Abstract
We used eye tracking to investigate lexical processing in aphasic participants by examining the fixation time course for rhyme (e.g., carrot – parrot) and cohort (e.g., beaker – beetle) competitors. Broca’s aphasic participants exhibited larger rhyme competition effects than age-matched controls. A reanalysis of previously reported data (Yee, Blumstein, & Sedivy, 2008) confirmed that Wernicke’s aphasic participants exhibited larger cohort competition effects. Individual-level analyses revealed a negative correlation between rhyme and cohort competition effect size across both groups of aphasic participants. Computational model simulations were performed to examine which of several accounts of lexical processing deficits in aphasia might account for the observed effects. Simulation results revealed that slower deactivation of lexical competitors could account for increased cohort competition in Wernicke’s aphasic participants; auditory perceptual impairment could account for increased rhyme competition in Broca's aphasic participants; and a perturbation of a parameter controlling selection among competing alternatives could account for both patterns, as well as the correlation between the effects. In light of these simulation results, we discuss theoretical accounts that have the potential to explain the dynamics of spoken word recognition in aphasia and the possible roles of anterior and posterior brain regions in lexical processing and cognitive control.
Keywords: spoken word recognition, aphasia, eye tracking, computational models, growth curve analysis
Introduction
It has been long recognized that individuals with aphasia have lexical processing impairments. Particular focus has been on the two classical clinical types, Broca’s and Wernicke’s aphasia, with the goal of understanding the neurobiological substrates of word recognition. Individuals with Broca’s aphasia typically have left frontal lesions including the inferior frontal gyrus (IFG). In contrast, individuals with Wernicke’s aphasia typically have left posterior lesions including the posterior portion of the superior temporal gyrus (STG) and often extending into inferior parietal areas. Although the mapping between clinical diagnosis and underlying neuropathology is far from perfect, as we will discuss, there is a rich literature demonstrating that individuals diagnosed with Broca’s and Wernicke’s aphasia exhibit different patterns of performance in lexical processing tasks. These differences have led to considerable debate about the nature of the functional deficits of these individuals and their underlying neurobiological substrates.
Given that word recognition calls on many different aspects of cognitive processing, impairments of any of these aspects could give rise to word recognition deficits. Much research has investigated potential deficits at different points in the processing stream. A classic method for investigating lexical access is semantic priming using the lexical decision paradigm. In general, individuals with Broca’s and Wernicke’s aphasia both show semantic priming in a lexical decision task, suggesting that they are able to map sound structure onto the lexicon and access lexical semantic representations (e.g., Blumstein, Milberg, & Shrier, 1982; Milberg & Blumstein, 1981). However, their priming is not completely normal. Several studies have demonstrated that in Broca’s aphasia, priming fails to emerge under some conditions in which controls exhibit priming, e.g., with acoustic modifications and at various interstimulus intervals (Milberg, Blumstein & Dworetzky, 1988; Misiurski, Blumstein, Rissman, & Berman, 2005; Prather, Zurif, Stern, & Rosen, 1992; Utman, Blumstein, & Sullivan, 2001). In Wernicke’s aphasia, in contrast, priming has emerged under more conditions than normal; for example, they show an equal magnitude of priming when a real word prime is changed to a phonologically similar nonword (e.g., gat-dog and wat-dog show as much priming as does cat-dog), whereas control participants show a graded reduction in priming (cat > gat > wat) (Milberg et al., 1988).
Although it is clear that Broca’s and Wernicke’s aphasia both cause deficits in lexical processing, there is disagreement about the exact nature of the deficit. The current work aims to provide a fuller picture of lexical processing in aphasia by combining computational modeling with data from an eye-tracking paradigm (the “visual world paradigm”) that provides finegrained information about the time course of lexical activation.
Five accounts of lexical processing deficits in aphasia
Several theories have been proposed to account for the lexical processing impairments in aphasia. Here we briefly state the central hypothesis of each and discuss some challenges in distinguishing between them.
Degree of activation. The overall activation in the lexicon is reduced in Broca’s aphasia and increased in Wernicke’s aphasia (Milberg, Blumstein, & Dworetzky, 1987; Milberg et al., 1988; Utman et al., 2001; McNellis & Blumstein, 2001; Blumstein & Milberg, 2000; Janse, 2006).
Time course of lexical activation. In Broca’s aphasia, lexical activation is delayed or slowed, leading to a later-than-normal rise time (Prather et al., 1992, 1997; Swinney, Zurif, & Nicol, 1989; Swinney, Prather, & Love, 2000). In Wernicke’s aphasia, a failure to inhibit lexical competitors leads to delayed deactivation in the lexical-semantic network (Prather et al., 1997).
Short-term memory. Aphasic individuals have deficits in short-term memory (or working memory) that cause difficulty maintaining the activation of lexical representations (R. Martin, Breedin, & Damian, 1999; see also N. Martin & Gupta, 2004). This account is related to, but distinct from, the time course account.
Auditory perceptual impairments. Word processing deficits are downstream consequences of perceptual impairments that cause difficulty identifying and/or discriminating speech sounds (e.g., Caplan, Gow, & Makris, 1995). As we shall discuss below, evidence from both aphasia and neuroimaging challenge this view. Nonetheless, it may be worthwhile to examine whether it can account for the eye tracking data, which in some instances can be more sensitive to subtle acoustic/phonological aspects of spoken word recognition than conventional methods such as priming (e.g., Allopenna, Magnuson, & Tanenhaus, 1998; McMurray, Tanenhaus & Aslin, 2002).
Cognitive control. Trouble selecting among competing alternatives produces lexical-semantic impairments in aphasic individuals, particularly those with damage to the left inferior frontal gyrus (IFG) (Gotts & Plaut, 2002; Jeffries & Lambon Ralph, 2006; Jeffries, Patterson, & Lambon Ralph, 2008; Novick, Trueswell, & Thompson-Schill, 2005).
Although the hypothesized underlying deficits differ, each of the above accounts can arguably explain the patterns that individuals with Broca’s and Wernicke’s aphasia exhibit in semantic priming. In Broca’s aphasia, reduced activation or delayed activation could make priming difficult to detect. A short-term memory deficit predicts reduced lexical activation and, consequently, reduced priming, as found in Broca’s aphasia. Difficulty selecting among competing alternatives could also be argued to account for fragile priming, particularly under conditions of lexical competition. Likewise, in Wernicke’s aphasia, increased activation and delayed deactivation could both account for priming occurring in more circumstances than normal. The perceptual impairment hypothesis can predict either the Broca’s pattern of reduced priming because perceptual noise typically reduces activation (i.e., perceptual certainty) or the Wernicke’s pattern of priming for a wider set of stimuli because perceptual noise reduces differentiation among similar speech sounds.
Eye-Tracking Studies of Spoken Word Processing
Prior studies using the visual world paradigm (VWP) with unimpaired participants have shown that fixations are time locked to fine-grained details of ongoing speech, allowing estimates of the time course of processing at phonetic, lexical, and sentence level time scales (for a review, see Tanenhaus, Magnuson, Dahan, & Chambers, 2000). In VWP studies participants are typically presented with a four-picture display and asked to “pick up” (i.e., move with a computer mouse) one of the objects in the display (the target). If the name of one of the distractor objects is an onset (“cohort”) competitor of the target word (e.g., beaker – beetle), participants are initially more likely to fixate on this object than on objects with phonologically unrelated names. For example, if asked to “Pick up the beaker,” participants are more likely to fixate a beetle than an unrelated distractor such as a carriage. If the name of one of the distractor objects is an offset (“rhyme”) competitor (e.g., beaker – speaker), participants also tend to fixate this object more than phonologically unrelated objects, though the competition tends to be weaker and later (Allopenna et al., 1998; Desroches, Joanisse, & Robertson, 2006; Magnuson, Tanenhaus, Aslin, & Dahan, 2003; for demonstrations of cohort and rhyme competition effects in other paradigms see Andruski, Blumstein, & Burton, 1994; Connine, Blasko, & Titone, 1993; Marslen-Wilson & Zwitserlood, 1989; Slowiaczek, Nusbaum, & Pisoni, 1987). Furthermore, as a word unfolds, the likelihood that a participant will fixate on its corresponding picture – and on its phonological competitors – closely matches each word’s lexical activation as predicted by simulations of the TRACE model of speech perception (McClelland & Elman, 1986). This correspondence suggests that participants’ fixations are tightly linked to lexical activation. Eye movements therefore have the potential to provide detailed information about lexical activation in individuals with Broca’s and Wernicke’s aphasia, thus shedding new light on their lexical processing deficits.
The Time Course of Spoken Word Activation in Aphasia
In a recent eye tracking study, Yee, Blumstein, and Sedivy (2008) found that individuals with Broca’s aphasia failed to show a reliable cohort competitor effect and individuals with Wernicke’s aphasia showed an abnormally large cohort competitor effect. Integrating these results with the existing literature is challenging because many studies of lexical activation in aphasic individuals have explored rhyme, rather than cohort activation (e.g., Milberg et al., 1988; Misiurski et al., 2005; Utman et al., 2001). Thus, one of the goals of the current study was to use the VWP to explore the time course of rhyme activation in Broca’s and Wernicke’s aphasia.. Combined with the existing eye-tracking data on cohort competition (Yee et al., 2008), this will supply critical data to help distinguish between the various accounts for lexical processing deficits in Broca’s and Wernicke’s aphasia.
A second goal was to utilize a more sophisticated data analysis technique, Growth Curve Analysis (GCA). GCA is a multilevel modeling framework that is expressly designed for the assessment of change over time (Singer & Willet, 2003), and which has been extended to the analysis of fixation time course data (Magnuson, Dixon, Tanenhaus, & Aslin, 2007; Mirman, Dixon, & Magnuson, 2008). GCA (described in more detail below) provides a powerful statistical method for quantifying differences in time course, allowing a better characterization of the competitor effects displayed by aphasic participants. In addition, GCA provides a method for analyzing time course differences at the individual level (rather than by group), which is particularly important for analyzing data from a relatively small number of participants, each of whom has unique lesions and may have unique impairments. Since these same participants also completed the cohort competition experiment (Yee et al., 2008), we also used GCA to re-analyze the cohort competition data in order to examine the pattern of rhyme and cohort competitor activation across individuals.
We will be presenting the results of two different analytical approaches. The first is a group analysis comparing control participants to Broca’s and Wernicke’s aphasic participants. This analysis is motivated by the long history of experimental and theoretical work that grouped individuals according to this diagnostic taxonomy and will allow our results to be integrated with the existing literature. However, it is also widely recognized that these clinical groupings may include individuals with different underlying neural pathologies, making it difficult to make claims about the neural systems underlying their language impairments. Thus, our second analysis disregards the diagnostic categories. Instead, we examine the relationship between individual participants’ rhyme and cohort effect sizes. Importantly, GCA allows both kinds of analyses to be performed within a single statistical framework.
Our third goal was to use a single computational framework to evaluate five different accounts of lexical processing impairments in aphasia. In the past, these accounts (described above) have generally been evaluated independently rather than compared to each other. We used the TRACE model of speech perception and spoken word recognition (McClelland & Elman, 1986) to implement the different accounts (manipulating individual parameters in TRACE in accordance with each). We then simulated competitor effects and evaluated the extent to which the different accounts can accommodate the eye tracking data.
This report is structured as follows: first, we present a VWP experiment examining rhyme competition effects in Broca’s and Wernicke’s aphasic and control participants and conduct group analyses using GCA. We then use GCA to re-analyze data from a previously reported experiment on cohort competition in these same participants (Yee et al., 2008). Next we use GCA to quantify individual participants’ rhyme and cohort competition effect sizes and examine the correlations between those effects. Finally, we present computational implementations of each of five accounts of lexical processing impairments in aphasia to evaluate the extent to which each account can capture the observed effects.
