Abstract
Heat shock proteins have been implicated as endogenous activators for dendritic cells (DCs). Without tissue distress or death, these intracellular molecules are inaccessible to surface receptor(s) on DCs, possibly to avoid uncontrolled DC activation and breakdown of immunologic tolerance. We herein addressed this hypothesis in transgenic mice by enforcing cell surface expression of gp96, a ubiquitous heat shock protein of the endoplasmic reticulum. Although a pan-specific promoter is used for transgene expression, neither the expression level nor the tissue distribution of the endogenous gp96 was altered by this maneuver. However, cell surface gp96 induced significant DC activations and spontaneous lupus-like autoimmune diseases, even though the development/functions of lymphocytic compartments were unaltered. Using a bone marrow chimera approach, we further demonstrated that both DC activation and autoimmunity elicited by cell surface gp96 are dependent on the downstream adaptor protein MyD88 for signaling by Toll/IL-1 receptor family. Our study not only established the proinflammatory property of cell surface gp96 in vivo, but also suggested a chronic stimulation of DCs by gp96 as a pathway to initiate spontaneous autoimmune diseases.
Dendritic cells (DCs) play critical roles in tolerance as well as in immunity (1, 2). Detailed understanding of factor(s) and condition(s) leading to such divergent states of DCs is critical in understanding immune responses against pathogens and tumors, as well as self antigens (3–5). DCs express pattern recognition receptors such as Toll-like receptors (TLRs) on the cell surface to sense microbial products (6). Recent studies suggest the presence of multiple receptors (7) including CD91 (8, 9), TLR2, and TLR4 (10) on DCs for the heat shock protein (HSP) gp96 of the endoplasmic reticulum. These findings imply that the export of gp96 in vivo might be an important signal for DC activation and thus could serve as a switch for DCs from maintaining tolerance to inducing immunity. However, no studies have directly addressed this question.
We have now tested this hypothesis by genetically targeting gp96 onto cell surfaces in a transgenic mouse model, 96tm-Tg. We found that these mice had hyperfunctional DCs and developed spontaneous lupus-like autoimmune diseases. Moreover, both DC activation and the development of autoimmune diseases in 96tm-Tg mice were dependent on MyD88, an important downstream adaptor protein for signaling by Toll/IL-1 receptor family (6, 11, 12).
Materials and Methods
Mice and Genotyping. The 96tm-Tg mice were generated by microinjection of purified ApaL I DNA fragment into C57BL/6 fertilized eggs (Fig. 1A). An immediate early promoter from cytomegalovirus was used to drive 96tm expression. Founder mice were detected by genomic PCR and Southern blot. All other mice came from The Jackson Laboratory, except for OT-I and MyD88-/- mice, which were kindly provided by L. Lefrançois (University of Connecticut, Farmington) and R. Medzhitov (Yale University, New Haven, CT), respectively. Animal experiments were carried out according to an approved protocol and the established guidelines of the University of Connecticut.
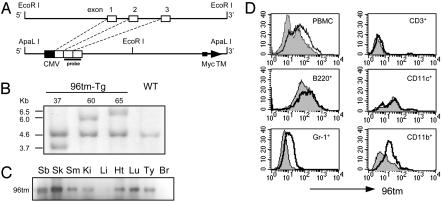
Fig. 1.
Generation of cell surface gp96-expressing transgenic mice. (A) Schematic diagram of the 5′ EcoRI fragment of gp96 gene, and a transcription unit of full-length gp96 cDNA (only the first three exons were indicated for comparison with the genomic sequence) fused with a Myc tag, followed by a transmembrane domain (TM), for production of 96tm-Tg mice in a C57BL/6 background. (B) Southern blot analysis of EcoRI-digested genomic DNA from three independent 96tm-Tg mouse lines (indicated as 37, 60, and 65). The probe used here corresponds to exon 2 and exon 3 of gp96 gene, as indicated in Fig. 1 A. (C) A total of 4 mg of tissue lysates of small bowel (Sb), skin (Sk), skeletal muscle (Sm), kidney (Ki), liver (Li), heart (Ht), lung (Lu), thymus (Ty), and brain (Br) from 96tm-Tg mice were immunoprecipitated with 5 μg of anti-gp96 Ab followed by a Western blot analysis with a mAb against Myc tag. There are no detectable 96tm from any tissues or organs in WT mice (data not shown). (D) FACS analysis of cell surface expression of gp96 by peripheral blood mononuclear cells (PBMC) and their subsets, from 96tm-Tg mice (open histogram) and WT mice (shaded histogram), by using a monospecific anti-mouse gp96 Ab, followed by an FITC-labeled secondary Ab. Background staining of Tg and WT cells by an isotype control Ab were identical (data not shown). Three experiments were performed with similar findings.
