Abstract
Although sleep deprivation is known to heighten pain sensitivity, the mechanisms by which sleep modifies nociception are largely unknown. Few studies of sleep-pain interactions have utilized quantitative sensory testing models that implicate specific underlying physiologic mechanisms. One possibility, which is beginning to receive attention, is that differences in sleep may alter the analgesic effects of distraction. We utilized the heat-capsaicin nociceptive model to examine whether self-reported habitual sleep duration is associated with distraction analgesia, the degree of secondary hyperalgesia and skin flare, markers implicating both central and peripheral processes that heighten pain. Twenty-eight healthy participants completed three experimental sessions in a randomized within subjects design. In the pain only condition, pain was induced for approximately 70-minutes via application of heat and capsaicin to the dorsum of the non-dominant hand. Verbal pain ratings were obtained at regular intervals. In the distraction condition, identical procedures were followed, but during heat-capsaicin pain, subjects played a series of video games. The third session involved assessing performance on the video games (no capsaicin). Participants indicated their normal self-reported habitual sleep duration over the past month. Individuals who slept less than 6.5 hours/night in the month prior to the study experienced significantly less behavioral analgesia, increased skin flare and augmented secondary hyperalgesia. These findings suggest that reduced sleep time is associated with diminished analgesic benefits from distraction and/or individuals obtaining less sleep have a reduced ability to disengage from pain-related sensations. The secondary hyperalgesia finding may implicate central involvement, whereas enhanced skin flare response suggests that sleep duration may also impact peripheral inflammatory mechanisms.
Keywords: Sleep, Pain, Capsaicin, Distraction, Flare, Secondary Hyperalgesia
Introduction
Poor sleep, including reduced total sleep time, is a well-established consequence of chronic pain. However, prospective studies indicate that sleep disturbance is associated with risk for developing pain complaints (Gupta et al. 2007; Kaila-Kangas et al. 2006; Mikkelsson et al. 1999; Siivola et al. 2004), and following painful injury, has been associated with development of persistent post-injury pain (Castillo et al. 2006; Smith et al. 2008). Self-reported sleep duration the preceding night predicts next-day pain frequency (Edwards et al. 2008), and experimental manipulations suggest sleep fragmentation increases pain sensitivity and may alter descending noxious control systems implicated in the pathophysiology of chronic pain (Lentz et al. 1999; Smith et al. 2007). One process by which sleep might alter pain perception is via effects on higher order cognitive processes know to modulate pain, such as distraction.
Distraction reduces pain in laboratory settings, during medical procedures, and in the context of ongoing chronic pain (McCaul and Haugtvedt 1995; Piira et al. 2002; Simmons et al. 2004). Although sleep deprivation studies document that reduced sleep impairs neuropsychological performance (Durmer and Dinges 2005), only one study has evaluated whether sleep restriction alters distraction analgesia, an important theoretical question, as these phenomena may share underlying psychophysical mechanisms. This innovative, laser-evoked potential study found that sleep restriction diminished the analgesic effect of distraction, suggesting alterations in sleep may impair higher order attentional modulation of pain (Tiede et al. 2009). It is unclear from this work whether these findings generalize to longer lasting pain states that are more typical of chronic pain or to individual variation in sleep time. Sleep restriction has been shown to increase pro-inflammatory cytokines and spontaneous pain complaints (Haack et al. 2007; Irwin et al. 2006; Irwin et al. 2008; Opp 2005). Inflammation is an underlying factor in a number of chronic pain conditions and is linked to sleep disturbance. Thus, understanding the interaction between pain, sleep and inflammation is of strong clinical importance.
The heat-capsaicin experimental nociceptive model may provide insight into peripheral and central processes involved in the initiation and maintenance of some chronic pain conditions. When capsaicin is coupled with heat, it activates TRPV1 receptors to evoke a burning sensation, a localized skin flare response and an area of secondary hyperalgesia. The flare response is an index of neurogenic inflammation (Holzer 1988; Holzer 1991; Veronesi et al. 1999). Secondary hyperalgesia takes place in unaffected skin surrounding the heat-capsaicin stimulus and is an indirect measure of central sensitization (Treede et al. 1992). The heat-capsaicin nociceptive model has yet to be applied to understand the mechanisms by which sleep impacts the pain experience and the extent to which it alters distraction analgesia. Differences in sleep duration and how they affect flare and secondary hyperalgesia (both consequences of the capsaicin procedure) may also be of importance, considering recent reports suggesting association between sleep and inflammatory pain states (Simpson and Dinges 2007). In the current project, we report new findings from a secondary analysis of a distraction-analgesia study (Campbell et al. 2010). Here we examine the relationships of self-reported habitual sleep duration with distraction analgesia, skin flare, secondary hyperalgesia, and daily pain complaints.
Methods
A total of 32 healthy individuals, recruited through posted flyers, completed this study. One individual was dropped for failing to complete the sleep questionnaire and an additional three participant’s data were dropped as significant outliers (greater than 2 standard deviations from the mean). Thus 28 individuals (48% female) were included in the current analyses. All study-related procedures were approved by the Johns Hopkins Hospital Institutional Review Board. Eligibility criteria included having no pain condition or medical disorders, active alcohol or drug abuse problem, or use of narcotics, antidepressants, anticonvulsants, and muscle relaxants. Verbal and written informed consent was obtained upon arrival, after which participants completed a health history questionnaire, which included an item querying average sleep duration in the past month. Participants then underwent one of three psychophysical noxious testing sessions, of the same length, in a randomized order. After each capsaicin session, the area of flare and secondary hyperalgesia were measured as described below. Sessions were scheduled at least one week apart. Participants were provided with monetary compensation for their participation.
