Abstract
Chronically elevated plasma angiotensin II (AngII) causes a salt-sensitive form of hypertension that is associated with a differential pattern of peripheral sympathetic outflow. This “AngII-salt sympathetic signature” is characterized by a transient reduction in sympathetic nervous system activity (SNA) to the kidneys, no change in SNA to skeletal muscle, and a delayed activation of SNA to the splanchnic circulation. Studies suggest that the augmented sympathetic influence on the splanchnic vascular bed increases vascular resistance and decreases vascular capacitance, leading to hypertension via translocation of blood volume from the venous to the arterial circulation. This unique sympathetic signature is hypothesized to be generated by a balance of central excitatory inputs and differential baroreceptor inhibitory inputs to sympathetic premotor neurons in the rostral ventrolateral medulla. The relevance of these findings to human hypertension and the future development of targeted sympatholytic therapies are discussed.
Keywords: Neurogenic hypertension, Sympathetic nerve activity, Salt, Angiotensin II, Splanchnic nerve activity
Introduction
High blood pressure (hypertension) continues at epidemic levels in the United States. Despite the growing arsenal of antihypertensive drugs, the fraction of patients responding adequately to traditional drug therapy remains unacceptably low [1]. The reason is likely to be that multiple physiological abnormalities driven by interactions between genetic, behavioral, and environmental factors can cause hypertension. There is now indisputable evidence that increased sympathetic nervous system activity (SNA) is one such physiological abnormality. Although pharmacotherapy targeting the sympathetic nervous system has important clinical utility in the management of hypertension, its use is complicated by the fact that currently available drugs block not only sympathetic control of arterial pressure but also many other functions, resulting in unwanted adverse effects. One of many important questions in the field has been whether it is possible to reduce SNA in a more regionally selective way (using drugs or other methods) and still lower blood pressure. The exciting recent demonstration in human patients of long-term antihypertensive responses to a novel, device-based method of selective renal denervation [2•] suggests that the answer is yes. Much older studies in humans showing sustained lowering of blood pressure after selective denervation of the splanchnic organs [3] also support this concept. When combined with clear evidence that SNA in human hypertensives is not increased globally, but rather in regionally specific ways [4, 5], these results suggest that a more refined approach to sympathoinhibition could be a very effective and well tolerated clinical antihypertensive therapy.
The mechanisms involved in the augmentation of peripheral sympathetic tone in neurogenic forms of hypertension, and the importance of the sympathetic outflow paths to the various cardiovascular end-organs in maintaining an elevated blood pressure during hypertension, remain a subject of intense debate. In this regard, experimental animal models of hypertension have provided significant insight into the impact of renal sympathetic nerves on long-term blood pressure regulation [6, 7]. Evidence from models of salt-sensitive neurogenic hypertension suggests, however, that the involvement of the renal sympathetic nerves is not necessarily a universal mechanism [8, 9•]. For example, our own studies have shown that increased renal sympathetic activity is not a critical factor when hypertension is induced in rats by chronic infusion of angiotensin II (AngII) combined with high intake of sodium chloride (AngII-salt hypertension) [9•, 10•, 11, 12]. Instead, recent studies implicate increased SNA to the splanchnic organs in the pathogenesis of AngII-salt hypertension. They suggest further that dietary salt (an established factor in human hypertension) and sodium-sensing sites in the brain are critical drivers of neurogenic activation in AngII-salt hypertension. Given the heightened interest in regionally selective sympatholytic therapy for hypertension, these findings have potentially significant implications for the future development and refinement of such treatment modalities. The purpose of this article is to review studies supporting the hypothesis that AngII-salt hypertension is largely driven by synergistic actions of circulating AngII and dietary salt on the brain, increasing SNA specifically to the splanchnic vascular bed. The potential of new therapies aimed at targeting neural control of the splanchnic vascular bed as a treatment for human hypertension are also discussed.
Angiotensin II–Induced Activation of the Sympathetic Nervous System Is Dependent on Salt Intake
Hypertension caused by infusion of AngII in animals involves multiple control systems whose influence on arterial pressure is dependent on the dose of AngII and on the presence of other factors such as salt intake [13, 14]. Doses of AngII that increase arterial pressure slowly over a course of days to weeks produce what is commonly referred to as the “slow pressor AngII” model. It is thought that the hypertension is mediated, at least in part, by an elevated level of SNA [15–17]. It has long been known that the severity of AngII-induced hypertension is directly dependent on the prevailing level of salt intake, and more recent studies suggest that the level of sympathoactivation is also dependent on the salt intake [9•, 18]. Thus, it is important to keep in mind that neurogenic mechanisms may be less important in “AngII-induced” hypertension in animals subjected to a normal or low salt intake than these mechanisms would be in those subjected to a high salt intake (“AngII-salt” hypertension).
