Abstract
Current evidence suggests that Alzheimer’s disease (AD) is a multi-factorial disease that starts with accumulation of multiple proteins. We have previously proposed that inhibition of γ-secretase may impair membrane recycling causing neurodegeneration starting at synapses (Sambamurti et al., 2006). We also proposed familal AD (FAD) mutations increase Aβ42 by inhibiting γ-secretase. Herein, we discuss the failure of Eli Lilly’s γ-secretase inhibitor, semagacestat, in clinical trials in the light of our hypothesis, which extends the problem beyond toxicity of Aβ aggregates. We elaborate that γ-secretase inhibitors lead to accumulation of amyloid precursor protein (APP) C-terminal fragments (CTFs) that can later be processed by γ-secretase to yields bursts of Aβ to facilitate aggregation. Although we do not exclude a role for toxic Aβ aggregates, inhibition of γ-secretase can affect numerous substrates other than APP to affect multiple pathways and the combined accumulation of multiple peptides in the membrane may impair its function and turnover. Taken together, protein processing and turnover pathways play an important role in maintaining cellular homeostasis and unless we clearly see consistent disease-related increase in their levels or activity, we need to focus on preserving their function rather than inhibiting them for treatment of AD and similar diseases.
Introduction
Alzheimer’s disease (AD) is a major public health problem affecting a large fraction of the elderly population (Hebert et al. 2003). Unfortunately there are currently no proven therapies that delay the onset or prevent the progression of AD. However, multiple genetic, epigenetic, environmental, life style factors such as exposure to metals, smoking, high cholesterol, diabetes and elevated homocysteine have been identified as potentially valuable targets for modifying risk (Bhat 2010, Lahiri et al. 2008, Sambamurti et al. 2004). Historically, the molecular era of research on AD starts with the seminal studies that identified the sequences of the major component of the senile plaque (SP) core as the 39-42 amino acid (aa) amyloid or A4 peptide (Aβ) (Glenner & Wong 1984b, Glenner et al. 1984, Glenner & Wong 1984a, Masters et al. 1985, Wong et al. 1985) and neurofibrillary tangles (NFTs) as the microtubule associated protein, Tau (MAPT) (Baudier & Cole 1987, Bancher et al. 1987, Wood et al. 1986, Pollock et al. 1986, Nukina & Ihara 1986, Montejo de Garcini et al. 1986, Kosik et al. 1986, Ihara et al. 1986, Grundke-Iqbal et al. 1986b, Grundke-Iqbal et al. 1986a, Delacourte & Defossez 1986, Brion et al. 1986). Screens of cDNA libraries detected eight isoforms of the amyloid protein precursor (APP) ranging in size from 677-770 aa (Goldgaber et al. 1987a, Goldgaber et al. 1987b, Kang et al. 1987, Robakis et al. 1987, Tanzi et al. 1987, Monning et al. 1992). Thus, Aβ starts as a much larger protein that is subsequently proteolytically processed by multiple pathways. MAPT, on the other hand, had been discovered previously as a promoter of microtubule assembly (Weingarten et al. 1975). Although MAPT was found in several dementias and has been linked genetically to tauopathy-associated dementias, an early genetic association between APP and familial AD (FAD) brought APP to the center of AD research (Goate et al. 1991, Mullan et al. 1992). These genetic studies were complemented by biochemical findings that levels of longer 42 aa forms of Aβ increase in FAD mutant cells. In addition, a major risk factor in AD is the ε4 allele of Apolipoprotein E (ApoE4). Several studies suggest that ApoE facilitates Aβ deposition and that its ε4 isoform has a higher affinity for Aβ. Based on studies showing that these longer forms of Aβ readily aggregate into neurotoxic oligomers and fibrils in vitro, the fundamental hypothesis to describe the origin of AD has been that Aβ initiates a toxic cascade that causes AD (Hardy and Selkoe, 2002).
This hypothesis has propelled the pharmaceutical industry to treat AD by reducing Aβ. Because AD has helped investigators defined a new phenomenon of protein aggregation and deposition that has been repeatedly observed in other neurodegenerative disorders such as Parkinson’s disease (PD) and Huntington’s disease (HD), it is hoped that the treatment paradigms developed for AD can be extended to other diseases.
Several agents were thus developed to reduce Aβ aggregates in animal models, including one specific for Aβ42, but all of these lack efficacy in disease treatment (e.g. Alzhemed™, Flurizan, http://www.alzforum.org/drg/drc/default.asp). However, it is important to note that most of these treatments failed to detect changes in Aβ in the cerebrospinal fluid (CSF), leading scientists to speculate that the problem has been one of dosing (Green et al. 2009, Imbimbo 2009). Two agents that clearly reduce Aβ in clinical trials are a vaccine against deposited amyloid - AN-1792, and a γ-secretase inhibitor (Portelius et al. 2010, Gilman et al. 2005). The former was removed from clinical trials when patients developed encephalitis due to the vaccine side effects although the approach is still being pursued using an alternative strategy of passive immunization (Klyubin et al. 2008, Bard et al. 2003). Also, in patients who did not acquire encephalitis, only showed marginal disease arrest was observed (Gilman et al. 2005). More recently, Eli Lily has stopped trials using its γ-secretase inhibitor – semagacestat – as this drug performed less well than placebo (Extance 2010, Panza et al. 2010). Major arguments in the AD field are that drug failure is due to effects on an alternative and critical target, Notch, shifting the treatment focus to more selective inhibitors that preferentially inhibit APP processing while avoiding Notch. It is also important to note that function of APP and the role played by the secretases is not known making effects on the target unclear. Recent studies suggest that APP may play a role in innate immunity, a response affected by γ-secretase inhibition (Lanz et al. 2006, Jayadev et al. 2010).
When assessing the failure of these drugs in human clinical trials, one must consider several problems for achieving efficacy in AD drug development. First, we must extend research beyond amyloid-lowering agents, determining the best time for treatment along with alternative risk factors and target AD markers more proximal to the observed neurodegeneration.
First, the role of protein aggregation in degeneration and mechanisms of toxicity have not been fully established. Therefore, we will first discuss the evidence that supports the amyloid hypothesis and continue to argue in support of an alternative hypothesis: the failure of γ-secretase processing leads to AD pathogenesis. This theory is based on our findings that Aβ42 levels actually increase upon reducing γ-secretase (Refolo et al. 1999, Sambamurti et al. 2006, Marlow et al. 2003). The major prediction of this hypothesis is that inhibitors of γ-secretase will actually worsen rather than attenuate neurodegeneration in AD, because of the enzyme’s important role in maintaining membrane homeostasis, failure of which, is the cause of AD-associated neurodegeneration. Such worsening of dementia was indeed observed in the trial (Extance 2010).
Other points to consider are that Aβ dyshomeostasis is an early phenomenon and the dementia may be driven by other yet to be discovered lesions as well as dysfunction of other cell components such as APOE, MAPT, TDP43, synuclein, etc (Wollmer 2010, Gabelle et al. 2010). Thus, multiple proteins are misprocessed in AD, and this may lead to dementia by multiple pathways. Although these downstream events are critical to AD pathogenesis, we will be focusing on the Aβ-related pathways to keep the discussion focused on drug development against this target.
A third problem is one of timing. For example, a known problem in genetic deficits in amino acid metabolism that leads to mental retardation is that compensation for the defect after the mental retardation sets in does not restore mental function (Orendac et al. 2003). Similarly, one may need to treat the problem of Aβ accumulation before the cascade of events cause a failure of processing and function of other proteins that are ultimately catastrophic. Although it has been reported that AD pathology predates dementia by 10 years or longer, this remains controversial due to its high variability and lack of longitudinal studies that are now underway through ADNI (Caroli & Frisoni 2010). However, it is important to recognize that dementia advances quite rapidly once it begins, suggesting that the progression of dementia may be determined by factors other than Aβ (Tarawneh & Holtzman 2009).
In short, given the recently reported failure of semagacestat and its withdrawal from clinical trials, it is critical to reevaluate the beneficial and detrimental effects of various Aβ-reducing treatment strategies and discuss other potential targets for treatment.
Aβ and APP processing
APP is a large type-I, single-pass integral membrane glycoprotein with a large N-terminal ectodomain, a single transmembrane domain and a cytoplasmic domain of 47 aa (Hardy & Selkoe 2002, Sambamurti et al. 2006, Sambamurti et al. 2002b, Sambamurti et al. 2002a). Alternative splicing of three exons (7, 8, 15) within the ectodomain region of a single gene on chromosome 21 generates eight isoforms of APP (695–770 aa) that are differentially expressed in different cell types (Sambamurti et al. 2002b, Sambamurti et al. 2002a). The major neuronal form is APP695, which defines neuron-specific alternative splicing, lacks exon 7: a Kunitz protease inhibitor (KPI; 289-344) seen in APP751 and APP770 and exon 8: the OX2 (345-363) seen in APP770. Exon 15 (637-654) is also differentially spliced and is absent in lymphocyte APP (LAPP) that is absent in neurons (Sandbrink et al. 1994). Loss of this 18–aa domain generates a novel motif that gets decorated by chondroitin sulfate glycosaminoglycans (GAGs) (Robakis et al. 1993).
To generate Aβ, APP is cleaved on the N-terminal side of the Aβ sequence by β-secretase to secrete the ectodomain fragment sAPPβ and the remaining membrane-bound CTFβ is further processed by γ-secretase within the membrane to secrete Aβ (Fig 1). However, this pathway accounts for the turnover of only a small fraction of APP and the majority (>90%) of APP is processed by an alternate pathway, α-secretase, between residues 16 and 17 of Aβ to secreted derivative, sAPPα, that preclude Aβ formation (Haass & Selkoe 1993, Sambamurti et al. 1992b, Anderson et al. 1991, Esch et al. 1990). This event is very rapid and occurs in the secretory pathway of the trans-Golgi network and on the cell surface (Sambamurti et al. 1992a, Sambamurti et al. 1992b).
