Abstract
This paper proposes a novel hypothetical model integrating formerly discrete theories of stress appraisal, neurobiological allostasis, automatic cognitive processing, and addictive behavior to elucidate how alcohol misuse and dependence are maintained and re-activated by stress. We outline a risk chain in which psychosocial stress initiates physiological arousal, perseverative cognition, and negative affect that, in turn, triggers automatized schema to compel alcohol consumption. This implicit cognitive process then leads to attentional biases toward alcohol, subjective experiences of craving, paradoxical increases in arousal and alcohol-related cognitions due to urge suppression, and palliative coping through drinking. When palliative coping relieves distress, it results in negative reinforcement conditioning that perpetuates the cycle by further sensitizing the system to future stressful encounters. This model has implications for development and implementation of innovative behavioral interventions (such as mindfulness training) that disrupt cognitive-affective mechanisms underpinning stress-precipitated dependence on alcohol.
Keywords: stress, alcohol dependence, implicit cognition, allostasis, mindfulness
INTRODUCTION
Alcohol dependence remains prevalent despite a century of intervention efforts. Even with apparently efficacious behavioral and pharmacological treatments, relapse following treatment is the norm, and long-term recovery rates are low. According to the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), 28.4% of persons ever treated for alcohol problems remain dependent on alcohol and 19.1% continue to exhibit alcohol abuse or subclinical dependence symptoms over the past year [1]. Hence, certain risk chains leading to the development and maintenance of alcohol dependence may be intractable to extant interventions. One such pathway may involve positive feedback loops between stress appraisal, emotion dysregulation, physiological arousal, implicit cognition, and palliative coping with alcohol. As components of this stress-initiated risk chain may be malleable to novel behavioral therapies targeting cognitive-affective mediators of pathogenic gene-environment interactions, further explication of the pathways underpinning stress-precipitated alcohol dependence appears warranted.
The etiology of alcohol use disorders is multifactorial, involving interactions between genetic, environmental, interpersonal, and individual factors. Over time, as alcohol consumption becomes compulsive, automatic appetitive behaviors begin to supersede controlled, volitional alcohol use [2]. Once patterns of recurrent, heavy drinking in response to stress and negative affect are established, self-regulatory cognitive control mechanisms are hijacked by the addictive process, and consumption of alcohol is continued despite willful intent to abstain. Even repeated exposure to aversive consequences may be insufficient to prevent drinking in the alcohol dependent individual. The question of why alcohol consumption persists in spite of, and perhaps, due to, stress has been the subject of theory and scientific investigation.
Stress appears to be a key mechanism underlying alcohol dependence, intensifying alcohol consumption and precipitating relapse; indeed, persons who drink alcohol to cope with stress and negative affect evidence significantly higher rates of lifetime and current alcohol dependence symptoms than persons who drink for other reasons [3], and increases in stress can precipitate a shift towards heavy and more frequent alcohol consumption [4]. Epidemiological evidence for the link between stress and drinking behavior has been found through analyses of NESARC data. Among adult past-year drinkers, 72.5% reported experiencing at least one stressful life event in the past year, and 23.2% had experienced 3 to 5 such stressors [4]. Drinkers who reported experiencing six or more stressful life events had consumed more than three times the amount of daily ethanol and evidenced more than thrice the frequency of heavy drinking compared to drinkers who had not experienced life stressors in the past year [4]. Each experience of a past-year stressful life event was associated with an increase in frequency of heavy drinking by 24% for men and 13% for women, and increases in stress were associated with heavier patterns of alcohol consumption [4]. Congruent with these findings, an event-history analysis of urban, young adults found that both distal and proximal exposure to stressful life events significantly predicted onset of alcohol dependence in a linear and additive fashion even after controlling for socioeconomic status and history of psychiatric disorder, implicating a possible causal role for life stress in the etiology of alcohol use disorders [5]. Clearly, life stress is prevalent among alcohol users, and is an important correlate of heavy drinking and alcohol dependence.
Early motivational theories posited a relationship between alcohol consumption and stress. The tension reduction hypothesis, originating from animal experiments [6], claims that stressful life circumstances motivate alcohol consumption, and under such aversive or conflict-laden conditions, alcohol decreases anxiety, which then reinforces subsequent alcohol consumption [7]. This theory parallels clinical observations that alcohol is often used to “self-medicate” aversive cognitive-emotional and psychophysiological sequelae of the stress response [8]. Yet, despite its initial popularity, the tension reduction hypothesis lost favor because there was little agreement regarding the conditions under which alcohol dampens the stress response, and some aversive conditions actually decrease alcohol consumption [e.g., 9]. Inconsistent evidence of tension reduction-related drinking motivations in humans has been attributed to differences in alcohol expectancies, that is, beliefs about alcohol’s supposedly ameliorative effect on distress [10].
In an influential paper addressing the putative stress-response-dampening effects of alcohol in humans, Levenson et al. [11] raised the possibility that cognitive factors might mediate the pharmacological effects of alcohol on physiological reactivity. Concomitantly, sons of male alcoholics have been shown to exhibit heightened autonomic stress responses that are dampened by the effects of alcohol [12]; such stress-response dampening has been shown to be highly correlated with executive function deficits indicative of prefrontal cortical dysfunction in descendants of alcoholic probands [13]. Pihl, Peterson, and Finn [14] hypothesized that persons who drink alcohol to reduce stress have neurocognitive tendencies towards misattributing threatening significance to novel stimuli, resulting in augmented arousal, while exhibiting attenuated responses to stimuli that require sustained attention for processing.
The relationship between attentional factors and stress-response-dampening was addressed in Steele and Joseph’s attention-allocation model [15]. This model posited that drinking reduces stress via alcohol myopia, that is, a pharmacologically-induced impairment in controlled cognitive processing coupled with a narrowed attentional focus onto immediate internal and external cues. Such myopia is hypothesized to reduce capacity for cognitive processing of stressful content in the face of a demanding task, and to limit attention to proximal stimuli rather than to future threats. Hence, this model predicts that alcohol consumption will reduce stress when attention to stressors is restricted or divided by task demands, a prediction that has been supported by several studies [15–18]. However, evidence suggests that, even without attentional manipulations, moderately high doses of alcohol can robustly reduce negative emotion [19]. Recent research has helped to reconcile this incongruity: using a social stress induction, alcohol was shown to exert direct stress-response-dampening effects on heart rate, galvanic skin response, and subjective anxiety, but the effects of drinking on stress-induced skin conductance responses were partially mediated by differences on a sustained attention task [20]. Hence, although the neuropharmacological properties of alcohol contribute to its anxiolytic effects, cognitive processes appear to be an important link in the association between stress and alcohol consumption.
Building on such earlier work, we argue that alcohol dependence is maintained, in part, by automatic and implicit cognitive processes which subvert and bypass the conscious desire to abstain from alcohol. We contend that stress and negative affect play a large role in activating appetitive automaticity and allostatic dysregulation underpinning alcohol dependence and relapse. We propose that the risk chain linking these pathogenic mechanisms may be explicated by a conceptual framework that integrates a transactional stress-coping model [21] with an allostatic model of alcohol dependence [22], a cognitive processing model of craving and compulsive alcohol use [23], and an affective processing model of negative reinforcement [24]. This integrated framework, which builds on our earlier conceptual model of stress, metacognition, and coping [25], describes a cybernetic system [26], that is, an informational circuit in which the causal flow loops back upon itself, with the output of the circuit (e.g., relapse) becoming its own input (i.e., a stressor) in further iterations of the cycle.
This article presents a new conceptual integration of formerly discrete theories of stress appraisal, neurobiological allostasis, automatic cognitive processing, and addictive behavior to explain how alcohol dependence is maintained and re-activated by stress. This conceptual framework has implications for development and implementation of innovative behavioral interventions that disrupt mechanisms underpinning stress-exacerbated dependence on alcohol.