The salt-sensitive nature of AngII-induced hypertension often has been ignored in the literature, perhaps accounting for the contradictory conclusions about the role of the sympathetic nervous system in the model. Two different methods for assessing the role of the sympathetic nervous system in AngII-induced hypertension have commonly been employed: 1) changes in the depressor response to ganglionic blockade, and 2) changes in tissue or plasma norepinephrine (NE) concentration and turnover. These indices serve as an indicator for AngII-induced changes in peripheral SNA, which can be generated at any level of the neuraxis. Changes in responses to ganglion blockade suggest changes in SNA effects on arterial pressure. However, it is important to note that changes in plasma NE and NE turnover do not necessarily reflect changes in SNA that directly affect arterial pressure. Our group and others have reported a delay of 5 to 7 days in the increase in the acute depressor response to ganglionic blockade in AngII-induced [19] and AngII-salt [18] models—a finding consistent with the hypothesis that AngII hypertension is due, in part, to delayed activation of peripheral sympathetic outflow. In contrast, tissue NE been reported to be regionally decreased in AngII-induced rats [20], and plasma NE was found to be unchanged in AngII-induced rabbits [20]. A factor that may explain these disparate findings is that the level of sympathetic outflow measured by these indices during AngII-induced hypertension is highly dependent on the level of dietary salt intake. Our studies have demonstrated that both an increase in the response to ganglionic blockade and a parallel increase in whole-body NE spillover is present in rats on a high-salt diet but not a normal-salt diet. These increases are observed only after 5 to 7 days of AngII administration [9•, 18]. These findings suggest that administration of AngII combined with a high-salt diet leads to delayed enhancement of peripheral sympathetic outflow that contributes to, but does not exclusively cause, the associated hypertension.
The Splanchnic Vascular Bed Is The Critical Neural Target in Angiotensin II–Salt Hypertension
Another potential explanation for the disparate findings between laboratories regarding the contribution of the sympathetic nervous system to AngII-induced hypertension is a focus on the kidney as the most important sympathetic effector organ in long-term blood pressure regulation. This long-standing view stems from the kidney's role in the regulation of blood volume, which has been hypothesized to be directly linked to the long-term control of arterial pressure [21]. The concept is supported by reports that renal denervation prevents some forms of experimental neurogenic hypertension [22, 23], as well as by recent studies showing that renal denervation results in sustained decreases in arterial pressure in humans with drug-resistant hypertension [24]. However, it is important to note that these studies have not demonstrated that renal denervation decreases arterial pressure secondary to loss of efferent neural control of kidney function and subsequent changes in blood volume. To the contrary, there is a building consensus that the response of arterial pressure to renal denervation is due to destruction of sensory fibers from the kidney, resulting in decreased SNA to other vascular beds such as skeletal muscle, as was recently reported in humans [25•].
In regard to the contribution of renal nerves specifically to AngII-induced hypertension, a number of studies have consistently found that renal SNA is decreased in this model, regardless of salt intake. Indirect assessment of renal SNA in dogs suggested that it was decreased in Ang II-induced hypertension [12], a finding that was later confirmed in rabbits in the first study to record SNA directly over a period of weeks [11]. We recently reported similar results using direct long-term recording of renal SNA in AngII-salt rats [10•]. In addition, renal denervation does not prevent AngII-salt hypertension in the rat [9•] or AngII-induced hypertension in the rabbit [19]. Although these observations have been used as an argument against the role of the sympathetic nervous system in AngII-induced hypertension [26], this view assumes that the kidney is the only neural target that can result in hypertension and disregards the contribution to the pathogenesis of neurogenic hypertension of changes in SNA to nonrenal vascular beds.
We have addressed this issue by using a number of indirect and direct methods to assess the relative importance of SNA to renal and nonrenal vascular beds in AngII-salt hypertension. Based on direct, long-term recording of lumbar SNA and hindlimb NE spillover [10•], as well as lumbar sympathectomy (Fink, unpublished observation), we conclude that SNA to skeletal muscle does not contribute to AngII-salt hypertension. On the other hand, in contrast to the finding that renal denervation has no effect on this model, denervation of the splanchnic vascular bed by celiac ganglionectomy (CGX) markedly attenuates the neurogenic phase of AngII-salt hypertension [9•]. This finding is consistent with an earlier study in which direct recording of splanchnic SNA revealed that it was increased in rats with AngII-induced hypertension compared with normotensive controls [27]. Collectively, these studies demonstrate that SNA is differentially regulated in AngII-salt rats and, more importantly, suggest that the splanchnic vascular bed is the primary target of the sympathetic nervous system in this model of hypertension.
How Does Increased Splanchnic Nerve Activity Lead to Hypertension in Angiotensin II–Salt Rats?