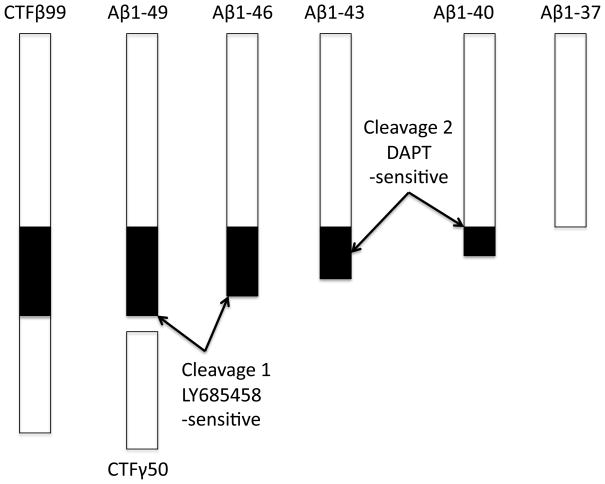
Figure 1. Pathways of APP proteolysis.
The full-length APP is a type-I integral membrane protein. It is cleaved in the exocytoplasmic domain at the start of Aβ by β-secretase and between residues 16-17 of Aβ by α secretase to generate secreted derivatives sAPPβ and sAPPα and C-terminal fragments CTFβ and CTFα. γ-Secretase cleaves CTFβ at the membrane cytoplasm boundary (ε site) to CTFγ and Aβ49, and subsequently to Aβ40 and Aβ42. Most secreted Aβ ends at residue 40 but a small percentage is longer terminating at residues 42 and 43. CTFα is similarly processed by γ-secretase (not shown). The predicted CTFγ is not seen probably due to its rapid turnover in the cell.
The C-terminal fragments CTFβ and CTFα generated by β- and α-secretase cleavages remain in the membrane and are processed by γ-secretase to generate secreted derivatives Aβ and Aα (Aβ17-40/42; aka P3) (Haass et al. 1993) and CTFγ (aka CTFε or AICD) (Pinnix et al. 2001) of 50 residues (Sastre et al. 2001, Gu et al. 2001). The Aβ and Aα fragments predominantly end at residue 40, while other forms, including Aβ42 are present at much lower levels (Seubert et al. 1992, Cai et al. 1993). The major importance of this finding has been that longer Aβ42 are increased as a general rule for most of the FAD mutations on APP (Suzuki et al. 1994).
The increase in Aβ42 was further supported and expanded to include FAD mutations linked to the critical γ-secretase subunit presenilin(s), PS1 and PS2 (Sherrington et al. 1995, Borchelt et al. 1996, Duff et al. 1996). These studies also confirmed a connection between APP and MAPT, as FAD mutations lead to AD earlier in life, with a richer neuropathological lesion load of typical plaques and tangles, even though the mutations only directly affect Aβ42 production before the development of pathology.
Amyloid aggregation and toxicity
A large body of literature has focused on amyloid neurotoxicity, a phenomenon first discovered by Yankner and colleagues in 1990 (Yankner et al. 1990). It was proposed that aggregates of Aβ induce toxic oxidative stress (Pappolla, Am J Pathol 1992). At that time, several well-known investigators questioned the data (Podlisny et al. 1992) and an entire issue of the journal Neurobiology of aging was dedicated to resolve the controversy (Yankner 1992). The conclusion of these studies was that amyloid is indeed toxic but that the toxicity varied wildly between preparations (May et al. 1992). Many studies showed that Aβ readily forms oligomers and larger aggregates that are toxic to cells in culture (Walsh & Selkoe 2004, Chromy et al. 2003, Lambert et al. 1994, Glabe 2008, Pike et al. 1991).
The aggregation of synthetic Aβ in vitro has been studied since the identification of the peptide in senile plaques but evolved into a larger discipline with the discovery that soluble oligomers rather than large insoluble aggregates of Aβ are toxic (Klein et al. 2001). This theory explains the neuronal deficiency in APP transgenic mouse models that occurs long before apparent amyloid deposition is observed (Reed et al. 2009). Furthermore, the theory also accounts for the role of FAD mutations due to the greater tendency of the longer Aβ42 species to aggregate, compared to the shorter Aβ40. Finally, this theory can be supported by the generally accepted view that the ApoE4 facilitates aggregation of Aβ and therefore increases the risk of AD (Fryer et al. 2005, Bales et al. 1999). This concept has gained substantive support from most investigators who suggested that Aβ oligomers can affect multiple pathways in the brain such as insulin signaling, NMDA receptor activity, mitochondrial copy number and function and toxicity due to oxidative stress-mediated damage to neurons (Smith et al. 1998, Pappolla et al. 1998, Casadesus et al. 2007). The presence of toxic oligomers has been demonstrated in cell cultures and in the brain (Pappolla et al. 1998). The exact nature of the toxic oligomer is, however, still being debated and has ranged from dimer, trimer, tetramer, to dodecamer (Walsh et al. 2001, Reed et al. 2009, Freir et al. 2010). Nevertheless, the oligomers appear to induce a broad range of deficiencies, including effects on LTP formation and memory that can be specifically targeted by pharmacological manipulation. One group suggests that low concentrations of Aβ monomer and oligomer normally facilitates synaptic plasticity and memory but high concentrations lead to dysfunction and dementia (Fa et al. 2010, Origlia et al. 2009). A transgenic mouse model that expresses Aβ oligomers lends additional support to this idea (Tomiyama et al. 2010, Yankner 1992).
Mechanisms of Aβ-induced toxicity remain rather unclear complicating the identification of downstream targets and their relevance to AD. Several targets have been discovered such as the insulin receptor, NMDA receptor, RAGE receptor, and ERAB (Klein 2002, Viola et al. 2008, Verdier et al. 2004, Origlia et al. 2008, He et al. 2000). Interestingly, the ERAB protein that binds Aβ may enter mitochondria that provide additional possibilities for its disruption of cellular energy metabolism (Sambamurti & Lahiri 1998). It has been suggested that intracellular rather than extracellular Aβ may actually be the cause of its toxicity (Tseng et al. 2004).
More recently, evidence suggests that a pyroglutamate form of Aβ is the toxic species rather than simply the levels of the normal peptide (Wirths et al. 2010). This has resulted in yet another target glutaminyl cyclase, the enzyme responsible for generating the pyroglutamate residue (Morawski et al. 2010).
The amyloid hypothesis
The extremely early development of dementia and AD pathology in FAD also suggests that this pathology may be driven simply by Aβ accumulation (Sambamurti et al. 2002b, Hardy & Selkoe 2002). The genetic link between Aβ42 and AD combined with the observed neurotoxicity in vitro form the basis for the amyloid hypothesis. Briefly, toxicity of Aβ or its aggregates is considered to be the key cause of AD and this toxicity is believed to sequentially induce neuronal dysfunction, MAPT phosphorylation, NFT formation and neurodegeneration. The actual toxic agent has been a source of considerable controversy with theories shifting over time to include senile plaques to Aβ aggregates of various types and the monomer. It has been argued that certain forms of Aβ accumulate early in AD and induce the formation of NFTs by affecting the signaling cascade. Others suggest that Aβ oligomers ranging from the dimer to the dodecamer are the toxic species that cause neurodegeneration in AD (Reed et al. 2009, Shankar et al. 2009). Also, some argue that microglia prevent oligomer toxicity but may induce toxicity when clearing plaques (Shah et al. 2010).
One of the early concerns of the amyloid hypothesis has been that senile plaques are frequently found in cognitively normal elderly individuals and the plaque count does not correlate with dementia (Terry 1998, Terry 1996). However, better correlation is observed when the plaque is limited to the neuritic kind and when soluble amyloid is measured (Parvathy et al. 2001). A significant advance that arose due to this controversy is an attempt to identify the toxic form of amyloid that may be responsible for AD pathogenesis.
Adhering to the amyloid hypothesis, one can readily find a number of potential targets for disease treatment (Table 1). Two key targets for inhibition in the synthesis pathway are β-secretase and γ-secretase. In addition, targets for stimulation are, α-secretase, Aβ degradation, and clearance pathways.
Table 1.
Major drug targets based on the amyloid hypothesis
| Target | +/− | Examples | Side effects of specific drug |
|---|---|---|---|
| APP synthesis | − | Posiphen; Antisense; siRNA | Limited! APP is redundant |
| β-secretase | − | CTS21166 | BACE1 has multiple substrates |
| γ-secretase* | − | semagacestat | Multiple targets, Toxic substrate accumulation |
| α-secretase | + | Benzolactam | Complex and not yet defined. Target dependent |
| Aβ degradation | + | TPA, UPA | Limited! May be very specific. |
| Aβ clearance | + | ApoE, LRP | Complex and not yet defined. Target dependent |
| Aβ aggregation | − | PBT-2 | Limited! May be very specific. |
| Vaccination | + | Vaccination; Passive immunization | Inflammation |
| Aβ toxicity | − | Anti-Tau; -oxidants; -inflammatory | Complex and not yet defined. Target dependent |
+= Stimulate; −= Inhibit
Alternate hypothesis: Inhibitor may cause AD!
Beta Secretase
The amount of Aβ generated is apparently regulated by the extent of APP cleavage by β-secretase making it a target for inhibiting Aβ production (Gandhi et al. 2004, Citron et al. 1992). The enzyme was identified by five groups as a novel single-pass, type-I integral membrane aspartyl protease and was variously named as BACE-1, memapsin-2 or Asp-2 by the different groups (Yan et al. 1999, Vassar et al. 1999, Sinha et al. 1999, Hussain et al. 1999, Lin et al. 2000). Although memapsin-2 for membrane aspartyl protease is the most biochemically appropriate name, the most popular name in literature is BACE-1. The enzyme is not inhibited by the traditional aspartyl protease inhibitor, pepstatin, and apparently has a large catalytic pocket and has been a challenge to inhibit in vivo (Ghosh et al. 2008, Maillard et al. 2007). Moreover, BACE-1 is an evolutionarily conserved enzyme and the combined deletion of BACE-1 and its homologue BACE-2 increases neonatal lethality (Dominguez et al. 2005, Venugopal et al. 2008).