THE HYPOTHETICAL MODEL: AN OVERVIEW
According to our integrated conceptual framework (depicted in Figure 1), the risk chain leading to stress-precipitated alcohol dependence involves a network of interlocking causal pathways between stress-reactivity, implicit cognitive operations, maladaptive cognitive control strategies, and reinforcement contingencies that organize and drive the appetitive, motivational states and drug-seeking behaviors that characterize this disorder. In brief, repeated alcohol misuse in the context of stress and negative affect establishes automatic alcohol-use action schemas that impel continued misuse of alcohol when primed by stress through automatized sequences of context-dependent behavior. Conscious inhibition of alcohol-use schemas manifests as the subjective experience of craving, a factor which drives alcohol use by amplifying psychophysiological arousal. Psychosocial stress and negative affect evoke automatic and non-automatic cognitive operations implicated in alcohol dependence, leading to increased motivation to consume alcohol as a means of palliative coping. In turn, such palliative coping through alcohol is sustained through negative reinforcement. Continuation of this self-perpetuating cycle, which may be conceptualized as a positive feedback loop, leads to an allostatic cycle of growing dependence on alcohol fueled by increasingly heightened sensitivity to stress. Hereafter we describe this model in full detail.
Figure 1.
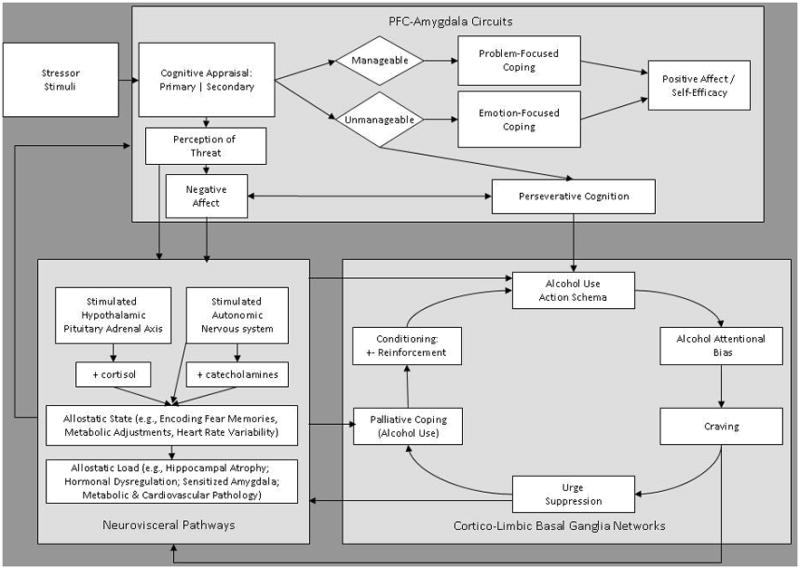
An Integrated Biopsychosocial Model of Automaticity, Allostasis, and Stress-Precipitated Alcohol Dependence
AN INTEGRATED BIOPSYCHOSOCIAL MODEL OF AUTOMATICITY, ALLOSTASIS, AND STRESS-PRECIPITATED ALCOHOL DEPENDENCE
Stress and cognitive appraisal activate the risk chain
Though some models of stress and addiction treat stress exposure as a monolithic concept, stress is a multicomponent process modulated by biopsychosocial factors. Among the numerous factors that influence the stress process, cognitive appraisal may be viewed as a central governor of the system. Although the stress concept derived from the physical sciences, biological organisms subjected to stressors are quite unlike inorganic objects which deform predictably and systematically under the external force of a load. Organisms actively construct their phenomenological experiences by coupling with the medium of the environment according to their own self-organizing structure [27]; in other words, an animal with “a nervous system perceives the world according to its own linkages and activities, not as a readout of some objective reality” [28]. Thus, humans encounter environmental stimuli and consciously and unconsciously appraise the meaning of events and situations in their according to their perceived relevance to self and others, perceptions which are shaped by the historical, sociocultural, and environmental context in which the individual is embedded.
This self-organizing evaluative process, known as appraisal, may fundamentally modulate physiological stimulus-response (S-R) relationships, allowing for substantial behavioral variation in the organism’s adaptation to the environment. Indeed, although an extensive range of diverse stimuli may activate the common set of cortical, sub-cortical, neuroendocrine, and autonomic systems involved in the stress response, appraisal accounts for qualitative and quantitative differences in stress reactivity within and between individuals. Exposed to the same stressor, one individual may respond with depression and helpless apathy, another with violence and rage, and a third with optimism and constructive, prosocial action.
A wide array of life events may initiate the stress process. In the face of war, economic recession, and ecological crisis, individuals and communities bear the brunt of societal and global catastrophes, compounded by the daily hassles of living. Vulnerable populations who are less able to cope due to a lack of social, economic, or cognitive-emotional resources evidence the adverse consequences of stress through mental and physical illness. Hence, stress-induced pathology presents a serious problem for a society increasingly subject to global and local strains, especially so for its most vulnerable members.
Furthermore, just as stress aggravates other forms of pathology, psychosocial stressors may exacerbate alcohol misuse. Individuals who experience social, legal, or work-related stressors report significantly more frequent heavy-drinking days than those persons who do not face such stressors [4]. Given the association between stress and heavy drinking and the higher prevalence of social, legal, and job-related stressors among poor persons, it follows that socioeconomic disadvantage is a significant correlate of higher levels of alcohol consumption. For example, past-year prevalence of alcohol dependence was highest among persons making less than $20,000 a year in 2002, and the odds of meeting criteria for alcohol dependence in the past year and over one’s lifetime are greatest for those with lower incomes [29]. Moreover, among poor persons, levels of job-related stress were positively correlated with higher quantity and frequency of alcohol consumption [4]. Although survey research cannot rule out the possibility of reciprocal causation, and clearly, heavy drinking may impede social and occupational functioning, it appears as if socioeconomic forces exert strain upon individuals that may result in stress-precipitated alcohol consumption.
Whether precipitated by psychosocial or physical stressors, the stress process initiates with a primary appraisal of stimuli for risk value. Appraisals may be automatic, executed without intention and performed without conscious deliberation [30]; for example, a meta-analysis has shown that predictions about the intent and future behaviors of others are typically made in less than 30 seconds [31]. Such rapid and unconscious appraisals may utilize hardwired reflexes, nondeclarative memory, and implicit cognitive operations, in contrast to intentional appraisal processes that rely upon declarative memory and propositional reasoning [32]. Implicit appraisals of threatening stimuli (e.g., angry facial expressions) facilitate survival and may have been naturally selected for during human evolution [33]. When a given stimulus is appraised as challenging, harmful, or threatening, an activation of physiological systems involved in the stress response co-occurs with the subjective experience of distress [21]. Subsequently, a cognitive process of secondary appraisal determines the sufficiency of available resources and coping options to meet the demands of the actual or potential threat.
From a biological perspective, appraisal may involve relaying visual, auditory, and somatic information about a stimulus from the thalamus to sensory processing areas of the cortex, activating affective processing circuits involving the amygdala, medial temporal lobe, and medial prefrontal and orbitofrontal cortices [34]. This neural circuitry appears to compute the hedonic or threat value of the stimulus according to previously established stimulus-reinforcement contingencies. For example, based on past experiences (e.g., previous encounters with strangers), a stimulus (e.g., facial expression of a passer-by on the street) is judged to be threatening, innocuous, or even rewarding. This computation may be modulated by prefrontal-amygdala circuits involving the ACC, prelimbic, and medial prefrontal cortices, which appear to temper and regulate stress reactivity through cognitive processing [34]. In addition, inputs from the prefrontal cortex (PFC) and hippocampus may provide the amygdala with information about the stimulus context, allowing for differentiation between stimuli that in one context would be appraised as benign and in another, as dangerous. Stress appraisals discriminating threatening from benign stimuli (e.g., snakes from flowers) can be made within 50 milliseconds [33]. Backwards masking experiments, involving brief presentation of a target stimulus immediately followed by a mask of random noise, show threat appraisals can occur without consciousness via implicitly conditioned, subcortical thalamic-amygdala pathways [35, 36]. Although stress appraisal is subserved by activity in cortical and limbic regions, it exerts downstream effects on the body through a number of pathways described below.