The splanchnic vascular bed receives 25% to 30% of the total cardiac output and has a major role in blood storage, as 25% to 30% of the total circulating blood volume is in the splanchnic vasculature [28]. As such, an increase in sympathetic tone to its arterioles leads to a significant rise in total peripheral resistance, and an increase in sympathetic tone to its small veins and venules results in a significant mobilization of blood to the central veins and an enhanced cardiac output via a rise in right atrial filling. The combined effect leads to a redistribution of blood volume from the splanchnic venous circulation to the systemic arterial vascular compartment, causing a rise in arterial pressure. The importance of sympathetic control of the splanchnic vascular bed, and vascular capacitance in particular, in both experimental and human hypertension has been recently reviewed [28]. Here we summarize evidence for a contribution of sympathetic control of this vascular bed specifically to AngII-salt hypertension.
Studies by King and Fink demonstrated an enhanced venomotor tone, as quantified by measurement of mean circulatory filling pressure (MCFP) and blood volume, in AngII-salt hypertensive rats, suggesting that decreased vascular capacitance contributes to this model [29]. Importantly, they found that the increase in venomotor tone did not occur in rats on a normal-salt diet and was reversed by ganglionic blockade [29]. Moreover, in addition to attenuating hypertension, CGX also prevents the increase in MCFP in AngII-salt rats, suggesting that the combination of AngII and a high-salt diet activates sympathetic activity to the capacitance vessels of the splanchnic vascular bed. In addition to neural control of splanchnic capacitance vessels, continuous measurement of arterial pressure and superior mesenteric blood flow in AngII-salt rats indicates that resistance vessels are also constricted by splanchnic SNA [30]. AngII administration results in a sustained increase in splanchnic vascular resistance in rats ingesting a high-salt diet but not in rats on a low-salt diet [30]. Interestingly, the increase in splanchnic vascular resistance is mediated by non-neurogenic mechanisms during the first 5 days of AngII but thereafter appears to be largely neurogenic. These studies are consistent with the hypothesis that splanchnic SNA plays a prominent role in the pathogenesis of AngII-salt hypertension. Although we hypothesize that this role is due to an increase in splanchnic SNA, as has been reported for AngII-induced hypertension [27], the possibility that AngII-salt amplifies the release of NE and/or the splanchnic vascular responsiveness to NE remains to be investigated.
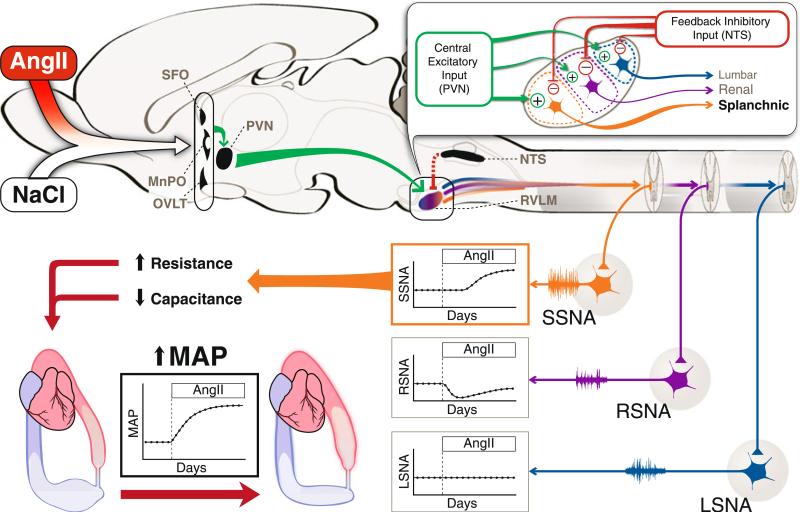
Taken together, these studies provide the first evidence for a neurogenic model of hypertension in which a nonrenal vascular bed is the primary target of the sympathetic nervous system. But a question still remains, particularly in the light of the dogma that all forms of hypertension are caused by reduced renal excretion of sodium and water and subsequent blood volume expansion [21]: How does increased splanchnic SNA cause hypertension in the absence of an increase in blood volume? As shown in Fig. 1 and described in detail in a recent “Exchange of Views” [31•], we hypothesize that the increase in SNA to the splanchnic vascular bed redistributes blood volume from the venous compartment to the arterial compartment, resulting in increased arterial pressure. We also predict that in order for this increase in arterial pressure to be sustained, the “renal function curve” resets secondary to this redistribution of blood volume [31•].
Fig. 1.