Antibodies, Chemicals, and Other Reagents. Most Abs came from BD Pharmingen. Carboxyfluorescein diacetate-succinimidyl ester was obtained from Molecular Probes. Ig levels were determined by a sandwich ELISA kit from Southern Biotechnology Associates. SIINFEKL was synthesized by Genemed Synthesis. Trinitrophenol (TNP)-Ficoll and TNP-keyhole limpet hemocyanin were obtained from Biosearch. Antibodies against double-stranded DNA and TNP were detected by ELISA. All cytokines were measured by ELISA unless otherwise indicated.
Histology. Tissues were fixed and stained with hematoxylin and eosin by standard methods. For electron microscopy, kidneys were prefixed with 3% glutaraldehyde, fixed in osmium tetroxide, embedded in resin, sectioned, stained with uranyl acetate and lead citrate, and examined by using transmission electron microscopy.
Immunohistochemistry. Cryosections of kidneys were blocked with normal goat serum, stained with FITC-conjugated goat anti-mouse Ig or anti-mouse C3 (ICN). Sections were also stained with horseradish peroxidase-labeled Abs against mouse IgA, IgG2a, and IgG2b (Southern Biotechnology Associates), followed by 3-amino-9-ethylcarbazole peroxidase substrate (Vector Laboratories).
Immunofluorescence for Detection of Antinuclear Antibodies (ANA). ANAs were measured by indirect immunofluorescence by using HEp-2-coated slides (INOVA Diagnostics, San Diego).
Mixed Lymphocyte Reaction. Splenic DCs were purified by CD11c magnetic beads (Miltenyi Biotec, Auburn, CA), irradiated (15 Gy), and cocultured at 37°C with 1.5 × 105 purified allogeneic CD4+ T cells per well in U-bottom 96-well plates. Fifty-six hours later, cells were pulsed overnight with 1 μCi (1 Ci = 37 GBq) of [3H]thymidine, harvested, and determined for 3H incorporation by a liquid scintillation counter (Microplate Scintillation & Luminescence Counter, Packard).
DC Maturation Assay. Bone marrow (BM)-derived immature DCs from WT or MyD88-/- mice were stimulated with CT-26 colon cancer cell lines with or without cell surface gp96 for 20 h, as described (13). DCs were then stained with Abs against CD11c and CD86, followed by fluorescence-activated cell sorter (FACS, Becton Dickinson) analysis. Supernatants were collected for assay of cytokines by ELISA kits from Endogen (Cambridge, MA).
BM Transplantation. BM cells (2 × 106) were injected via tail vein into WT mice, or 96tm-Tg mice that were lethally irradiated (550 × 2 cGy with an interval of 4 h) 24 h before transfer.
Statistical Analysis. Student's t test was used for statistical analysis. Values of P < 0.05 were considered to represent statistically significant differences.
Results
Development of Spontaneous Autoimmune Diseases by 96tm-Tg Mice. To address the immunologic significance of cell surface gp96 in vivo, we generated a transgenic mouse, 96tm-Tg, by using the same cytomegalovirus promoter-driven construct that has been used previously to target gp96 onto cell surfaces of tumor cells (13) (Fig. 1). Three distinct lines with different sites of integrations were generated, and the transgene penetrates in each line according to a predicted Mendelian fashion. 96tm protein was expressed in the gut, skin, skeletal muscle, kidney, heart, lung, and thymus, but it was undetectable in the liver and brain (Fig. 1C). By FACS analysis using a gp96-specific Ab, we found that 96tm is mainly expressed by Gr-1+ granulocytes and CD11b+ monocytes in the peripheral blood. There was no or little expression of 96tm by CD11c+ cells and T and B lymphocytes (Fig. 1D). The expression of endogenous gp96 is comparable between WT and 96tm-Tg mice, and no 96tm was detectable in the serum of Tg mice by ELISA, indicating that surface gp96 did not shed significantly (data not shown).