More recent studies have identified critical substrates for BACE-1 including neuregulin, LRP1, Klotho, sialyl transferase and voltage-regulated sodium channels (Li et al. 2010, Wong et al. 2005). In addition, a large number of substrates were identified by proteomic analysis recently (Hemming et al. 2009). These studies suggest that total inhibition of BACE1 in vivo may lead to unwanted reduction in the processing/turnover of a number of proteins that may lead to their toxic accumulation.
Although the nonspecific effects such as the impairment of other targets of BACE1 and γ-secretase may be considered to be critical for drug development, it is important to note that the partial inhibition of this type of processing may be well tolerated. For instance BACE1 heterozygous knockout mice, which express only 50% of the wild type activity are healthy (Roberds et al. 2001). Indeed, initial reports suggested that even 100% knockout in homozygous mice was tolerated: although, this finding was challenged in subsequent studies (Willem et al. 2006). Given that one can freely titrate the inhibitor dose, one may need to only partially inhibit APP processing to reduce Aβ below a critical threshold. A benefit of inhibiting BACE1 is that, in addition to Aβ, levels of CTFβ will also be inhibited. Several studies have suggested that CTFβ is also very toxic and will therefore be considered a second target (Neve et al. 1990).
A major caveat in this analysis is that BACE1 is an enzyme conserved from Dictyostelium, C. elegans and Drosophila to man, complicating the idea that it is an enzyme designed to constitutively generate large amounts of a toxic peptide. One would imagine that natural selection would eliminate this toxic property by mutating either the substrate or the enzyme in a variety of organisms. However, Aβ and BACE1 target sequences on APP are remarkably conserved from reptiles to man (Table 2; excerpted from an NCBI Blast search of the NR database; http://blast.ncbi.nlm.nih.gov/Blast.cgi). Therefore, Aβ continuously generated and bound to other secreted proteins in vivo may either have a conserved function, or at least be a harmless secreted waste product. The cause of neurodegeneration may then be either the unnatural accumulated forms of the peptide, loss of some binding partner, or alternative metabolites whose homeostasis fails along with APP.
Table 2.
Sequence surrounding the β- and α-secretase - cleavage sites are conserved
| −14 to −1 | β-site 1-16 | α-site 17-28 | 29–40 | |
|---|---|---|---|---|
| LTNIKTEEISEVKM | DAEFRHDSGYEVHHQK | LVFFAEDVGSNK | GAIIGLMVGGVV | Homo1 |
| LTNVKTEEVSEVKM | DAEFRHDSGYEVHHQK | LVFFAEDVGSNK | GAIIGLMVGGVV | Gallus2 |
| LTNVKTEEISEVKM | DAEFRHDSGYEVHHQK | LVFFAEDVGSNK | GAIIGLMVGGVV | Chelydra3 |
| LTNIKTEEISEVKM | DSEYRHDTAYEVHHQK | LVFFAEEVGSNK | GAIIGLMVGGVV | Xenopus4 |
| LTGIKTEEIAELKM | ETEFQQDSGYEVHHQK | LVFFPKDVGSNK | GAIIGLMVGGVV | Narke5 |
| VTGKSMEAVPELRM | ETEDRQSTEYEVHHQK | LVFFAEDVGSNK | GAIIGLMVGGVV | Tetraodon6 |
Human,
Chicken,
Turtle,
Frog,
Electric ray,
Pufferfish
Alpha Secretase
The absolute identity of α-secretase remains unclear, although most evidence points to redundant processing by multiple members of the adamalysin family (ADAM-9, 10, 12, 17, 19). This pathway was referred to as the “good pathway” as it was technically anti-amyloidogenic and Aβ is bisected into two pieces after residue 16. Nevertheless, it is important to note that inhibition of this activity does not increase Aβ (Gandhi et al. 2004). However, inducible forms of α-secretase were detected early during the characterization of APP processing pathways (Caporaso et al. 1992). These studies found that stimulation of inducible α-secretase cleavage reduced the yield of Aβ, suggesting that agents such as muscarinic receptor agonists or protein kinase C activators may be used to constitutively increase sAPPα at the expense of sAPPβ and Aβ (Nitsch et al. 2000, Nitsch et al. 1992). These drugs are currently being tested in clinical trials (www.Alz.org). Still, as part of a powerful signaling cascade that may affect various critical cellular pathways, this approach may be challenging to consistently implement. Multiple membrane proteins are processed by α-secretase like pathways, including most of the targets described listed in the β-secretase section. The pathway includes key developmental genes such as Notch and appears to be exquisitely regulated. While stimulating this process, it may be critical not to disturb the carefully regulated network of α-secretase substrates. Finally, although Aβ is traditionally treated as the toxic protein, truncated Aβ (17-40/42) generated after α- and γ-secretase cleavages are also reported to be toxic by forming calcium channels (Jang et al. 2010).
γ-secretase
Intramembrane proteolysis of several type-I integral membrane glycoproteins is mediated by γ-secretase, a multi-subunit protease complex containing several integral membrane proteins. Four essential subunits of this enzyme have been identified: Presenilin (PS1/PS2); anterior pharynx (APH-1); presenilin enhancer (PEN-2); nicastrin (NCT). Increasing the concentration of the four subunits in animal cells increases γ-secretase activity but increasing PS1 or PS2 alone does not (Marlow et al. 2003, Edbauer et al. 2003). Reconstitution of the four subunits in yeast, which normally lacks γ-secretase, generates the active enzyme, suggesting that these subunits are sufficient for activity, although other regulatory subunits may be present (Edbauer et al. 2003). The most well known substrate of γ-secretase is APP, which generates the secreted Aβ40 and 42 peptides from CTFβ as described earlier. The literature suggests that this essential enzyme gives rise to two toxic peptides, Aβ and CTFγ/AICD (Ghosal et al. 2009, Pimplikar 2009). An important recent alternative theory is that accumulation of CTFγ/AICD causes neuronal dysfunction and MAPT hyperphosphorylation (Ghosal et al. 2009, Pimplikar 2009). However, it is important to note that the APP intracellular domain fragment expressed is actually 59 residues long rather than the normally found 50-residue fragment.
Presenilin: Gain or loss of function
The major catalytic subunit of γ-secretase is PS1/PS2, which bears multiple mutations that cause FAD (Sambamurti et al. 2006, Sambamurti et al. 2002a). For a number of years, the argument has been that these mutations cause a novel gain of function to increase the ratio of Aβ42/Aβ40. However, a number of groups questioned the gain of function theory based on the hypofunction of mutant PS1 in C. elegans (Levitan et al. 1996). Some suggested that PS1 has alternative activities that may be compromised in AD such as a critical role in endocytosis (Tamboli et al. 2008, Ikeuchi et al. 2003).
Our studies using antisense RNA to inhibit PS1 expression showed that when PS1 levels got reduced to a level before detection, Aβ42 actually increases without significantly changing Aβ40 (Refolo et al. 1999). In contrast, when γ-secretase activity was increased by coexpressing all four subunits of the enzyme, Aβ42/Aβ40 ratios were reduced, an effect that is opposite of that seen by transfection of FAD mutation PS1 (Marlow et al. 2003).
All these studies indicate that FAD mutations lead to loss of function. Moreover, the loss of function appears to affect γ-secretase processing rather than an alternative function of PS1 or PS2.
Mechanism of γ-secretase cleavage
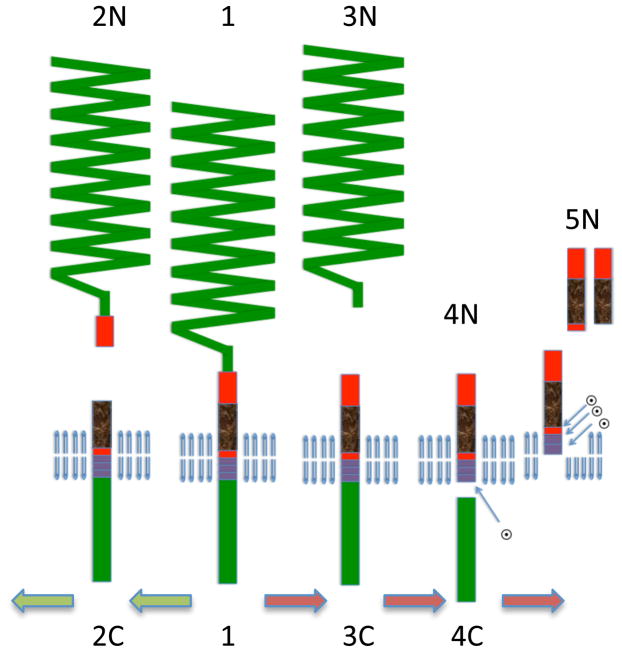
Several groups showed that γ-secretase cleavage proceeds sequentially (Fig 2) by first cleaving at the membrane cytoplasmic junction of APP to generate Aβ of 49 residues (Xu 2009, Takami et al. 2009). This fragment is rapidly cleaved to Aβ46 by the same enzyme (Zhao et al. 2004). Interestingly, these cleavages are inhibited by transition state inhibitors such as LY-685,458 but N-[N-(3,5-difluorophenacetyl)-L-alanyl]-(S)-phenylglycine methyl ester; DAPT, allow the first two cleavages to proceed resulting in the accumulation of Aβ46, which is subsequently sequentially processed to Aβ40 and Aβ42 (Zhao et al. 2010).
Figure 2. Model for γ-secretase processing of APP.
The final step of intramembrane processing is mediated by γ-secretase, which is a multisubunit integral membrane protein. Cleavage of membrane protein is enigmatic due to the lack of water within the membrane for hydrolysis. The proposed model takes advantage of the knowledge that γ-secretase is a multicatalytic enzyme that cleaves CTFα/β multiple times within the membrane and these cleavages are differentially sensitive to two different inhibitors: LY-685,458 and DAPT. The hypothesis suggests that after cleaving at the membrane cytoplasmic boundary, the transmembrane domain is still bound to the first LY-685,458 – sensitive active site of the enzyme and is subsequently free floating within the membrane. At this stage, it is susceptible to cleavage at the membrane - medium interface where it is cleaved by the second DAPT-sensitive active site. Thus, LY-685,458 blocks all cleavages whereas DAPT only blocks the cleavage from Aβ46 to Aβ43 and beyond. Each of these cuts are slightly ragged, but normally follow a pattern of cleaving three residues from the first cleavage.