Pain Alone Session
A thick piece of non-porous dressing was applied to the skin at one of two randomized dorsal hand locations. Prior to application, a 6.25 cm2 hole was cut into the center of it (used to standardize the area of capsaicin cream application). Approximately 0.35 g of 10% capsaicin cream was applied inside this hole and evenly spread on the skin. The area was then covered by Tegaderm transparent dressing (3M Health Care, St. Paul, MN, USA). Topical capsaicin-induced pain varies strongly as a function of skin temperature and is most reliably measured with the application of heat (Dirks et al. 2003), thus a peltier-device heating element (Medoc US, Minneapolis, MN, USA) was strapped on the area with Velcro wrist straps. This device was held at a constant temperature of 38°C during the session. Pain is rated as moderately intense using this method and peaks at 15–20 min post-application, plateauing for approximately 1 hour afterwards (Anderson et al. 2002; Bencherif et al. 2002). Participants provided pain intensity ratings every five minutes on a 0–100 scale for 70 minutes. Following completion of the session, the capsaicin cream was removed from the skin and the area of flare and secondary hyperalgesia were measured as described below.
Pain + Distraction Session
Identical capsaicin and thermal device application procedures were followed during this session. Subjects began the distraction task - a series of video games that required a high level of attention twenty minutes following capsaicin and thermo deapplication. The distraction task consisted of 3 video games, each played for 15 minutes in a randomized order, with performance (i.e., scores) monitored and monetary incentives provided. The video games are arcade-style and award points for advancing through more difficult levels. These video games are conceptually similar to a visual stroop task, which requires sustained attention and working memory as subjects respond to a series of presented stimuli (Roelofs et al. 2003). In light of findings suggesting that more engaging distraction tasks produce greater distraction analgesia (Hoffman et al. 2003), and given that previous studies of distraction’s effect on pain have used only fairly brief applications of noxious stimuli and distraction (e.g., most noxious stimuli and the concurrent distraction task last for 1–4 minutes), we enhanced sustained engagement in the video game task by offering monetary incentives for successful performance (i.e., increasing cash payment for higher scores). Participants again provided pain intensity ratings, on a 0 to 100 scale, once every 5 minutes. Participants were instructed to rate their perceived pain every five minutes while continuing to play the video games, in order to maximize attention toward the distractor task. Following completion of the session, the capsaicin cream was removed from the skin and measures of flare and secondary hyperalgesia were obtained.
Distraction Alone Session
In the final session, the distraction task was conducted and no capsaicin was applied to the skin. Because participants did not undergo a pain-induction procedure during this session, no pain ratings were obtained. This session was included to assess any differences in video game performance between painful and non-painful trials.
Questionnaires
Sleep Duration in the past month
Due to recent evidence suggesting a link between sleep duration and pain perception (Edwards et al. 2008; Kelly et al. 2010), participants completed the sleep duration item from the Pittsburgh Sleep Quality Index (PSQI (Buysse et al. 1989)). Specifically, they responded to the following question, “During the past month, how many hours of actual sleep did you get at night? (This may be different than the number of hours you spent in bed.) Strong correlations have been noted between subjective sleep questions and more objective actigraphy measures in assessing night time sleep duration (Lockley et al. 1999).
Mood Questions
Given the relationship between mood and pain (Connelly et al. 2007; Tang et al. 2008) and potentially sleep (Argoff 2007; O’Brien et al. 2010; Palermo and Kiska 2005), participants were asked to describe their current mood prior to the testing sessions on a 10-point scale (anchored by “Extremely Good” to “Extremely Bad”).
Daily Pain Questions
Although all participants were healthy and pain free, subjects were asked to list the number of times they had painful occurrences over the past month, as well as the intensity of their pain during the episodes (including headache, muscle pain, dental pain, etc.) on a 0–100 severity scale.
Post Distraction Questions
At the end of each of the two distraction sessions, participants were asked to rate their level of attention focused on the video games on a 0–100 scale (0 = not at all distracted, 100 = completely distracted). During the Pain + Distraction session, participants also rated the degree to which playing the video games reduced the amount of pain they felt, as well as how much the pain interfered with performance on the video games.
Area of flare and secondary hyperalgesia measurements
Flare
Following removal of the capsaicin, the area of redness, or “flare,” initiated by the capsaicin procedure was traced from the back of the hand on to a sheet of acetate paper and measured using a digital planimeter (Planix 10S). Flare is the neurogenic inflammatory response (axon reflex vasodilation) associated with capsaicin and is commonly identified as the area of primary hyperalgesia (Carter 1991; Dray 1992).
Secondary Hyperalgesia
Assessment of secondary hyperalgesia was performed at and around the site of the thermode/capsaicin application. The area of secondary hyperalgesia was quantified with a normally non-painful mechanical probe (32mN force; mechanical hyperalgesia) by stimulating the skin distant from the treated area and slowly moving inward until the participant indicated the stimulus had become noxious or until the subject reported a definite change in sensation (i.e., burning, tenderness, more intense pricking). This marking was done along 8 radial arms (rostral-caudal, lateral-medial, etc.) in steps of 5 mm at intervals of approximately 1 second, and the borders were marked with a felt pen. The distances were traced to a sheet of acetate paper for later measurement and calculation of surface area similar to Flare described above (Frymoyer et al. 2007; Mathiesen et al. 2006).