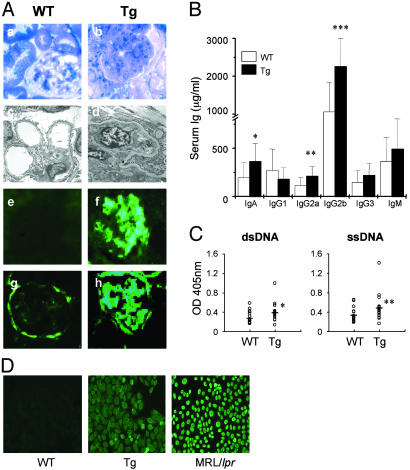
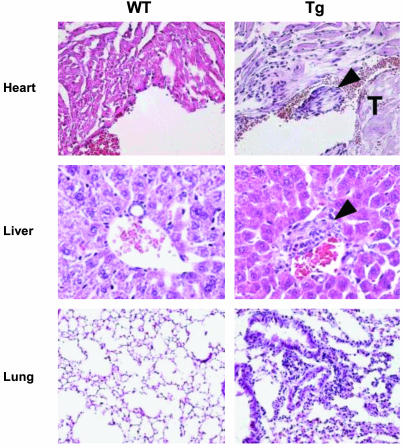
Despite no apparent gross abnormalities, histological examinations demonstrated severe glomerulonephritis in 96tm-Tg mice of all three lines after mice reached >20 weeks of age (Fig. 2 and data not shown), evidenced by protein deposition in the glomerular basal membrane, thickened/collapsed capillary loops, mesangial expansion, and diffuse proliferation of endothelial and mesangial cells. Perhaps due to relatively well-reserved glomerulobasilar membrane, 96tm-Tg mice did not have heavy proteinuria. Nevertheless, further analysis by immunofluorescence microscopy revealed diffuse deposition of immune complexes in the glomeruli (Fig. 2 A). Isotype analysis showed depositions of IgA as well as IgG (Fig. 7, which is published as supporting information on the PNAS web site). These findings are indistinguishable from type IV diffuse proliferative nephritis, associated with systemic lupus erythematosus (SLE) (14). Like SLE, 96tm-Tg mice had significant hypergammaglobulinemia (Fig. 2B), high titers of Abs against both double-stranded DNA and single-stranded DNA (Fig. 2C), and ANAs (Fig. 2D). There were also signs of systemic inflammations, such as pneumonitis, endocarditis, and hepatitis (Fig. 3). The presence of gp96 on cell surface, however, does not induce production of Abs against gp96 itself (data not shown).
Fig. 2.
96tm-Tg mice develop lupus-like autoimmune diseases. (A) Kidney sections of 96tm-Tg37 mice or WT littermates were analyzed by electron microscopy (a and b, ×600; c ×5,000; d, ×6,000) and immunofluorescence light microscopy after staining with Ab against mouse Ig (e and f, ×200), and mouse C3 (g and h, ×200). Both 96tm-Tg60 and 96tm-Tg65 mice have similar kidney diseases (data not shown). (B) Quantitative analysis of serum Ig isotypes from age-matched 96tm-Tg mice (n = 11) and WT littermates (n = 9) by ELISA. *, P = 0.0457; **, P = 0.0328; ***, P = 0.00249. (C) Sera from 96tm-Tg mice (n = 19) and WT mice (n = 20) were determined for the presence of IgG against double-stranded DNA and single-stranded DNA. Dashes represent the mean value within each group. *, P = 0.027; **, P = 0.029. (D) Indirect immunofluorescence assay for the presence of ANA. At least five mice per group were examined. Sera from MRL/lpr mice were used as a positive control.
Fig. 3.
Systemic inflammation associated with cell surface expression of gp96. Five-micrometer tissue sections from 96tm-Tg mice and WT littermates were stained with hematoxylin and eosin. Representative fields of heart, liver, and lung were shown at ×200 (liver) and ×100 (heart and lung) magnifications. The presence of endocardial thrombi (T) formation in 96tm-Tg mice is indicated. Arrow depicts areas of infiltration with inflammatory cells. Increased cellularity and diffuse alveolar wall thickenings associated with the lungs of 96tm-Tg mice are shown.