This finding fits with our hypothesis that CTFα/β is cleaved close to the membrane cytoplasmic junction first, where it can access the water from the cytoplasm for hydrolysis (Sambamurti et al. 2006). The remaining transmembrane domain floats freely within the membrane to be cleaved at the membrane/lumen junction. Unlike the original cut-expose-cut model (Murphy et al. 2002), the protein will not require extraction from the membrane for cleavage.
An early finding is that inhibition of γ-secretase results in substrate accumulation in the membrane and yields more cleavage products upon incubation of these membranes (McLendon et al. 2000). In addition, our unpublished results (Venugopal and Sambamurti) show that CTFα/β turnover is extremely slow upon inhibition of γ-secretase, suggesting that the cell lacks efficient alternative pathways for its disposal. Further, initial long exposure followed by removal of γ-secretase from the medium allows the accumulated CTFα/β to turnover suggesting that the pathway may generate Aβ from accumulated substrate (Sharma, Barnwell and Sambamurti, in preparation). This may explain the large increase in Aβ reported in later time points after γ-secretase inhibition in human clinical trials (Siemers et al. 2007). Indeed this type of generation of Aβ in bursts may facilitate seeding of the Aβ oligomers and fibrils providing yet another Aβ-related toxic mechanism by partial or intermittent γ-secretase inhibition.
Several studies have suggested that loss of γ-secretase activity increases the frequency of cancer through unknown mechanisms (Das et al. 2004, Roperch et al. 1998). Recent studies suggest that mutations that dramatically lower γ-secretase activity may be linked to an unusually aggressive form of Acne inversa (Wang et al. 2010).
Thus, even if AD is triggered by accumulation of toxic Aβ oligomers, γ-secretase inhibition may be a poor option to pursue for disease treatment given that it may actually facilitate the formation of Aβ aggregates and given its large number of essential substrates.
Mouse models
Despite the failure of transgenic mice to truly model the pathology of AD with progressive synaptic loss followed by neurodegeneration of cholinergic and hippocampal neurons, the partial pathology showing amyloid deposition as plaques and behavior deficits has made it a useful model for at least partly understanding the role of Aβ and PS1/PS2 mutations in neuronal dysfunction (Games et al. 1995, Cheng et al. 2007).
Limitations of the current animal models to study AD
A major argument against the amyloid hypothesis has been that senile plaques develop in transgenic mice without concomitant neurofibrillary tangle formation or neurodegeneration. However, one needs to appreciate that the mice express mutant forms of APP and PS1 that are linked to FAD, and therefore failure to find appropriate neurodegeneration in these mice simply indicates that the model is unable to develop AD pathology and cannot prove or disprove any hypothesis. Part of the problem with animal models is that evolution retains the physiologically critical elements across various phyla, but pathology does not have to be conserved unless mandated by limitations of the shared design.
In addition to mutations that modulate APP processing to influence Aβ42 yield, a simple duplication of the APP gene resulting in a 1.5-fold increase in its copy number has been a reported to be a cause of FAD (Rovelet-Lecrux et al. 2007). This also explains the nearly inevitable AD pathology in older Down’s syndrome patients. It is important to note a caveat at this stage: although APP transgenic mice expressing high levels of amyloid fail to show the characteristic loss of cholinergic neurons, trisomic mice that model Down’s syndrome do show this loss (Lockrow et al. 2009, Salehi et al. 2006). Furthermore, the loss of cholinergic neurons can be prevented if one of the copies of APP is knocked out, suggesting that factors other than APP in the mouse chromosome 16 (syntenic with 21 in humans) may facilitate the APP-mediated neurodegeneration. Interestingly, all these changes appear to be mediated by a simple 1.5-fold increase in APP and its CTFs without any obvious deposition of amyloid. This mouse model also shows an APP dose-dependent increase in degeneration of the locus coeruleus beta-adrenergic neurons (Heneka et al. 2002), which has recently been recognized an important pathway that is lost early in AD. Interestingly, cholinergic and adrenergic neurons appear to play a parallel role in memory and one may compensate for the other in young animal models (Wenk et al. 1987). This neuronal pathway may therefore be targeted like the cholinergic pathway for treatment of AD.
Certain caveats associated with the aforementioned animal models exist and should be emphasized. First, the mechanism of accelerated aging in these models are either unknown or associated with specific targeted genetic manipulations that may not fully recapitulate the processes that occur during physiological aging. APP regulation differs between human and the widely used animal models. For example, the APP5′-UTR, including the important CAGA box, differs significantly between plaque-forming and non plaque-forming species, among 26 different species studied (Maloney et al. 2004). In addition, polymorphisms of the APP and APOE promoters implicated in AD etiology do not have corresponding homologous sequences in the mouse or rat gene promoters (Lahiri et al. 2005). In other words, sufficient evidence exists to indicate the folly of merely presuming that AD associated gene regulation at any level would be identical
An alternative model suggesting that γ-secretase inhibition causes AD
We have previously proposed that failure of γ-secretase is the cause of AD in families carrying FAD mutations (Sambamurti et al 2006). We will discuss some of the aspects of the hypothesis below.
Partial γ-secretase inhibition by FAD mutations
An important part of γ-secretase processing of APP is that several inhibitors act quite anomalously showing increases in Aβ42 at low doses. Indeed, it was initially proposed that different enzyme generate Aβ42 and Aβ40 (Figueiredo-Pereira et al. 1999). However, this was ruled out as subsequent studies found that several inhibitors actually increase Aβ42 at low doses (Durkin et al. 1999). This phenomenon was confirmed when we determined that PS1 antisense RNA reduces PS1 and increases Aβ42 while slightly and non-significantly reducing Aβ40 (Refolo et al. 1999), and others found that knocking PS1 out results in a complete loss of γ-secretase cleavage and Aβ40 as well as Aβ42 (De Strooper et al. 1998). Finally, we found that a combination of limiting subunits – Aph1, Pen2 and nicastrin – resulted in increased γ-secretase processing but also reduced AB42/Aβ40 ratios (Marlow et al. 2003).
As described earlier, recent studies have found that γ-secretase actually includes two independent activities that are inhibited by different compounds. The same enzyme however sequentially cleaves CTFβ in increments of three until it reaches Aβ40/Aβ42 as repeatedly demonstrated by multiple groups (Takami et al. 2009, Xu 2009). Sequential cleavage of Aβ by γ-secretase adds further support to the partial γ-secretase inhibition hypothesis and supports a second new hypothesis regarding the mechanism of intramembrane proteolysis (Fig 2). Briefly, it takes four cleavage steps from 49-46-43-40 to Aβ40 but only three steps to Aβ42 (48/49-45/46-42). These cleavages yield products that are predominantly trapped within the membrane/γ-secretase cleft and are in constant equilibrium with the lumen/medium. When the peptide gets released into the medium it is diluted and therefore no longer part of the cleavage reaction. Therefore, slow or impaired second site cleavage will result in an increase in Aβ42/43, which will be released before it gets cleaved further to Aβ40.
One study of a large number of FAD mutants showed that some mutations reduce CTFγ but others do not have such dramatic effects (Walker et al. 2005). These findings can be readily explained by the presence of two different catalytic activities in γ-secretase that may be independently affected by the FAD mutations, as discussed earlier.
Thus the idea of using γ-secretase as a treatment target may be ideal, if one proposes that it is the enzyme that generates two toxic peptides However, if we were to work from the perspective that it is an essential enzyme with numerous substrates ranging from Notch and Cadherin to Klotho and neuregulin, we will need to be cautious about using inhibitors to treat the disease (Sambamurti et al. 2006). Even if one finds an inhibitor that does not cleave Notch, it may still inhibit the processing of other essential substrates. The failure of treatment may readily find scapegoats in targets other than Notch and the importance of the enzyme in preventing AD may be lost.
We have proposed that since large amounts of APP are synthesized and turned over by this pathway continuously, and the protein is preferentially trafficked into axons and dendrites, its metabolites may simply accumulate in nerve endings and prevent the recovery and reuse of synaptic membranes. This should in turn result in traffic jam of membrane vesicles with reduced recycling capabilities. The failure to recycle membranes may then lead to the synaptic dysfunction observed in AD even before loss of cells. Axonal transport failure may then lead to MAPT accumulation in the cell body where it is mislocalized and deposited as NFTs (Sambamurti et al. 2006).
Other hypotheses pertaining to γ-secretase dysfunction
The basis of our theory that γ-secretase impairment causes AD is that direct antisense RNA-mediated inhibition of PS1 synthesis led to an increase in the Aβ42/40 ratio (Refolo et al. 1999) and drop in the Aβ42/Aβ40 ratio upon reconstitution of γ-secretase activity by coexpression of all four subunits (Marlow et al. 2003). The notion was supported by findings that FAD mutant PS1 was unable to complement sel12 mutations in C. elegans (Levitan et al. 1996).
Several groups suggested that PS1 had functions independent of γ-secretase such as intracellular adhesion and capacitative calcium entry that may explain its hypofunction while simultaneously explaining a gain of function with respect to γ-secretase activity (Saura et al. 2004, Yoo et al. 2000, Singh et al. 2001). Thus a theory was promulgated that presenilin loss of function causes neurodegeneration, but this dysfunction may be due to multiple functions of the protein (Shen & Kelleher 2007). The discussion was supported by findings that loss of PS1 or nicastrin, two γ-secretase subunits, leads to neuronal loss and behavior deficits in mice (Saura et al. 2005, Tabuchi et al. 2009, Lambert et al. 2001). This proposed hypothesis includes discussion suggesting that γ-secretase hypofunction may also be a basis for AD but that additional loss of alternative function may also play a role. The key points in this hypothesis are also highlighted by other studies showing that FAD mutations affect cadherin processing by γ-secretase and affect the yield of the critical memory-related factor – CREB (Marambaud et al. 2003).