Stress-evoked activity in the amygdala results in concurrent activation of the hypothalamic-pituitary-adrenal (HPA) axis, the locus coeruleus, and the autonomic nervous system. Perception of threat triggers a neuroendocrine cascade from HPA axis, initiated by the central amygdala signaling the paraventricular nucleus of the hypothalamus to release corticotrophin-releasing hormone (CRH), stimulating the pituitary to secrete beta-endorphin and adrenocorticotropin (ACTH) which, in turn, trigger release of cortisol from the adrenal cortex [37]. Cortisol exerts effects on nearly every cell of the body to redirect regulatory processes to meet the perceived challenge; these changes include mobilizing cellular energy resources via induction of liver enzymes, decreasing digestion, modulating the trafficking of immune cells, and influencing inflammatory processes through cytokine production [38, 39]. Cortisol also facilitates the encoding of fear-based memories by influencing neurotransmission between the amygdala and hippocampus [40]. Such hormonal regulation is comparatively slow, occurring over periods of several minutes, hours, or days [41].
In addition to these slower hormonal responses, stress appraisal activates the locus coeruleus (LC) in the brainstem and the adrenals to release the catecholamines nonadrenaline and adrenaline, which increase heart rate, blood pressure, and blood flow to skeletal muscles and the brain during the “fight-or-flight response” [42, 43]. This stress-evoked survival response is also mediated through the rapid response (occurring within seconds) of sympathetic and parasympathetic neurons of the autonomic nervous system (ANS), which link PFC, amygdala, brainstem, viscera, and periphery to innervate muscle groups, drive and modulate the pacemaker of the heart, effect gastric contractions, stimulate sweat gland activity, and regulate shifts in body temperature [41]. Under typical conditions, the ANS is tonically inhibited by prefrontal cortical regions such as ACC, orbitofrontal, ventromedial, and insular cortices, but is disinhibited during threat perception to mobilize the body into defensive action [44].
If, during this complex cognitive process of appraisal, available resources (e.g., individual, familial, or communal) are deemed insufficient to address the challenge presented by the threatening stimulus, then biopsychosocial consequences of stress may result. Prolonged or repeated stress activation may lead to an allostatic state, a chronic deviation of self-regulatory mechanisms from their normal mode of operation that leads to heightened sensitivity to threat and vulnerability to future stressors [45]. Among allostatic mechanisms at work is a feed-forward cycle between the amygdala and the HPA axis, whereby the amygdala triggers the hypothalamus to release cortisol from the adrenal glands that, in turn, impairs hippocampal function while sensitizing the amygdala, leading to greater cortisol release during repeated exposures to the stressor [40]. Release of stress hormones also impairs PFC function, which inhibits successful emotion regulation and heightens future stress reactivity [46, 47]. This state of hyperarousal results in allostatic load, a “wear and tear” on the body involving consequences such as hippocampal atrophy [40], as well as neuroendocrine and cardiovascular [48] dysregulation. Hence, recurrent stress exerts deleterious effects on the body via a prolonged state of arousal that may ultimately result in drinking as a means of relieving distress if unchecked by effective coping efforts.
Problem- and emotion-focused coping ameliorate stress
Once an event is appraised as stressful, the individual may utilize problem- and emotion-focused coping efforts to deal with the stressor. Problem-focused coping consists of strategic attempts to manage or resolve the stressful event by gathering information, making decisions, and resolving conflict. Positive emotion can be generated when, as a result of successful coping efforts, the stressor event is resolved favorably; however, biopsychosocial distress intensifies when coping attempts are unsuccessful and the stressor is not resolved [21]. Lack of a favorable resolution may lead to deployment of emotion-focused coping efforts to manage the distress itself (e.g., positive reappraisal, a cognitive-affective regulatory strategy of re-interpreting the stressor event as benign or meaningful). Positive reappraisal, which appears to engage the PFC and anterior cingulate cortex (ACC) to inhibit activation of the amygdala [49], may attenuate negative emotions via the re-construal of the stressor event as meaningful and growth-promoting.
Positive reappraisal is an active coping strategy, rather than a defense mechanism used to repress or deny [50]. Unlike suppression of negative emotions which can cause increased sympathetic nervous system activation [51], positive reappraisal does not typically lead to physiological or psychosocial complications [52]. In addition, positive reappraisal is often the first step toward a reengagement with the stressor event. For instance, a person stricken with a non-fatal heart attack might positively reappraise the event as an opportunity to change their lifestyle and subsequently begin to make changes in diet and exercise behaviors. Alternatively, a person who has recovered from cancer might view their survival of the disease as evidence of their strength and resilience, and they might decide to dedicate their life to helping others make similar recoveries. Hence, positive reappraisal is often an adaptive rather than an avoidant strategy. However, in the absence of adaptive coping, stress often leads to perseveration.
Perseverative cognition exacerbates stress
The stress response often results in perseverative cognition, a maladaptive process of fruitlessly maintaining a cognitive representation of the stressor in the absence of adaptive coping behavior [37]. Such perseveration may involve activation of circuits including the PFC, hippocampus, and extended amygdala, whereby computations about present environmental stimulus contingencies are colored by past aversively conditioned relations stored in explicit memory systems [34]. Regions in PFC that appear to provide top-down governance of the amygdala during stress appraisal may become impaired during anxiety states, leading to amplified threat perception [53]. Perseverative cognitive styles such as catastrophizing (i.e., exaggeration of the threat value of a stimulus) or rumination (the experience of recurrent, intrusive negative thoughts about an event), result in runaway positive feedback loops between cognitive stress-appraisal processes, negative affect, and sustained activation of the ANS and its visceral efferents. Protracted activation of this pathway disrupts homeostasis of body systems through cortisol- and catecholamine- mediated stress-responses [37]. In the case of an alcohol dependent individual early in the process of recovery, this activation compounds the physiological distress of conditions such as alcohol withdrawal.
Stress primes impulsive alcohol consumption via allostasis
Allostatic load from chronic, cognitively-driven, negative affective states may dysregulate stress and reward neurocircuitry within the extended amygdala, moving the brain reward set point from its normal level, resulting in decreased sensitivity to reward and increased sensitivity to punishment or aversive states [22]. As natural rewards lose their reinforcing value and aversive emotional states intensify under stress, this shift in reward and punishment thresholds may elicit increased consumption of alcohol as a means of maintaining a hedonic equilibrium. The attempt to achieve a hedonic state comes at a cost: continued alcohol consumption further shifts the reward set point in the brain, compounding stress-induced insensitivity to positively-valenced experiences while exacerbating reactivity to punishment, stress, and other aversive states. Alcohol-related increases in reward threshold are thought to be subserved by decreased activity of neurochemical systems implicated in the rewarding effects of alcohol (e.g., GABAergic, opioid, dopamingeric, serotonergic, and glutamatergic systems), while alcohol-related increases in stress reactivity are thought to be mediated by heightened activity of corticotrophin-releasing factor (CRF) stress systems and decreased activity of anxiolytic neuropeptide Y (NPY) systems [54].
Stress-induced dysregulation of hedonic processing may be particularly pernicious among alcohol dependent individuals, who tend to favor immediate gratification and discount delayed rewards [55]. This cognitive process of impulsive decision-making, as evidenced by choosing smaller, immediate rewards over larger, delayed rewards, may be mediated by increased activation in posterior parietal cortex, dorsal PFC, and parahippocampal gyrus regions [56], or a combination of decreased activity and structural abnormalities in orbitofrontal cortex [57]. Given their tendency toward impulsivity exacerbated by a possible neurobiological shift of the reward set point, abstinent alcohol dependent individuals may experience involitional relapse under stress as a means of achieving hedonic allostasis, despite potential future consequences of use. In this case, the impulse to drink may be subserved by automatic cognitive processes.
Automatic alcohol use schemata regulate addictive behavior
Stress can induce perseverative cognition and intense affective experiences of worry and dysphoria that may evoke alcohol use action schema, automatized, associative networks which are thought to encode information for the nonvolitional execution of alcohol-use behaviors [23]. Indeed, stress has been shown to bias responses toward habitual behaviors that are resistant to changes in outcome contingencies [58]. Frequent drinking in response to stressors initially leads to formation of behavior-outcome associations [59] as the palliative effects of alcohol negatively reinforce drinking behaviors. At first, such stress-precipitated drinking may stem from explicit expectancies that alcohol will provide relief from stress [60] based on past experience of the rewarding and hedonic effects of alcohol. Over time, repeated drinking under stressful circumstances can lead to stimulus-response habits which may not be affected by aversive consequences. For example, among rats, self-administration of alcohol is rendered undeterred or insensitive to conditioned aversion (e.g. illness due to alcohol being contaminated with lithium chloride) [61]. This finding from basic science parallels observations of intractable drinking in the face of severe, stress-inducing consequences such as loss of a spouse or job.