Central modulation of sympathetic outflow by circulating angiotensin II (AngII) and salt (NaCl). Circulating AngII and salt stimulates neurons in the forebrain circumventricular organs (subfornical organ, SFO; organum vasculosum of the lamina terminalis, OVLT), which leads to activation of neurons in the hypothalamic paraventricular nucleus (PVN) through direct or indirect projections via the median preoptic nucleus (MnPO). Benzamil-sensitive sodium channels are thought to be involved in transducing this salt signal. PVN activation leads to enhanced central drive to sympathetic premotor neurons in the rostral ventrolateral medulla (RVLM). The degree of enhanced central drive is hypothesized to be specific to different vascular beds, depicted as specific sets of RVLM neurons targeting the splanchnic, renal, or lumbar vascular regions. During the initial stage of hypertension, when direct vascular actions of AngII are the main contributor to the increase in mean arterial pressure, feedback inhibitory input from peripheral baroreceptors relayed through the nucleus of the solitary tract (NTS) counteracts the central drive in the RVLM. This inhibitory drive is thought to be strongest to RVLM neurons that modulate renal sympathetic nerve activity (RSNA), and the level of inhibition is coupled to the temporal pattern and degree of baroreceptor adaptation during the course of hypertension. The different level of excitatory and inhibitory input to specific populations of RVLM neurons results in a gradual rise in splanchnic SNA (SSNA), an initial decrease and gradual recovery of RSNA, and no change in lumbar SNA (LSNA). The gradual rise in SSNA and its influence on the splanchnic vascular bed, which stores a large proportion of total blood volume and receives a significant percentage of cardiac output, is thought to be the main contributor to the gradual rise in mean arterial pressure (MAP) during AngII-salt hypertension. A high SSNA causes vasoconstriction in arterioles, small veins, and venules, which contributes to an increase in total peripheral resistance and leads to reduced vascular capacitance. The combined effect mobilizes unstressed blood volume from the venous compartment into the arterial compartment, ultimately reflected as a rise in MAP
Angiotensin II–Salt Hypertension Is Dependent on Sodium-Sensitive Pathways in the Brain
The observation that the neurogenic component of AngII-induced hypertension is exacerbated by a high-salt diet suggests a synergistic interaction between circulating AngII and sodium-sensitive pathways that affect sympathetic activity [14]. We recently reported that the neurogenic phase of AngII-salt hypertension is abolished by intracerebroventricular (ICV) administration of the sodium channel blocker benzamil [32]. ICV benzamil or its analogue amiloride also prevents and/or reverses other salt-sensitive models of hypertension [33]. The type of sodium channel in the brain that is blocked by ICV benzamil is currently unknown, but the epithelial sodium channel (ENaC) has been proposed as a likely candidate [33]. ENaCs, which have a major role in modulating sodium transport in renal tubules, are modulated by circulating AngII [34] and play a critical role in regulating sodium balance and arterial pressure.
The fact that ENaCs may exist in the brain raises the possibility that salt-sensitive hypertension caused by administration of sodium-retaining hormones, such as AngII and aldosterone, combined with a high-salt diet may be due to increased activity of ENaCs (and therefore sodium transport) in the brain rather than in the renal tubules. “Benzamil-blockable” channels in the brain have been proposed to play a role in the hypertensive response to increased sodium concentration in cerebrospinal fluid [35] and neurogenic forms of salt-sensitive hypertension. However, the three subunits of ENaC have not been shown to be co-localized at the cellular level within the brain, and their precise location within sympathoregulatory pathways remains to be established. We hypothesize that ENaCs (or other benzamil-blockable sodium channels) are an important component of an osmosensitive (or sodium-sensitive) neural pathway originating in the lamina terminalis of the forebrain. Based on our observation that ICV benzamil blocks the activation of PVN neurons in DOCA-salt rats [33], we propose that activity in this pathway can drive increased SNA via inputs to the hypothalamic paraventricular nucleus (PVN) and sympathetic premotor neurons in the brainstem.
Central and Peripheral Mechanisms that Shape the “AngII-Salt Sympathetic Signature”
As described above, SNA in AngII-salt rats is not uniformly increased to all vascular beds but rather is differentially expressed over time. The “AngII-salt sympathetic signature” is shown in Fig. 1. In contrast to SNA to the hind limbs (lumbar SNA), which remains unchanged throughout the AngII administration period [10•], renal SNA initially decreases in response to AngII administration but gradually returns to control levels [10•]. Based on indirect measures, we hypothesize that the neurogenic phase of AngII-salt hypertension is due to a delayed activation of splanchnic SNA [9•, 18, 30].
What is the clinical significance of a disease-specific sympathetic signature in regard to new treatments? Primarily, it points to the development of novel sympatholytic therapies targeting sites within the neuraxis that are responsible for region-specific increases in SNA associated with hypertension and other diseases such as heart failure. To reach that goal, it would be desirable to understand the central and peripheral mechanisms that shape various signatures. For example, why in AngII-salt rats is renal SNA decreased transiently and the increase in splanchnic SNA delayed in onset? More specifically, what ultimately drives splanchnic SNA in this model of hypertension, and how does that lead to the cardiovascular responses that result in hypertension? Answering these questions will provide the framework required for the development of organ-specific or region-specific sympatholytic therapies that effectively treat hypertension and other diseases associated with regional sympathetic hyperactivity.