Reliance on behavior or neurodegeneration as endpoints of genetic manipulation in AD mouse models is problematic, as they may be broadly affected by a number of factors such as loss of one or more essential functions of presenilin, altered blood supply, neurotransmitter imbalance, inflammation, etc. Thus, a lot of nonspecific pathologies may lead to behavior deficits, which may not represent a result of logical development of AD-specific pathology. Behavior improvement in mice has been repeatedly demonstrated with γ-secretase inhibitors and other Aβ42-centric treatments, without their successful translation to humans (Spilman et al. 2008, Comery et al. 2005, Lambert et al. 2001). Nevertheless, these findings indicate that the behavioral impairment in mice that are engineered to express high levels of Aβ42 is due primarily to Aβ-dependent pathology.
Recent studies have identified mutations and variants in γ-secretase subunits that may provide some insight into the role of this enzyme in AD (Wang et al. 2010, Coolen et al. 2006, Coolen et al. 2005). The Coolen et al. study is noteworthy and suggests that γ-secretase loss leads to specific neuronal deficits as Aph-1b loss leads to specific apomorphine (dopamine agonist) hypersensitivity.
The role of reduction in γ-secretase activity in AD has been questioned as the γ-secretase hypofunction mutations (frameshifts, nonsense) observed on PS-1, PEN-2 and NCT were not associated with an increase in incidence of AD (although further studies are needed to exclude disease association) and acne inversa was not observed in AD (Wang et al. 2010, Kelleher & Shen 2010).
How can we reconcile these findings with our model of membrane protein homeostasis and PS1 loss of function? It is critical to note that few of these acne inversa patients are very old and hence, more studies are needed to confirm any association and the study did not report any activity measurements (Wang et al. 2010). Loss of function mutations in the heterozygous state do not normally induce a large loss of activity in multi-subunit enzymes of this type as the defective proteins are not incorporated into the complex, but only affect the rate of assembly. In contrast, missense mutant proteins do incorporate into the complexes and generate defective enzymes. Extreme loss of γ-secretase is incompatible with life leading to embryonic lethality and is therefore unlikely to be encountered in adults. Moreover, the reported mutations are likely to produce significant levels of truncated proteins that may inappropriately interact with substrates or other proteins to produce dysfunctional protein complexes. Acne inversa or hidradenitis suppurativa occurs in skin creases due to blockage of sweat glands and inflammation, likely caused by accumulation such defective protein complexes. Alternatively, mutations may increase susceptibility to acne inversa by developmental failure of sweat glands and sebaceous glands within hair follicles. Further, detailed analysis is definitely needed to understand the biochemical basis of the observed acne inversa phenotype and its relationship to AD.
Secondly, because multiple forms of γ-secretase manifest due to the existence of PS1, PS2 and multiple variants of its Aph-1 subunits, and have different substrates in different tissues, the behavior of these mutations can vary considerably with organ systems, a factor that also needs additional investigation. Heterozygous knockout PS1, PS2 and other γ-secrtase subunit mice are indeed viable. Recent studies indicate that even a complete loss of NCT allows generation of active γ-secretases (Zhao et al. 2010).
Third, our hypothesis is not dependent on developmental losses that arise due to loss of γ-secretase function, but a malfunction that occurs in older people who still need an active enzyme to maintain membrane function and homeostasis in the brain. APP, in our opinion, is a key player because it is highly expressed in the synapse and has a tendency to strongly interact to form membrane-associated complexes with cytoplasmic and extracellular factors that cannot be easily cleared. We propose that along with APP, other proteins such as lipoprotein receptors also fail, overwhelming the alternative maintenance mechanisms that preserve synaptic integrity. Intermittent failure of γ-secretase due to physiological or environmental factors that overload the enzyme could actually foster amyloid deposition, as the substrate, CTFβ, will accumulate in the absence of its rapid turnover, and yield bursts of Aβ that are concentrated in membranes, to promote seeding and aggregation. In particular, the long axons need to be supplied by an active microtubule system with a local management of membranes at the presynaptic terminals that will be stressed by such a failure resulting in a large biochemical requirement for synaptic membrane replenishment. This in turn will lead to disruption of the microtubule-MAPT structure in axons. The difference in AD susceptibility of different species may simply be a reflection of the large expansion of the human brain leading to reduced adaptability to such extreme biochemical requirements. Several factors that may be explained by this hypothesis include MAPT failure, the association with the ApoE ε4 allele and the loss of brain cholesterol associated with AD (Sambamurti et al. 2006).
Predictions of the γ-secretase inhibition model for AD
Some of the original predictions of this model were as follows:
Membranes from dystrophic neurons should show an accumulation of APP CTFs
We should see accumulation of other γ-secretase substrates in the degenerating brain.
FAD mutations should disrupt or inhibit other functions of γ-secretase substrates such as cholesterol homeostasis.
Chronic sublethal inhibition of γ-secretase should cause neurodegeneration in appropriate models systems to augment AD dementia.
The failure of the recent Ely Lily clinical trial provides validation for this hypothesis by the conducting the experiment and proving proof for prediction number 4. Although evidence does exist for the other prediction, we need more studies to provide direct support.
Several factors such as high cholesterol diets are believed to cause AD and a number of studies support the idea that cholesterol increases amyloid deposition (Pappolla et al. 2002, Refolo et al. 2001, Refolo et al. 2000, Sambamurti et al. 2004). We have found that isoprenoids that are synthesized in the cholesterol biosynthesis pathway are necessary for γ-secretase assembly (Zhou et al. 2008). High cholesterol is known to inhibit the synthesis of mevalonic acid, which is the precursor of isoprenoids and may therefore impair γ-secretase assembly. These predictions need to be tested in animal models and in human clinical samples. The major brain cholesterol transporter is ApoE and may play a role in this regulation. Moreover, the LDL and LRP receptors that are known to transport ApoE are also γ-secretase substrates that may create imbalances in the enzyme-substrate ratios.
Conclusion
AD is a complex neurodegenerative disease that takes a number of years to develop. Current animal models are limited, as they do not truly follow the complex neuropathology and neurodegeneration observed in subjects with AD. Indeed, a number of people with AD neuropathology are cognitively normal raising the possibility that the lesions do not necessarily lead to disease in everyone. The transgenic mice may be better models of this type of pathological aging. The amyloid hypothesis is complicated by the identification of multiple toxic forms of Aβ and multiple toxic domains on APP (Fig 3). Furthermore, multiple toxic proteins found in other neurodegenerative diseases also accumulate in AD and may contribute to the neurodegeneration in the disease (Fig 4). The major support for the amyloid hypothesis comes from the increased production of Aβ42 by FAD mutations and toxicity of oligomeric aggregates. Our analysis in this article indicates why this may not be a gain of pathological function, but may actually represent inhibition of γ-secretase activity and therefore a loss of function series of mutations. While we are not contradicting the amyloid hypothesis, we propose a modified version of the amyloid hypothesis that takes a broader view of misprocessed proteins and suggests that neurotoxicity may involve a host of γ-secretase substrates beyond amyloid. We propose that loss of γ-secretase activity leads to accumulation of several membrane protein fragments to different degrees, depending on the extent of α- and β-secretase like processing of these substrates. Individually this increase may be small and undetectable except for highly expressed and processed proteins like APP, but the combined accumulation of membrane-bound stubs will likely make the membrane unavailable for recovery and recycle, and ultimately lead to synaptic dysfunction. In short, the pathological pathway in AD is complex, and like cancer, we may see the unraveling of multiple mechanisms of degeneration with benefits that will extend to multiple diseases in the process. However, the cautionary note may be that we may need to expand the basic research in the area enormously to tackle the complex problem and limit translational studies to those that can be well validated.
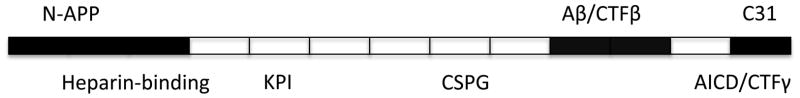
Figure 3. Multiple toxic domains and metabolites of APP have been defined.
Multiple cleavage sites have been defined on APP and these appear to generate a number of toxic proteins such as n-APP, Aβ and C31 shown above the APP molecule in the schematic diagram (not to scale). Even the major cleavage products such as CTFγ/AICD have been defined as toxic in animal models. These potentially toxic fragments demonstrate that the toxicity of APP may arise due to improper turnover and cleavage rather than due to the normal turnover pathways and argue against disrupting normal turnover mechanisms. APP is an important multifunctional protein with the key known functional domains shown below the molecule.
Figure 4. Faulty protein turnover can lead to toxic accumulation of a number of proteins.
A number of proteins are known to accumulate in senile plaques and neurofibrillary tangles in subpopulations of patients with AD. Several of these proteins are known to accumulate in other degenerative diseases and have mutations linked to the disease. Some of the accumulated proteins become toxic after microglial processing whereas others are degraded to inactive products by microglia as shown for plaque amyloid. None of these proteins are neurotoxic in the young or even most of the health aging populations. Thus, rather than focusing on toxic products one may need to find ways to preserve the proteolytic clearance pathways that maintain secreted, membrane and cytoplasmic protein homeostasis.
Acknowledgments
The authors thank the funding from NIH intramural and extramural (AG023055, AG022103, AG18379, and AG18884) and Alzheimer’s Association IIRG-10-173180. The authors thank Dr. Jennifer Schnellmann of the MUSC library science and informatics center for reading and editing the document.
Dedication: The authors thank the former reviews editor, Dr. Mark Smith for inviting this review as one of his last actions prior to his untimely demise. The scientific community will miss him for his great sense of humor and challenging ideas.
Footnotes
This research does not represent a conflict of interest for the authors.