PART 1: EYE TRACKING
Rhyme Competition
As described above, the experiment was designed to provide a richer description of the time course of lexical processing in aphasia. To complement the cohort competition data (Yee et al., 2008), the current experiment focuses on the time course of rhyme competition in Broca’s aphasic, Wernicke’s aphasic, and control participants. Follow-up analyses then examine the relative strength of cohort and rhyme competition for each participant.
Methods
Participants
Twelve college-aged and twelve older control participants were recruited from the Brown University community and surrounding area and were paid for their participation. The older control participants (7 males, 5 females) were matched in age to the aphasic participants (average age 67). All participants were paid for their participation.
The aphasic participants included six participants diagnosed with Broca’s aphasia and five diagnosed with Wernicke’s aphasia. Classification was based on performance on the Boston Diagnostic Aphasia Exam (BDAE) (Goodglass and Kaplan, 1972). The BDAE provides a profile of language abilities and impairments across a range of language functions including measures of speech output (e.g., articulation, phrase length, articulatory agility, grammatical form), auditory comprehension (e.g., word discrimination, verbal commands, yes-no questions, word categories, and complex ideational material), naming, repetition, and paraphasia (sound substitutions and word substitutions). Diagnosis was made by review of performance on the BDAE and consensus by a team of researchers after evaluation of the individual participant.
The aphasic participants all had unilateral lesions resulting from cerebrovascular accident, and did not have an associated dementia or memory deficit (e.g., Korsakoff). None had a significant history of other neurological or psychiatric illness or drug/alcohol abuse. All were literate in English, had English as the native language, and had normal hearing in the speech frequencies. All were several years post-stroke. Data from one Wernicke’s aphasic participant were excluded because of right visual field neglect; all other participants had normal or corrected to normal vision and no known oculomotor deficits. The response times of two participants (one with Broca’s and one with Wernicke’s aphasia) were more than three standard deviations above the mean of the rest of the participants in their groups. Each also had an error rate more than two standard deviations above the mean of the rest of his group. As a result, these two participants were not included in the analyses. All of the remaining participants (five Broca’s and three Wernicke’s) were able to understand the experimental task and performed well above chance on five practice trials. Further information about the characteristics of these eight aphasic participants is provided in Table 1. All five of the Broca’s aphasic participants had lesions involving anterior areas, and four of these lesions involved Broca’s area (left IFG); however, it is unclear whether the fifth participant’s lesion extended into the IFG. All of the Wernicke’s aphasic participants had lesions that included the temporal lobe.
Table 1.
Diagnostic information about aphasic participants. Z-scores are included for word discrimination (WD), body part identification (BP), commands (COM), and complex ideational material (CIM) subtests of the Boston Diagnostic Aphasia Examination including, as well as overall auditory comprehension and fluency z-scores.
| ID | Gender | Age at Testing |
Years Post Onset |
WD | BP | COM | CIM | Auditory Comp z-score |
Fluency | Lesion |
|---|---|---|---|---|---|---|---|---|---|---|
| B01 | F | 58 | 6 | .78 | .78 | .98 | 1.36 | +0.97 | +0.80* | Lesion in anterior left MCA distribution centered on the Sylvian fissure and involving both grey and white matter; some extension into left temporal and inferior parietal area. |
| B02 | M | 67 | 9 | .96 | .78 | .98 | .34 | +0.77 | +0.50 | Large left frontal infarct corresponding to occlusion of the anterior branches of the MCA (lateral frontal, frontal operculum; less severe in the motor cortex, caudate, putamen, and anterior limb of the internal capsule). |
| B03 | M | 60 | 18 | .96 | .78 | .98 | 1.10 | +0.95 | +0.57 | Left hemisphere lesion involving caudate and globus pallidus, anterior internal capsule to medial temporal cortex and insula, and anterior PVWM. |
| B04 | M | 74 | 18 | .96 | .78 | .14 | 1.10 | +0.75 | +0.20 | Left MCA infarct involving Broca's area with deep extension involving subcallosal fasciculus. Patchy posterior lesion across temporal isthmus with superior extension to pre-motor and sensory cortex. |
| B05 | F | 61 | 16 | .96 | .97 | .77 | 1.10 | +0.95 | +0.86* | Large left insular lesion extending to anterior temporal lobe, sparing both Wernicke’s area and the anterior region of Broca’s area. |
| W01 | M | 44 | 4 | .30 | .60 | −1.54 | −.94 | −0.39 | +0.85 | Cerebral infarct involving branches of the left MCA with primary involvement of the anterior left temporal lobe, adjacent frontal lobe and basal ganglia. |
| W02 | M | 70 | 7 | .66 | .22 | .77 | −.18 | +0.37 | +0.93 | Hemorrhagic infarct in the left temporal and parietal lobes extending into the basal ganglia-internal capsule region. Superior extension into the sensory cortex and the white matter and periventricular white matter deep to the lower pre-motor, motor and sensory cortex areas. |
| W03 | F | 75 | 3 | .66 |
.41 | −.49 | .08 | +0.17 | +0.85 | Left hemisphere lesion involves the subcortical temporal isthmus, the most posterior portion of Wernicke’s area, and the white matter deep to Wernicke’s area. Superior extension of lesion involves the supramarginal and angular gyri and the white matter deep to these areas. |
The indicates that participants B01 and B05 had been previously diagnosed as Broca’s aphasic, but showed improved fluency in their speech production with occasional runs of output longer than 4 words, though they still showed deficits in articulation and had the nonfluent speech output deficits typical of Broca’s aphasia; thus, we describe them as recovered Broca’s aphasics.
Apparatus
An SMI EyeLink I head-mounted eye tracker was used to monitor participants' eye movements. A camera imaged the participant’s left eye at 250 Hz. Stimuli were presented with PsyScript, a freely available language for scripting psychology experiments (Bates & D’Oliveiro, 2003) on a 15 inch ELO touch-sensitive monitor. The aphasic participants were tested in their homes (6) or at Brown University (2). The young and older control participants were tested at Brown University.
Materials
A female local-area speaker (E.Y.) read each target word in isolation with sentence-final intonation. The stimuli were recorded in a sound-treated room. Twelve two-syllable pictureable nouns served as target words. For each of these target words, there existed a pictureable noun that was a rhyme competitor (e.g., carrot - parrot). All rhyme competitors shared everything but their initial consonant (11 of 12) or initial consonant cluster (1 of 12) with the target. Average target duration was 539 ms. Each critical trial display included a target picture, a rhyme competitor picture, and two pictures that were phonologically and semantically unrelated to the target and its cohort and rhyme competitors1. In all critical trials, object positions, including the positional relationship between the target and the related item, were counterbalanced so that each object type was equally likely to appear in each corner of the display.
The names of the unrelated pictures in each critical trial were frequency-matched with the name of the phonologically related picture. To ensure that the pictures in critical trials clearly represented what they were intended to represent, picture-name correspondence pre-tests were conducted. Participants who did not participate in the eye-tracking study were presented with each picture and a label (either its intended name or a randomly selected name), and were asked to judge whether they matched. To ensure a high degree of picture-name correspondence, at least 15 of the 16 participants had to agree that the intended name matched the picture. A few of the pictures did not meet this criterion and were replaced with new pictures. These were presented to at least five new participants who were asked to label each picture. If more than one of the participants did not provide the intended label, the picture was replaced with a new picture and re-normed in the same way.
Mean fixation proportion over time to the two unrelated pictures served as the baseline against which to compare mean fixation proportions over time to the related picture (this average provides the most appropriate estimate of the baseline likelihood of looking at an image that is unrelated to the target). Twelve distractor trials were included in which the names of two of the objects in the display rhymed, but in which neither related object was the target. Thus, even if any participants noticed that some of the objects were related, they could not then predict that the target would be one of the related objects.
The testing session included 185 trials: 12 critical trials, 12 distractor trials, 96 filler trials and 5 practice trials. An additional 60 trials were included in the testing session as part of the three experiments (cohort competition, semantic competition, and cohort-mediated semantic competition) described in Yee et al. (2008). Thus, data from both the rhyme and cohort competitor experiments were collected in the same testing session. Data from the cohort competitor trials are re-analyzed below. Participants completed the testing in approximately 30–45 minutes. Fitting and calibrating the eyetracker required an additional 10–15 minutes. Trial order was randomized for each participant.
Procedure
Participants were presented with a 3 × 3 array with four pictures on it, one in each corner (see Figure 1). Each cell in the array was approximately 2 × 2 inches. Participants were seated at a comfortable distance (about 18 inches) from a touch-sensitive monitor, with the monitor at eye height. Therefore, each cell in the grid subtended about 6.4 degrees of visual angle. The eye tracker is accurate to less than one degree of visual angle. One second after the display appeared, a red square appeared in the center of the screen. Participants were instructed to touch the red square when it appeared. Touching the red square caused it to disappear and triggered a sound file naming one of the objects. The red square was included in the procedure to decrease the likelihood that participants would be fixating on one of the pictures at word onset. After the participant selected one of the pictures by touching it on the screen, the screen went blank and the trial ended. There were 5 practice trials, during and/or after which the instructions were repeated as necessary. Prior to any critical trials there were also 8 filler trials to further accustom participants to the task.
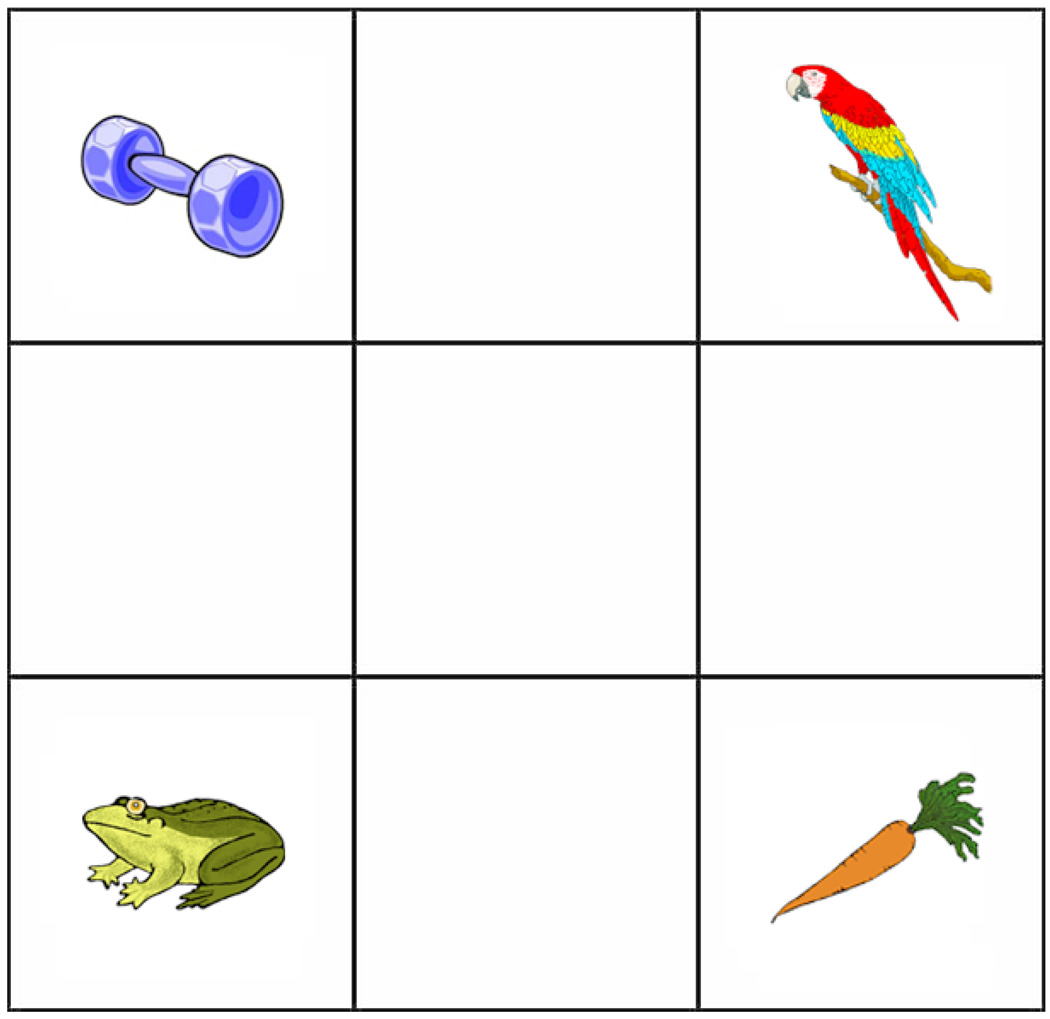
Figure 1.