Figure 1 shows our current working hypothesis about the generation of the AngII-salt sympathetic signature. Although AngII-induced increases in peripheral sympathetic “tone” can be generated at any level of the neuraxis, significant evidence exists that AngII acts primarily in the brain to increase SNA activity ([15–17]. The mechanism of the sympathoexcitatory actions of circulating AngII, as well as the synergistic interaction with salt intake, likely involves forebrain circumventricular organs, which (owing to their poor blood-brain barrier) act as the key sensory sites linking blood-borne chemical signals to central autonomic pathways [36]. For example, the subfornical organ (SFO) is responsive to both circulating AngII and osmotic stimuli (ie, increased plasma sodium), and studies suggest that it may contribute to AngII-induced hypertension [37, 38]. In addition to the SFO, the organum vasculosum of the lamina terminalis (OVLT) has been proposed to be a central osmoreceptor and is known to transduce a number of osmotic stimuli into sympathoexcitatory responses [39]. Both the SFO and OVLT send projections to the median preoptic nucleus (MnPO), which acts as an integration and relay site with known excitatory projections to the PVN [40]. Neuronal activity in the PVN drives SNA by at least two pathways: excitatory projections to sympathetic premotor neurons in the rostral ventrolateral medulla (RVLM), and direct projections to preganglionic neurons in the spinal cord [39]. We hypothesize that circulating AngII and salt act in the SFO and OVLT to drive convergent sympathoexcitatory pathways in the brain that include the MnPO, PVN, and RVLM [14].
The RVLM, which is regarded as the major sympathetic premotor nucleus controlling peripheral vasomotor tone and blood pressure [41], has been reported to have a topo-graphical organization based on each vascular bed [42]. This provides a neuroanatomic substrate for generation of differential sympathetic outflow patterns such as that observed in AngII-salt hypertension. Represented in Fig. 1 (inset) are single sympathetic premotor RVLM neurons that drive lumbar, renal, and splanchnic SNA. Each neuron receives a central excitatory input driven by the forebrain structures mentioned above; this input ultimately activates the PVN-RVLM pathway. In addition, each RVLM neuron also receives an inhibitory input that is driven by signals from aortic and carotid arterial baroreceptor afferents to the nucleus tractus solitarius (NTS), which are relayed via the caudal ventrolateral medulla [42]. The output of each spinally projecting RVLM neuron is then determined by the strength of these excitatory (centrally driven) and inhibitory (baroreceptor) inputs.
Although this is a highly simplified scheme, it serves as a starting point to understand how the AngII-salt sympathetic signature may be generated. We hypothesize that time-dependent changes in both central (excitatory) and baroreceptor (inhibitory) inputs ultimately drive the AngII-salt sympathetic signature as follows: All of our studies to date suggest that hypertension during the first 5 days of AngII-salt treatment is the result of nonneurogenic mechanisms such as direct vasoconstriction, consistent with our observations that SNA to the splanchnic, renal, and hindlimb vascular beds is not increased during this time [13]. The transient decrease in renal SNA is consistent with a strong input from arterial baroreceptors during the initial phase of AngII-salt hypertension, but, as a result of baroreceptor adaptation, renal SNA returns to control levels at steady-state. The fact that renal SNA is not increased once baroreceptor adaptation has occurred suggests that the central excitatory drive to RVLM neurons that target renal preganglionic neurons is relatively weak. This hypothesis is supported by two observations: first, surgical denervation of arterial baroreceptors results in a more rapid increase in arterial pressure during the initial phase but has no effect on the steady-state level of arterial pressure in AngII-induced hypertension [43, 44]; and second, the AngII-induced decrease in renal SNA does not occur in baroreceptordenervated animals [43]. It is also interesting to note that chronic electrical stimulation of arterial baroreceptors is effective in decreasing arterial pressure in dogs with obesity-induced hypertension [45], in which renal nerves are critical to hypertension development [46], but not in dogs with AngII-induced hypertension [47]. In contrast to renal-targeted RVLM neurons, which we propose are predominantly controlled by baroreceptor input, RVLM neurons regulating SNA to the hindlimb appear to be equally controlled by central excitatory and peripheral inhibitory inputs in AngII-salt treated rats. This scheme is suggested by the observation that in both rats and humans, acute AngII administration has no effect on SNA to skeletal muscle when baroreceptor input is intact, but increases SNA in the absence of baroreceptor input [48]. Finally, we hypothesize that the AngII-salt–induced increase in splanchnic SNA is the result of a delayed increase in central excitatory input to RVLM neurons that control splanchnic preganglionic neurons, and that baroreceptor input to these neurons is weaker than the input to RVLM neurons controlling renal and lumbar SNA.
The hypothesis presented in Fig. 1 is supported by the studies reviewed above but remains to be fully tested by further investigation. We acknowledge that there are several other potential mechanisms for the generation of the AngII-salt sympathetic signature. For example, it may also be generated exclusively by central pathways such that the excitatory inputs (and possibly centrally driven inhibitory inputs) are differentially expressed to drive splanchnic, renal, and lumbar SNA. Future studies are needed to address these possibilities.