Data Reduction and Analysis
Participants were coded based on self-reported habitual sleep duration in to ≤ 6.5 hours/night sleep duration and > 6.5 hours/night sleep duration groups. This cut point was selected based on studies demonstrating that individuals reporting less than 6.5 hours of total sleep at night are at increased risk for negative health-related outcomes, including all-cause mortality (Bonnet and Arand 1995; Kripke et al. 2002). Due to the relatively small sample size, we were unable to further divide the sample into “normal” and “long sleepers” (e.g. only one person, 3.2% of the sample, reportedly obtained > 8.5 hours). Following the 20-minute ramp-up period, pain ratings were averaged, by 15 minute increments, into early (minutes 21 to 35), middle (36 to 51) and late (52 to 67) ratings by session (see Figure 1). Repeated measures analysis of variance (ANOVA) was conducted on pain ratings during the Pain Alone and Pain + Distraction sessions in order to examine the effects of sleep group on behavioral analgesia. All analyses controlled for catastrophizing, a persistently negative mental set related to pain, as we have recently shown that the time course of distraction may vary by pain catastrophizing (Campbell et al. 2010). Sleep group served as a between-subjects independent variable, while Session and Time were within-subjects factors. ANOVAs were also conducted to evaluate any differences in self-reported mood, and degree of attention to the video games reported by each group during the Pain + Distraction session and the Distraction Alone session. Analyses also examined participants’ self-reported distraction induced analgesia, perceived interference by pain on game performance, and actual game performance. Benign daily pain responses, including pain severity, and frequency of pain were examined between the ≤ 6.5 hour sleep duration and the > 6.5 hour sleep duration groups. Finally, the area of flare and secondary hyperalgesia were compared across sleep groups.
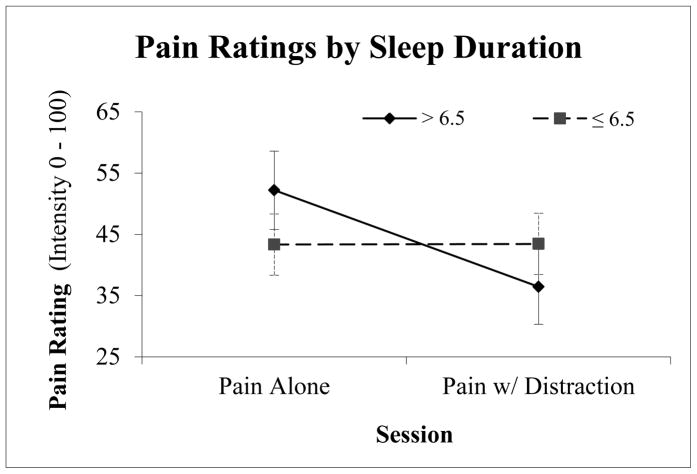
Figure 1.
Pain intensity ratings for the Pain Alone and Pain w/Distraction sessions for individuals sleeping more and less than 6.5 hours/night; error bars represent standard error.
Results
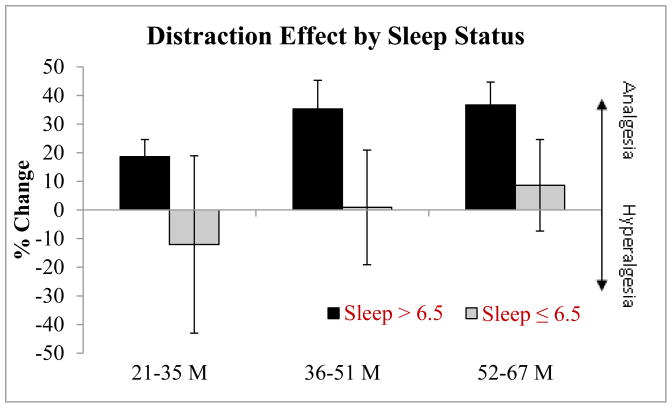
Demographic data for the two sleep groups are presented in Table 1; there were no significant differences in sex, age or ethnicity between the two groups. No overall difference between the two sleep groups emerged for ratings of pain intensity in the capsaicin alone (M=43.33, SD = 24.95 vs. M=48.25, SD 27.93 for the self-reported habitual ≤ 6.5 hr sleep duration and > 6.5 hr sleep duration groups respectively) or capsaicin with distraction sessions (M=43.44, SD=24.95; M=33.96, SD = 25.78 respectively; p’s > .05). A significant main effect of condition was observed (F(1,25) = 6.1, p = .02), suggesting the distraction task was successful, with ratings of capsaicin plus distraction (M = 39.9, SE = 3.1) substantially lower than capsaicin alone pain ratings (M = 47.4, SE = 4.3). A significant main effect of time also emerged (F(1,24) = 3.3, p = .05), with ratings subtly reducing over the three time points (M = 44.5, SD = 3.5; M = 43.9, SD = 3.6; M = 42.9, SD = 3.4, respectively). As hypothesized, a condition by group interaction emerged, such that participants reporting > 6.5 hours of sleep per night received significantly greater behavioral analgesia than those who reported ≤ 6.5 hours per night(F(1,25) = 5.3, p = .03), see Figure 1. These values are presented as a percent reduction from pain alone ratings in Figure 2. Self-reported ratings of mood prior to the sessions did not differ by group (p > .05). Ratings of the degree of attention focused on game play, perceived interference in game play due to pain, as well as objective video game performance (measured through individual’s video game scores) did not differ as a function of sleep (p’s > 0.05) or with pain (p > 0.05). Individuals reporting ≤ 6.5 hours per night over the preceding month reported a significantly greater number of daily pain experiences (M = 10.3, SD = 9.3) when compared to those sleeping > 6.5 hours (M= 2.5, SD = 2.1; F(1,23) = 12.1, p = .002). The intensity of daily pain was also significantly different between sleep groups, with those in the self-reported < 6.5 hr sleep duration reporting greater intensity (M = 29.5, SD = 22.5 vs. M = 12.3, SD = 16.3). No relationship was observed between daily pain frequency or intensity in pain alone or pain plus distraction ratings (p > .05). Interestingly, behavioral analgesia (calculated by subtracting pain ratings during video game play from pain alone ratings) was significantly associated with the frequency of daily pain (r = −.59, p = .002), such that the more analgesia received from distraction the less frequent the bouts of pain.
Table 1.