Example display from Experiment. The target object is carrot, parrot is the rhyme competitor, frog and dumbbell are unrelated objects.
Growth Curve Analysis
Under the GCA approach to analyzing visual world eye tracking data (Mirman et al., 2008), there are two (or more) hierarchically related submodels to capture the data pattern. The first submodel, usually called level-1, is a model of the overall time course of fixation without distinguishing between participants or types of objects that are fixated (target, competitor, or unrelated). It captures the effect of time on fixation proportions using fourth-order orthogonal polynomials. A fourth-order polynomial is necessary to capture the rise and fall of fixation probabilities over the course of a trial. Orthogonal polynomials are transformations of natural polynomials that make the individual time terms independent (i.e., remove the correlation between, for example, linear and quadratic time), thus allowing a more precise evaluation of differences in dynamics of processing. Specifically, the intercept term reflects average overall fixation proportion (note that on this approach, the intercept term does not stand for the y-intercept, but rather is the average y-value of the modeled curve), the linear term reflects a monotonic change in fixation proportion (similar to a linear regression of fixation proportion as a function of time), and the quadratic term reflects an increase followed by a decrease in fixation proportion. The cubic and quartic terms tend to capture minor details in the asymptotic tails of the fixation proportion curves and are not informative for typical VWP experiments (see Mirman et al., 2008, for details). Note that effects on the intercept term are equivalent to the standard visual world paradigm comparisons of overall fixation proportion; thus GCA contains both the standard analysis and more sophisticated time course comparisons.
The second set of models, called level-2, captures participant and object type effects, that is, the effects of interest. These models describe (potentially) each level-1 model term as a function of population means, fixed effects, and random effects. The fixed effects are straightforward effects of participant and object type. These fixed effects capture overall differences between objects types (e.g., more fixation of parrot than frog when carrot is the target), participant groups (e.g., slower fixation time course for aphasic participants than controls), and participant group by object type interactions (i.e., the differences in competition effects across participant groups). The random effects (sometimes also called “residual effects”) are the deviation for an individual participant in a particular condition from the predicted performance based on the mean for that condition (across participants) and the mean for that participant (across conditions). In other words, random effects capture an individual participant’s effect size for a particular manipulation. As such, random effects provide a way to quantify individual participant effect sizes, which is a critical step in evaluating individual differences.
Results
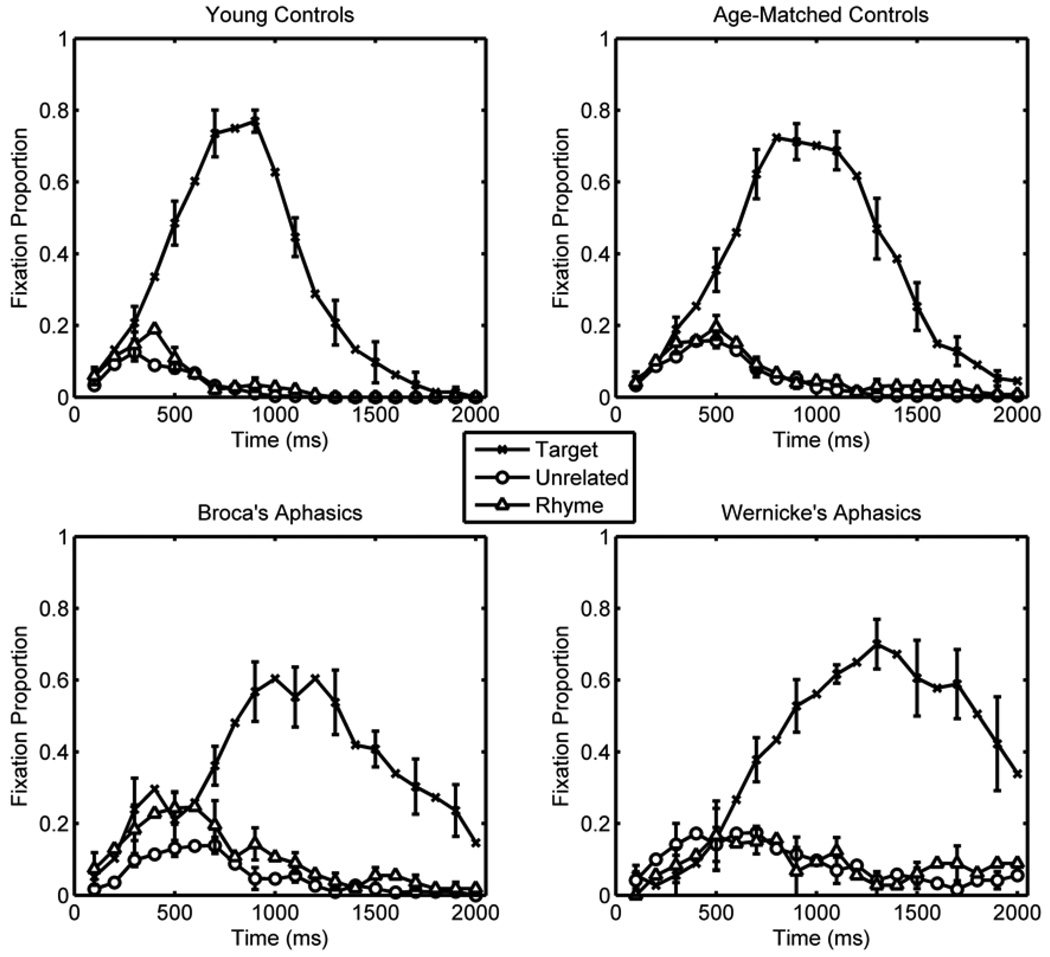
Participants selected the correct picture in critical trials with a very high degree of accuracy: young controls: 100%; age-matched controls: 98%; Broca’s aphasic participants: 97%; Wernicke’s aphasic participants: 96%. Only correct-response trials were included in the fixation analyses; thus, comprehension failures could not bias the fixation data. Figure 2 plots the mean fixation time course for the target, rhyme competitor, and the average of the two unrelated pictures (from target onset to 2000 ms after onset) in rhyme competitor trials for each of the four participant groups (young controls, age-matched controls, Broca’s aphasic participants and Wernicke’s aphasic participants).
Figure 2.
Average fixation time course for young control (top left), age-matched control (top right), Broca’s aphasic (bottom left), and Wernicke’s aphasic (bottom right) groups. Error bars represent ±1SE and are shown on every other data point.
Young and age-matched controls
Nine trials (6.3%) did not provide any data because there were no eye movements after the onset of the target word, so these trials were excluded. Data from young and age-matched controls were used to test whether the materials and procedures of the current experiment replicate the previous finding of rhyme competition in typical adults (Allopenna et al., 1998). To that end, data from young and age-matched controls were analyzed separately using GCA on the time course data from word onset to 2000 ms after word onset. The critical effects of interest were of object type (rhyme vs. unrelated) on level-1 time terms. For young controls, GCA revealed a marginally significant effect of object type on the intercept (i.e., different mean curve heights; B = 0.013, t(11) = 2.12, p < 0.1) and a significant effect on the linear term (i.e., different overall linear slopes; B = −0.053, t(11) = 3.02, p < 0.01). Effects of object type on other terms were not reliable (all t < 1.0, all p > 0.4). For age-matched controls, GCA revealed a significant effect of object type on the intercept (B = 0.0159, t(11) = 2.21, p < 0.05) and no reliable effects on other terms (all t < 1.0, all p > 0.6). The positive effect on the intercept indicates that control participants fixated the rhyme competitor more than the unrelated object. For young controls, the fixation time course difference between rhyme and unrelated objects was stronger on the linear term, which was negative because the largest fixation curve difference between rhyme and unrelated objects occurred relatively early (the maximum difference was approximately 400 ms after word onset) and the difference became smaller as the trial proceeded. For the present purpose, the critical finding is that the materials and procedures used in the Experiment produce a small rhyme competition effect in young and age-matched controls. We now turn to the critical comparisons of age-matched control and aphasic groups.
Aphasic participants and age-matched controls
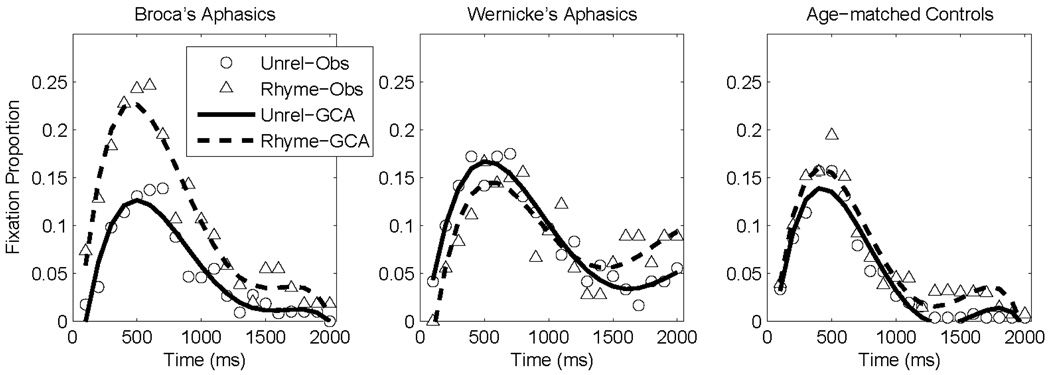
The data for this analysis were composed of rhyme and unrelated fixation time courses for the 12 age-matched controls, 5 Broca’s aphasic participants, and 3 Wernicke’s aphasic participants. The level-2 model contained effects of object type (rhyme vs. unrelated), participant group (age-matched controls vs. Broca’s aphasic participants vs. Wernicke’s aphasic participants), and the critical competitor-x-group interaction (difference in rhyme competition among participant groups). This interaction term reflects the extent to which the difference between rhyme and unrelated fixation time courses differed between participant groups. The age-matched controls served as the comparison group and coefficients were estimated for the two aphasic groups relative to the control group. Fixation data and curve fits for these three groups are plotted in Figure 3.
Figure 3.
Rhyme competition behavioral data (symbols) and growth curve analysis model fits (lines).
For the critical competitor-x-group interaction, the Broca’s aphasic group had a significantly higher intercept term (B = 0.036, t(34) = 2.1, p < 0.05) and a marginally lower linear term (B = − 0.12, t(736) = 1.7, p < 0.1) relative to age-matched controls. The effect on the intercept term indicates that the difference in overall fixation of rhyme vs. unrelated objects was larger for the Broca’s aphasic group than for the control group. The negative effect on the linear term reflects the relative earliness of the larger rhyme competition effect. That is, the rhyme competition effect is larger for Broca’s aphasic participants than age-matched controls early in the time course and decreases through the course of a trial. The Wernicke’s aphasic group did not differ significantly from the control group on any of the time terms (all t < 1.6, all p > 0.13). In a direct comparison of Broca’s and Wernicke’s aphasic groups, the Wernicke’s aphasic group had a marginally lower intercept term (B = −0.053, t(12) = 1.8, p < 0.1), a significantly higher linear term (B = 0.25, t(288) = 2.6, p < 0.05), and no significant difference in the quadratic term (B = 0.0043, t(288) = 0.1, p > 0.9). These results indicate that Broca’s aphasic participants exhibited a larger rhyme competition effect than Wernicke’s aphasic participants or age-matched controls. That is, Broca’s aphasic participants exhibited a greater difference between rhyme and unrelated competition fixation time courses than Wernicke’s aphasic participants or age-matched controls. Table 2 presents the full GCA results for the rhyme competition data.