Effects of Cell Surface gp96 on Hematopoiesis and T and B Cell Functions. We next studied whether 96tm affected normal development of the hematopoietic system, to possibly account for the observed autoimmune phenotype. We found no significant differences of cellularity, or distribution of hematopoietic cells of various lineages between WT and Tg mice (Fig. 8A, which is published as supporting information on the PNAS web site). 96tm-Tg mice do not have significant lymphoadenopathy or splenomegaly (data not shown). Further phenotypical analysis showed no significant increase of T or B cells that express activation markers including CD44, CD69, and CD25 (Table 1, which is published as supporting information on the PNAS web site). 96tm-Tg and WT littermates mounted similar levels of IgG and IgM responses against TNP-Ficoll, a T cell-independent antigen (Fig. 8B). Moreover, we have crossed 96tm-Tg mice with Vα2β5 T cell receptor transgenic mice specific for Kb-SIINFEKL (OT-I mice). We found no apparent impacts of cell surface gp96 on the development and function of OT-I (Fig. 8 C and D). We conclude that cell surface expression of gp96 in vivo does not cause developmental defect of the immune system, and there are no apparent intrinsic abnormalities with either B or T lymphocytes.
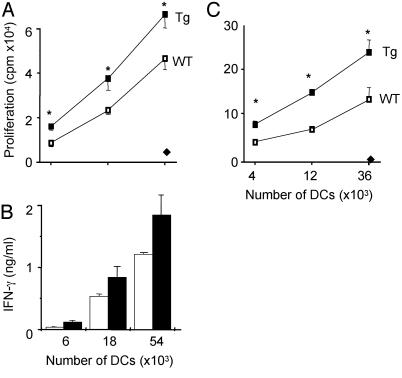
DCs from 96tm-Tg Mice Were Hyperfunctional. We then focused our attention on gp96–DC interactions because gp96 has been shown to mature DCs in vitro (13, 15, 16). In the steady state, DCs are critically important in maintaining tolerance (3–5). We suspected that chronic 96tm–DC interaction in 96tm-Tg mice might lead to a switch of DC function from tolerance to immunity, resulting in priming of autoreactive T cells and development of autoimmune diseases. To test this possibility, we compared the efficiency of DCs from WT and 96tm-Tg mice to stimulate naive CD4+ and CD8+ T cells. Freshly isolated splenic CD11c+ DCs from 96tm-Tg mice stimulated allogeneic naive CD4+ T cells more efficiently than WT DCs, as measured by T cell proliferation (Fig. 4A) and secretion of IFN-γ (Fig. 4B).
Fig. 4.
Enhanced T cell stimulatory functions by DCs from 96tm-Tg mice. (A and B) Freshly isolated CD11c+ splenic DCs from 96tm-Tg mice were tested for their ability to stimulate allogeneic naive CD4+ T cells to proliferate by [3H]thymidine incorporation (A) and to produce IFN-γ by ELISA (B). Four independent experiments were performed with similar findings. (A and C) * represents a statistically significant difference between WT and Tg DCs (P < 0.05). Controls represent results obtained by using syngeneic T cells (♦). (C) Purified CD11c+ splenic cells from WT or 96tm-Tg mice were pulsed with SIINFEKL, fixed with paraformaldehyde, and cocultured at the indicated number with naive OT-I T cells for 40 h. Proliferation during the last 20 h was measured by [3H]thymidine incorporation.
To study the roles of Tg DCs on activating CD8+ T cells, we pulsed CD11c+ splenic DCs from 96tm-Tg or WT mice with a saturating amount of SIINFEKL. Peptide-loaded DCs were then washed, fixed, and used for stimulation of OT-I T cells in vitro. We found once again that Tg DCs were more efficient than WT DCs in stimulating CD8+ T cells (Fig. 4C). This experiment also ruled out any soluble factors that might confer for the hyperactive function of Tg DCs. Such an activity was not due to the costimulatory capacity of cell surface gp96 itself as reported by others (17) because anti-gp96 Ab was unable to block this activity (data not shown), and there were no significant 96tm expressions by CD11c+ DCs (Fig. 1 and data not shown).