Demographic information for sleep duration groups
| Group | Sex (%female) | Age (std. dev.) | Ethnicity % |
|---|---|---|---|
| ≤ 6.5 (n=9) | 67% | 27.9 (6.5) | 33% non-Hispanic white |
| > 6.5 (n=19) | 42% | 25.3 (6.7) | 58% non-Hispanic white |
Figure 2.
Percent change from Pain Alone to Pain + Distraction ratings for individuals sleeping more and less than 6.5 hours/night (calculated by subtracting pain rating under distraction from the pain alone condition, divided by pain alone, times 100: higher change indicates greater analgesic effect); error bars represent standard error.
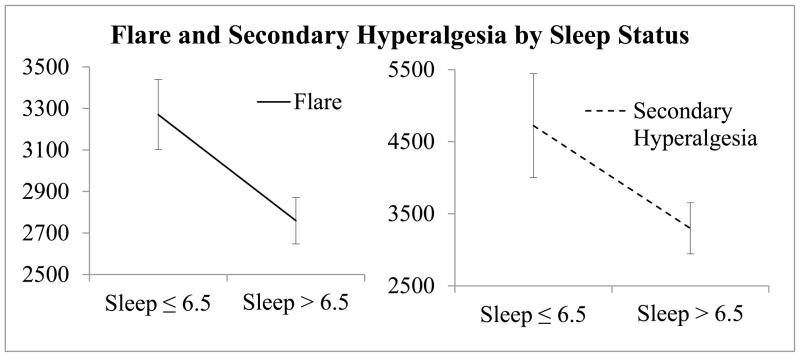
No differences emerged between the areas of flare or secondary hyperalgesia between Pain Alone and Pain plus Distraction sessions; therefore, the area of flare and secondary hyperalgesia were averaged across sessions. Those reporting ≤ 6.5 hour sleep duration had significantly larger areas of flare when compared to individuals in the > 6.5 hour sleep duration group (p = .02).
Individuals in the self-reported ≤ 6.5 hour sleep duration group also experienced a larger area of secondary hyperalgesia (p = .05), see Figure 3 for a graphical representation. The area of flare was associated with secondary hyperalgesia in > 6.5 hour sleepers only (r = .58, p = .01).
Figure 3.
Mean Flare and Secondary Hyperalgesia measurements (in mm2) for individuals sleeping more and less than 6.5 hours/night; error bars represent standard error.
The areas of flare and secondary hyperalgesia were significantly associated with the intensity of daily pain reported outside the laboratory (r = .47, p = .03 and r = .48, p = .02 respectively) and secondary hyperalgesia was additionally correlated to the frequency of pain reports (r = .5, p = .02).
Discussion
This study suggests that distraction, specifically video game play, is associated with reductions in heat-capsaicin pain ratings over an extended period (approximately 45 minutes) and that self-reported habitual sleep duration of ≤ 6.5 hours interacts with the degree of distraction analgesia such that this group derived less benefit from distraction, especially during the first two thirds of the procedure. This finding was particularly driven by the observation that within the 45 minute session, individuals with lower self-reported habitual sleep durations were delayed in experiencing the pain reducing effects of distraction. Additionally, these data are the first to report that less sleep is associated with secondary hyperalgesia, a marker of spinal facilitation implicated in the maintenance of a chronic pain, especially neuropathic pain states. Finally, we also found self-reported habitual sleep duration to be related to the degree of local skin flare, suggesting that sleep may also alter the pain experience by impacting peripheral processes involved in neurogenic inflammation. Reports of daily pain were also associated with sleep, behavioral analgesia, flare and secondary hyperalgesia, highlighting the potential clinical utility of the heat-capsaicin nociceptive model. Indices of mood, attention and perceived interference did not differ between the two groups and these results do not appear to be attributable to the possible effects of insufficient sleep on video game performance.
Distraction Analgesia and Sleep Duration
With respect to the distraction analgesia finding, a prominent daytime feature of sleep deprivation/disruption is diminished attention/vigilance (Lim and Dinges 2008) and poor cognitive performance on higher order tasks invoking working memory and executive functions (Bonnet 1985), processes which are presumably involved in active efforts to focus attention in the presence of salient information (nociception) that competes for cognitive processing/resources. Our finding that distraction analgesia is associated with reduced self-reported habitual sleep duration is consistent with recent findings (Tiede et al. 2009) that experimentally-induced shortened sleep (achieved through sleep restriction) was associated with weaker ability to disengage attention from pain. Interestingly, in the current study we did not find sleep-related differences in self-reported attention directed to the video game, or in video game performance scores, indicating that factors other than attention, perhaps working memory, may have been contributing to the effect of sleep duration on pain responses.
The mechanism by which short sleep duration is associated with distraction analgesia will require further investigation. Neuroimaging studies of distraction-related tasks, such as hypnosis and virtual reality completed during experimental noxious stimulation, have demonstrated that distraction reduces pain-related activation in both cortical and subcortical regions, broadly including the anterior cinlguate, insula, primary and secondary somatosenory cortices, and the thalamus (Valet et al. 2004). The location(s) and cerebral processes involved in the distraction analgesia effect, however, remain largely unknown, though some have speculated that distraction is likely to engage various descending nociceptive modualtory systems (Johnson 2005) in the medullar brain stem, including the periaquaductal grey and spinal dorsal horn (Bantick et al. 2002; Tracey et al. 2002). Both serotonergic and opioidergic neurotransmission play a primary role in these systems (Petrovic et al. 2000; Tracey et al. 2002). Prior work by our group investigated the possibility that experimental sleep fragmentation might impair endogenous opioid-dependent descending nociceptive inhibition. We found that experimental sleep fragmentation impaired diffuse noxious inhibitory controls wherein a tonic painful stimulus reduced the percept of a phasic noxious stimulus (Smith et al. 2007), a phenomenon thought to reflect descending inhibitory capacity.