References
- Anderson JP, Esch FS, Keim PS, Sambamurti K, Lieberburg I, Robakis NK. Exact cleavage site of Alzheimer amyloid precursor in neuronal PC-12 cells. Neurosci Lett. 1991;128:126–128. doi: 10.1016/0304-3940(91)90775-o. [DOI] [PubMed] [Google Scholar]
- Bales KR, Verina T, Cummins DJ, et al. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancher C, Lassmann H, Budka H, Grundke-Iqbal I, Iqbal K, Wiche G, Seitelberger F, Wisniewski HM. Neurofibrillary tangles in Alzheimer’s disease and progressive supranuclear palsy: antigenic similarities and differences. Microtubule-associated protein tau antigenicity is prominent in all types of tangles. Acta Neuropathol. 1987;74:39–46. doi: 10.1007/BF00688336. [DOI] [PubMed] [Google Scholar]
- Bard F, Barbour R, Cannon C, et al. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudier J, Cole RD. Phosphorylation of tau proteins to a state like that in Alzheimer’s brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem. 1987;262:17577–17583. [PubMed] [Google Scholar]
- Bhat NR. Linking cardiometabolic disorders to sporadic Alzheimer’s disease: a perspective on potential mechanisms and mediators. J Neurochem. 2010;115:551–562. doi: 10.1111/j.1471-4159.2010.06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Brion JP, Flament-Durand J, Dustin P. Alzheimer’s disease and tau proteins. Lancet. 1986;2:1098. doi: 10.1016/s0140-6736(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Cai XD, Golde TE, Younkin SG. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993;259:514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Caporaso GL, Gandy SE, Buxbaum JD, Ramabhadran TV, Greengard P. Protein phosphorylation regulates secretion of Alzheimer beta/A4 amyloid precursor protein. Proc Natl Acad Sci U S A. 1992;89:3055–3059. doi: 10.1073/pnas.89.7.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroli A, Frisoni GB. The dynamics of Alzheimer’s disease biomarkers in the Alzheimer’s Disease Neuroimaging Initiative cohort. Neurobiol Aging. 2010;31:1263–1274. doi: 10.1016/j.neurobiolaging.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesus G, Smith MA, Basu S, Hua J, Capobianco DE, Siedlak SL, Zhu X, Perry G. Increased isoprostane and prostaglandin are prominent in neurons in Alzheimer disease. Mol Neurodegener. 2007;2:2. doi: 10.1186/1750-1326-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng IH, Scearce-Levie K, Legleiter J, et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282:23818–23828. doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]
- Chromy BA, Nowak RJ, Lambert MP, et al. Self-assembly of Abeta(1-42) into globular neurotoxins. Biochemistry. 2003;42:12749–12760. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Comery TA, Martone RL, Aschmies S, et al. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2005;25:8898–8902. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen MW, van Loo KM, van Bakel NN, Ellenbroek BA, Cools AR, Martens GJ. Reduced Aph-1b expression causes tissue- and substrate-specific changes in gamma-secretase activity in rats with a complex phenotype. FASEB J. 2006;20:175–177. doi: 10.1096/fj.05-4337fje. [DOI] [PubMed] [Google Scholar]
- Coolen MW, Van Loo KM, Van Bakel NN, Pulford DJ, Serneels L, De Strooper B, Ellenbroek BA, Cools AR, Martens GJ. Gene dosage effect on gamma-secretase component Aph-1b in a rat model for neurodevelopmental disorders. Neuron. 2005;45:497–503. doi: 10.1016/j.neuron.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Das I, Craig C, Funahashi Y, et al. Notch oncoproteins depend on gamma-secretase/presenilin activity for processing and function. J Biol Chem. 2004;279:30771–30780. doi: 10.1074/jbc.M309252200. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Delacourte A, Defossez A. Alzheimer’s disease: Tau proteins, the promoting factors of microtubule assembly, are major components of paired helical filaments. J Neurol Sci. 1986;76:173–186. doi: 10.1016/0022-510x(86)90167-x. [DOI] [PubMed] [Google Scholar]
- Dominguez D, Tournoy J, Hartmann D, et al. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem. 2005;280:30797–30806. doi: 10.1074/jbc.M505249200. [DOI] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, et al. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Durkin JT, Murthy S, Husten EJ, Trusko SP, Savage MJ, Rotella DP, Greenberg BD, Siman R. Rank-order of potencies for inhibition of the secretion of abeta40 and abeta42 suggests that both are generated by a single gamma-secretase. J Biol Chem. 1999;274:20499–20504. doi: 10.1074/jbc.274.29.20499. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Extance A. Alzheimer’s failure raises questions about disease-modifying strategies. Nat Rev Drug Discov. 2010;9:749–751. doi: 10.1038/nrd3288. [DOI] [PubMed] [Google Scholar]
- Fa M, Orozco IJ, Francis YI, Saeed F, Gong Y, Arancio O. Preparation of oligomeric beta-amyloid 1-42 and induction of synaptic plasticity impairment on hippocampal slices. J Vis Exp. 2010 doi: 10.3791/1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo-Pereira ME, Efthimiopoulos S, Tezapsidis N, Buku A, Ghiso J, Mehta P, Robakis NK. Distinct secretases, a cysteine protease and a serine protease, generate the C termini of amyloid beta-proteins Abeta1-40 and Abeta1-42, respectively. J Neurochem. 1999;72:1417–1422. doi: 10.1046/j.1471-4159.1999.721417.x. [DOI] [PubMed] [Google Scholar]
- Freir DB, Fedriani R, Scully D, Smith IM, Selkoe DJ, Walsh DM, Regan CM. Abeta oligomers inhibit synapse remodelling necessary for memory consolidation. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelle A, Portet F, Berr C, Touchon J. Neurodegenerative dementia and parkinsonism. J Nutr Health Aging. 2010;14:37–44. doi: 10.1007/s12603-010-0007-z. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Refolo LM, Sambamurti K. Amyloid precursor protein compartmentalization restricts beta-amyloid production: therapeutic targets based on BACE compartmentalization. J Mol Neurosci. 2004;24:137–143. doi: 10.1385/JMN:24:1:137. [DOI] [PubMed] [Google Scholar]
- Ghosal K, Vogt DL, Liang M, Shen Y, Lamb BT, Pimplikar SW. Alzheimer’s disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc Natl Acad Sci U S A. 2009;106:18367–18372. doi: 10.1073/pnas.0907652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Gemma S, Tang J. beta-Secretase as a therapeutic target for Alzheimer’s disease. Neurotherapeutics. 2008;5:399–408. doi: 10.1016/j.nurt.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283:29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984a;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984b;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW, Quaranta V, Eanes ED. The amyloid deposits in Alzheimer’s disease: their nature and pathogenesis. Appl Pathol. 1984;2:357–369. [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science. 1987a;235:877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Goldgaber D, Lerman MI, McBride WO, Saffiotti U, Gajdusek DC. Isolation, characterization, and chromosomal localization of human brain cDNA clones coding for the precursor of the amyloid of brain in Alzheimer’s disease, Down’s syndrome and aging. J Neural Transm Suppl. 1987b;24:23–28. [PubMed] [Google Scholar]
- Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986a;261:6084–6089. [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986b;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Misonou H, Sato T, Dohmae N, Takio K, Ihara Y. Distinct intramembrane cleavage of the beta-amyloid precursor protein family resembling gamma-secretase-like cleavage of Notch. J Biol Chem. 2001;276:35235–35238. doi: 10.1074/jbc.C100357200. [DOI] [PubMed] [Google Scholar]
- Haass C, Hung AY, Schlossmacher MG, Teplow DB, Selkoe DJ. beta- Amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J Biol Chem. 1993;268:3021–3024. [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993;75:1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- He XY, Merz G, Yang YZ, Pullakart R, Mehta P, Schulz H, Yang SY. Function of human brain short chain L-3-hydroxyacyl coenzyme A dehydrogenase in androgen metabolism. Biochim Biophys Acta. 2000;1484:267–277. doi: 10.1016/s1388-1981(00)00014-7. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hemming ML, Elias JE, Gygi SP, Selkoe DJ. Identification of beta-secretase (BACE1) substrates using quantitative proteomics. PLoS One. 2009;4:e8477. doi: 10.1371/journal.pone.0008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O’Banion MK, Weinberg G, Klockgether T, Feinstein DL. Noradrenergic depletion potentiates beta -amyloid-induced cortical inflammation: implications for Alzheimer’s disease. J Neurosci. 2002;22:2434–2442. doi: 10.1523/JNEUROSCI.22-07-02434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I, Powell D, Howlett DR, et al. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- Ihara Y, Nukina N, Miura R, Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. J Biochem. 1986;99:1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T, Dolios G, Kim SH, Wang R, Sisodia SS. Familial Alzheimer disease-linked presenilin 1 variants enhance production of both Abeta 1-40 and Abeta 1-42 peptides that are only partially sensitive to a potent aspartyl protease transition state inhibitor of “gamma-secretase”. J Biol Chem. 2003;278:7010–7018. doi: 10.1074/jbc.M209252200. [DOI] [PubMed] [Google Scholar]
- Imbimbo BP. Why did tarenflurbil fail in Alzheimer’s disease? J Alzheimers Dis. 2009;17:757–760. doi: 10.3233/JAD-2009-1092. [DOI] [PubMed] [Google Scholar]