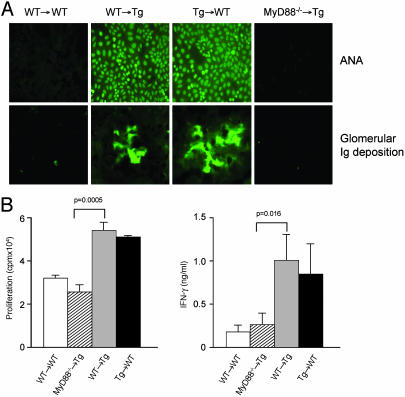
Effects of 96tm Expression by Nonhematopoietic Tissues on Autoimmunity. Our data therefore is consistent with the hypothesis that the primary consequence of cell surface gp96 expression in 96tm-Tg mice is the chronic activation of DCs, which resulted in activation of self-reactive T cells and the breakdown of peripheral tolerance. If so, 96tm-Tg mice, reconstituted with hematopoietic system from WT mice should be expected to manifest signs of autoimmune diseases as a result of stimulations of WT DCs, in trans, by 96tm on nonhematopoietic cells. To test this hypothesis, we reconstituted lethally irradiated 6-week-old 96tm-Tg mice with BM from WT C57BL/6 mice. Approximately 98% donor chimerism was achieved based on analysis of splenocytes using a congenic marker (data not shown). Four months later, we found that WT → Tg mice developed both ANA and glomerulonephritis whereas no autoimmunity was evident with WT → WT chimeras (Fig. 5A). Conversely, Tg → WT chimeric mice also developed autoimmunity, suggesting that the autoimmune phenotype associated with 96tm-Tg mice is due to the stimulation of the immune system by extracellular gp96, expressed by either nonhematopoietic parenchyma tissues (as in the case of WT → Tg mice) or hematopoietic cells (Tg → WT mice). The development of lupus-like diseases in WT recipient mice also further ruled out the possibility that the immune deregulation in 96tm-Tg mice is due to abnormal thymic environment as a result of cell surface gp96 expression.
Fig. 5.
Immunopathology associated with 96tm-Tg mice depends on a MyD88-mediated pathway. (A) Mice were lethally irradiated (550 cGy × 2) and transplanted intravenously with BM from WT, 96tm-Tg, or MyD88-/- mice. Four months after the transplantation, sera were collected from recipient mice and tested for the presence of ANA. Kidney sections were stained for the presence of Ig deposition by immunofluorescence assay by using Ab against mouse Ig. All fields are magnified ×200. There were five mice per group. Data from one representative mouse per group is shown. (B) Freshly isolated splenic CD11c+ DCs (b haplotype) from various chimeric mice were tested for their abilities to stimulate allogeneic naive CD4+ T cells (d haplotype) to proliferate by [3H]thymidine incorporation (Left) and to secrete IFN-γ by ELISA (Right).
MyD88 Is Critical for 96tm-Induced DC Activation and Autoimmune Diseases. Having established that the autoimmune phenotype did not require gp96 expression on the hematopoietic system, we then expanded the use of bone marrow chimeras to study the downstream signaling requirements associated with cell surface gp96 in vivo. The only signaling receptors described for gp96 so far are TLR2/4 (10), which transduces signals primarily via the cytosolic intermediate adaptor molecule MyD88 (11, 18). We chose to address the contribution of MyD88 in our system. Six-week-old 96tm-Tg mice were lethally irradiated and transplanted intravenously with WT or MyD88-/- BM. Four months after the transplantation, we found that MyD88-/- → Tg mice, in contrast to WT → Tg mice, failed to develop autoimmune diseases (Fig. 5A), evidenced by no detectable ANA and complete lack of glomerular deposition of immune complexes. Our results demonstrated that the breakdown of tolerance by gp96 in vivo depended on a MyD88-mediated pathway.
Our functional study earlier suggests that the pathogenicity of autoimmune diseases in 96tm-Tg mice is a DC-driven process. If so, we envisaged that the lack of autoimmunity in MyD88-/- → Tg mice is due to the inability of 96tm to activate MyD88-/- DCs. As predicted, the development of diseases in BM chimeric mice correlated precisely with increased DC functions in activating T cells (Fig. 5B). DCs from WT → Tg mice hyperstimulated T cells whereas DCs from MyD88-/- → Tg mice stimulated T cells only modestly even though they have been exposed in vivo to 96tm.
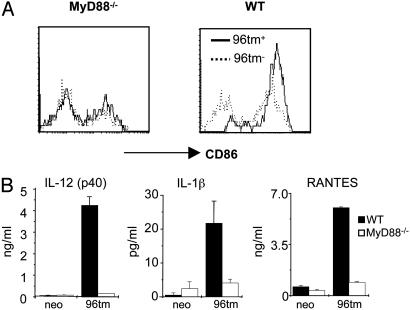
To further delineate the roles of MyD88 in 96tm-DC interactions, we incubated 96tm-expressing cells with BM-derived DCs from MyD88-/- mice. We found that MyD88 is essential for 96tm to activate DCs in vitro, evidenced by complete abrogation of up-regulation of CD86 (Fig. 6A) and production of cytokines [IL-1β, IL-12 p40, and RANTES (regulated upon activation, normal T cell expressed and secreted)] after stimulation of MyD88-/- DCs with 96tm (Fig. 6B).
Fig. 6.