Sleep Duration and Daily Pain
In the current sample of young, healthy individuals, self-reported habitual sleep duration of ≤ 6.5 was associated with a greater number of daily pain experiences and greater severity of pain when compared to individuals in the > 6.5 sleep duration group, which is consistent with prior studies suggesting that less sleep is associated with enhanced daily pain (Edwards et al. 2008; Onen et al. 2001). Interestingly behavioral analgesia (the amount of pain reduction under the distracted condition) was significantly associated with the frequency of pain over the preceding month; with greater distraction effects corresponding to less frequent bouts of pain. This suggests that the ability to engage in distraction may share mechanisms with and/or impact the experience of every day pain.
Sleep Duration and Hyperemia
Perhaps even more striking, was the robust finding that getting ≤ 6.5 hours of sleep was associated with increased hyperemia. The heat/capsaicin induced flare response involves neurogenic inflammation (Holzer 1998). Capsaicin is a selective excitotoxin activating vanilloid receptors localized on thin afferent terminals that elicit a burning pain sensation (Caterina et al. 1997). Specifically, stimulation of these primary afferent fibers elicits the release of: 1) calcitonin gene-related peptide (CGRP), 2) substance P and 3) neurokinin A (NKA) (Holzer 1998). These substances promote dilation of arterioles, sensitize primary afferents, mediate venular permeability, and promote recruitment of proinflammatory substances such as IL-B and prostaglandins (Coutaux et al. 2005). The overall function of this process is presumably to maintain homeostasis and promote tissue repair at the site of trauma; however, enhanced neurogenic inflammation is believed to play a role in the pain complaints of diseases such as rheumatoid arthritis (Frieri 2003; Levine et al. 1987; Schaible et al. 2002). Although we are not aware of any other studies which have utilized capsaicin to explore possible associations between neurogenic inflammation and sleep, a prior sleep deprivation study by Lentz and colleagues (Lentz et al. 1999) reported that selective slow wave sleep deprivation was associated with reactive erythema to a mechanical skin fold task in women. Exploration of specific neurochemical pathways by which sleep duration modulates neurogenic inflammation could have important functional implications for the role of sleep in homeostasis.
Experiments in healthy young adults demonstrate that even mild, short-term sleep loss activates inflammatory signaling (Irwin et al. 2008) and elevations in pro-inflammatory cytokines (Haack et al. 2007). For example, Haack and colleagues found that reducing sleep duration to four hours per night across 10 days produced elevated IL-6 and CRP levels when compared to individuals sleeping 8 hours. They also found that IL-6 was significantly associated with spontaneous pain ratings. In another recent study of middle-aged adults, a short habitual sleep time was associated with increased circulating CRP and IL-6 (Miller et al. 2009). Future work should assess how systemic inflammation interacts with local neurogenic inflammation and vice versa. It may be that individual differences in inflammatory processes could predispose individuals to develop chronic inflammatory pain conditions, and that clinical examination using the capsaicin nociceptive model may eventually be used as a simple and inexpensive method for identifying individuals at higher risk.
Sleep Duration and Secondary Hyperalgesia
In addition to positive associations between sleep duration and hyperemia, we also found sleep duration to be associated with enhanced secondary hyperalgesia, i.e. the region of enhanced mechanical sensitivity extending beyond the heat-capsaicin treated skin. Considerable experimental evidence suggests that sleep deprivation is associated with mechanical and thermal hyperalgesia in healthy subjects (Lautenbacher et al. 2006). Furthermore, we have shown that insomnia diagnosis is related to mechanical and thermal hyperalgesia in temporomandibular joint disorder, an idiopathic clinical pain condition (Smith et al. 2009). To our knowledge, the present study is the first to directly measure an association between sleep and secondary hyperalgesia. Unlike other quantitative sensory testing studies of sleep (Lautenbacher et al. 2006; Onen et al. 2001), capsaicin-induced secondary hyperalgesia implicates enhanced sensitization or hyperexcitability of dorsal horn inter neurons (Lamotte et al. 1991; Lamotte et al. 1992; Torebjork et al. 1992) and provides a functional marker for sensory activity at the spinal level (Morris et al. 1998). Such neuroplastic changes in the spinal cord are modulated by the complex interplay of peripheral and supraspinal inputs, and are believed to play a prominent role in the maintenance of chronic pain states (Cervero et al. 2003; Cervero and Laird 1996; Treede et al. 1992). The heat-capsaicin model has previously been validated as a practical, reliable tool for investigating experimental secondary hyperalgesia (Harding et al. 2001). Interestingly, secondary hyperalgesia may also be influenced by inflammatory mediators (Ma and Quirion 2007). Overall, the present data suggest that sleep disturbance may be associated with a generalized disturbance of nociceptive modulation at the level of the spinal cord. We cannot, however, determine from these data whether supraspinal processes, which are known to regulate second order spinal afferents, are also associated with reduced sleep. Morris and colleagues have shown that both Rheumatoid arthritis and Fibromyalgia patients display increased capsaicin-induced secondary hyperalgesia, suggesting enhanced spinal activity in these conditions (Morris et al. 1998). The authors suggest that this enhanced spinal activity is an important determinant of clinical features in these disorders. Future studies should examine the degree to which and what specific sleep parameters impact secondary hyperalgesia and whether experimentally modifying sleep impacts secondary hyperalgesia.
Endogenous opioids are also thought to be, at least in-part, responsible for analgesia produced by distraction. This is another potential explanation for the connection between daily pain and sleep, distraction, flare and secondary hyperalgesia. Fragmented sleep appears to affect the endogenous opioid nociceptive-inhibitory system, as assessed through DNIC (diffuse noxious inhibitory controls; a quantitative sensory testing technique thought to reflect endogenous opioid tone) (Smith et al. 2007) and this in turn may increase daily pain (Edwards et al. 2003) and potentially distraction effects. Goffaux and colleagues (Goffaux et al. 2007) found that secondary hyperalgesia was strongly influenced by descending signals that were presumably cortical in origin. Thus, endogenous opioid alterations may enhance facilitation of spinal cord pain processing, which may increase secondary hyperalgesia (Goffaux et al. 2007).