Table 2.
Rhyme competition GCA results. The Estimates are for the critical competitor-by-group interaction terms (Standard errors for the Estimates are in parentheses). Left section shows results for the Broca’s aphasic group relative to age-matched controls, middle section shows results for the Wernicke’s aphasic group relative to age-matched controls, right section shows direct comparison of Broca’s and Wernicke’s aphasic groups.
| Broca’s Aphasic | Wernicke’s Aphasic | Broca’s vs. Wernicke’s | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | t | p < | Estimate | t | p < | Estimate | t | p < | |
| Intercept | 0.036 (0.017) | 2.1 | 0.05 | −0.018 (0.021) | 0.9 | n.s. | −0.053 (0.030) | 1.8 | 0.1 |
| Linear | −0.12 (0.071) | 1.7 | 0.1 | 0.13 (0.086) | 1.5 | n.s. | 0.25 (0.097) | 2.6 | 0.05 |
| Quadratic | 0.0069 (0.042) | 0.2 | n.s. | 0.011 (0.051) | 0.2 | n.s. | 0.0043 (0.063) | 0.1 | n.s. |
Discussion
The critical findings were that Broca’s aphasic participants exhibited a significantly larger rhyme competition effect than age-matched controls or Wernicke’s aphasic participants and that there was no statistically reliable difference in rhyme competition between Wernicke’s aphasic participants and age-matched controls. These results replicate a preliminary study (Yee, Blumstein & Sedivy, 2000) in which 3 participants with Broca’s aphasia were found to have a higher peaking rhyme competitor effect than did 12 young controls (individuals with Wernicke’s aphasia were not tested in that study). This large rhyme competitor effect for Broca’s aphasic participants is also consistent with Utman et al.’s (2001) finding that individuals with Broca’s aphasia show greatly reduced priming for acoustically modified unvoiced-onset primes that have a voiced-onset lexical competitor (e.g., pear, which has bear as a competitor). Utman et al. interpreted this result to mean that the prime’s lexical competitors (which were rhyme competitors) were highly active and hence competed with the prime, reducing its activation and the subsequent priming effect. The present study provides more direct evidence that individuals with Broca’s aphasia have highly active rhyme competitors.
The present results may appear to be incompatible with Milberg at al.’s (1988) finding that individuals with Wernicke’s aphasia exhibit greater rhyme-based semantic priming than do individuals with Broca’s aphasia. Specifically, for Wernicke’s, but not for Broca’s aphasic participants, a reliable priming effect was found for targets that were semantically related to words that rhymed with a non-word prime (e.g., gat and wat primed dog, presumably via the activation of cat). A critical distinction between the two studies, however, is that in Milberg et al. all primes were non-words. Hence, the prime did not perfectly match any lexical representation, and should lead to diffuse activation of many lexical representations, some of which would not be semantically related to the target (gat would activate gap, gash, bat, sat, and many other words in addition to cat). The dynamics of resolving this ambiguity and how this diffuse activation interacts with the task demands of semantic priming is not a trivial issue and may have led to this difference in outcome.
The finding that Broca’s aphasic participants exhibited an abnormally large rhyme competitor effect and Wernicke’s aphasic participants failed to exhibit a rhyme competitor effect stands in stark contrast to the same participants’ cohort competitor effects (Yee et al., 2008), which showed the exact opposite pattern: a larger-than-control cohort competition effect for Wernicke’s aphasic participants and an absent cohort competition effect for Broca’s aphasic participants. In order to provide comparable analyses of rhyme and cohort competition effects, we used GCA to re-analyze the cohort competition data.
Re-Analysis of Cohort Competition Data
The cohort competition data reported in Yee et al. (2008; Experiment 2) were re-analyzed using GCA. The cohort competition experiment was conducted in the same session as the rhyme competition experiment, and testing procedure and materials were the same, except that the cohort competitors and targets shared onsets (e.g., hammer – hammock, see Yee et al., 2008 for full stimulus list), which were defined as either the entire first syllable (10 of 12 items) or the onset and vowel of the first syllable (2 of 12 items). Data were from the same 12 age-matched control, 5 Broca’s aphasic, and 3 Wernicke’s aphasic participants. Similar to the rhyme competitor trials, accuracy rates were high (young control: 99%; age-matched control: 99%; Broca’s aphasic: 98%; Wernicke’s aphasic: 100%) and only correct-response trials were included in the fixation analyses.
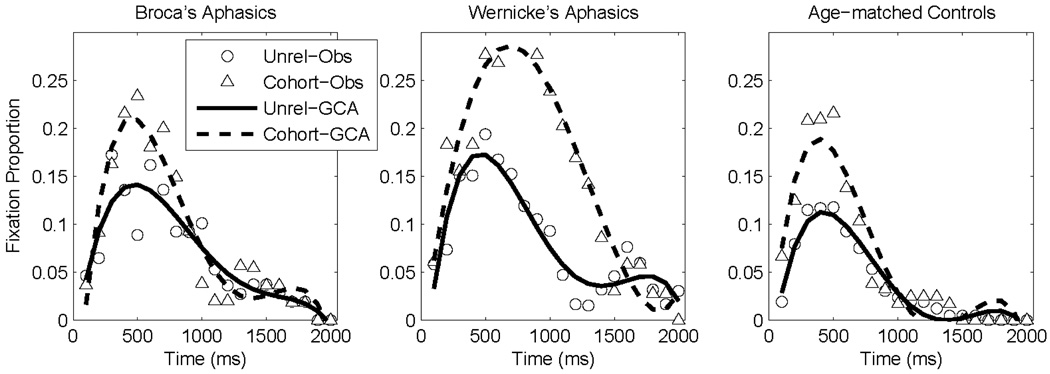
The growth curve model was structured the same way as for the rhyme competition experiment, with level-2 effects of object type (cohort vs. unrelated), participant group (age-matched control vs. Broca’s aphasic vs. Wernicke’s aphasic), and the critical object-x-group interaction (difference in cohort competition among participant groups). Similar to the rhyme competition data, this interaction term captures the difference in cohort competition between age-matched control, Broca’s aphasic, and Wernicke’s aphasic groups. The age-matched control group served as the comparison group and coefficients were estimated for the two aphasic groups relative to the control group. Behavioral data and curve fits for these three groups are plotted in Figure 4.
Figure 4.
Cohort competition behavioral data (symbols) and growth curve analysis model fits (lines).
For the critical object-x-group interaction, the Broca’s aphasic group did not differ reliably (all t < 1.2, all p > 0.2) from the age-matched controls (who showed a significant cohort competitor effect on the intercept [B = 0.023, t(11) = 2.9, p < 0.01] and linear [B = −0.11, t(11) = 5.6, p < 0.0001] terms). The Wernicke’s aphasic group had a significantly larger intercept term compared to age-matched controls (B = 0.050, t(34) = 2.4, p < 0.05), no significant difference in the linear term (B = −0.0093, t(736) = 0.2, p > 0.85), and a significantly more negative quadratic term (B = −0.28, t(736) = 4.0, p < 0.0001). The effect on the intercept term indicates overall greater cohort competition for the Wernicke’s aphasic group relative to age-matched control group. Because competitor fixation curves follow an inverted U shape, the level-1 quadratic term is negative; thus, the negative effect on the quadratic term for Wernicke’s aphasic group relative to age-matched control group indicates a steeper (i.e., even more negative) rise and fall rate of cohort competition for Wernicke’s aphasic group.
In a direct comparison of Broca’s and Wernicke’s aphasic groups, the Wernicke’s aphasic group had a marginally higher intercept term (B = 0.057, t(12) = 2.0, p < 0.1), no significant difference in the linear term (B = −0.057, t(288) = 0.9, p > 0.35), and a significantly more negative quadratic term (B = −0.24, t(288) = 2.8, p < 0.01). These results indicate that the Wernicke’s aphasic group exhibited a larger cohort competition effect than the Broca’s aphasic group. That is, individuals with Wernicke’s aphasia exhibited a greater difference between cohort and unrelated competition fixation time courses than individuals with Broca’s aphasia. Overall, these GCA results replicate the results reported by Yee et al. (2008): Broca’s aphasic participants exhibited a numerically reduced cohort effect, but it did not differ reliably from age-matched controls, whereas Wernicke’s aphasic participants exhibited a larger cohort competition effect than age-matched controls and Broca’s aphasic participants. Table 3 presents the full GCA results for the cohort competition data.
Table 3.
Cohort competition GCA results. The Estimates are for the critical competitor-by-group interaction terms (Standard errors for the Estimates are in parentheses). Left section shows results for the Broca’s aphasic group relative to age-matched controls, middle section shows results for the Wernicke’s aphasic group relative to age-matched controls, right section shows direct comparison of Broca’s and Wernicke’s aphasic groups.
| Broca’s Aphasic | Wernicke’s Aphasic | Broca’s vs. Wernicke’s | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | t | p < | Estimate | t | p < | Estimate | t | p < | |
| Intercept | −0.0072 (0.017) | 0.4 | n.s. | 0.050 (0.021) | 2.4 | 0.05 | 0.057 (0.029) | 2.0 | 0.1 |
| Linear | 0.047 (0.041) | 1.2 | n.s. | −0.0093 (0.050) | 0.2 | n.s. | −0.057 (0.064) | 0.9 | n.s. |
| Quadratic | −0.044 (0.057) | 0.8 | n.s. | −0.28 (0.070) | 4.0 | 0.0001 | −0.24 (0.084) | 2.8 | 0.01 |
The combined rhyme and cohort competition data indicate that Broca’s aphasic participants exhibit larger rhyme competition effects and Wernicke’s aphasic participants exhibit larger cohort competition effects. Although the complementary effects (reduced rhyme competition for Wernicke’s aphasic participants and reduced cohort competition for Broca’s aphasic participants) did not differ reliably from controls, they were numerically smaller (demonstrated most clearly by the negative intercept estimates in Tables 2 and 3). This pattern could arise from two separate impairments: one that is associated with Broca’s aphasia and causes greater rhyme competition (and possibly weaker cohort competition) and another that is associated with Wernicke’s aphasia and causes the opposite pattern. Alternatively, this pattern could arise from impairment along a single dimension such that Broca’s aphasia is associated with a shift from normal in one direction, resulting in increased rhyme competition, and Wernicke’s aphasia is associated with a shift in the other direction, resulting in increased cohort competition. This alternative deserves consideration because the distinction between Broca’s and Wernicke’s aphasia may not fully capture the differences between individuals with aphasia (e.g., Schwartz, Dell, Martin, Gahl, & Sobel, 2006). A single dimension account predicts that there should be a negative correlation between rhyme and cohort effect sizes across individuals.
To evaluate the plausibility of a single dimension account we conducted a mini-case-series type of analysis in which we investigated whether there is a relationship between rhyme and cohort effect sizes across individual participants. One benefit of analyzing rhyme and cohort effect sizes at the level of individual participants is that this approach avoids analyses that rely on comparisons of small groups. Traditional cognitive neuropsychology relies on discovering dissociations of function between a participant or participant group A on cognitive function X and participant or participant group B on function Y. The case series approach also allows investigation of the relationship between impairment of function X and Y -- which can provide new insights and advance development of theories of brain-behavior relationships, particularly when combined with computational modeling (e.g., Patterson & Plaut, 2009).