Such schemata may arise out of a history of repeated alcohol consumption in much the same way that other overlearned behavioral repertoires become automatized. S-R habits are established through repetition. After hundreds of repetitions of consistent responses to a given stimulus, attending and responding to that stimulus become automatic, leading to rapid processing in neural circuits involved in response execution [62]. Automaticity requires the consistent training of associations without varying S-R relationships [63]. During formation of automatic habits, a neurobiological shift occurs in which behaviors that were originally guided by associative networks involving PFC regions become controlled by sensorimotor cortico-basal ganglia networks [64]. Addictive consumption of alcohol appears to derive, in part, from an automatized stimulus-response habit.
Automaticity underlying compulsive drinking may be compounded by changes in brain reward circuits resulting from repeated alcohol consumption. Specifically, dopaminergic neuroadaptations in the nucleus accumbens and ventral striatum appear to result in sensitization to the rewarding effects of alcohol and alcohol-related cues [65]. Such heightened incentive salience may impart compulsivity to alcohol-seeking behaviors, motivating the alcohol dependent person to drink despite countervailing reasons to remain abstinent. Thus, cues such as the sight of a bar, an advertisement in a magazine, or a familiar “drinking buddy” can reflexively trigger the desire to consume alcohol, long after one has gone through withdrawal and even after extended periods of abstinence. Once alcohol has been obtained, it may be consumed automatically, guided by implicit cognitive schemata.
Alcohol use action schemata may be subserved by neural circuits between the dorsal cingulate cortex, hippocampus, amygdala, striatum, and nucleus accumbens where conditioning and context-encoding neural projections motivate appetitive behavior [66]. The rapid, automatic processing of addiction-related stimuli (including negative affective states and environmental-contextual stimulus configurations) via implicit schema may trigger conditioned appetitive and behavioral responses without deployment of conscious decision-making processes. Hence, the alcohol dependent person may find him or herself consuming alcohol without consciousness of the motive or intent to drink, in much the same way as other complex thought-action repertoires such as goal-pursuit can be engaged without conscious volition by conditioned contextual cues [30].
A body of research suggests that alcohol dependent, alcohol abusing, and heavy-drinking individuals process alcohol cues differently than neutral cues. Indeed, a meta-analysis of 17 studies found that persons with alcohol use disorders evidence significantly slowed cognitive processing of alcohol-related stimuli [67]. However, slowed reaction times to alcohol cues on the addiction Stroop task may alternately index exogenous engagement of attention, capture of cognitive resources, or elicitation of subjective craving; all three processes may result in cognitive load which may impede goal-directed behavior [68]. However, whether such disruption of cognitive resources contributes to alcoholics’ self-reported difficulty in using coping skills to resist alcohol cravings remains to be tested. Regardless, such automatic cognitive processes appear to exert a significant influence on drinking. Implicit memory associations of alcohol with positive outcomes (e.g., providing relief from stress) are among the strongest predictors of future drinking behavior, even after controlling for lifetime alcohol use, explicit alcohol expectancies, and sociodemographic and personality variables [69]. Automatic alcohol approach associations have been associated with urge to drink after exposure to alcohol [70]. Moreover, stress and negative affect seem to facilitate automatic processing of alcohol cues. In contrast to problem drinkers who reported low levels of psychiatric distress, among problem drinkers high in psychiatric distress, negative affective words primed responses (i.e., speeded reaction times) to alcohol words [71]. Similarly, using the addiction Stroop task, Stewart and colleagues found that persons who drank alcohol to cope with stress exhibited priming effects to negative mood cues on alcohol words [72]. Hence, as stress biases behavioral strategies toward habitual responding, persons with well-established alcohol use action schemata may be subject to involuntary and implicit cognitive processing of alcohol cues that in turn impel alcohol consumption.
Alcohol attentional bias is linked to craving
Engagement of alcohol use action schemata may result in automatic processing of salient stimuli, manifested as an involuntary attentional bias towards alcohol cues. On visual probe tasks, heavy drinkers compared to light social drinkers preferentially attend to alcohol-relevant stimuli, evidencing decreased reaction times to probes replacing alcohol photographs relative to those replacing neutral photos [68]. In heavy drinkers, this bias occurs for alcohol cues presented for durations of 500 ms and 2000 ms, and not for stimuli presented only for 200 ms [73]. By contrast, abstinent alcohol abusers evidenced an attentional bias for alcohol-related photos presented for 50 ms, but showed no bias for photos presented for 500 ms [74]. Perceptual research finds that shifting attention to a visual cue requires ~50 ms [75], whereas disengaging attention from one cue and shifting it to another location in space requires ~150 ms [76]. Hence, alcohol attentional biases are observed during both maintenance/disengagement of attention as well as during initial orienting processes.
Alcohol attentional bias is robustly and positively correlated with craving [77]. The relation between alcohol attentional biases and subjective craving may be causal; persons trained to attend to alcohol cues for 500 ms with a modified visual probe task experienced increased cravings and consumed significantly more beer compared to persons trained to attend to neutral stimuli [78]. Although the processes by which addiction-related attentional biases influence alcohol dependence have not been adequately detailed, it is evident that subjective urges to drink and drinking behavior itself are modulated by attention. Among persons who drink alcohol to cope, stress intensifies alcohol attentional bias and concomitant experience of craving [79].
Across several studies, attentional bias appears to be proportional to the frequency and quantity of alcohol consumed by drinkers [68]. Additionally, alcohol attentional bias as measured by the addiction Stroop task predicts relapse in alcohol abusers [80] and alcohol consumption at a 6-month follow-up [81]. Due to ambiguity in interpreting results from the addiction Stroop task, it is unclear how to account for this predictive relationship. Nevertheless, whether through the invocation of automatic, conditioned responses, preferential attending to alcohol cues, or diversion of cognitive resources away from maintenance of normal daily activity and thought processes, alcohol-related attentional biases are associated with addictive behavior and may foster maintenance of alcohol dependence or promote relapse.
Craving is associated with thwarted automatic impulses, autonomic arousal, and dysphoria
There appears to be a positive feedback loop between alcohol attentional biases and the experience of craving, such that preferential attending to alcohol cues drives craving, which then magnifies the attentional bias [79]. Craving itself is a multifaceted phenomenon, involving cognitive processes, negative affect, neurobiological circuits involved in withdrawal and reward, contextual learning, and socially-driven alcohol expectancies. Theorists debate whether craving is the subjective correlate of classically conditioned alcohol withdrawal [82], the cognitive interpretation of alcohol cue-related physiological arousal [83], the expectation or anticipation of the rewarding effects of alcohol [84], or the cognitive, affective, and physiological reactivity resulting from impeded automatized alcohol-use sequences [23]. Here we focus on Tiffany’s [23] hypothesis that alcohol dependent persons in recovery experience craving when they attempt to block or inhibit an automatic impulse to consume alcohol triggered by external (e.g., the sight of one’s favorite drink) or internal (e.g., an emotional state) cues.
Given that brain areas such as the orbitofrontal cortex and ACC are implicated in cognitive control and volitional inhibition of urge impulses [85], cognitive processing models of craving [86] would predict increased activity in these brain regions when alcohol dependent individuals attempt to abstain from drinking in the face of alcohol cues. In fact, craving has been associated with increased activity in orbitofrontal cortex [57, 87] whereas efforts to inhibit addictive urges have also been shown to evoke ACC activity [88]. Moreover, heightened activation of PFC has been observed among abstinent alcohol dependent persons during exposure to alcohol-related stimuli [89] who presumably were attempting to regulate appetitive urges towards alcohol during a cue-exposure paradigm. Although the relation of such neural activations to drinking behavior remains unspecified, the intensity of alcohol cue-induced activations in medial PFC, ACC, and striatal brain regions has been shown to significantly predict alcohol intake during subsequent relapse in previously abstinent alcohol dependents [90].