Limitations
The current study includes several limitations that should be noted when interpreting the results. Sleep was not manipulated; therefore causal interpretations cannot be made. All participants were young, healthy individuals undergoing laboratory-based pain induction procedures, which might limit generalizabilty. Although a number of studies have provided evidence for the clinical relevance of such studies in general (Fillingim and Lautenbacher 2004), and topical capsaicin in particular (Morris et al. 1998; Morris et al. 1997), and several studies note that distraction techniques may be quite useful in acute pain settings, some controversy exists over the efficacy of distraction in chronic pain conditions (Thorn 2004). Snijders and colleagues (Snijders et al. 2010) recently found that a computerized divided attention distractor task failed to attenuate pain ratings in chronic, unexplained pain patients. Thus, the relevance of this work to acute clinical pain and persistent pain conditions is unknown. A major limitation of this study is that sleep was assessed using a simple question of duration over the last month. Although self-reported sleep parameters such as duration are frequently used and have been validated against more objective measures (Lockley et al. 1999; Usui et al. 1999), other studies have found these measures to differ (Buysse et al. 2008; Van Den Berg et al. 2008). Future study is warranted to replicate these novel associations with polysomnography and/or other more objective measures of sleep such as actigraphy. Another limitation may be that the current study did not assess sleepiness in participants prior to undergoing testing. A recent study found that sleepiness-alertness was associated with analgesic efficacy (Steinmiller et al. 2010), thus it is unclear whether the current results may have been influenced by this factor. Another limitation may be that the current study did not examine blood pressure in response to capsaicin or video game play. A study by Raudenbush and colleagues found that video game play was associated with increased pulse, particularly during action, fighting, sports and boxing games (Raudenbush et al. 2009). Because we did not measure systolic or diastolic pressure or heart rate, the current study is unable to determine whether distraction analgesia may have been related to alterations in blood pressure. A final limitation may be that the distraction task was not long enough. It is unknown whether a longer follow-up period would have unveiled differences in sleep groups over extended periods of time. The current analysis suggests that distraction analgesia was increasing over time in both groups. The current study is unable to assess longer term effects of distraction and any potential difference in sleep duration groups at extended periods.
Summary and Future Directions
Despite these limitations, our findings provide evidence that shorter self-reported habitual sleep duration may reduce or delay the onset of distraction-induced analgesia. Future studies might benefit from examination of the differential physiological effects of sleep and distraction to gain a more thorough understanding of the mechanisms contributing to the differential analgesic effects of distraction depending on sleep duration. Manipulations involving an opioid antagonist and experimental sleep deprivation or restriction may be a useful next step in elucidating the underlying mechanisms by which sleep, and which aspects of sleep, shapes the experience of pain and analgesia. Clinically, it is noteworthy that it may be useful to start a distraction task earlier in patients obtaining insufficient sleep, or work to improve sleep in these patients before medical procedures in order to allow time for these analgesic processes to engage. Collectively, additional study seems warranted to characterize the potentially complex relationships between sleep duration, distraction, inflammation, and potentially endogenous opioid reactivity.
Acknowledgments
This work was supported by NIH grants AT001433 and F32 NS063624
Footnotes
Disclosures: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Anderson WS, Sheth RN, Bencherif B, Frost JJ, Campbell JN. Naloxone increases pain induced by topical capsaicin in healthy human volunteers. Pain. 2002 Sep;99(1–2):207–16. doi: 10.1016/s0304-3959(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Argoff CE. The coexistence of neuropathic pain, sleep, and psychiatric disorders: a novel treatment approach. Clin J Pain. 2007 Jan;23(1):15–22. doi: 10.1097/01.ajp.0000210945.27052.b3. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002 Feb;125(Pt 2):310–9. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Bencherif B, Fuchs PN, Sheth R, Dannals RF, Campbell JN, Frost JJ. Pain activation of human supraspinal opioid pathways as demonstrated by [11C]-carfentanil and positron emission tomography (PET) Pain. 2002 Oct;99(3):589–98. doi: 10.1016/S0304-3959(02)00266-X. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Effect of sleep disruption on sleep, performance, and mood. Sleep. 1985;8(1):11–9. doi: 10.1093/sleep/8.1.11. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. We are chronically sleep deprived. Sleep. 1995 Dec;18(10):908–11. doi: 10.1093/sleep/18.10.908. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008 Dec 15;4(6):563–71. [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Campbell CM, Witmer K, Simango M, Carteret A, Loggia ML, Campbell JN, et al. Catastrophizing delays the analgesic effect of distraction. Pain. 2010 May;149(2):202–7. doi: 10.1016/j.pain.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RB. Topical Capsaicin in the Treatment of Cutaneous Disorders. Drug Development Research. 1991;22:109–123. [Google Scholar]