Clinical Significance: A Model for the Development of Region-Specific Sympatholytic Therapies
Findings from studies designed to test or challenge the hypothesis shown in Fig. 1 will provide new knowledge useful for the development of novel “targeted sympathetic blockade” therapies that are tailored to treat disease-specific sympathetic signatures. With the recent report of a catheter-based device for denervation of human kidneys to treat drug-resistant hypertension [2•], such an approach is no longer purely theoretical. The design concept for this device was based on the theory that neurogenic hypertension is due to increased sympathetic activity to the kidneys. This hypothesis initially appeared to be supported by the fact that arterial pressure is reduced for 2 years following a single renal denervation procedure. However, in one case report in which SNA to skeletal muscle was measured before and after renal denervation, it was found to be reduced from 56 bursts per minute before the procedure to 41 bursts per minute 1 month later and 19 bursts per minute 1 year after the procedure [25•]. This observation, coupled with the fact that the magnitude of the decrease in whole-body NE spillover following renal denervation cannot be explained by loss of renal efferent activity alone, suggests that the procedure also decreases SNA to nonrenal vascular beds [25•]. That decrease may be secondary to destruction of sympathoexcitatory renal afferent nerves and may account for a major portion of the antihypertensive response to the procedure. This idea implies that an important direction for the future may be the development of region-specific sympatholytic therapies that affect non-renal vascular beds. These therapies could target either the central pathways responsible for region-specific increases in SNA or the peripheral pathways, such as sympathetic ganglia.
Based on studies of the AngII-salt model reviewed here, the splanchnic vascular bed may be a logical target for such therapies. Indeed, before the development of antihypertensive drugs, surgical denervation of the splanchnic vascular bed was an effective treatment for hypertension, although it was associated with numerous adverse effects [3], which may have resulted from the radical nature of these extensive surgical procedures. Several improved methods now exist for blocking the celiac ganglion [49] or splanchnic nerves [50] to treat cancer pain in humans using minimally invasive endoscopic approaches. Therefore, based on the recent success of renal denervation to treat human hyper-tension, it reasonable to again investigate targeting neural control of the splanchnic circulation as well.
Conclusions
Investigations of the AngII-salt model of hypertension provide new insight into the concept of disease-specific sympathetic signatures and, more specifically, the contribution of sympathetic activity to the splanchnic vascular bed in the pathogenesis of salt-sensitive hypertension. This novel perspective opens possibilities for the development of new therapies designed to treat neurogenic forms of hypertension and other diseases associated with increased sympathetic activity. Further studies are needed to establish the extent to which increased splanchnic nerve activity contributes to other experimental models of hypertension. The recent clinical success of targeted renal nerve ablation for the treatment of uncontrolled hypertension has proven that selective targeting of peripheral nerves is a viable therapeutic option in hypertension. As such treatment modalities gain momentum and a larger cohort is included in trials, the celiac plexus may prove to be a desirable target for treatment of some forms of human hypertension.
Footnotes
Disclosure Conflicts of interest: J. Osborn: None; G. Fink: Payment for expert testimony on behalf of Daiichi-Sankyo in a patent case; M. Kuroki: None.
Contributor Information
John W. Osborn, Department of Integrative Biology and Physiology, University of Minnesota, 6-125 Jackson Hall, Minneapolis, MN 55455, USA
Gregory D. Fink, Department of Pharmacology and Toxicology, Michigan State University, East Lansing, MI 48824, USA
Marcos T. Kuroki, Department of Integrative Biology and Physiology, University of Minnesota, 6-125 Jackson Hall, Minneapolis, MN 55455, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Chobanian AV. The hypertension paradox—More uncontrolled disease despite improved therapy. N Engl J Med. 2009;361:878–97. doi: 10.1056/NEJMsa0903829. [DOI] [PubMed] [Google Scholar]
- 2 •.Symplicity HTN-2 Investigators: Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [This study demonstrated long-lasting decreases in arterial pressure in patients with drug-resistant hypertension following bilateral renal denervation by radiofrequency ablation. This new technology suggests that regional sympathetic ablation may be a new direction for the development of devices to treat hypertension.] [DOI] [PubMed] [Google Scholar]
- 3.Smithwick RH. Splanchnicectomy for essential hypertension; results in 1, 266 cases. JAMA. 1953;152(16):1501–4. doi: 10.1001/jama.1953.03690160001001. [DOI] [PubMed] [Google Scholar]