Individual-Level Analysis of Rhyme and Cohort Competition Effects In addition to fixed effects of object type, group, etc., growth curve analysis models can (as described above) include individual participant-by-condition random effects. To evaluate individual-level differences, the following analyses were based on individual-by-object-type random effects from a growth curve model that included no participant fixed effects. By leaving out participant fixed effects, the entirety of individual differences were shifted to the random effects. Consequently, random effects for a given model term (intercept, linear, quadratic, etc.) quantified the extent to which each individual is different from the mean model term for each object type (competitor and unrelated). In other words, we created a growth curve model of overall average rhyme and cohort effects, leaving the random effects to quantify the deviation of each individual from the overall mean. The difference between competitor and unrelated object random effects for each participant is a GCA measure of effect size for each participant. Random effects for this analysis were chosen on the basis of which terms showed reliable group differences in the group analyses. Namely, we examined random effects on the intercept and quadratic terms for cohort competition and we examined random effects on the intercept term for rhyme competition. To evaluate the magnitude of cohort and rhyme competition, we focused on the difference between each participant’s random effect for the competitor and the unrelated objects (i.e., competitor minus unrelated).
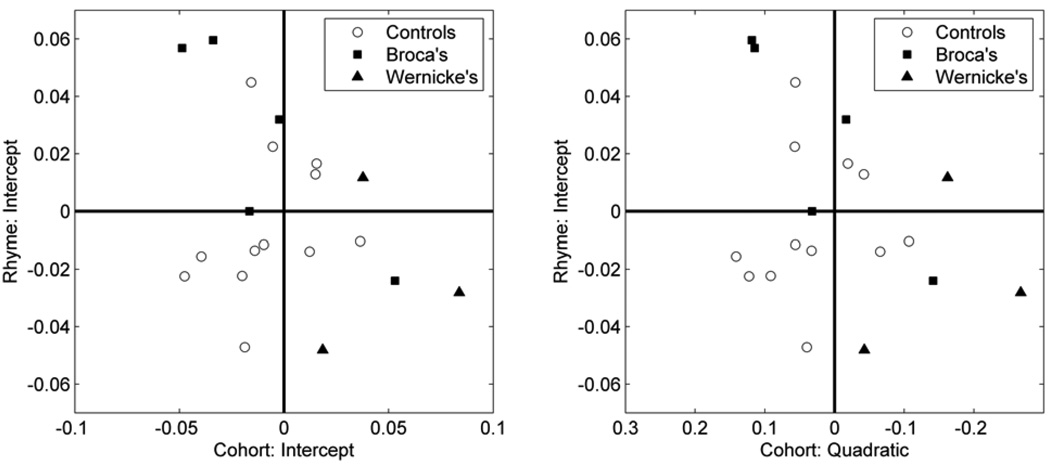
Figure 5 shows individual participant’s random effect difference (competitor – unrelated) for the cohort competition effect (horizontal axis; intercept term in left panel, quadratic term in right panel) and the rhyme competition effect (vertical axis). Each participant group is shown in a different symbol and the plot is divided into quadrants to highlight deviations from the mean. Note that for the cohort quadratic term (right panel) a large cohort effect corresponds to a more negative quadratic term, so the horizontal axis has been reversed to keep the intuitive interpretation of effect size direction (i.e., larger effects on the right). The overall correlation between cohort and rhyme competition effect sizes was not significant (rhyme intercept and cohort intercept: r = −0.32, p > 0.15; rhyme intercept and cohort quadratic: r = 0.30, p > 0.2). However, Figure 5 shows that the aphasic participant cohort and rhyme competition effect sizes (filled symbols) fall along a diagonal from the upper left to the lower right, reflecting a negative relationship between cohort and rhyme competition effects. That is, those aphasic participants that exhibited stronger cohort competition effects also tended to exhibit weaker rhyme competition effects and vice versa. Indeed, the high correlation between cohort and rhyme competition effect sizes for the 8 aphasic participants was reliable when measured by the rhyme intercept and cohort intercept terms (r = −0.76, p < 0.05) and approached significance when measured by the rhyme intercept and cohort quadratic terms (r = 0.70, p = 0.053; as mentioned above, larger competition effects are positive for the intercept term and negative for the quadratic term, thus this positive correlation coefficient is indicative of a negative relationship between effect sizes). This result suggests that a single dimension may account for the different behavioral performance of Broca’s and Wernicke’s aphasiac participants on spoken word recognition.
Figure 5.
Distribution of effect sizes. Open circles are age-matched controls, filled squared are Broca’s aphasic participants, filled triangles are Wernicke’s aphasic participants. The rhyme effect size is quantified using the intercept random effects. The cohort effect size is quantified using the intercept (left panel) and quadratic random effects (right panel). Note that for the quadratic the horizontal axis (cohort competition effect size) is reversed because a more negative quadratic term indicates a larger cohort competition effect.
Given the relatively small number of aphasic participants, there may be an elevated risk of Type 1 error for the effect size correlation analysis. To evaluate this possibility we conducted a permutation test: From our full sample of 20 participants (12 control and 8 aphasic), we repeatedly (10,000 times) selected random independent subsamples of 8 participants and tested the correlation between cohort and rhyme effect sizes for those 8 participants. Then we compared how many of those subsamples produced correlations of equal or greater magnitude than the correlations for the actual 8 aphasic participants. This method uses the observed distribution of cohort-rhyme effect size pairs to assess the statistical significance of the correlation found for aphasic participants. Less than 5% (i.e., p < 0.05) of the permutations produced correlations greater than the actual aphasic participant correlation (rhyme intercept and cohort intercept terms: p = 0.0245; rhyme intercept and cohort quadratic terms: p = 0.0385). This result demonstrates that the correlation between cohort and rhyme effects in the aphasic participant data is unlikely to have occurred by random sampling from an undifferentiated group of aphasic and control participants.
We now turn to consideration of the proposed accounts of aphasic processing impairments that were described in the introduction, and directly test them in a series of simulations using the TRACE model (McClelland & Elman, 1986). In these simulations we varied model parameters in accordance with different accounts and examined whether the model produces the observed pattern of cohort and rhyme competition effects.
PART 2: COMPUTATIONAL MODELING
Simulations
As discussed above, verbal formulations of a given theory can be interpreted to make conflicting predictions and multiple theories can seem to make the same prediction. Computational modeling provides a concrete way to test a theory by implementing its proposed mechanisms and letting them interact in simulations. Like a behavioral experiment, computational models require the researcher to operationalize the theory and to specify all of the implementational details. Simulations then allow empirical testing of whether the theory, as instantiated in the model, accounts for the behavioral data. Such empirical theory testing is valuable because it provides a way of shifting the discussion away from intuitions about whether a theory would or would not account for the data to concrete details of how the theory would need to be instantiated in order to account for the data.
As a general rule, all theories including computational models are subject to the principle of parsimony: models should be as simple as possible, while still allowing the same computational framework to be re-used as much as possible (i.e., one should not create unique, toy models for every simulation). Following this principle, we conducted simulations with the TRACE model of speech perception (McClelland & Elman, 1986) to test whether the five different theories reviewed in the introduction can account for the eye tracking data. We chose the TRACE model because it provides the greatest breadth and depth of coverage of behavioral phenomena (for a review of computational models of spoken word recognition see Magnuson, Mirman, & Harris, in press), including the phenomenon under investigation here – the pattern of cohort and rhyme competition in typical adults (Allopenna et al., 1998). Furthermore, TRACE has directly manipulable processing parameters, which can be used to produce a concrete implementation of each of the five accounts of lexical processing impairments in aphasia reviewed above.
The TRACE model consists of simple processing units organized into three layers: an acoustic/articulatory feature layer, where input is represented in seven banks of units corresponding to values along each feature dimension (e.g., voicing); a phoneme layer, where each unit corresponds to a particular phoneme (/b/, /p/, etc.); and a lexical layer, where each unit corresponds to a particular word (beaker, carrot, etc.). Activation of a processing unit reflects the state of combined evidence within the system for the presence of that linguistic unit. Mutually consistent units on different levels (e.g., /b/ as the first phoneme in a spoken word, beaker as the identity of the word) activate each other via feedforward and feedback excitatory connections, and mutually inconsistent units within the same level (e.g., /b/ and /p/ as the first phoneme) compete through mutually inhibitory connections. When input is presented at the feature layer, it is propagated to the phoneme layer and then to the lexical layer. Processing proceeds gradually with between-layer feedforward and feedback excitation and within-layer competitive inhibition. Activation flow is bi-directional: both bottom-up (features to phonemes to words) and top-down (words to phonemes to features). The processing dynamics of the TRACE model are governed by experimenter-set parameters such as the strength of bottom-up and top-down connections, unit decay rates, etc. A standard set of parameters was defined by McClelland and Elman (1986) in the original description of the TRACE model and has been used since then to account for dozens of phenomena in speech perception and spoken word recognition, including cohort and rhyme competition effects (Allopenna et al., 1998). In the present simulations we take the standard parameter set as the model of typical (control) spoken word recognition and examine whether theoretically-motivated changes to specific parameters produce the observed aphasic patterns of cohort and rhyme competition.
Methods
The simulations were carried out using the jTRACE implementation of the TRACE model (Strauss, Harris, & Magnuson, 2007; available at http://maglab.psy.uconn.edu/jtrace). The lexicon was a slightly expanded version of the standard TRACE lexicon and consisted of 220 words. A few words were added to the standard lexicon so that it would include three test sets of target-cohort-rhyme-unrelated quartuples: beaker-beetle-speaker-parrot, casket-castle-basket-speaker, and carrot-careeb2-parrot-basket. The rhyme competitor from one target word was used as the unrelated object for another target word so that subtle lexical neighborhood differences would not obscure rhyme competition effects. Each simulation consisted of presenting the target word and letting the activation propagate through the model for 60 cycles, by which point the model had “recognized” the target word in the sense that, under the standard parameter set, the probability of selecting the correct picture from the set of four alternatives was over 99%. At each processing cycle, four-alternative-forced-choice fixation probabilities for the target and each of the three objects (cohort, rhyme, and unrelated) were computed using the Luce (1959) choice rule:
Where p(Ri) is the proportion of fixations to image i, ai is the activation level of word i, and j indexes the set of alternatives; in this case, the four images on the screen. Activations were based on processing in the entire lexicon; thus, all words could influence activations. The Luce rule provides a decision mechanism analogous to the choice task our participants faced: although any word might become active in memory, there were only four behavioral “outlets”, corresponding to the four objects depicted in the visual display. Under the Luce rule, words with higher activation are more likely to be fixated and all fixation proportions add to 1.0. The constant k is a response selection parameter that determines the slope of the nonlinear relationship between activation and fixation probability. We take “response selection” to refer to the process by which active lexical representations determine behavioral responses (i.e., fixations). The k parameter determines the slope of the relationship between (relative) lexical activation and response probability. This slope can be considered an implementation of “response selectivity” in the sense that a steeper slope corresponds to higher selectivity (i.e., high sensitivity to differences in lexical activation) and a shallower slope corresponds to lower selectivity (i.e., lower sensitivity to differences in lexical activation). The Luce choice rule is the standard method of linking model activations to behavioral responses (e.g., McClelland & Elman, 1986), including fixation behavior measured in human participants in the VWP (e.g., Allopenna et al., 1998; Dahan, Magnuson, & Tanenhaus, 2001). Following Dahan et al. (2001), the default value of k was set to 7 (the same value has been used in simulations of several VWP experiments; see Magnuson et al., in press, for a review).