- Castillo RC, MacKenzie EJ, Wegener ST, Bosse MJ. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006 Oct;124(3):321–9. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997 Oct 23;389(6653):816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JM. Mechanisms of allodynia: interactions between sensitive mechanoreceptors and nociceptors. Neuroreport. 1996 Jan 31;7(2):526–8. [PubMed] [Google Scholar]
- Cervero F, Laird JM, Garcia-Nicas E. Secondary hyperalgesia and presynaptic inhibition: an update. Eur J Pain. 2003;7(4):345–51. doi: 10.1016/s1090-3801(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Connelly M, Keefe FJ, Affleck G, Lumley MA, Anderson T, Waters S. Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. Pain. 2007 Sep;131(1–2):162–70. doi: 10.1016/j.pain.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutaux A, Adam F, Willer JC, Le BD. Hyperalgesia and allodynia: peripheral mechanisms. Joint Bone Spine. 2005 Oct;72(5):359–71. doi: 10.1016/j.jbspin.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Dirks J, Petersen KL, Dahl JB. The heat/capsaicin sensitization model: a methodologic study. J Pain. 2003 Apr;4(3):122–8. doi: 10.1054/jpai.2003.10. [DOI] [PubMed] [Google Scholar]
- Dray A. Neuropharmacological mechanisms of capsaicin and related substances. Biochem Pharmacol. 1992 Aug 18;44(4):611–5. doi: 10.1016/0006-2952(92)90393-w. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005 Mar;25(1):117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008 Jul;137(1):202–7. doi: 10.1016/j.pain.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Ness TJ, Weigent DA, Fillingim RB. Individual differences in diffuse noxious inhibitory controls (DNIC): association with clinical variables. Pain. 2003 Dec;106(3):427–37. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Lautenbacher S. The Importance of Quantitative Sensory Testing in the Clinical Setting. In: Lautenbacher S, Fillingim RB, editors. Pathophysiology of pain perception. New York: Kluwer Academic Plenum Publishers; 2004. [Google Scholar]
- Frieri M. Neuroimmunology and inflammation: implications for therapy of allergic and autoimmune diseases. Ann Allergy Asthma Immunol. 2003 Jun;90(6 Suppl 3):34–40. doi: 10.1016/s1081-1206(10)61658-4. [DOI] [PubMed] [Google Scholar]
- Frymoyer AR, Rowbotham MC, Petersen KL. Placebo-controlled comparison of a morphine/dextromethorphan combination with morphine on experimental pain and hyperalgesia in healthy volunteers. J Pain. 2007 Jan;8(1):19–25. doi: 10.1016/j.jpain.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Goffaux P, Redmond WJ, Rainville P, Marchand S. Descending analgesia--when the spine echoes what the brain expects. Pain. 2007 Jul;130(1–2):137–43. doi: 10.1016/j.pain.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Gupta A, Silman AJ, Ray D, Morriss R, Dickens C, Macfarlane GJ, et al. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology (Oxford) 2007 Apr;46(4):666–71. doi: 10.1093/rheumatology/kel363. [DOI] [PubMed] [Google Scholar]
- Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007 Sep 1;30(9):1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding LM, Murphy A, Kinnman E, Baranowski AP. Characterization of secondary hyperalgesia produced by topical capsaicin jelly--a new experimental tool for pain research. Eur J Pain. 2001;5(4):363–71. doi: 10.1053/eujp.2001.0253. [DOI] [PubMed] [Google Scholar]
- Hoffman HG, Richards T, Coda B, Richards A, Sharar SR. The illusion of presence in immersive virtual reality during an fMRI brain scan. Cyberpsychol Behav. 2003 Apr;6(2):127–31. doi: 10.1089/109493103321640310. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–68. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991 Jun;43(2):143–201. [PubMed] [Google Scholar]
- Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998 Jan;30(1):5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006 Sep 18;166(16):1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008 Sep 15;64(6):538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. How does distraction work in the management of pain? Curr Pain Headache Rep. 2005 Apr;9(2):90–5. doi: 10.1007/s11916-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Kaila-Kangas L, Kivimaki M, Harma M, Riihimaki H, Luukkonen R, Kirjonen J, et al. Sleep disturbances as predictors of hospitalization for back disorders-a 28-year follow-up of industrial employees. Spine. 2006 Jan 1;31(1):51–6. doi: 10.1097/01.brs.0000193902.45315.e5. [DOI] [PubMed] [Google Scholar]
- Kelly GA, Blake C, Power CK, O’keeffe D, Fullen BM. The Association Between Chronic Low Back Pain and Sleep: A Systematic Review. Clin J Pain. 2010 Sep 8; doi: 10.1097/AJP.0b013e3181f3bdd5. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002 Feb;59(2):131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Lamotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol Lond. 1992;448:749–64. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006 Oct;10(5):357–69. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999 Jul;26(7):1586–92. [PubMed] [Google Scholar]
- Levine JD, Goetzl EJ, Basbaum AI. Contribution of the nervous system to the pathophysiology of rheumatoid arthritis and other polyarthritides. Rheum Dis Clin North Am. 1987;13:369–83. [PubMed] [Google Scholar]
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999 Sep;8(3):175–83. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- Ma W, Quirion R. Inflammatory mediators modulating the transient receptor potential vanilloid 1 receptor: therapeutic targets to treat inflammatory and neuropathic pain. Expert Opin Ther Targets. 2007 Mar;11(3):307–20. doi: 10.1517/14728222.11.3.307. [DOI] [PubMed] [Google Scholar]
- Mathiesen O, Imbimbo BP, Hilsted KL, Fabbri L, Dahl JB. CHF3381, a N-methyl-D-aspartate receptor antagonist and monoamine oxidase-A inhibitor, attenuates secondary hyperalgesia in a human pain model. J Pain. 2006 Aug;7(8):565–74. doi: 10.1016/j.jpain.2006.02.004. [DOI] [PubMed] [Google Scholar]
- McCaul KD, Haugtvedt C. Attention, distraction and cold pressor pain. J Personal Soc Psychol. 1995;43(1):154–62. doi: 10.1037//0022-3514.43.1.154. [DOI] [PubMed] [Google Scholar]