MyD88 is required by 96tm to activate DCs in vitro. WT or MyD88-/- BMDCs were cocultured with 96tm+ or 96tm- cells for 20 h, followed by FACS analysis of expression of cell surface CD86 (A) or ELISA measurement for the releases of IL-1β, IL-12 p40, and RANTES (regulated upon activation, normal T cell expressed and secreted) by DCs (B).
Discussion
We have, genetically, addressed the immunomodulating properties of HSP gp96 in vivo. By solely targeting gp96 onto cell surfaces without manipulation of the level of endogenous gp96, we demonstrated that direct access of gp96 to the immune system induced spontaneous lupus-like autoimmune diseases. Mechanistic study revealed that the induction of autoimmunity is mainly a DC-driven process because there are no obvious alterations of intrinsic T or B cell development/functions in 96tm-Tg mice and because DCs from these mice are more efficient for the activation of both CD4+ and CD8+ T cells. Our results have thus uncovered two aspects of HSP gp96 in vivo. First, extracellular gp96 could serve as an endogenous DC activator on an organismal level. Second, chronic activation of DCs by gp96 may cause breakdown of peripheral tolerance, resulting in lupus-like autoimmune diseases.
96tm can mature DC efficiently in vitro (Fig. 6). DCs from 96tm-Tg mice, however, do not express significantly higher levels of CD80, CD86, or other conventional costimulatory molecules than DCs from WT mice (data not shown) even though these DCs were hyperfunctional in stimulating T cells (Fig. 4). Similarly, CD11c+ cells from tertiary tissues (lung, liver, and skin) of Tg mice also do not express more abundant maturation markers, including MHC II, based on FACS analysis of CD11c+ single cell suspension and immunohistology (data not shown). Studies are needed to determine whether there is increased production of proinflammatory cytokines including IL-6 and IL-12 by Tg DCs in vivo. There are several possible explanations for the discrepancy between in vitro and in vivo data. For in vitro experiments, DCs from WT mice were pulsed with 96tm+ cells acutely. By contrast, DCs in Tg mice were stimulated with 96tm chronically, all of the way from their ontogeny. During this time, fully matured DCs might have been eliminated. It is equally possible that chronically stimulated DCs in 96tm-Tg mice have modulated the expression level or function of receptors for gp96 so that only partial maturation can be achieved on further stimulation by gp96. A similar phenomenon, termed DC exhaustion, has been described for LPS-mediated DC activation (19).
We demonstrated that gp96-elicited autoimmune phenotype depends on MyD88 (Fig. 5). This finding suggests again that the immune deregulation of 96tm-Tg mice is primarily a DC-driven process. Lethally irradiated mice transplanted with MyD88-/- BM (MyD88-/- → Tg) were fully reconstituted (data not shown), consistent with the report that the hematopoietic system of MyD88-/- mice is developmentally normal (20). In these chimeric mice, 96tm was expressed abundantly by nonhematopoietic tissues, which would allow MyD88-/- DCs to interact with 96tm. Yet splenic DCs from MyD88-/- → Tg mice did not have increased ability to stimulate T cells, and these mice did not develop autoimmune diseases (Fig. 5B). By contrast, both DC hyperfunction and autoimmunity were readily detectable in WT → Tg mice. The differences between MyD88-/- → Tg and WT → Tg mice also ruled out the possibility that a residual hematopoietic system of Tg origin in these chimeric mice is responsible for the breakdown of immunologic tolerance.
MyD88 is essential for signaling by Toll/IL-1 receptor family including all TLRs, IL-1β, IL-18, and other poorly studied orphan receptors (11, 12, 21, 22). Even though gp96 in the endoplasmic reticulum acts as a molecular chaperone for both TLR2 and TLR4 (23), whether extracellular gp96 can serve as an endogenous ligand for TLRs remains controversial (10, 24). 96tm-Tg model should be useful for genetically pinpointing the signaling receptors for gp96 and for understanding of the roles of gp96 in immune system in vivo.