- Jang H, Arce FT, Ramachandran S, Capone R, Azimova R, Kagan BL, Nussinov R, Lal R. Truncated beta-amyloid peptide channels provide an alternative mechanism for Alzheimer’s Disease and Down syndrome. Proc Natl Acad Sci U S A. 2010;107:6538–6543. doi: 10.1073/pnas.0914251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev S, Case A, Eastman AJ, Nguyen H, Pollak J, Wiley JC, Moller T, Morrison RS, Garden GA. Presenilin 2 is the predominant gamma-secretase in microglia and modulates cytokine release. PLoS One. 2010;5:e15743. doi: 10.1371/journal.pone.0015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Shen J. Genetics. Gamma-secretase and human disease. Science. 2010;330:1055–1056. doi: 10.1126/science.1198668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein WL. Abeta toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, et al. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28:4231–4237. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Ge YW, Maloney B, Wavrant-De Vrieze F, Hardy J. Characterization of two APP gene promoter polymorphisms that appear to influence risk of late-onset Alzheimer’s disease. Neurobiol Aging. 2005;26:1329–1341. doi: 10.1016/j.neurobiolaging.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Zawia NH, Greig NH, Sambamurti K, Maloney B. Early-life events may trigger biochemical pathways for Alzheimer’s disease: the “LEARn” model. Biogerontology. 2008;9:375–379. doi: 10.1007/s10522-008-9162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Stevens G, Sabo S, et al. Beta/A4-evoked degeneration of differentiated SH-SY5Y human neuroblastoma cells. J Neurosci Res. 1994;39:377–385. doi: 10.1002/jnr.490390404. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Viola KL, Chromy BA, et al. Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J Neurochem. 2001;79:595–605. doi: 10.1046/j.1471-4159.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- Lanz TA, Karmilowicz MJ, Wood KM, et al. Concentration-dependent modulation of amyloid-beta in vivo and in vitro using the gamma-secretase inhibitor, LY-450139. J Pharmacol Exp Ther. 2006;319:924–933. doi: 10.1124/jpet.106.110700. [DOI] [PubMed] [Google Scholar]
- Levitan D, Doyle TG, Brousseau D, Lee MK, Thinakaran G, Slunt HH, Sisodia SS, Greenwald I. Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1996;93:14940–14944. doi: 10.1073/pnas.93.25.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Bo H, Zhang XC, Hartsuck JA, Tang J. Predicting memapsin 2 (beta-secretase) hydrolytic activity. Protein Sci. 2010;19:2175–2185. doi: 10.1002/pro.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc Natl Acad Sci U S A. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockrow J, Prakasam A, Huang P, Bimonte-Nelson H, Sambamurti K, Granholm AC. Cholinergic degeneration and memory loss delayed by vitamin E in a Down syndrome mouse model. Exp Neurol. 2009;216:278–289. doi: 10.1016/j.expneurol.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard MC, Hom RK, Benson TE, et al. Design, synthesis, and crystal structure of hydroxyethyl secondary amine-based peptidomimetic inhibitors of human beta-secretase. J Med Chem. 2007;50:776–781. doi: 10.1021/jm061242y. [DOI] [PubMed] [Google Scholar]
- Maloney B, Ge YW, Greig N, Lahiri DK. Presence of a “CAGA box” in the APP gene unique to amyloid plaque-forming species and absent in all APLP-1/2 genes: implications in Alzheimer’s disease. FASEB J. 2004;18:1288–1290. doi: 10.1096/fj.03-1703fje. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Marlow L, Canet RM, Haugabook SJ, Hardy JA, Lahiri DK, Sambamurti K. APH1, PEN2, and Nicastrin increase Abeta levels and gamma-secretase activity. Biochem Biophys Res Commun. 2003;305:502–509. doi: 10.1016/s0006-291x(03)00797-6. [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PC, Gitter BD, Waters DC, Simmons LK, Becker GW, Small JS, Robison PM. beta-Amyloid peptide in vitro toxicity: lot-to-lot variability. Neurobiol Aging. 1992;13:605–607. doi: 10.1016/0197-4580(92)90064-5. [DOI] [PubMed] [Google Scholar]
- McLendon C, Xin T, Ziani-Cherif C, et al. Cell-free assays for gamma-secretase activity. FASEB J. 2000;14:2383–2386. doi: 10.1096/fj.00-0286fje. [DOI] [PubMed] [Google Scholar]
- Monning U, Konig G, Banati RB, Mechler H, Czech C, Gehrmann J, Schreiter-Gasser U, Masters CL, Beyreuther K. Alzheimer beta A4-amyloid protein precursor in immunocompetent cells. J Biol Chem. 1992;267:23950–23956. [PubMed] [Google Scholar]
- Montejo de Garcini E, Serrano L, Avila J. Self assembly of microtubule associated protein tau into filaments resembling those found in Alzheimer disease. Biochem Biophys Res Commun. 1986;141:790–796. doi: 10.1016/s0006-291x(86)80242-x. [DOI] [PubMed] [Google Scholar]
- Morawski M, Hartlage-Rubsamen M, Jager C, et al. Distinct glutaminyl cyclase expression in Edinger-Westphal nucleus, locus coeruleus and nucleus basalis Meynert contributes to pGlu-Abeta pathology in Alzheimer’s disease. Acta Neuropathol. 2010;120:195–207. doi: 10.1007/s00401-010-0685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Uljon SN, Golde TE, Wang R. FAD-linked mutations in presenilin 1 alter the length of Abeta peptides derived from betaAPP transmembrane domain mutants. Biochim Biophys Acta. 2002;1586:199–209. doi: 10.1016/s0925-4439(01)00098-9. [DOI] [PubMed] [Google Scholar]
- Neve RL, Dawes LR, Yankner BA, Benowitz LI, Rodriguez W, Higgins GA. Genetics and biology of the Alzheimer amyloid precursor. Prog Brain Res. 1990;86:257–267. doi: 10.1016/s0079-6123(08)63182-9. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Deng M, Tennis M, Schoenfeld D, Growdon JH. The selective muscarinic M1 agonist AF102B decreases levels of total Abeta in cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol. 2000;48:913–918. [PubMed] [Google Scholar]
- Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- Nukina N, Ihara Y. One of the antigenic determinants of paired helical filaments is related to tau protein. J Biochem. 1986;99:1541–1544. doi: 10.1093/oxfordjournals.jbchem.a135625. [DOI] [PubMed] [Google Scholar]
- Orendac M, Zeman J, Stabler SP, Allen RH, Kraus JP, Bodamer O, Stockler-Ipsiroglu S, Kvasnicka J, Kozich V. Homocystinuria due to cystathionine beta-synthase deficiency: novel biochemical findings and treatment efficacy. J Inherit Metab Dis. 2003;26:761–773. doi: 10.1023/B:BOLI.0000009963.88420.c2. [DOI] [PubMed] [Google Scholar]
- Origlia N, Capsoni S, Cattaneo A, Fang F, Arancio O, Yan SD, Domenici L. Abeta-dependent Inhibition of LTP in different intracortical circuits of the visual cortex: the role of RAGE. J Alzheimers Dis. 2009;17:59–68. doi: 10.3233/JAD-2009-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origlia N, Righi M, Capsoni S, et al. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. J Neurosci. 2008;28:3521–3530. doi: 10.1523/JNEUROSCI.0204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Imbimbo BP, et al. REVIEW: gamma-Secretase inhibitors for the treatment of Alzheimer’s disease: The current state. CNS Neurosci Ther. 2010;16:272–284. doi: 10.1111/j.1755-5949.2010.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappolla MA, Omar RA, Kim KS, Robakis NK. Immunohistochemical evidence of oxidative stress in Alzheimer's disease. Am J Pathol. 1992;140:621–628. [PMC free article] [PubMed] [Google Scholar]
- Pappolla MA, Chyan YJ, Omar RA, Hsiao K, Perry G, Smith MA, Bozner P. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer’s disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. Am J Pathol. 1998;152:871–877. [PMC free article] [PubMed] [Google Scholar]
- Pappolla MA, Smith MA, Bryant-Thomas T, Bazan N, Petanceska S, Perry G, Thal LJ, Sano M, Refolo LM. Cholesterol, oxidative stress, and Alzheimer’s disease: expanding the horizons of pathogenesis. Free Radic Biol Med. 2002;33:173–181. doi: 10.1016/s0891-5849(02)00841-9. [DOI] [PubMed] [Google Scholar]
- Parvathy S, Davies P, Haroutunian V, et al. Correlation between Abetax-40-, Abetax-42-, and Abetax-43-containing amyloid plaques and cognitive decline. Arch Neurol. 2001;58:2025–2032. doi: 10.1001/archneur.58.12.2025. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. Aggregation-related toxicity of synthetic beta-amyloid protein in hippocampal cultures. Eur J Pharmacol. 1991;207:367–368. doi: 10.1016/0922-4106(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. Int J Biochem Cell Biol. 2009;41:1261–1268. doi: 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnix I, Musunuru U, Tun H, Sridharan A, Golde T, Eckman C, Ziani-Cherif C, Onstead L, Sambamurti K. A novel gamma -secretase assay based on detection of the putative C-terminal fragment-gamma of amyloid beta protein precursor. J Biol Chem. 2001;276:481–487. doi: 10.1074/jbc.M005968200. [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Stephenson DT, Frosch MP, Lieberburg I, Clemens JA, Selkoe DJ. Synthetic amyloid beta-protein fails to produce specific neurotoxicity in monkey cerebral cortex. Neurobiol Aging. 1992;13:561–567. doi: 10.1016/0197-4580(92)90056-4. [DOI] [PubMed] [Google Scholar]
- Pollock NJ, Mirra SS, Binder LI, Hansen LA, Wood JG. Filamentous aggregates in Pick’s disease, progressive supranuclear palsy, and Alzheimer’s disease share antigenic determinants with microtubule-associated protein, tau. Lancet. 1986;2:1211. doi: 10.1016/s0140-6736(86)92212-9. [DOI] [PubMed] [Google Scholar]
- Portelius E, Dean RA, Gustavsson MK, Andreasson U, Zetterberg H, Siemers E, Blennow K. A novel Abeta isoform pattern in CSF reflects gamma-secretase inhibition in Alzheimer disease. Alzheimers Res Ther. 2010;2:7. doi: 10.1186/alzrt30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MN, Hofmeister JJ, Jungbauer L, et al. Cognitive effects of cell-derived and synthetically derived Abeta oligomers. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refolo LM, Eckman C, Prada CM, Yager D, Sambamurti K, Mehta N, Hardy J, Younkin SG. Antisense-induced reduction of presenilin 1 expression selectively increases the production of amyloid beta42 in transfected cells. J Neurochem. 1999;73:2383–2388. doi: 10.1046/j.1471-4159.1999.0732383.x. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Pappolla MA, LaFrancois J, et al. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987;84:4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis NK, Vassilacopoulou D, Efthimiopoulos S, Sambamurti K, Refolo LM, Shioi J. Cellular processing and proteoglycan nature of amyloid precursor proteins. Ann N Y Acad Sci. 1993;695:132–138. doi: 10.1111/j.1749-6632.1993.tb23041.x. [DOI] [PubMed] [Google Scholar]