Each of the five accounts of lexical processing impairment in aphasia was instantiated as a parameter change in the TRACE model as outlined in Table 4. We strove to achieve the most sound implementation of each account possible in TRACE. Although a better performing implementation of a particular account might be possible if we designed a model from scratch, this would undermine the ability to directly compare the different accounts (a primary motivation for implementing them in a common framework), and would distance these simulations from the broader literature on spoken word recognition.
Table 4.
Summary of simulation design.
| Account | jTRACE Parameter |
Default Value |
Tested Range | Rationale |
|---|---|---|---|---|
| Degree of activation of lexical candidates |
rest.w | −0.01 | −0.025 – 0.005 | This parameter determines the rest activation of word units, which corresponds to the baseline activation of lexical candidates. |
| Time course of activation | ||||
| (a) Slowed lexical activation |
Attention* | 1.0 | 0.4 – 1.2 | This parameter determines lexical units’ responsiveness to input, when it is less than 1.0, activation will be slowed. |
| (b) Deactivation of competitors |
gamma.w | 0.03 | 0.02 – 0.04 | This parameter determines the strength of lexical inhibition; that is, the extent to which the target will be able to deactivate its competitors. |
| Working memory | decay.w | 0.05 | 0.05 – 0.13 | This parameter determines the rate of decay of lexical unit activation. Faster decay reflects difficulty maintaining activation, that is, an impairment of working memory. |
| Cognitive control | k | 7 | 3–11 | This parameter determines the slope of the nonlinear relationship between lexical activation and response (fixation) probability. |
| Auditory perceptual impairment |
Input Noise |
0.0 | 0.0 – 0.6 | This parameter determines the magnitude of noise added to the model input, reflecting a relatively low-level auditory perceptual impairment. |
The ranges of values were selected to provide an adequate test of each theory by providing sufficient testing of the effect of manipulating each parameter. In general, a broad range of both increases and decreases of each parameter were tested. However, we did not implement changes if they were inconsistent with the theories they were intended to test (e.g., working memory is predicted to be reduced for individuals with aphasia, not increased). Further, some changes were not possible (e.g., the default level of noise is 0, so it is not possible to make it lower).
For each value of each parameter, three VWP trials were simulated by presenting the target and computing a predicted time course of fixation proportions for the four critical words. The complete simulation results comprise 5400 observations across 4 independent factors: manipulated parameter (6 parameters), parameter value (5 levels), object type (3: cohort, rhyme, and unrelated), and time (60 cycles). To compress this extremely large data set into a digestible form, cohort and rhyme competition effect sizes were computed as the difference in average predicted fixation proportion (i.e., response probability) between the critical competitor (cohort or rhyme) and the unrelated object. Although this approach greatly simplifies the time course data, it is analogous to the intercept term in GCA, which captured critical group and individual differences. This data compression also makes it possible to summarize a large number of simulations in a way that reveals the consequences of each parameter change on the magnitude of cohort and rhyme competition effects.
Results
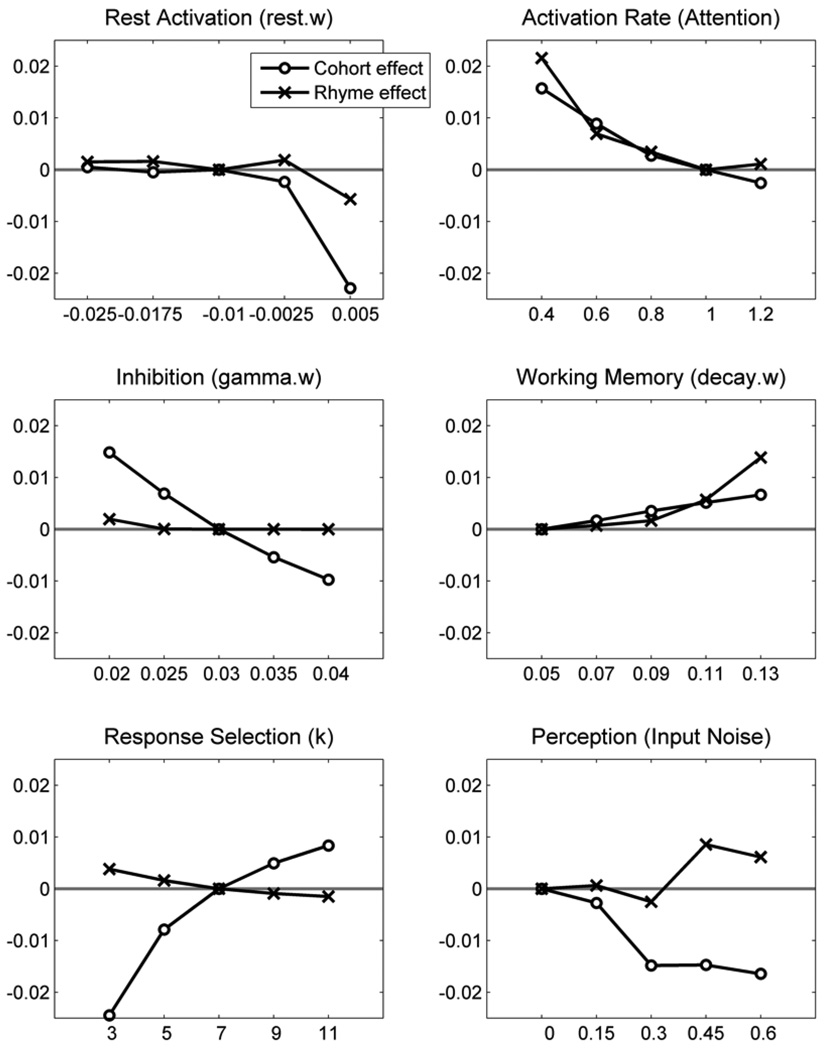
Figure 6 shows the effects of manipulating each critical parameter on the cohort and rhyme competition effect sizes (zero represents the effect size under the default parameter set, that is, the effect for controls).
Figure 6.
Simulation results. Each panel shows the effect of changing a single parameter on the magnitude of the cohort competition effect (circles) and rhyme competition effect (x’s). The effect size under the default parameter set is denoted by the gray line at 0, positive values indicate an increased competition effect, negative values indicate a decreased competition effect.
For the degree of lexical activation hypothesis, manipulations of baseline lexical activation (rest.w, top left panel in Figure 6) had virtually no effect on competition effect sizes until the rest activation became higher than 0, at which point cohort and rhyme competition effects were reduced to the point of being nearly eliminated. This discontinuity arises because in TRACE, units with activation less than 0 do not interact. When rest activation is higher than 0, the target word can start inhibiting competitors sooner, thus making the overall competition effect smaller. Critically, the pattern of reduced cohort and rhyme competition does not match data from either of the aphasic groups.
To instantiate the time course of lexical activation hypothesis as it pertains to Broca’s aphasia (i.e., delayed activation), we manipulated activation gain, thus slowing lexical units’ responsiveness to input (this parameter was initially implemented by Mirman, McClelland, Holt, & Magnuson, 2008, as a possible model of lexical attention so it is labeled “Attention” in jTRACE; for other uses of activation gain to account for individual differences see Gotts & Plaut, 2002; Kello & Plaut, 2003; Kello, 2003; Kello, Sibley, & Plaut, 2005). This caused both cohort and rhyme effects to increase because it took longer for the target word to become active enough to completely inhibit the competitors (top right panel in Figure 6). The pattern of increased cohort and rhyme competition does not match either of the aphasic groups.
To instantiate the time-course hypothesis as it pertains to Wernicke’s aphasia (i.e., delayed deactivation) we manipulated the inhibition parameter to decrease lexical inhibition (gamma.w, middle left panel in Figure 6). This increased both cohort and rhyme effects because the target was less able to inhibit competitor activation. The increase was much greater for cohort than rhyme competitors. The difference arose because cohort competitors compete with the target when it is only beginning to become active, thus weaker inhibitory links allow cohorts to become much more active. Rhyme activation happens later, at a point when the target has already become active enough to inhibit competitors even if inhibition strength is reduced. Furthermore, because cohort competitors are more active when inhibition is reduced, the rhyme competitor must overcome that additional source of inhibition; thus, inhibition strength has minimal effects on the magnitude of the rhyme competition effect. The Wernicke’s aphasic group exhibited larger cohort competition effects than age-matched controls and their rhyme effect did not reliably differ from that of controls (though it was numerically smaller). Thus, this parameter provides a possible, though imperfect, match to the Wernicke’s aphasic data.
To simulate a working memory deficit, the model’s ability to maintain lexical activations was reduced by increasing the rate of lexical unit decay (middle right panel in Figure 6). This particularly affected target activation because, in TRACE, units that are more active experience a stronger pull of decay (i.e., the effect of decay is proportional to activation). As a result, cohort and rhyme competitors experienced less inhibition from the target and could become more active, producing larger cohort and rhyme competition effects. At moderately high decay rates, reduced target activation allowed slightly larger cohort effects, but since the rhyme had to compete with both the target and the cohort, it did not benefit as much. At very high decay rates, target and cohort activations were already beginning to decline when the rhyme was getting active, so the rhyme competition effect could become much larger. Neither of these patterns is a good match to the behavioral data.
To simulate the perceptual impairment hypothesis, we increased noise in the input to TRACE. This caused an increase in the rhyme competition effect and a decrease in the cohort competition effect (bottom right panel in Figure 6). These data represent averages of 5 simulations for each stimulus quartet at each noise level, so that the results would not be due to idiosyncratic effects of a particular noise sample (note that for simulations with other parameters, the results are deterministic, and so multiple runs are not required). By increasing bottom-up ambiguity, noise slowed down the dynamics of activation and competition at the lexical level, thus allowing the rhyme competitors to become more active and reducing the onset advantage that cohort competitors normally enjoy. This pattern is similar to the Broca’s aphasic pattern of increased rhyme effects.