Our study suggests that chronic stimulations by extracellular HSPs may constitute a novel mechanism for autoimmune diseases. The autoimmune features of 96tm-Tg mice, including hypergammaglobulinemia, antinuclear antibody, glomerulonephritis, and multiorgan inflammations, closely resemble SLE in humans (25). Interestingly, “ectopic” expressions of HSPs have been reported in many systems (26). In particular, the cell surface expression of a cytosolic HSP90 (a paralogue of gp96) has been linked to active SLE in humans (27). The hallmark of SLE is the dysregulation of the immune system, resulting in polyclonal activation of T and B cells, leading to multiorgan inflammation/damages from immune complex deposition and infiltrations with inflammatory cells (28). Although lupus-like diseases can be caused by defects in many pathways of the immune system, studies suggest that one of the mechanisms for SLE is the defect in peripheral B or T cell tolerance as a result of chronic stimulation/activation of APCs. For example, chronic activation of Langerhan's cells by ectopic CD40 ligand expression in the murine epidermis led to not only chronic skin inflammations, but also systemic autoimmunity, including immune complex-mediated glomerular nephritis (29). Activation by genomic DNAs by means of TLR-9 has been implicated in the generation of autoantibodies against Ig (30). Peripheral monocytes from patients with active, but not quiescent, SLEs were found to differentiate into active DCs more readily, due to higher serum levels of type I IFN (31). These studies are thus consistent with the notion that chronic activations of DCs alone may provoke autoimmunity (32–34), which suggests that silencing of DCs in vivo may have therapeutic value against autoimmune diseases.
Conclusions
We have genetically demonstrated the proinflammatory properties of HSP gp96 in vivo. Manipulation of tissue distribution and subcellular localizations of HSP expression might circumvent the difficulties in performing genetic study of HSPs due to their essential housekeeping functions. More importantly, in response to stress, HSPs not only change in the level of expression, but also undergo subcellular redistributions. We propose that the trafficking of HSPs to the extracellular milieu, either actively or passively, in the situation of tissue stress, is a critical signal for the activation of DCs in vivo, which in turn contributes to the initiation and fate determination of adaptive immunity against pathogens as well as self antigens (26).
Supplementary Material
Acknowledgments
We are indebted to Dr. P. Zhang for help in electron microscopy and to members from the Immunology Graduate Program, University of Connecticut School of Medicine, for helpful inputs. This work was supported in part by National Institutes of Health Grants CA90337 and CA100191 (to Z.L.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DC, dendritic cell; TLR, Toll-like receptor; HSP, heat shock protein; ANA, antinuclear antibody; BM, bone marrow; FACS, fluorescence-activated cell sorter; SLE, systemic lupus erythematosus; TNP, trinitrophenol.
References
- 1.Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245-252. [DOI] [PubMed] [Google Scholar]
- 2.Shortman, K. & Liu, Y. J. (2002) Nat. Rev. Immunol. 2, 151-161. [DOI] [PubMed] [Google Scholar]
- 3.Steinman, R. M. & Nussenzweig, M. C. (2002) Proc. Natl. Acad. Sci. USA 99, 351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu, K., Iyoda, T., Saternus, M., Kimura, Y., Inaba, K. & Steinman, R. M. (2002) J. Exp. Med. 196, 1091-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belz, G. T., Behrens, G. M., Smith, C. M., Miller, J. F., Jones, C., Lejon, K., Fathman, C. G., Mueller, S. N., Shortman, K., Carbone, F. R. & Heath, W. R. (2002) J. Exp. Med. 196, 1099-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janeway, C. A., Jr., & Medzhitov, R. (2002) Annu. Rev. Immunol. 20, 197-216. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava, P. K. (2002) Nat. Rev. Immunol. 2, 185-194. [DOI] [PubMed] [Google Scholar]