- Mikkelsson M, Sourander A, Salminen JJ, Kautiainen H, Piha J. Widespread pain and neck pain in school children. A prospective one-year follow-up study. Acta Paediatr. 1999 Oct;88(10):1119–24. doi: 10.1080/08035259950168199. [DOI] [PubMed] [Google Scholar]
- Miller MA, Kandala NB, Kivimaki M, Kumari M, Brunner EJ, Lowe GD, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009 Jul 1;32(7):857–64. [PMC free article] [PubMed] [Google Scholar]
- Morris V, Cruwys S, Kidd B. Increased capsaicin-induced secondary hyperalgesia as a marker of abnormal sensory activity in patients with fibromyalgia. Neuroscience Letters. 1998 Jul 10;250(3):205–7. doi: 10.1016/s0304-3940(98)00443-1. [DOI] [PubMed] [Google Scholar]
- Morris VH, Cruwys SC, Kidd BL. Characterisation of capsaicin-induced mechanical hyperalgesia as a marker for altered nociceptive processing in patients with rheumatoid arthritis. Pain. 1997 Jun;71(2):179–86. doi: 10.1016/s0304-3959(97)03361-7. [DOI] [PubMed] [Google Scholar]
- O’Brien EM, Waxenberg LB, Atchison JW, Gremillion HA, Staud RM, McCrae CS, et al. Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clin J Pain. 2010 May;26(4):310–9. doi: 10.1097/AJP.0b013e3181c328e9. [DOI] [PubMed] [Google Scholar]
- Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001 Mar;10(1):35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- Opp MR. Cytokines and sleep. Sleep Med Rev. 2005 Oct;9(5):355–64. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Kiska R. Subjective sleep disturbances in adolescents with chronic pain: relationship to daily functioning and quality of life. J Pain. 2005 Mar;6(3):201–7. doi: 10.1016/j.jpain.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000 Mar;85(1–2):19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- Piira T, Taplin JE, Goodenough B, von Baeyer CL. Cognitive-behavioural predictors of children’s tolerance of laboratory-induced pain: implications for clinical assessment and future directions. Behav Res Ther. 2002 May;40(5):571–84. doi: 10.1016/s0005-7967(01)00073-0. [DOI] [PubMed] [Google Scholar]
- Raudenbush B, Koon J, Cessna T, McCombs K. Effects of playing video games on pain response during a cold pressor task. Percept Mot Skills. 2009 Apr;108(2):439–48. doi: 10.2466/PMS.108.2.439-448. [DOI] [PubMed] [Google Scholar]
- Roelofs J, Peters ML, Vlaeyen JW. The modified Stroop paradigm as a measure of selective attention towards pain-related information in patients with chronic low back pain. Psychol Rep. 2003 Jun;92(3 Pt 1):707–15. doi: 10.2466/pr0.2003.92.3.707. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann N Y Acad Sci. 2002 Jun;966:343–54. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- Siivola SM, Levoska S, Latvala K, Hoskio E, Vanharanta H, Keinanen-Kiukaanniemi S. Predictive factors for neck and shoulder pain: a longitudinal study in young adults. Spine. 2004 Aug 1;29(15):1662–9. doi: 10.1097/01.brs.0000133644.29390.43. [DOI] [PubMed] [Google Scholar]
- Simmons D, Chabal C, Griffith J, Rausch M, Steele B. A clinical trial of distraction techniques for pain and anxiety control during cataract surgery. Insight. 2004 Oct;29(4):13–6. [PubMed] [Google Scholar]
- Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev. 2007 Dec;65(12 Pt 2):S244–S252. doi: 10.1111/j.1753-4887.2007.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007 Apr 1;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- Smith MT, Klick B, Kozachik S, Edwards RE, Holavanahalli R, Wiechman S, et al. Sleep onset insomnia symptoms during hospitalization for major burn injury predict chronic pain. Pain. 2008 Sep 15;138(3):497–506. doi: 10.1016/j.pain.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Peterson S, et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009 Jun 1;32(6):779–90. doi: 10.1093/sleep/32.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders TJ, Ramsey NF, Koerselman F, van GJ. Attentional modulation fails to attenuate the subjective pain experience in chronic, unexplained pain. Eur J Pain. 2010 Mar;14(3):282–10. doi: 10.1016/j.ejpain.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Steinmiller CL, Roehrs TA, Harris E, Hyde M, Greenwald MK, Roth T. Differential effect of codeine on thermal nociceptive sensitivity in sleepy versus non sleepy healthy subjects. Exp Clin Psychopharmacol. 2010 Jun;18(3):277–83. doi: 10.1037/a0018899. [DOI] [PubMed] [Google Scholar]
- Tang NK, Salkovskis PM, Hodges A, Wright KJ, Hanna M, Hester J. Effects of mood on pain responses and pain tolerance: an experimental study in chronic back pain patients. Pain. 2008 Aug 31;138(2):392–401. doi: 10.1016/j.pain.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Thorn BE. Cognitive Therapy for Chronic Pain: A Step-by-step Guide. Guilford Press; 2004. [Google Scholar]
- Tiede W, Magerl W, Baumgartner U, Durrer B, Ehlert U, Treede RD. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2009 Oct 26; doi: 10.1016/j.pain.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Torebjork HE, Lundberg LE, Lamotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol Lond. 1992;448:765–80. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002 Apr 1;22(7):2748–52. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Usui A, Ishizuka Y, Obinata I, Okado T, Fukuzawa H, Kanba S. Validity of sleep log compared with actigraphic sleep-wake state II. Psychiatry Clin Neurosci. 1999 Apr;53(2):183–4. doi: 10.1046/j.1440-1819.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004 Jun;109(3):399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Van Den Berg JF, Van Rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008 Sep;17(3):295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- Veronesi B, Oortgiesen M, Carter JD, Devlin RB. Particulate matter initiates inflammatory cytokine release by activation of capsaicin and acid receptors in a human bronchial epithelial cell line. Toxicol Appl Pharmacol. 1999 Jan 1;154(1):106–15. doi: 10.1006/taap.1998.8567. [DOI] [PubMed] [Google Scholar]