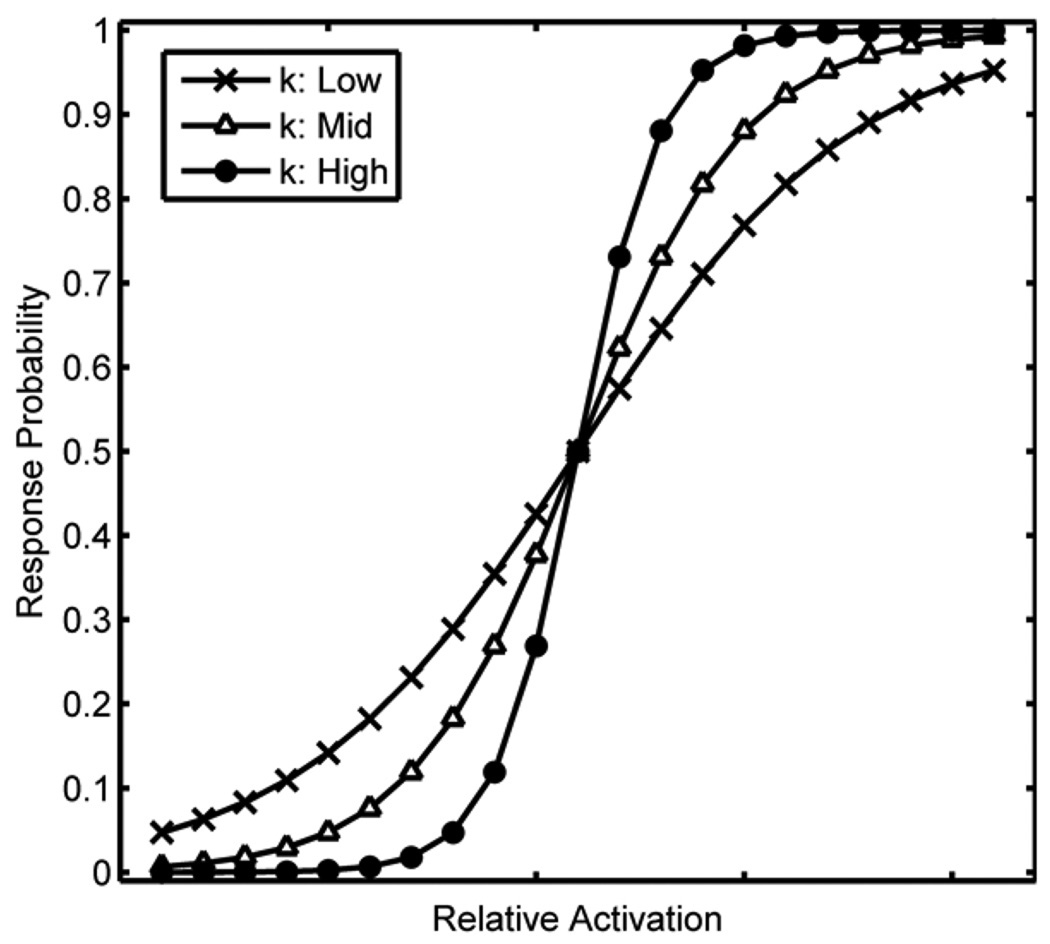
For the cognitive control hypothesis, manipulation of the k parameter in the Luce choice rule resulted in opposite effects on cohort and rhyme competition effect sizes (bottom left panel in Figure 6). When k was low, the rhyme competition effect was larger and the cohort competition effect was smaller; when k was high, the cohort competition effect was larger and the rhyme competition effect was smaller. The k parameter controls the (nonlinear) slope of the relationship between (relative3) activation and response probability as schematically depicted in Figure 7. When k is high, the nonlinearity is exaggerated and approaches a threshold function; when k is low, the nonlinearity is reduced and approaches a linear relationship. This change in slope has opposite effects on cohort and rhyme competition because the activation of cohort and rhyme competitors (relative to other response options) falls on opposite sides of the cross-over point: rhyme competitors have relatively low activation and cohort competitors have relatively high activation. As k increases, the probability of fixating a cohort competitor increases and the probability of fixating a rhyme competitor decreases. This result indicates that a cognitive control deficit in selecting among competing alternatives is a promising account of the negative correlation between cohort and rhyme competition effect sizes found for the 8 aphasic participants (collapsing across diagnostic categories). However, this result must be interpreted cautiously because high k would also predict faster responses in the behavioral task (i.e., shorter time to touch the target object picture) and low k would predict slower responses, but all aphasic participants responded more slowly than age-matched controls.
Figure 7.
Schematic representation of the effect of k on the relationship between relative activation and response probability.
Discussion
Six possible implementations covering five different accounts of aphasic lexical processing deficits were tested by manipulating parameters that control the dynamics of processing in the TRACE model of speech perception. The effects of changes in parameters on the magnitude of cohort and rhyme competition effects were compared to behavioral data. Changes in resting activation, rate of activation, and rate of decay did not produce the pattern of cohort and rhyme competition found for aphasic participants. A reduction in deactivation of competitors (reduced lexical inhibition) provided a partial match to the Wernicke’s aphasic pattern and an increase in input noise provided a partial match to the Broca’s aphasic pattern. Changes in response selectivity led to opposite effects on cohort and rhyme competition, consistent with a single-dimension account of the negative correlation between cohort and rhyme competition effects found for the group of 8 aphasic participants.
Under the classical separation of Broca’s and Wernicke’s aphasia as independent impairments with different lesion loci, it is sensible to consider different underlying causes. As such, slower deactivation of lexical competitors (as implemented by reduced lexical inhibition) matched the Wernicke’s aphasic pattern of increased cohort competition effects with minimal difference in rhyme competition. An auditory perceptual impairment (as implemented by increased input noise) matched the Broca’s aphasic pattern of increased rhyme effects. It also predicted that Broca’s aphasic participants would have reduced cohort competition, which was consistent with a numerical, though not statistically reliable, tendency.
Turning to the neurobiology, these accounts predict that individuals with Wernicke’s aphasia should have damage in brain regions associated with lexical inhibition (resulting in slower deactivation of competitors) and individuals with Broca’s aphasia should have damage in brain regions associated with perceptual processing of speech. It is necessary to be tentative in attempting to account for these results in terms of lesion location because some of the aphasic participants’ lesions were quite large, and because fine-grained analyses of these participants’ lesions were not possible. Nevertheless, individuals with Broca’s aphasia typically have damage to frontal brain regions and there is evidence that frontal speech production regions are involved in speech perception (e.g., Wilson, Saygin, Sereno, & Iacobini, 2004; Yuen, Davis, Brysbaert, & Rastle, 2010; see also Lotto, Hickok, & Holt, 2009, for a different view of the perception-production link) and that IFG is recruited in resolving competition in phonological and word processing tasks (Blumstein, Myers, & Rissman, 2005; Blumstein, 2007; Myers, 2007; Righi, Blumstein, Mertus, & Worden, 2010; Snyder, Feigenson, & Thompson-Schill, 2007). However, Broca’s aphasia is not specifically associated with speech perception impairments (e.g., Blumstein et al., 1977; Blumstein, 2001), and in fact, it is Wernicke’s aphasia and posterior superior temporal regions that are more commonly associated with auditory perceptual aspects of speech processing (Boatman, 2004; Caplan et al., 1995; see also Hickok & Poeppel, 2000; Zatorre, Belin, & Penhune, 2002). Furthermore, several studies have failed to find a relationship between auditory perception impairments and auditory language comprehension abilities in aphasia (e.g., Baker, Blumstein, & Goodglass, 1981; Basso, Casati, & Vignolo, 1977; Blumstein et al., 1977). In sum, although input noise causes the TRACE model to produce a Broca’s aphasic-like pattern and reduced lexical inhibition causes a Wernicke’s aphasic-like pattern, the typical neurobiology of Broca’s and Wernicke’s aphasia is exactly opposite to these accounts: frontal damage in Broca’s aphasics would predict deficits in resolving competition rather than deficits in speech perception, and posterior damage in Wernicke’s aphasia would predict deficits in speech perception rather than deficits in resolving competition. Given this inconsistency with the neuroanatomy, perceptual impairment and slowed deactivation of competitors are not very promising accounts of Broca’s and Wernicke’s aphasia.
A stronger anatomical argument can be made for an impairment of response selection (as implemented by changes in the k parameter governing response selectivity in the decision rule). With regard to Broca’s aphasic participants, this deficit is consistent with the view that the IFG is particularly important for selecting among competing alternatives (e.g., Thompson-Schill, D'Esposito, Aguirre, & Farah, 1997; Snyder et al., 2007). (We speculate about a possible anatomical link between abnormal response selection and the posterior regions typically affected in Wernicke’s aphasia in the General Discussion). Furthermore, the effect of k on the nonlinear relationship between activation and response probability can be recast as a gain manipulation that bears a strong computational similarity to the neuromodulatory account proposed by Gotts and Plaut (2002) to account for “access/refractory” semantic impairments in aphasic individuals. In addition, Lambon Ralph and colleagues (Jeffries & Lambon Ralph, 2006; Jeffries et al., 2008; see also Hodgson & Lambon Ralph, 2008) have argued that the semantic deficits in aphasia are due to executive control deficits rather than core semantic knowledge deficits. Thus, the present results suggest that executive control deficits may also provide the most parsimonious account of spoken word recognition deficits in aphasia. This account also provides an intriguing alternative because it was the only one that captured the negative correlation between cohort and rhyme competition effects across the 8 aphasic participants.
GENERAL DISCUSSION
The dynamics of lexical processing in aphasia were examined using the visual-world eye-tracking paradigm. The results revealed greater rhyme competition (e.g., carrot – parrot) for Broca’s aphasic participants than age-matched controls and Wernicke’s aphasic participants. Rhyme competition for Wernicke’s aphasic participants did not differ from age-matched controls. The opposite pattern was found for cohort competition (e.g., beaker – beetle): Wernicke’s aphasic participants exhibited larger cohort competition effects than age-matched controls or Broca’s aphasic participants, who did not differ from age-matched controls (Yee et al., 2008). One possible interpretation of this finding is consistent with the approach conventionally taken in the literature: individuals with Broca’s and Wernicke’s aphasia have two different impairments that give rise to different patterns of cohort and rhyme competition. However, analysis of individual participant data revealed that cohort and rhyme competition effects were negatively correlated in this aphasic participant sample, suggesting that the effects may not be independent. Therefore, an alternative interpretation is that a single dimension, with opposing patterns of disruption, could underlie the lexical processing deficits observed in these aphasic participants.
To test proposed accounts of lexical processing deficits in aphasia, we conducted simulations of the TRACE model using manipulations of processing parameters as implementations of the proposed accounts. In the interests of parsimony, a model of impaired processing should build on a strong model of unimpaired processing, making TRACE an excellent candidate for testing accounts of aphasia. The simulations revealed that three accounts were compatible with aphasic participants’ lexical processing abnormalities. A reduction in lexical inhibition increased the cohort competition effect with minimal effect on the rhyme competition effect -- a reasonable match to Wernicke’s aphasic group’s pattern. An increase in input noise increased the rhyme competition effect and reduced the cohort competition effect -- a reasonable match to the Broca’s aphasic group’s pattern. Changes in response selectivity (k) led to opposite effects on cohort and rhyme competition, capturing the correlation pattern found for the complete group of 8 aphasic participants. Other implementations failed to produce any of the behavioral data patterns. Consequently, the simulations lend support to accounts that propose reduced deactivation of competitors in Wernicke’s aphasia, auditory perceptual impairments in Broca’s aphasia (though independent data challenge the auditory perceptual impairments account), or differentially impaired response selectivity in both groups.
The response selectivity account is particularly promising because it can account for the correlation between aphasic participants’ cohort and rhyme competition effects. For Broca’s aphasic participants, this account fits well with the view that the left IFG is important for response selection: since individuals with Broca’s aphasia tend to have damage to the left IFG, their response selectivity (modeled by k in the TRACE simulations) is reduced, giving rise to larger rhyme competition effects and smaller cohort competition effects. Individuals with Wernicke’s aphasia tend to have posterior, rather than frontal, lesions; for these individuals the model indicates an increase in response selectivity. One possible account of this pattern is that frontal and posterior regions form a dynamic balance, such that, for unimpaired individuals, behavior is the nonlinear outcome of a dynamic combination of representation activation driven by posterior regions and response selection driven by frontal regions (for converging functional neuroimaging evidence that posterior temporal brain regions are involved in meaning activation and prefrontal brain regions are involved in response selection see Bedny, McGill, & Thompson-Schill, 2008; Noppeney, Phillips, & Price, 2004). As a result, frontal damage reduces the impact of response selection processes and leads to a behavioral pattern that primarily reflects posterior activation patterns; that is, the normal nonlinearity of response selection is reduced and response probability approaches a linear relationship to activation. In contrast, on this account, impaired activation resulting from posterior damage tilts the balance in the opposite direction, producing a pattern in which slight differences (in either direction) in activation are exaggerated by response selection. This leads to a hyper-selective threshold-like behavioral pattern characterized by strong response to moderately activated representations (e.g., cohort competitors) and minimal response to weakly activated representations (e.g., rhyme competitors).
This account also presents an intriguing perspective on the fluent/nonfluent distinction between Wernicke’s and Broca’s aphasia. On the response selection view, the slow, telegraphic speech associated with Broca’s aphasia may be a result of difficulty selecting words for production, especially function words, which have minimal semantic and contextual support (for a related view see also Novick et al., 2005). Conversely, the fluent but meaningless speech associated with Wernicke’s aphasia may be a consequence of an overabundance of words being selected for production. Future work is needed to test these interesting possibilities. Similarly, due to the absence of fine-grained lesion information and the fact that several participants had large lesions, the anatomical conjectures should be treated as well-motivated hypotheses rather than conclusions. Further behavioral and computational testing in the context of fine-grained lesion analysis is required to assess whether this dynamic balance hypothesis can account for the full range of lexical processing impairments in individuals with aphasia.