- 4.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:995–1055. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 5.Esler M, Lambert E, Schlaich MP. Chronic activation of the sympathetic nervous system is the dominant contributor to systemic hypertension. J Appl Physiol. 2010;109(6):1996–8. doi: 10.1152/japplphysiol.00182.2010. [DOI] [PubMed] [Google Scholar]
- 6.DiBona GF. Sympathetic nervous system and kidney in hypertension. Curr Opin in Nephrol and Hypertension. 2002;11:197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 7.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77(1):75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 8.Osborn JL, Roman RJ, Ewens JD. Renal nerves and the development of Dahl salt-sensitive hypertension. Hypertension. 1988;11:523–8. doi: 10.1161/01.hyp.11.6.523. [DOI] [PubMed] [Google Scholar]
- 9 •.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50(3):547–56. doi: 10.1161/HYPERTENSIONAHA.107.090696. [This the first report that clearly demonstrates that experimental neurogenic hypertension is driven by increased sympathetic nerve activity to a nonrenal vascular bed; (ie, the splanchnic circulation).] [DOI] [PubMed] [Google Scholar]
- 10 •.Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension. 2010;55:644–51. doi: 10.1161/HYPERTENSIONAHA.109.145110. [This is the first report in any species of direct long-term recording of sympathetic nerve activity (SNA) to two vascular beds in a model of cardiovascular disease. Although the AngII-salt model is neurogenically driven, SNA decreased to the kidneys and remained unchanged to the hind limb.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What Sets the Long-Term Level of Renal Sympathetic Nerve Activity. A Role for Angiotensin II and Baroreflexes? Circ Res. 2003;92:1339–6. doi: 10.1161/01.RES.0000078346.60663.A0. [DOI] [PubMed] [Google Scholar]
- 12.Lohmeier TE, Hildbrand DA. Renal Nerves Promote Sodium Excretion in Angiotensin-Induced Hypertension. Hypertension. 1998;31:429–34. doi: 10.1161/01.hyp.31.1.429. [DOI] [PubMed] [Google Scholar]
- 13.Osborn JW, Fink GD. Region-specific changes in sympathetic nerve activity in angiotensin II-salt hypertension in the rat. Exp Physiol. 2010;95(1):61–8. doi: 10.1113/expphysiol.2008.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9(3):228–35. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 15.Brody MJ, Haywood JR, Touw KB. Neural mechanisms in hypertension. AnnRevPhysiol. 1980;42:441–53. doi: 10.1146/annurev.ph.42.030180.002301. [DOI] [PubMed] [Google Scholar]
- 16.Fink GD. Long-term sympathoexcitatory effect of angiotensin II: A mechanism of spontaneous and renovascular hypertension. Clin Exp Pharm and Phys. 1997;24(1):91–5. doi: 10.1111/j.1440-1681.1997.tb01789.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferrario CM. Neurogenic actions of angiotensin II. Hypertension. 1983;5(suppl V):V73–9. doi: 10.1161/01.hyp.5.6_pt_3.v73. [DOI] [PubMed] [Google Scholar]
- 18.King AJ, Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1262–7. doi: 10.1152/ajpregu.00819.2007. [DOI] [PubMed] [Google Scholar]
- 19.Burke SL, Evans RG, Moretti JL, Head GA. Levels of renal and extrarenal sympathetic drive in angiotensin II-induced hypertension. Hypertension. 2008;51(4):878–83. doi: 10.1161/HYPERTENSIONAHA.107.100800. [DOI] [PubMed] [Google Scholar]
- 20.Kline RL, Chow K- Y, Mercer PF. Does enhanced sympathetic tone contribute to angiotensin II hypertension in rats? Eur J Pharmacol. 1990;184:109–18. doi: 10.1016/0014-2999(90)90671-r. [DOI] [PubMed] [Google Scholar]
- 21.Guyton AC, Coleman TG, Cowley Jr AW, Manning Jr RD, Norman RA JR, Ferguson JD. A systems analysis approach to understanding long-range arterial blood pressure control and hypertension. Circulation Res. 1974;35:159–69. [Google Scholar]
- 22.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol. 2005;289(4):H1519–28. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 23.DiBona GF. Neural control of renal function: Cardiovascular implications. Hypertension. 1989;13:539–48. doi: 10.1161/01.hyp.13.6.539. [DOI] [PubMed] [Google Scholar]
- 24.Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler M. Renal denervation as a therapeutic approach for hypertension: Novel implications for an old concept. Hypertension. 2009;54:1195–201. doi: 10.1161/HYPERTENSIONAHA.109.138610. [DOI] [PubMed] [Google Scholar]
- 25 •.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler M. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361(9):932–4.. doi: 10.1056/NEJMc0904179. [In this case report, the effect of bilateral renal denervation on muscle sympathetic nerve activity (SNA) was investigated. This activity was found to decrease dramatically, suggesting that the decrease in arterial pressure following renal denervation may be due to a decrease not in renal SNA, but in SNA to other vascular beds.] [DOI] [PubMed] [Google Scholar]
- 26.Navar LG. Counterpoint: Activation of the intrarenal reninangiotensin system is the dominant contributor to systemic hypertension. J Appl Physiol. 2010;109:1998–2000. doi: 10.1152/japplphysiol.00182.2010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luft FC, Wilcox CS, Unger T, Kuhn R, Demmert G, Rohmeiss P, et al. Angiotensin-induced hypertension in the rat: Sympathetic nerve activity and prostaglandins. Hypertension. 1989;14:396–403. doi: 10.1161/01.hyp.14.4.396. [DOI] [PubMed] [Google Scholar]
