highly PD-relevant neurotoxic mechanism (Complex I inhibition). This impairment was not reversed by supplemental anti-oxidant or bioenergetic molecules, and DJ-1 did not modulate astrocytic GSH or HO-1. More extensive assessments of the astrocyte conditioned media will therefore be needed to identify the specific astrocyte-released factors that are active in our model. We believe that pursuing this in future studies will be important to the identification and development of astrocyte-based, disease-modifying therapies against environmental toxicant exposures and PD.
Supplementary Material
Acknowledgments
We acknowledge the technical assistance of Bethann E. Gabris. This work was supported by NINDS K08NS055736 and American Parkinson Disease Association George C. Cotzias Fellowship grants to DAH.
Abbreviations used
- ABAM
antibiotic-antimycotic
- CoQ
coenzyme Q10
- DIV
days in vitro
- DMEM
Dulbecco’s modified Eagle media
- FCS
fetal calf serum
- FPX
fenpyroximate
- FZQ
fenazaquin
- GFAP
glial fibrillary acidic protein
- GPx1
glutathione peroxidase 1
- GSH
glutathione
- GSHee
glutathione ethyl ester
- HO-1
heme oxygenase-1
- ICC
immunocytochemistry
- LAC
lactic acid
- LC
lactacystin
- MAP2
microtubule associated protein 2
- mDJwt
mouse wild-type DJ-1 cDNA
- NAC
N-acetyl-L-cysteine
- 3-NPA
3-nitroproprionic acid
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- PD
Parkinson’s disease
- PQ
paraquat
- PYR
pyridaben
- Rot
rotenone
- siRNA
small interfering RNA
- siDJ#2
anti-mouse DJ-1 siRNA
- siNS
non-silencing siRNA
- SUC
succinic acid
- VitE
vitamin E
Footnotes
The authors have no financial conflicts of interest to declare.
References
- Aleyasin H, Rousseaux MW, Phillips M, et al. The Parkinson’s disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc Natl Acad Sci U S A. 2007;104:18748–18753. doi: 10.1073/pnas.0709379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Mateos E, Perier C, Zhang L, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Chen H, Weisskopf MG, O’Reilly E, McCullough ML, Calle EE, Schwarzschild MA, Thun MJ. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- Ballard PA, Tetrud JW, Langston JW. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): seven cases. Neurology. 1985;35:949–956. doi: 10.1212/wnl.35.7.949. [DOI] [PubMed] [Google Scholar]
- Benvenisti-Zarom L, Regan RF. Astrocyte-specific heme oxygenase-1 hyperexpression attenuates heme-mediated oxidative injury. Neurobiol Dis. 2007;26:688–695. doi: 10.1016/j.nbd.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Canet-Aviles RM, Sherer TB, et al. Intersecting pathways to neurodegeneration in Parkinson’s disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22:404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Blackinton J, Lakshminarasimhan M, Thomas KJ, Ahmad R, Greggio E, Raza AS, Cookson MR, Wilson MA. Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. J Biol Chem. 2009;284:6476–6485. doi: 10.1074/jbc.M806599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson’s disease--is there a link? Environ Health Perspect. 2006;114:156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Ravagna A, Colombrita C, Scapagnini G, Guagliano E, Calvani M, Butterfield DA, Giuffrida Stella AM. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J Neurosci Res. 2005;79:509–521. doi: 10.1002/jnr.20386. [DOI] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, et al. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol Dis. 2009;34:279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer-and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience. 1993;52:1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J. Microtubule: a common target for parkin and Parkinson’s disease toxins. Neuroscientist. 2006;12:469–476. doi: 10.1177/1073858406293853. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23:6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gash DM, Rutland K, Hudson NL, et al. Trichloroethylene: Parkinsonism and Complex 1 mitochondrial neurotoxicity. Ann Neurol. 2008;63:184–192. doi: 10.1002/ana.21288. [DOI] [PubMed] [Google Scholar]
- Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B. Well-water consumption and Parkinson’s disease in rural California. Environ Health Perspect. 2009;117:1912–1918. doi: 10.1289/ehp.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junn E, Taniguchi H, Jeong BS, Zhao X, Ichijo H, Mouradian MM. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc Natl Acad Sci U S A. 2005;102:9691–9696. doi: 10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintworth H, Newhouse K, Li T, Choi WS, Faigle R, Xia Z. Activation of c-Jun N-terminal protein kinase is a common mechanism underlying paraquat- and rotenone-induced dopaminergic cell apoptosis. Toxicol Sci. 2007;97:149–162. doi: 10.1093/toxsci/kfm029. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Rebert CS, Irwin I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey. Brain Res. 1984;292:390–394. doi: 10.1016/0006-8993(84)90777-7. [DOI] [PubMed] [Google Scholar]
- Liu F, Nguyen JL, Hulleman JD, Li L, Rochet JC. Mechanisms of DJ-1 neuroprotection in a cellular model of Parkinson’s disease. J Neurochem. 2008a;105:2435–2453. doi: 10.1111/j.1471-4159.2008.05333.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Nguyen JL, Hulleman JD, Li L, Rochet JC. Mechanisms of DJ-1 neuroprotection in a cellular model of Parkinson’s disease. J Neurochem. 2008b doi: 10.1111/j.1471-4159.2008.05333.x. [DOI] [PubMed] [Google Scholar]
- Makar TK, Nedergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJ. Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J Neurochem. 1994;62:45–53. doi: 10.1046/j.1471-4159.1994.62010045.x. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Thimmulappa R, Navas-Acien A, et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Matz P, Turner C, Weinstein PR, Massa SM, Panter SS, Sharp FR. Heme-oxygenase-1 induction in glia throughout rat brain following experimental subarachnoid hemorrhage. Brain Res. 1996;713:211–222. doi: 10.1016/0006-8993(95)01511-6. [DOI] [PubMed] [Google Scholar]
- Meulener M, Whitworth AJ, Armstrong-Gold CE, Rizzu P, Heutink P, Wes PD, Pallanck LJ, Bonini NM. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Mullett SJ, Hinkle DA. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol Dis. 2009;33:28–36. doi: 10.1016/j.nbd.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaso K, Kitayama M, Mizuta E, Fukuda H, Ishii T, Nakashima K, Yamada K. Co-induction of heme oxygenase-1 and peroxiredoxin I in astrocytes and microglia around hemorrhagic region in the rat brain. Neurosci Lett. 2000;293:49–52. doi: 10.1016/s0304-3940(00)01491-9. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Youngster SK, Kindt MV, Heikkila RE. MPTP, MPP+ and mitochondrial function. Life Sci. 1987;40:721–729. doi: 10.1016/0024-3205(87)90299-2. [DOI] [PubMed] [Google Scholar]
- Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem. 2005;280:42026–42035. doi: 10.1074/jbc.M508628200. [DOI] [PubMed] [Google Scholar]
- Ramachandiran S, Hansen JM, Jones DP, Richardson JR, Miller GW. Divergent mechanisms of paraquat, MPP+, and rotenone toxicity: oxidation of thioredoxin and caspase-3 activation. Toxicol Sci. 2007;95:163–171. doi: 10.1093/toxsci/kfl125. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol Sci. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Shalat SL, Buckley B, Winnik B, O’Suilleabhain P, Diaz-Arrastia R, Reisch J, German DC. Elevated serum pesticide levels and risk of Parkinson disease. Arch Neurol. 2009;66:870–875. doi: 10.1001/archneurol.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzu P, Hinkle DA, Zhukareva V, et al. DJ-1 colocalizes with tau inclusions: a link between parkinsonism and dementia. Ann Neurol. 2004;55:113–118. doi: 10.1002/ana.10782. [DOI] [PubMed] [Google Scholar]