Craving also correlates with metabolic increases in dorsolateral PFC and the amygdala [91], suggesting that addictive urges are subserved by activation of integrated cognitive-emotional circuits that may drive the dysphoric states associated with the thwarted appetitive response. It is possible that alcohol cue-induced activations in PFC-amygdala emotion regulation circuits may result in the downstream cascade of autonomic responses that have been shown to co-occur with subjective craving, including decreased heart-rate variability and increased salivation [92], as well increased blood pressure and salivary cortisol levels [93]. Such cue-induced autonomic responses show a high concordance with the subjective experience of craving [94], which is characterized by a wide array of predominately aversive interoceptive responses [95], including increased heartbeat, tension, jitteriness, and restlessness. This constellation of physiological responses is a relatively undifferentiated aggregate of generalized autonomic arousal that co-occurs with the dysphoria of craving.
Suppressing the urge to drink intensifies craving and alcohol-related cognitions
In response to the disturbing thoughts and feelings that accompany craving, alcohol dependent persons in recovery may attempt to suppress the urge to drink as an expression of “willpower” [96]. Unwittingly, such efforts may only serve to enhance availability of alcohol-related cognitions and affective reactions to consciousness, as a body of research indicates that attempted suppression often results in an increased rate of the very thoughts and moods it is directed against, as well as heightened psychophysiological reactivity [97]. Indeed, heavy drinkers exhibited faster reaction times to alcohol-related statements than to control phrases after having been asked to suppress drinking urges subsequent to visual and olfactory alcohol cue-exposure [98]. Among alcohol dependents presented with an imaginal alcohol exposure script, thought suppression was inversely associated with tonic heart rate variability, such that individuals who tend toward suppression exhibit autonomic dysregulation indicative of impaired inhibitory control of perseverative cognition [99]. Hence, it appears as if the attempt to suppress the urge to drink paradoxically increases autonomic arousal and intrusive alcohol-related cognitions characteristic of craving, magnifying distress and enhancing the drive to consume alcohol.
Palliative coping through alcohol consumption is negatively reinforcing
For persons who drink to cope with stress and negative affect, alcohol consumption may be an attempt to allay the autonomic arousal and aversive emotional states that co-occur with stress and craving. In this light, alcohol use is a form of palliative coping [100]. This alcohol-mediated coping response may operate through two relatively-distinct neuropharmacologic pathways: via anxiolytic depressant effects that reduce sympathetic arousal [101], and via acutely rewarding effects that compensate for dysphoric emotions via endogenous opioid and dopamine release [87]. Although the some of the opioid and dopamingeric effects of alcohol consumption are positively reinforcing, alcohol’s psychopharmacological reduction of negative affect is theorized to operate through negative reinforcement [24]. Through both forms of reinforcement, alcohol consumption may become a conditioned response to endogenously-generated negative affect and exogenously encountered stressor stimuli.
When the addictive process is established, withdrawal from alcohol generates negative emotions and physiological distress, motivating the addict to imbibe more alcohol to relieve the discomfort. Ultimately, the negative reinforcement obtained from such palliative coping efforts bolsters associations between stress, perseverative cognition, negative affect, and alcohol use action schemata, such that when reactivated by subsequent stressors, these cognitive-affective stimulus configurations initiate and guide ensembles of automatized alcohol consumption behaviors. This pattern drives relapse into a self-destructive, downward spiral fueled by an increasing sensitivity and vulnerability to stressful life events.
Conclusion
Stress appraisals coupled with an actual or perceived lack of problem-solving resources result in neurophysiological arousal, perseverative cognition, and negative affect. This reactivity may in turn trigger automatized schemata to deploy sequences of maladaptive cognitive-behavioral processes, including attentional biases towards affectively-charged stimuli, the urge to alleviate distress, and palliative coping attempts to avoid the stressor or allay its impact through impulsive behavior. When palliative coping relieves distress, it results in negative reinforcement conditioning that perpetuates the cycle by further sensitizing the system to future stressful encounters.
Hypothetically, this stress-initiated risk chain may undergird multiple forms of psychopathological self-regulation failure, ranging from alcohol and drug misuse to sex and gambling addiction, obsessive-compulsive disorder, eating and mood disorders. These diverse conditions appear to share a common structure of stress-precipitated, automatic allostasis, where dysfunctional attempts to self-regulate in response to stressors perpetuate a system of runaway positive feedback loops that result in continued dysregulation. Whether an individual becomes adapted to exogenously obtained chemicals or to those generated within the nervous system during a particular mood, in either case that individual acclimates to a particular state of the mileu interior, and thereby becomes entrenched in a self-perpetuating cycle.
Thus, stress-maintained addiction is a form of adaptation, a learning process whereby a number of interlocking variables are maximized, resulting in runaway growth of the system and disruption of homeostasis. The essential systemic dependency of organism and environment, involving the organismic relationship to food, water, air, and other basic units of survival, is disrupted via the acquisition of a new dependency (i.e., dependence on drugs or alcohol). This acquisition involves a systemic change, an acclimation of the system to a new functional state. However, in the escalating symmetrical process of stress-precipitated addiction, the adaptation of the system does not solve but instead exacerbates the perceived problem, resulting in a positive feedback loop of increased addictive behavior, leading to a runaway state. The intake of the psychoactive substance recalibrates the bias of the system, setting it into an allostatic mode.
In this sense, addiction may be seen as a self-organizing system, operating to maximize and maintain its own organization without assistance from an external regulator [102]. Like other self-organizing or autopoeitic systems, stress-precipitated addiction is a multivariate process whose components (neurobiological and sociocognitive) dynamically interact to produce and preserve its internal coherence. Out of this dynamic interaction arises the emergent phenomenon of addiction itself: that is, the self-maintaining, continually recalibrating process relayed in the proverb: “first the man takes a drink, then the drink takes the drink, then the drink takes the man” [103]. Self-organizing systems maintain homeostasis through overarching negative feedback processes, and hence, only change as a result of perturbation from an outside source [26]. If the calamitous social, occupational, and health consequences of “hitting bottom” are of sufficient intensity, they may serve to perturb the otherwise stable, self-perpetuating system of addiction, eventuating in the dissolution of the autopoeitic unity of the addictive system. Similarly, if therapies target critical links of the risk chain, the resultant perturbation of the addictive system may lead to an adaptive reconfiguration. Whether precipitated by hitting bottom or fostered through treatment, the disassembly of behavioral routines, cognitive processes, and physiological responses underpinning addiction may ultimately lead to the shift towards sobriety.
The integrated biopsychosocial model of stress-precipitated alcohol dependence has implications for targeted forms of treatment, guiding the design of interventions that may disrupt the risk chain at multiple points. Therapies can be aimed at initial stress appraisal processes, leading to more accurate perception of situational demands and valid self-efficacy estimations. Clarified appraisals may attenuate subsequent stress reactions, interrupting preservative cognitive processes like catastrophizing and rumination, thereby preventing or lessening stress-precipitated alcohol consumption. Similarly, interventions could reduce the psychophysiological arousal and negative affective states triggered by stress appraisal. By inducing a parasympathetic “relaxation response,” stress-precipitated activation of the nervous system can be countered, preventing elicitation of downstream addictive processes. Emotion regulation through affect labeling, attentional refocusing, cognitive reappraisal, or metacognitive decentering from affectively-charged stimulus evaluations can attenuate the influence of emotionally-distressing stimuli on biobehavioral responses [104]. These forms of self-regulatory cognitive control mechanisms can be developed over time as a means of coping with distress, and appear to be subserved by the interaction of PFC structures (e.g., ACC, dorsolateral and medial PFC) with the amygdala and insula [49]. Thus, interventions that promote clarification of appraisals, disrupt perseverative cognition, and facilitate emotion regulation may prevent stressful encounters from precipitating or exacerbating the consumption of alcohol.