- 28.Fink GD, Arthur C. Corcoran Memorial Lecture. Sympathetic activity, vascular capacitance, and long-term regulation of arterial pressure. Hypertension. 2009;53(2):307–12. doi: 10.1161/HYPERTENSIONAHA.108.119990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King AJ, Fink GD. Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms. Hypertension. 2006;48:927–33. doi: 10.1161/01.HYP.0000243799.84573.f8. [DOI] [PubMed] [Google Scholar]
- 30.Kuroki M, Guzman P, Fink GD, Osborn JW. Chronic administration of angiotensin II (AngII) to rats consuming a high salt diet causes mesenteric vasoconstriction by increasing sympathetic tone. FASEB J. 2010;24:809–18. [Google Scholar]
- 31 •.Osborn JW, Averina VA, Fink GD. Current computational models do not reveal the importance of the nervous system in long-term control of arterial pressure. Exp Physiol. 2009;94(4):381–97. doi: 10.1113/expphysiol.2008.043281. [This paper reviews the theoretical framework for the hypothesis that neurogenic hypertension can result from increased sympathetic activity to nonrenal vascular beds and redistribution of total blood volume.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsch DM, Guzman P, Rodriguez M, Toney GM, Osborn JW. Effect of intracerebroventricular (ICV) and subcutaneous (SC) benzamil administration on AngII-salt hypertension. FASEB J. 2010;24:982–9. [Google Scholar]
- 33.Abrams JM, Osborn JW. A role for benzamil-sensitive proteins of the central nervous system in the pathogenesis of salt-dependent hypertension. Clin Exp Pharmacol Physiol. 2008;35(5–6):687–94. doi: 10.1111/j.1440-1681.2008.04929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the coritical collecting duct via AT1 receptors. J Am Soc Nephrol. 2002;13:1131–5. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 35.Wang HW, Amin MS, El-Shahat E, Huang BS, Tuana BS, Leenen FH. Effects of central sodium on epithelial sodium channels in rat brain. Am J Physiol Regul Integr Comp Physiol. 2010;299:R222–33. doi: 10.1152/ajpregu.00834.2009. [DOI] [PubMed] [Google Scholar]
- 36.Johnson AK, Loewy AD. Circumventricular organs and their role in visceral functions. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. Oxford University Press; New York: 1990. pp. 247–67. [Google Scholar]
- 37.Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol. 2005;288(2):H680–5. doi: 10.1152/ajpheart.00823.2004. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman M, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2005;95:210–6. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 39.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177(1):43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- 40.Stocker SD, Toney GM. Median preoptic neurones projecting to the hypothalamic paraventricular nucleus respond to osmotic, circulating Ang II and baroreceptor input in the rat. J Physiol. 2005;(Pt 2):568, 599–615. doi: 10.1113/jphysiol.2005.094425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypertension: role of rostral ventral lateral medulla. Curr Hypertens Rep. 2003;5:262–8. doi: 10.1007/s11906-003-0030-0. [DOI] [PubMed] [Google Scholar]
- 42.Guyenet P. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–46. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 43.Barrett CJ, Guild SJ, Ramchandra R, Malpas SC. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension. 2005;46(1):168–72. doi: 10.1161/01.HYP.0000168047.09637.d4. [DOI] [PubMed] [Google Scholar]
- 44.Cowley Jr AW, DeClue JW. Quantification of baroreceptor influence on arterial pressure changes seen in primary angiotensin-induced hypertension in dogs. Circulation Research. 1976;39:779–87. doi: 10.1161/01.res.39.6.779. [DOI] [PubMed] [Google Scholar]
- 45.Lohmeier TE, Dwyer TM, Irwin MAR, Kieval RS. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension. 2007;49(6):1307–14. doi: 10.1161/HYPERTENSIONAHA.107.087874. [DOI] [PubMed] [Google Scholar]
- 46.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–7. doi: 10.1161/01.hyp.25.4.893. [DOI] [PubMed] [Google Scholar]
- 47.Lohmeier TE, Dwyer TM, Hildbrand DA, Irwin MAR, Serdar DJ, Kieval RS. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension. 2005;46:1194–200. doi: 10.1161/01.HYP.0000187011.44201.2e. [DOI] [PubMed] [Google Scholar]
- 48.Xu L, Sved AF. Acute sympathoexcitatory action of angiotensin II in conscious baroreceptor-denervated rats. Am J Physiol. 2002;283:R451–9. doi: 10.1152/ajpregu.00648.2001. [DOI] [PubMed] [Google Scholar]
- 49.Levy MJ. New approaches, including targeting the ganglia. Gastrointest Endosc. 2009;69(2 suppl):S166–71. doi: 10.1016/j.gie.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 50.Kang CM, Lee HY, Yang HJ, Jang HJ, Gil YC, Kim KS, et al. Bilateral thoracoscopic splanchnicectomy with sympathectomy for managing abdominal pain in cancer patients. Am J Surg. 2007;194(1):23–9. doi: 10.1016/j.amjsurg.2006.11.018. [DOI] [PubMed] [Google Scholar]