- 8.Binder, R. J., Han, D. K. & Srivastava, P. K. (2000) Nat. Immunol. 1, 151-155. [DOI] [PubMed] [Google Scholar]
- 9.Basu, S., Binder, R. J., Ramalingam, T. & Srivastava, P. K. (2001) Immunity 14, 303-313. [DOI] [PubMed] [Google Scholar]
- 10.Vabulas, R. M., Braedel, S., Hilf, N., Singh-Jasuja, H., Herter, S., Ahmad-Nejad, P., Kirschning, C. J., Da Costa, C., Rammensee, H. G., Wagner, H. & Schild, H. (2002) J. Biol. Chem. 277, 20847-20853. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov, R., Preston-Hurlburt, P., Kopp, E., Stadlen, A., Chen, C., Ghosh, S. & Janeway, C. A., Jr. (1998) Mol. Cell 2, 253-258. [DOI] [PubMed] [Google Scholar]
- 12.Martin, M. U. & Wesche, H. (2002) Biochim. Biophys. Acta 1592, 265-280. [DOI] [PubMed] [Google Scholar]
- 13.Zheng, H., Dai, J., Stoilova, D. & Li, Z. (2001) J. Immunol. 167, 6731-6735. [DOI] [PubMed] [Google Scholar]
- 14.Masutani, K., Akahoshi, M., Tsuruya, K., Tokumoto, M., Ninomiya, T., Kohsaka, T., Fukuda, K., Kanai, H., Nakashima, H., Otsuka, T. & Hirakata, H. (2001) Arthritis Rheum. 44, 2097-2106. [DOI] [PubMed] [Google Scholar]
- 15.Basu, S., Binder, R. J., Suto, R., Anderson, K. M. & Srivastava, P. K. (2000) Int. Immunol. 12, 1539-1546. [DOI] [PubMed] [Google Scholar]
- 16.Singh-Jasuja, H., Scherer, H. U., Hilf, N., Arnold-Schild, D., Rammensee, H. G., Toes, R. E. & Schild, H. (2000) Eur. J. Immunol. 30, 2211-2215. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee, P. P., Vinay, D. S., Mathew, A., Raje, M., Parekh, V., Prasad, D. V., Kumar, A., Mitra, D. & Mishra, G. C. (2002) J. Immunol. 169, 3507-3518. [DOI] [PubMed] [Google Scholar]
- 18.Kawai, T., Adachi, O., Ogawa, T., Takeda, K. & Akira, S. (1999) Immunity 11, 115-122. [DOI] [PubMed] [Google Scholar]
- 19.Langenkamp, A., Messi, M., Lanzavecchia, A. & Sallusto, F. (2000) Nat. Immunol. 1, 311-316. [DOI] [PubMed] [Google Scholar]
- 20.Schnare, M., Barton, G. M., Holt, A. C., Takeda, K., Akira, S. & Medzhitov, R. (2001) Nat. Immunol. 2, 947-950. [DOI] [PubMed] [Google Scholar]
- 21.Medzhitov, R. (2001) Nat. Rev. Immunol. 1, 135-145. [DOI] [PubMed] [Google Scholar]
- 22.Takeda, K., Kaisho, T. & Akira, S. (2003) Annu. Rev. Immunol. 21, 335-376. [DOI] [PubMed] [Google Scholar]
- 23.Randow, F. & Seed, B. (2001) Nat. Cell Biol. 3, 891-896. [DOI] [PubMed] [Google Scholar]
- 24.Reed, R. C., Berwin, B., Baker, J. P. & Nicchitta, C. V. (2003) J. Biol. Chem. 278, 31853-31860. [DOI] [PubMed] [Google Scholar]
- 25.Tan, E. M., Cohen, A. S., Fries, J. F., Masi, A. T., McShane, D. J., Rothfield, N. F., Schaller, J. G., Talal, N. & Winchester, R. J. (1982) Arthritis Rheum. 25, 1271-1277. [DOI] [PubMed] [Google Scholar]
- 26.Liu, B., DeFilippo, A. & Li, Z. (2002) Mol. Cancer Ther. 1, 1147-1151. [PubMed] [Google Scholar]
- 27.Erkeller-Yuksel, F. M., Isenberg, D. A., Dhillon, V. B., Latchman, D. S. & Lydyard, P. M. (1992) J. Autoimmun. 5, 803-814. [DOI] [PubMed] [Google Scholar]
- 28.Shlomchik, M. J., Craft, J. E. & Mamula, M. J. (2001) Nat. Rev. Immunol. 1, 147-153. [DOI] [PubMed] [Google Scholar]
- 29.Mehling, A., Loser, K., Varga, G., Metze, D., Luger, T. A., Schwarz, T., Grabbe, S. & Beissert, S. (2001) J. Exp. Med. 194, 615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leadbetter, E. A., Rifkin, I. R., Hohlbaum, A. M., Beaudette, B. C., Shlomchik, M. J. & Marshak-Rothstein, A. (2002) Nature 416, 603-607. [DOI] [PubMed] [Google Scholar]
- 31.Blanco, P., Palucka, A. K., Gill, M., Pascual, V. & Banchereau, J. (2001) Science 294, 1540-1543. [DOI] [PubMed] [Google Scholar]
- 32.Ludewig, B., Junt, T., Hengartner, H. & Zinkernagel, R. M. (2001) Curr. Opin. Immunol. 13, 657-662. [DOI] [PubMed] [Google Scholar]
- 33.Shortman, K. & Heath, W. R. (2001) Nat. Immunol. 2, 988-989. [DOI] [PubMed] [Google Scholar]
- 34.Walker, L. S. & Abbas, A. K. (2002) Nat. Rev. Immunol. 2, 11-19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.