In addition, interventions could target alcohol use action schema and the ensembles of maladaptive cognitive-behavior processes that lead to addictive consumption of alcohol. Therapies may increase attention to drinking triggers and the presence of urges, enabling a skillful deployment of coping strategies. If, as Rohsenow and colleagues [105] observed, inattention to alcohol cues is correlated with increased drinking behaviors, then alcohol consumption may be decreased by enhancing attention to alcohol dependence triggers. Concomitantly, interventions could enable awareness of the engagement of alcohol use action schema when triggered by alcohol cues or negative affect, thereby allowing for the disruption of automatized drinking processes with a controlled coping response. Abstaining from use of alcohol requires the deployment of cognitive control mechanisms in stressful situations where affect regulation is needed. Psychosocial interventions might strengthen top-down cognitive control, thereby facilitating inhibition of alcohol use urges in the face of stress triggers. Additionally, stress-precipitated engagement of alcohol use action plans may also be interrupted by disengagement of attention from alcohol-relevant cues to allow for focus on neutral or health-promoting stimuli. Ultimately, repetitively engaging, disengaging, and moving attention away from alcohol-use triggers (including interoceptive data stemming from affective responses) toward innocuous or beneficial stimuli may weaken associative networks of alcohol use action schema. Lastly, treatment may help the alcohol dependent person to learn how to tolerate alcohol-related cognitions and craving without engaging in thought suppression. In so doing, alcohol cue-exposure may occur without the added burden of the post-suppression rebound effect, leading to the eventual extinction of conditioned appetitive responses.
Although a number of cognitive-behavioral therapies might leverage some of the aforementioned therapeutic mechanisms, one such intervention, mindfulness training, holds especial promise as a means of targeting the risk chain underpinning stress-precipitated alcohol dependence. Mindfulness training, which originates from Buddhist traditions but has been coopted by and translated for secular, Western clinicians, has been shown to exert significant, salutary effects on stress-related, biobehavioral conditions [106, 107]. Mindfulness involves self-regulation of a metacognitive form of attention: a nonreactive, non-evaluative monitoring of moment-by-moment cognition, emotion, perception, and physiological state without fixation on thoughts of past and future [25, 108]. A growing body of research suggests that mindfulness impacts stress, implicit cognition, and attentional processes [108–112]; hence, mindfulness training may comprehensively target a broad range of the pathogenic processes most central to stress-precipitated forms of alcohol dependence. Indeed, clinical research suggests that mindfulness-based treatments may improve therapeutic outcomes in substance-abusing populations [113–116].
Hypothetically, mindfulness training may target stress-precipitated alcohol dependence and relapse through a number of means, as delineated below. Mindfulness has been conceptualized as an awareness of stimuli without distortions and reactivity related to emotional valence [117]; hence, it is theoretically plausible that mindfulness training would increase the accuracy of primary and secondary stress appraisals [25] as well as facilitate cognitive reappraisal [118, 119], thereby reducing exaggerated stress reactions stemming from perseverative cognitive processes such as catastrophizing or rumination. Given that mindfulness training has been shown to decrease negative affectivity [120, 121] and stress-related pathology [122], mindful emotion regulation may attenuate stress reactivity or promote psychophysiological recovery from stressors, reducing the risk of stress-induced relapse. Second, mindfulness training may disrupt alcohol use action schema by increasing awareness of the presence of urges, enabling a skillful deployment of coping strategies. Mindfulness training may alert the individual to the engagement of alcohol use action schema when triggered by alcohol cues or negative affect, thereby disrupting automatized drinking processes with controlled cognitive operations. Third, mindfulness may facilitate exposure to alcohol-related cognitions and cravings without being subject to the paradoxical effects of urge suppression. Mindful exposure to alcohol cue-reactivity may prevent post-suppression rebound effects on the accessibility of alcohol-related thoughts. Indeed, changes in thought suppression have been shown to partially mediate the effects of mindfulness training on alcohol use and drinking consequences [123]. Fourth, because mindfulness training has been shown to potentiate attentional orienting functions [112] mindfulness-based interventions may facilitate disengagement of attention from alcohol-relevant cues, weakening alcohol attentional biases and thereby allowing for focus on neutral or health-promoting stimuli. Preliminary evidence has begun to substantiate some of these hypothesized pathways: a recent randomized controlled pilot trial of a mindfulness-oriented cognitive intervention for alcohol dependent individuals found that 10 weeks of mindfulness training reduced stress and thought suppression while modulating implicit alcohol attentional biases and facilitating heart-rate variability recovery from stress-primed alcohol cues [116]. More research is needed to test the model proposed in this paper.
The stress reaction and its addictive consequences, then, are not eventualities, for with sufficient intervention and training threat appraisals can give way to reappraisals of self-efficacy, acceptance, or a sense of coherence even in the face of grave adversity. The encounter with the stressor can be met with a sense of resourcefulness or with an attitude of benefit-finding, and in so doing, what would have otherwise been perceived as threatening becomes a navigable and meaningful challenge. Through the generative cognitive process of re-attributing the meaning of ambiguous stimuli, the individual can attend to constructions of reality wherein they have the wherewithal to adapt to and solve the problems in their lives. Surely, it is this ability that is articulated in the addict’s supplication for “the serenity to accept the things I cannot change, the courage to change the things I can, and the wisdom to know the difference.” The person recovering from alcohol dependence cannot avoid the ubiquity of stressors, but may be able to use problem-focused coping to manage them. Where problem-solving approaches fall short, emotion-focused strategies such as reappraisal and decentering can be employed, attenuating the affective reaction to the stressor. For the seriously and chronically dependent individual, attentional biases and cravings may automatically arise during the stress response, driving the impulse to relieve distress with alcohol. But herein may lay the power of volition, not in repressing the urge to drink, but in mindfully observing the addictive impulse as it arises, abides, and ceases without triggering alcohol consumption. In so doing, each moment of unanswered craving in the face of a stressor becomes an instance of extinction learning that can break the chains of risk and ultimately fuel the recovery process with a sense of empowerment.
Footnotes
Conflict of interest statement
We declare a lack of conflicts of interest in the development and publication of this manuscript. We had no financial and personal relationships with people or organizations that could inappropriately bias our work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Eric L. Garland, College of Social Work, Trinity Institute for the Addictions, Florida State University.
Charlotte A. Boettiger, University of North Carolina – Chapel Hill, Department of Psychology, Biomedical Research Imaging Center, Bowles Center for Alcohol Studies, Curriculum in Neurobiology.
Matthew O. Howard, University of North Carolina – Chapel Hill, School of Social Work.