- Sagara J, Makino N, Bannai S. Glutathione efflux from cultured astrocytes. J Neurochem. 1996;66:1876–1881. doi: 10.1046/j.1471-4159.1996.66051876.x. [DOI] [PubMed] [Google Scholar]
- Sagara JI, Miura K, Bannai S. Maintenance of neuronal glutathione by glial cells. J Neurochem. 1993;61:1672–1676. doi: 10.1111/j.1471-4159.1993.tb09802.x. [DOI] [PubMed] [Google Scholar]
- Sanchez M, Gastaldi L, Remedi M, Caceres A, Landa C. Rotenone-induced toxicity is mediated by Rho-GTPases in hippocampal neurons. Toxicol Sci. 2008;104:352–361. doi: 10.1093/toxsci/kfn092. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial Complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD. Anatomic and disease specificity of NADH CoQ1 reductase (Complex I) deficiency in Parkinson’s disease. J Neurochem. 1990;55:2142–2145. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- Shamoto-Nagai M, Maruyama W, Kato Y, Isobe K, Tanaka M, Naoi M, Osawa T. An inhibitor of mitochondrial Complex I, rotenone, inactivates proteasome by oxidative modification and induces aggregation of oxidized proteins in SH-SY5Y cells. J Neurosci Res. 2003;74:589–597. doi: 10.1002/jnr.10777. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, et al. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Richardson JR, Testa CM, Seo BB, Panov AV, Yagi T, Matsuno-Yagi A, Miller GW, Greenamyre JT. Mechanism of toxicity of pesticides acting at Complex I: relevance to environmental etiologies of Parkinson’s disease. J Neurochem. 2007;100:1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kommineni C, Jeffries BA, Castranova V, Tyurina YY, Tyurin VA, Serbinova EA, Fabisiak JP, Kagan VE. Redox cycling of phenol induces oxidative stress in human epidermal keratinocytes. J Invest Dermatol. 2000;114:354–364. doi: 10.1046/j.1523-1747.2000.00865.x. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Tyurina JY, Kawai K, Tyurin VA, Kommineni C, Castranova V, Fabisiak JP, Kagan VE. Selective peroxidation and externalization of phosphatidylserine in normal human epidermal keratinocytes during oxidative stress induced by cumene hydroperoxide. J Invest Dermatol. 2002;118:1008–1018. doi: 10.1046/j.1523-1747.2002.01759.x. [DOI] [PubMed] [Google Scholar]
- Smith TS, Parker WD, Jr, Bennett JP., Jr L-dopa increases nigral production of hydroxyl radicals in vivo: potential L-dopa toxicity? Neuroreport. 1994;5:1009–1011. doi: 10.1097/00001756-199404000-00039. [DOI] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi Y, Munemoto H, Ishikawa S, Matsumoto K, Iguchi-Ariga SM, Ariga H. DJ-1, a causative gene product of a familial form of Parkinson’s disease, is secreted through microdomains. FEBS Lett. 2008;582:2643–2649. doi: 10.1016/j.febslet.2008.06.043. [DOI] [PubMed] [Google Scholar]
- Ved R, Saha S, Westlund B, et al. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem. 2005;280:42655–42668. doi: 10.1074/jbc.M505910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waak J, Weber SS, Waldenmaier A, et al. Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. FASEB J. 2009;23:2478–2489. doi: 10.1096/fj.08-125153. [DOI] [PubMed] [Google Scholar]
- Wang XF, Cynader MS. Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochem. 2000;74:1434–1442. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- Wang XF, Li S, Chou AP, Bronstein JM. Inhibitory effects of pesticides on proteasome activity: implication in Parkinson’s disease. Neurobiol Dis. 2006;23:198–205. doi: 10.1016/j.nbd.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Wilk JB, Tobin JE, Suchowersky O, et al. Herbicide exposure modifies GSTP1 haplotype association to Parkinson onset age: the GenePD Study. Neurology. 2006;67:2206–2210. doi: 10.1212/01.wnl.0000249149.22407.d1. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhong N, Wang H, et al. The Parkinson’s disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum Mol Genet. 2005;14:1231–1241. doi: 10.1093/hmg/ddi134. [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.