- Roberds SL, Anderson J, Basi G, et al. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer’s disease therapeutics. Hum Mol Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- Roperch JP, Alvaro V, Prieur S, et al. Inhibition of presenilin 1 expression is promoted by p53 and p21WAF-1 and results in apoptosis and tumor suppression. Nat Med. 1998;4:835–838. doi: 10.1038/nm0798-835. [DOI] [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Frebourg T, Tuominen H, Majamaa K, Campion D, Remes AM. APP locus duplication in a Finnish family with dementia and intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 2007;78:1158–1159. doi: 10.1136/jnnp.2006.113514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A, Delcroix JD, Belichenko PV, et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Granholm AC, Kindy MS, Bhat NR, Greig NH, Lahiri DK, Mintzer JE. Cholesterol and Alzheimer’s disease: clinical and experimental models suggest interactions of different genetic, dietary and environmental risk factors. Curr Drug Targets. 2004;5:517–528. doi: 10.2174/1389450043345335. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Greig NH, Lahiri DK. Advances in the cellular and molecular biology of the beta-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2002a;1:1–31. doi: 10.1385/NMM:1:1:1. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Hardy J, Refolo LM, Lahiri DK. Targeting APP Metabolism for the Treatment of Alzheimer’s Disease. Drug Development Res. 2002b;56:211–227. [Google Scholar]
- Sambamurti K, Lahiri DK. ERAB contains a putative noncleavable signal peptide. Biochem Biophys Res Commun. 1998;249:546–549. doi: 10.1006/bbrc.1998.9178. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Refolo LM, Shioi J, Pappolla MA, Robakis NK. The Alzheimer’s amyloid precursor is cleaved intracellularly in the trans-Golgi network or in a post-Golgi compartment. Ann N Y Acad Sci. 1992a;674:118–128. doi: 10.1111/j.1749-6632.1992.tb27481.x. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Shioi J, Anderson JP, Pappolla MA, Robakis NK. Evidence for intracellular cleavage of the Alzheimer’s amyloid precursor in PC12 cells. J Neurosci Res. 1992b;33:319–329. doi: 10.1002/jnr.490330216. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Suram A, Venugopal C, Prakasam A, Zhou Y, Lahiri DK, Greig NH. A partial failure of membrane protein turnover may cause Alzheimer’s disease: a new hypothesis. Curr Alzheimer Res. 2006;3:81–90. doi: 10.2174/156720506775697142. [DOI] [PubMed] [Google Scholar]
- Sandbrink R, Masters CL, Beyreuther K. Beta A4-amyloid protein precursor mRNA isoforms without exon 15 are ubiquitously expressed in rat tissues including brain, but not in neurons. J Biol Chem. 1994;269:1510–1517. [PubMed] [Google Scholar]
- Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron MM, Teplow DB, Haass C. Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep. 2001;2:835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura CA, Chen G, Malkani S, et al. Conditional inactivation of presenilin 1 prevents amyloid accumulation and temporarily rescues contextual and spatial working memory impairments in amyloid precursor protein transgenic mice. J Neurosci. 2005;25:6755–6764. doi: 10.1523/JNEUROSCI.1247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shah P, Lal N, Leung E, Traul DE, Gonzalo-Ruiz A, Geula C. Neuronal and axonal loss are selectively linked to fibrillar amyloid-{beta} within plaques of the aged primate cerebral cortex. Am J Pathol. 2010;177:325–333. doi: 10.2353/ajpath.2010.090937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Leissring MA, Adame A, Sun X, Spooner E, Masliah E, Selkoe DJ, Lemere CA, Walsh DM. Biochemical and immunohistochemical analysis of an Alzheimer’s disease mouse model reveals the presence of multiple cerebral Abeta assembly forms throughout life. Neurobiol Dis. 2009;36:293–302. doi: 10.1016/j.nbd.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Siemers ER, Dean RA, Friedrich S, Ferguson-Sells L, Gonzales C, Farlow MR, May PC. Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-beta after inhibition of gamma-secretase. Clin Neuropharmacol. 2007;30:317–325. doi: 10.1097/WNF.0b013e31805b7660. [DOI] [PubMed] [Google Scholar]
- Singh N, Talalayeva Y, Tsiper M, et al. The role of Alzheimer’s disease-related presenilin 1 in intercellular adhesion. Exp Cell Res. 2001;263:1–13. doi: 10.1006/excr.2000.5098. [DOI] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PL, Siedlak SL, Tabaton M, Perry G. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem. 1998;70:2212–2215. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- Spilman P, Lessard P, Sattavat M, et al. A gamma-secretase inhibitor and quinacrine reduce prions and prevent dendritic degeneration in murine brains. Proc Natl Acad Sci U S A. 2008;105:10595–10600. doi: 10.1073/pnas.0803671105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Chen G, Sudhof TC, Shen J. Conditional forebrain inactivation of nicastrin causes progressive memory impairment and age-related neurodegeneration. J Neurosci. 2009;29:7290–7301. doi: 10.1523/JNEUROSCI.1320-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y. gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29:13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamboli IY, Prager K, Thal DR, et al. Loss of gamma-secretase function impairs endocytosis of lipoprotein particles and membrane cholesterol homeostasis. J Neurosci. 2008;28:12097–12106. doi: 10.1523/JNEUROSCI.2635-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Gusella JF, Watkins PC, et al. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tarawneh R, Holtzman DM. Critical issues for successful immunotherapy in Alzheimer’s disease: development of biomarkers and methods for early detection and intervention. CNS Neurol Disord Drug Targets. 2009;8:144–159. doi: 10.2174/187152709787847324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD. The pathogenesis of Alzheimer disease: an alternative to the amyloid hypothesis. J Neuropathol Exp Neurol. 1996;55:1023–1025. [PubMed] [Google Scholar]
- Terry RD. The cytoskeleton in Alzheimer disease. J Neural Transm Suppl. 1998;53:141–145. doi: 10.1007/978-3-7091-6467-9_12. [DOI] [PubMed] [Google Scholar]
- Tomiyama T, Matsuyama S, Iso H, et al. A mouse model of amyloid beta oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30:4845–4856. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BP, Kitazawa M, LaFerla FM. Amyloid beta-peptide: the inside story. Curr Alzheimer Res. 2004;1:231–239. doi: 10.2174/1567205043332045. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Venugopal C, Demos CM, Rao KS, Pappolla MA, Sambamurti K. Beta-secretase: structure, function, and evolution. CNS Neurol Disord Drug Targets. 2008;7:278–294. doi: 10.2174/187152708784936626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier Y, Zarandi M, Penke B. Amyloid beta-peptide interactions with neuronal and glial cell plasma membrane: binding sites and implications for Alzheimer’s disease. J Pept Sci. 2004;10:229–248. doi: 10.1002/psc.573. [DOI] [PubMed] [Google Scholar]
- Viola KL, Velasco PT, Klein WL. Why Alzheimer’s is a disease of memory: the attack on synapses by A beta oligomers (ADDLs) J Nutr Health Aging. 2008;12:51S–57S. doi: 10.1007/BF02982587. [DOI] [PubMed] [Google Scholar]
- Walker ES, Martinez M, Brunkan AL, Goate A. Presenilin 2 familial Alzheimer’s disease mutations result in partial loss of function and dramatic changes in Abeta 42/40 ratios. J Neurochem. 2005;92:294–301. doi: 10.1111/j.1471-4159.2004.02858.x. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Condron MM, Selkoe DJ, Teplow DB. In vitro studies of amyloid beta-protein fibril assembly and toxicity provide clues to the aetiology of Flemish variant (Ala692-->Gly) Alzheimer’s disease. Biochem J. 2001;355:869–877. doi: 10.1042/bj3550869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11:213–228. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi: 10.1126/science.1196284. [DOI] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk G, Hughey D, Boundy V, Kim A, Walker L, Olton D. Neurotransmitters and memory: role of cholinergic, serotonergic, and noradrenergic systems. Behav Neurosci. 1987;101:325–332. doi: 10.1037//0735-7044.101.3.325. [DOI] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Wirths O, Bethge T, Marcello A, et al. Pyroglutamate Abeta pathology in APP/PS1KI mice, sporadic and familial Alzheimer’s disease cases. J Neural Transm. 2010;117:85–96. doi: 10.1007/s00702-009-0314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmer MA. Cholesterol-related genes in Alzheimer’s disease. Biochim Biophys Acta. 2010;1801:762–773. doi: 10.1016/j.bbalip.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Wong CW, Quaranta V, Glenner GG. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985;82:8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HK, Sakurai T, Oyama F, et al. beta Subunits of voltage-gated sodium channels are novel substrates of beta-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. J Biol Chem. 2005;280:23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- Wood JG, Mirra SS, Pollock NJ, Binder LI. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986;83:4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. Gamma-secretase catalyzes sequential cleavages of the AbetaPP transmembrane domain. J Alzheimers Dis. 2009;16:211–224. doi: 10.3233/JAD-2009-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Bienkowski MJ, Shuck ME, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- Yankner BA. Commentary and perspective on studies of beta amyloid neurotoxicity. Neurobiol Aging. 1992;13:615–616. doi: 10.1016/0197-4580(92)90067-8. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Cheng I, Chung S, et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Zhao G, Liu Z, Ilagan MX, Kopan R. Gamma-secretase composed of PS1/Pen2/Aph1a can cleave notch and amyloid precursor protein in the absence of nicastrin. J Neurosci. 2010;30:1648–1656. doi: 10.1523/JNEUROSCI.3826-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Mao G, Tan J, Dong Y, Cui MZ, Kim SH, Xu X. Identification of a new presenilin-dependent zeta-cleavage site within the transmembrane domain of amyloid precursor protein. J Biol Chem. 2004;279:50647–50650. doi: 10.1074/jbc.C400473200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Suram A, Venugopal C, Prakasam A, Lin S, Su Y, Li B, Paul SM, Sambamurti K. Geranylgeranyl pyrophosphate stimulates gamma-secretase to increase the generation of Abeta and APP-CTFgamma. FASEB J. 2008;22:47–54. doi: 10.1096/fj.07-8175com. [DOI] [PMC free article] [PubMed] [Google Scholar]