References
- 1.Dawson DA, et al. Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction. 2005;100(3):281–92. doi: 10.1111/j.1360-0443.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- 2.Wiers RW, et al. The search for new ways to change implicit alcohol-related cognitions in heavy drinkers. Alcohol Clin Exp Res. 2006;30(2):320–31. doi: 10.1111/j.1530-0277.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- 3.Schroder KE, Perrine MW. Covariations of emotional states and alcohol consumption: evidence from 2 years of daily data collection. Soc Sci Med. 2007;65(12):2588–602. doi: 10.1016/j.socscimed.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson DA, Grant BF, Ruan WJ. The association between stress and drinking: modifying effects of gender and vulnerability. Alcohol Alcohol. 2005;40(5):453–60. doi: 10.1093/alcalc/agh176. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd DA, Turner RJ. Cumulative lifetime adversities and alcohol dependence in adolescence and young adulthood. Drug Alcohol Depend. 2008;93(3):217–26. doi: 10.1016/j.drugalcdep.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conger JJ. The effects of alcohol on conflict behavior in the albino rat. Q J Stud Alcohol. 1951;12(1):1–29. [PubMed] [Google Scholar]
- 7.Cappell H, Herman CP. Alcohol and tension reduction. A review. Q J Stud Alcohol. 1972;33(1):33–64. [PubMed] [Google Scholar]
- 8.Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4(5):231–44. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- 9.Caplan MA, Puglisi K. Stress and conflict conditions leading to and maintaining voluntary alcohol consumption in rats. Pharmacol Biochem Behav. 1986;24(2):271–80. doi: 10.1016/0091-3057(86)90350-3. [DOI] [PubMed] [Google Scholar]
- 10.Young RM, Oei TP, Knight RG. The tension reduction hypothesis revisited: an alcohol expectancy perspective. Br J Addict. 1990;85(1):31–40. doi: 10.1111/j.1360-0443.1990.tb00621.x. [DOI] [PubMed] [Google Scholar]
- 11.Levenson RW, et al. Alcohol and stress response dampening: pharmacological effects, expectancy, and tension reduction. J Abnorm Psychol. 1980;89(4):528–38. doi: 10.1037//0021-843x.89.4.528. [DOI] [PubMed] [Google Scholar]
- 12.Pihl RO, Finn P, Peterson J. Autonomic hyperreactivity and risk for alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(3–4):489–96. doi: 10.1016/0278-5846(89)90136-x. [DOI] [PubMed] [Google Scholar]
- 13.Peterson JB, Finn PR, Pihl RO. Cognitive dysfunction and the inherited predisposition to alcoholism. J Stud Alcohol. 1992;53(2):154–60. doi: 10.15288/jsa.1992.53.154. [DOI] [PubMed] [Google Scholar]
- 14.Pihl RO, Peterson J, Finn P. Inherited predisposition to alcoholism: characteristics of sons of male alcoholics. J Abnorm Psychol. 1990;99(3):291–301. doi: 10.1037//0021-843x.99.3.291. [DOI] [PubMed] [Google Scholar]
- 15.Steele CM, Josephs RA. Drinking your troubles away. II: An attention-allocation model of alcohol’s effect on psychological stress. J Abnorm Psychol. 1988;97(2):196–205. doi: 10.1037//0021-843x.97.2.196. [DOI] [PubMed] [Google Scholar]
- 16.Curtin JJ, et al. Alcohol and fear-potentiated startle: the role of competing cognitive demands in the stress-reducing effects of intoxication. J Abnorm Psychol. 1998;107(4):547–57. doi: 10.1037//0021-843x.107.4.547. [DOI] [PubMed] [Google Scholar]
- 17.Curtin JJ, et al. Alcohol affects emotion through cognition. Psychol Sci. 2001;12(6):527–31. doi: 10.1111/1467-9280.00397. [DOI] [PubMed] [Google Scholar]
- 18.Josephs RA, Steele CM. The two faces of alcohol myopia: attentional mediation of psychological stress. J Abnorm Psychol. 1990;99(2):115–26. doi: 10.1037//0021-843x.99.2.115. [DOI] [PubMed] [Google Scholar]
- 19.Donohue KF, et al. Intoxication level and emotional response. Emotion. 2007;7(1):103–12. doi: 10.1037/1528-3542.7.1.103. [DOI] [PubMed] [Google Scholar]
- 20.Sher KJ, et al. Stress-response-dampening effects of alcohol: attention as a mediator and moderator. J Abnorm Psychol. 2007;116(2):362–77. doi: 10.1037/0021-843X.116.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazarus R, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- 22.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 23.Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97(2):147–68. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- 24.Baker TB, et al. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 25.Garland EL. The meaning of mindfulness: A second-order cybernetics of stress, metacognition, and coping. Complementary Health Practice Review. 2007;12(1):15–30. [Google Scholar]
- 26.Bateson G. Steps to an ecology of mind. Chicago: The University of Chicago Press; 1972. [Google Scholar]
- 27.Maturana H, Varela F. The tree of knowledge: The biological roots of human understanding. Boston: Shambala; 1987. [Google Scholar]
- 28.Lewis MD. Personal pathways in the development of appraisal: A complex systems/stage theory perspective. In: Scherer KR, Schorr A, Johnstone T, editors. Appraisal processes in emotion. Oxford University Press; New York: 2001. [Google Scholar]
- 29.Hasin DS, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 30.Bargh JA, Chartrand TL. The unbearable automaticity of being. American Psychologist. 1999;54(7):462–479. [Google Scholar]
- 31.Ambady N, Rosenthal R. Psychological Bulletin. Vol. 111. 1992. Thin slices of expressive behavior as predictors of interpersonal consequences: A meta-analysis; pp. 256–274. [Google Scholar]
- 32.Ellsworth PC, Scherer KR. Appraisal processes in emotion. In: Davidson RJ, editor. Handbook of affective sciences. Oxford University Press; New York: 2002. pp. 572–595. [Google Scholar]
- 33.Ohman A, Wiens S. On the automaticity of autonomic responses in emotion: An evolutionary perspective. In: Davidson RJ, editor. Handbook of affective sciences. Oxford University Press; New York: 2002. pp. 256–275. [Google Scholar]
- 34.LeDoux J. Synapitc self. New York: Viking; 2002. [Google Scholar]
- 35.Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30(10):953–8. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Ohman A, et al. On the unconscious subcortical origin of human fear. Physiol Behav. 2007;92(1–2):180–5. doi: 10.1016/j.physbeh.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 37.Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res. 2006;60(2):113–24. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- 38.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama. 1992;267(9):1244–52. [PubMed] [Google Scholar]
- 39.Wolf JM, et al. Determinants of the NF-kappaB response to acute psychosocial stress in humans. Brain Behav Immun. 2009;23(6):742–9. doi: 10.1016/j.bbi.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 40.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 41.Janig W. The autonomic nervous system and its coordination by the brain. In: Davidson RJ, editor. Hanbook of affective sciences. Oxford University Press; New York: 2002. pp. 135–186. [Google Scholar]
- 42.Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress. 2002;5(1):55–8. doi: 10.1080/102538902900012345. [DOI] [PubMed] [Google Scholar]
- 43.Cannon WB. Organization of physiological homeostasis. Physiology Review. 1929;9 :399–431. [Google Scholar]
- 44.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33(2):81–8. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 45.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 46.Arnsten AF. The biology of being frazzled. Science. 1998;280(5370):1711–2. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- 47.Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55(4):362–8. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 48.Brosschot JF, Pieper S, Thayer JF. Expanding stress theory: prolonged activation and perseverative cognition. Psychoneuroendocrinology. 2005;30(10):1043–9. doi: 10.1016/j.psyneuen.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Folkman S. Positive psychological states and coping with severe stress. Soc Sci Med. 1997;45(8):1207–21. doi: 10.1016/s0277-9536(97)00040-3. [DOI] [PubMed] [Google Scholar]
- 51.Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. J Abnorm Psychol. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- 52.Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 53.Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 54.Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27(2):232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 55.Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96(1):73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- 56.Boettiger CA, et al. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27(52):14383–91. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dom G, et al. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. Br J Psychiatry. 2005;187:209–20. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- 58.Dias-Ferreira E, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–5. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 59.Elsner B, Hommel B. Effect anticipation and action control. J Exp Psychol Hum Percept Perform. 2001;27(1):229–40. doi: 10.1037//0096-1523.27.1.229. [DOI] [PubMed] [Google Scholar]
- 60.Cooper ML, et al. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 1995;69(5):990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- 61.Dickinson A, Wood N, Smith JW. Alcohol seeking by rats: action or habit? Q J Exp Psychol B. 2002;55(4):331–48. doi: 10.1080/0272499024400016. [DOI] [PubMed] [Google Scholar]
- 62.Schneider W, Chein JM. Controlled & automatic processing: behavior, theory, and biological mechanisms. Cognitive Science. 2003;27:525–559. [Google Scholar]
- 63.Shiffrin RM, Schneider W. Controlled and automatic human information processing. II. Perceptual learning, automatic attending and a general theory. Psychological Review. 1977;84(2):127–190. [Google Scholar]
- 64.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–76. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 65.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 67.Cox WM, Fadardi JS, Pothos EM. The addiction-stroop test: Theoretical considerations and procedural recommendations. Psychol Bull. 2006;132(3):443–76. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- 68.Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug and Alcohol Dependence. 2008;97(1–2):1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 69.Stacy AW. Memory activation and expectancy as prospective predictors of alcohol and marijuana use. J Abnorm Psychol. 1997;106(1):61–73. doi: 10.1037//0021-843x.106.1.61. [DOI] [PubMed] [Google Scholar]
- 70.Palfai TP, Ostafin BD. Alcohol-related motivational tendencies in hazardous drinkers: assessing implicit response tendencies using the modified-IAT. Behav Res Ther. 2003;41(10):1149–62. doi: 10.1016/s0005-7967(03)00018-4. [DOI] [PubMed] [Google Scholar]
- 71.Zack M, Toneatto T, MacLeod CM. Implicit activation of alcohol concepts by negative affective cues distinguishes between problem drinkers with high and low psychiatric distress. J Abnorm Psychol. 1999;108(3):518–31. doi: 10.1037//0021-843x.108.3.518. [DOI] [PubMed] [Google Scholar]
- 72.Stewart SH, et al. Affective priming of alcohol schema in coping and enhancement motivated drinkers. Cognitive Behaviour Therapy. 2002;31:68–80. [Google Scholar]
- 73.Field M, et al. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology (Berl) 2004;176(1):88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- 74.Noel X, et al. Time course of attention for alcohol cues in abstinent alcoholic patients: the role of initial orienting. Alcoholism: Clinical and Experimental Research. 2006;30(11):1871–7. doi: 10.1111/j.1530-0277.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 75.Duncan J, Ward R, Shapiro K. Direct measurement of attentional dwell time in human vision. Nature. 1994;369(6478):313–5. doi: 10.1038/369313a0. [DOI] [PubMed] [Google Scholar]
- 76.Theeuwes J, Chen CY. Attentional capture and inhibition (of return): the effect on perceptual sensitivity. Percept Psychophys. 2005;67(8):1305–12. doi: 10.3758/bf03193636. [DOI] [PubMed] [Google Scholar]
- 77.Field M, Mogg K, Bradley BP. Craving and cognitive biases for alcohol cues in social drinkers. Alcohol Alcohol. 2005;40(6):504–10. doi: 10.1093/alcalc/agh213. [DOI] [PubMed] [Google Scholar]
- 78.Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology (Berl) 2005;183(3):350–7. doi: 10.1007/s00213-005-0202-5. [DOI] [PubMed] [Google Scholar]
- 79.Field M, Powell H. Stress increases attentional bias for alcohol cues in social drinkers who drink to cope. Alcohol Alcohol. 2007;42(6):560–6. doi: 10.1093/alcalc/agm064. [DOI] [PubMed] [Google Scholar]
- 80.Cox WM, et al. Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug Alcohol Depend. 2002;68(3):237–43. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 81.Cox WM, Pothos EM, Hosier SG. Cognitive-motivational predictors of excessive drinkers’ success in changing. Psychopharmacology (Berl) 2007;192(4):499–510. doi: 10.1007/s00213-007-0736-9. [DOI] [PubMed] [Google Scholar]
- 82.Melchior CL, Tabakoff B. A conditioning model of alcohol tolerance. Recent Dev Alcohol. 1984;2:5–16. doi: 10.1007/978-1-4684-4661-6_1. [DOI] [PubMed] [Google Scholar]
- 83.Ludwig AM, Wikler A. “Craving” and relapse to drink. Q J Stud Alcohol. 1974;35(1):108–30. [PubMed] [Google Scholar]
- 84.Marlatt GA. Cognitive factors in the relapse process. In: Marlatt GA, Gordon JR, editors. Relapse prevention. Guilford Press; New York: 1985. pp. 128–200. [Google Scholar]
- 85.Knoch D, Fehr E. Resisting the power of temptations: the right prefrontal cortex and self-control. Ann N Y Acad Sci. 2007;1104:123–34. doi: 10.1196/annals.1390.004. [DOI] [PubMed] [Google Scholar]
- 86.Tiffany ST. Cognitive concepts of craving. Alcohol Res Health. 1999;23(3):215–24. [PMC free article] [PubMed] [Google Scholar]
- 87.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 88.Brody AL, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62(6):642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park MS, et al. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol. 2007;42(5):417–22. doi: 10.1093/alcalc/agl117. [DOI] [PubMed] [Google Scholar]
- 90.Grusser SM, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- 91.Grant S, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93(21):12040–5. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ingjaldsson JT, Thayer JF, Laberg JC. Craving for alcohol and pre-attentive processing of alcohol stimuli. Int J Psychophysiol. 2003;49(1):29–39. doi: 10.1016/s0167-8760(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 93.Fox HC, et al. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 94.Sinha R, et al. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170(1):62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- 95.Bergquist KL, Fox HC, Sinha R. Self-reports of interoceptive responses during stress and drug cue-related experiences in cocaine- and alcohol-dependent individuals. Exp Clin Psychopharmacol. 2010;18(3):229–37. doi: 10.1037/a0019451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bateson G. The cybernetics of “self”: A theory of alcoholism. Psychiatry. 1971;34(1):1–18. doi: 10.1080/00332747.1971.11023653. [DOI] [PubMed] [Google Scholar]
- 97.Wenzlaff RM, Wegner DM. Thought suppression. Annual Review of Psychology. 2000;51:59–91. doi: 10.1146/annurev.psych.51.1.59. [DOI] [PubMed] [Google Scholar]
- 98.Palfai TP, et al. Effects of suppressing the urge to drink on the accessibility of alcohol outcome expectancies. Behaviour Research and Therapy. 1997;35(1):59–65. doi: 10.1016/s0005-7967(96)00079-4. [DOI] [PubMed] [Google Scholar]
- 99.Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54(12):1427–36. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- 100.Olff M, Langeland W, Gersons BP. Effects of appraisal and coping on the neuroendocrine response to extreme stress. Neurosci Biobehav Rev. 2005;29(3):457–67. doi: 10.1016/j.neubiorev.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 101.Stritzke WG, Lang AR, Patrick CJ. Beyond stress and arousal: a reconceptualization of alcohol-emotion relations with reference to psychophysiological methods. Psychol Bull. 1996;120(3):376–95. doi: 10.1037/0033-2909.120.3.376. [DOI] [PubMed] [Google Scholar]
- 102.Bickel WK, Potenza MN. The forest and the trees: Addiction as a complex self-organizing system. In: Miller WR, Carroll KM, editors. Rethinking substance abuse. Guilford Press; New York: 2006. [Google Scholar]
- 103.Ludwig AM. Understanding the alcoholic’s mind: The nature of craving and how to control it. New York: Oxford University Press; 1988. [Google Scholar]
- 104.Gross JJ, Thompson RA, Gross JJ, editors. Handbook of Emotion Regulation. Guilford Press; New York: 2007. Emotion regulation: Conceptual foundations; pp. 3–26. [Google Scholar]
- 105.Rohsenow DJ, et al. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol. 1994;62(3):620–6. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- 106.Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA. 2008;300(11):1350–2. doi: 10.1001/jama.300.11.1350. [DOI] [PubMed] [Google Scholar]
- 107.Greeson JM. Mindfulness Research Update: 2008. Complement Health Pract Rev. 2009;14(1):10–18. doi: 10.1177/1533210108329862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lutz A, et al. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12(4):163–9. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang YY, et al. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci U S A. 2007;104(43):17152–6. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang YY, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci U S A. 2009;106(22):8865–70. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wenk-Sormaz H. Meditation can reduce habitual responding. Altern Ther Health Med. 2005;11(2):42–58. [PubMed] [Google Scholar]
- 112.Jha A, Krompinger J, Baime M. Mindfulness training modifies subsystems of attention. Cognitive, Affective, and Behavioral Neuroscience. 2007;7(2):109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- 113.Zgierska A, et al. Mindfulness meditation for alcohol relapse prevention: A feasibility pilot study. Journal of Addiction Medicine. 2008;2(3):165–173. doi: 10.1097/ADM.0b013e31816f8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bowen S, et al. Mindfulness meditation and substance use in an incarcerated population. Psychology of Addictive Behaviors. 2006;20(3):343–7. doi: 10.1037/0893-164X.20.3.343. [DOI] [PubMed] [Google Scholar]
- 115.Marcus MT, et al. Change in stress levels following mindfulness-based stress reduction in a therapeutic community. Addictive Disorders and their Treatment. 2003;2:63–68. [Google Scholar]
- 116.Garland EL, et al. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: Results from a randomized controlled pilot trial. Journal of Psychoactive Drugs. 2010;42(2):177–192. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brown KW, Ryan RM, Creswell JD. Mindfulness: Theoretical foundations and evidence for its salutary effects. Psychological Inquiry. 2007;18(4):211–237. [Google Scholar]
- 118.Garland EL, Gaylord S, Park J. The role of mindfulness in positive reappraisal. Explore (NY) 2009;5(1):37–44. doi: 10.1016/j.explore.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garland EL, et al. Upward spirals of positive emotions counter downward spirals of negativity: Insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical Psychology Review. 2010;30:849–864. doi: 10.1016/j.cpr.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Evans S, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2007 doi: 10.1016/j.janxdis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 121.Teasdale JD, et al. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psychol. 2002;70(2):275–87. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- 122.Grossman P, et al. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 123.Bowen S, et al. The role of thought suppression in the relationship between mindfulness meditation and alcohol use. Addict Behav. 2007 doi: 10.1016/j.addbeh.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