Limitations
With regard to the behavioral data, a primary limitation of the current study is the limited sample of eight aphasic participants. This was due to the usual challenges of recruiting and testing individuals with aphasia, exclusion criteria imposed by the eye-tracking testing paradigm (e.g., exclusion of participants with visual field neglect), and the need to connect to the existing literature by testing aphasic participants who fit the classic Broca’s or Wernicke’s profiles. Future studies should examine a broader range of individuals with aphasia to test whether the inverse relationship between rhyme and cohort competition holds in a larger and more diverse sample of aphasic participants and to evaluate whether the dynamic balance hypothesis is consistent with the underlying neuropathology.
With regard to the simulations, the primary limitation is the TRACE framework. TRACE is an excellent computational framework for the present simulations because it accounts for the broadest and deepest set of behavioral phenomena among implemented models of speech perception and spoken word recognition. Nevertheless, TRACE has several limitations, including lack of a learning mechanism (cf. Mirman, McClelland, & Holt, 2006), a brute-force approach to the representation of time (with dedicated units for each temporal section of the input), discretely specified representational units (features, phonemes, and words), and limited connections to the broader scope of language and cognitive processing (such as conceptual knowledge, visual processing, short-term memory, attention, etc.). Using other computational models to simulate the behavioral patterns we observed may lead to additional insights. Nonetheless, the present data provide important constraints on future development of more sophisticated computational models of speech perception and spoken word recognition.
Conclusion
In sum, tests of spoken word recognition using the visual world eye-tracking paradigm revealed a complex pattern of differences between aphasic and unimpaired spoken word recognition dynamics. Growth curve analysis provided a statistical technique for quantifying these differences and revealed both group and individual differences. Specifically, we observed larger rhyme effects for Broca’s aphasic participants compared to age-matched controls, larger cohort effects for Wernicke’s aphasic participants compared to age-matched controls, and a negative correlation between rhyme and cohort competition effect sizes across all aphasic participants. Importantly, conventional diagnostic-group analyses would have missed the individual-level negative correlation. Finally, several accounts of lexical processing impairments in aphasia were evaluated using simulations of the TRACE model. For systems as complex as language, intuition-based predictions are precarious because even a small set of interacting processes can produce unexpected outcomes; thus, simulations provide critical, concrete tests of theoretical accounts. Simulations revealed that (1) slower deactivation of lexical competitors could account for the Wernicke’s aphasic group’s pattern of increased cohort competition effects with minimal difference in rhyme competition; (2) auditory perceptual impairment produced a Broca’s aphasic-like pattern of increased rhyme effects; and (3) an impairment of selecting among competing alternatives could account for both patterns and was the only one that captured the negative correlation between cohort and rhyme competition effects across the 8 aphasic participants. Of these three accounts, the dynamic balance hypothesis related to the response selection account is perhaps the most promising due to its parsimony in accounting for the full range of observed differences in the time course of lexical processing in aphasia.
Appendix.
List of Experiment critical items
| Target | Target Freq | Rhyme | Rhyme Freq | Unrelated #1 | Unrelated #1 Freq | Unrelated #2 | Unrelatd #2 Freq |
|---|---|---|---|---|---|---|---|
| dollar | 3.20 | collar | 1.53 | vacuum | 1.51 | rabbit | 1.53 |
| kettle | 0.78 | medal | 1.15 | blouse | 1.15 | microphone | 1.18 |
| ruler | 1.38 | cooler | 1.15 | sailboat | 1.11 | spoon | 1.15 |
| walker | 1.64 | locker | 1.11 | flashlight | 1.11 | microscope | 1.11 |
| mountain | 2.38 | fountain | 1.51 | towel | 1.51 | peanut | 1.48 |
| honey | 1.64 | money | 3.36 | book | 2.99 | plant | 2.90 |
| jello | 0.30 | cello | 0.70 | lipstick | 0.78 | kite | 0.78 |
| nickel | 1.46 | pickle | 1.00 | sneaker | 1.00 | tack | 1.00 |
| tire | 1.96 | fire | 2.55 | newspaper | 2.76 | radio | 2.61 |
| candle | 1.48 | sandal | 0.78 | blender | 0.78 | wrench | 0.78 |
| carrot | 1.30 | parrot | 0.78 | dumbbell | 0.78 | frog | 0.78 |
| speaker | 2.04 | beaker | 0.60 | crayon | 0.70 | handbag | 0.60 |
| Mean | 1.63 | 1.35 | 1.35 | 1.32 | |||
Rhyme competitor’s frequency was matched to that of unrelated objects, t(11)=39, p=70 (paired t-test comparing frequency of competitor to average of the two unrelated objects).
Acknowledgments
This research was supported by F32HD052364 to DM, a Jacob K. Javits Fellowship to EY, NSF CAREER 0748684 to JSM, National Institutes of Health grants DC005765 to JSM, HD001994 and HD40353 to Haskins Labs, DC00314 to Brown University, and DC0081 to the Boston University School of Medicine, and the Moss Rehabilitation Research Institute. This material is the result of work supported with resources and the use of facilities at the Department of Veterans Affairs Medical Centers in Boston, MA and Providence, RI. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the NIH. We thank Kathy Kurowski and Cara Misiurski for assistance with participant testing, James Dixon for helpful suggestions regarding the growth curve analyses, and Ted Strauss for assistance with the simulations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A preliminary report on this work was presented at the 2008 Meeting of the Cognitive Neuroscience Society (April, 2008, San Francisco, CA).
Pilot studies and prior experiments (Yee & Sedivy, 2006) established that when they were unrelated to the target, the pictures that served as competitors did not draw more fixations than other unrelated pictures.
Due to the limited phonetic inventory of TRACE, the simplest way to create well-balanced quartuples was to make this a word in the lexicon.
Response probability is a function of the activation of a particular response option relative to other response options. Since k does not have a direct effect on activation patterns, these can be considered constant relative to the effect of k.
Contributor Information
Daniel Mirman, Moss Rehabilitation Research Institute, Elkins Park, PA 19027, USA.
Eiling Yee, Department of Psychology, University of Pennsylvania, Philadelphia, PA 19104, USA.
Sheila E. Blumstein, Department of Cognitive, Linguistic, and Psychological Sciences, Brown University Research Service, Department of Veterans Affairs Medical Center, Providence, RI 02908, USA Boston University Harold Goodglass Aphasia Research Center at the VA Boston Healthcare System, Boston, MA 02130, USA; Research Service, VA Boston Healthcare System, Boston, MA 02130, USA.
James S. Magnuson, Department of Psychology, University of Connecticut, Storrs, CT 06269, USA Haskins Laboratories, New Haven, CT 06511, USA.
References
- Allopenna PD, Magnuson JS, Tanenhaus MK. Tracking the time course of spoken word recognition using eye movements: Evidence for continuous mapping models. Journal of Memory & Language. 1998;38(4):419–439. [Google Scholar]
- Andruski JE, Blumstein SE, Burton M. The effect of subphonetic differences on lexical access. Cognition. 1994;52(3):163–187. doi: 10.1016/0010-0277(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Baker E, Blumstein SE, Goodglass H. Interaction between phonological and semantic factors in auditory comprehension. Neuropsychologia. 1981;19(1):1–15. doi: 10.1016/0028-3932(81)90039-7. [DOI] [PubMed] [Google Scholar]
- Basso A, Casati G, Vignolo LA. Phonemic identification defect in aphasia. Cortex. 1977;13(1):85–95. doi: 10.1016/s0010-9452(77)80057-9. [DOI] [PubMed] [Google Scholar]
- Bates TC, D’Oliveiro L. Psyscript: A Macintosh application for scripting experiments. Behavior Research Methods, Instruments & Computers. 2003;35(4):565–576. doi: 10.3758/bf03195535. [DOI] [PubMed] [Google Scholar]
- Bedny M, McGill M, Thompson-Schill SL. Semantic adaptation and competition during word comprehension. Cerebral Cortex. 2008;18(11):2574–2585. doi: 10.1093/cercor/bhn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein SE. Deficits of speech production and speech perception in aphasia. In: Berndt RS, editor. Handbook of Neuropsychology. 2nd edition. Vol. 3. Amsterdam, The Netherlands: Elsevier Science B.V.; 2001. [Google Scholar]
- Blumstein SE. Word recognition in aphasia. In: Gaskell G, editor. Oxford Handbook of Psycholinguistics. Oxford: Oxford University Press; 2007. [Google Scholar]
- Blumstein SE, Baker E, Goodglass H. Phonological factors in auditory comprehension in aphasia. Neuropsychologia. 1977;15(1):19–30. doi: 10.1016/0028-3932(77)90111-7. [DOI] [PubMed] [Google Scholar]
- Blumstein SE, Milberg WP. Language deficits in Broca’s and Wernicke’s aphasia: A singular impairment. In: Grodzinsky Y, Shapiro LP, Swinney D, editors. Language and the brain: Representation and processing. Foundations of neuropsychology series. San Diego, CA, US: Academic Press; 2000. pp. 167–183. [Google Scholar]
- Blumstein SE, Milberg W, Shrier R. Semantic processing in aphasia: Evidence from an auditory lexical decision task. Brain and Language. 1982;17(2):301–315. doi: 10.1016/0093-934x(82)90023-2. [DOI] [PubMed] [Google Scholar]
- Blumstein SE, Myers EB, Rissman J. The perception of voice-onset time: An fMRI investigation of phonetic category structure. Journal of Cognitive Neuroscience. 2005;17(9):1353–1366. doi: 10.1162/0898929054985473. [DOI] [PubMed] [Google Scholar]
- Boatman D. Cortical bases of speech perception: Evidence from functional lesion studies. Cognition. 2004;92(1–2):47–65. doi: 10.1016/j.cognition.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Caplan D, Gow D, Makris N. Analysis of lesions by MRI in stroke patients with acoustic-phonetic processing deficits. Neurology. 1995;45(2):293–298. doi: 10.1212/wnl.45.2.293. [DOI] [PubMed] [Google Scholar]
- Connine CM, Blasko DG, Titone D. Do the beginnings of spoken words have a special status in auditory word recognition? Journal of Memory and Language. 1993;32(2):193–210. [Google Scholar]
- Dahan D, Magnuson JS, Tanenhaus MK. Time course of frequency effects in spoken-word recognition: Evidence from eye movements. Cognitive Psychology. 2001;42:317–367. doi: 10.1006/cogp.2001.0750. [DOI] [PubMed] [Google Scholar]
- Desroches AS, Joanisse MF, Robertson EK. Specific phonological impairments in dyslexia revealed by eyetracking. Cognition. 2006;100(3):B32–B42. doi: 10.1016/j.cognition.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency analysis of English usage: Lexicon and grammar. Boston, MA, US: Houghton, Mifflin Co; 1982. [Google Scholar]
- Godfrey JJ, Holliman EC, McDaniel J. Switchboard: Telephone speech corpus for research and development. IEEE ICASSP. 1992:517–520. [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia, PA, US: Lea and Febiger; 1972. [Google Scholar]
- Gotts SJ, Plaut DC. The impact of synaptic depression following brain damage: A connectionist account of “access/refractory” and “degraded-store” semantic impairments. Cognitive, Affective & Behavioral Neuroscience. 2002;2(3):187–213. doi: 10.3758/cabn.2.3.187. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4(4):131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hodgson C, Lambon Ralph MA. Mimicking aphasic semantic errors in normal speech production: Evidence from a novel experimental paradigm. Brain and Language. 2008;104:89–101. doi: 10.1016/j.bandl.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Janse E. Lexical competition effects in aphasia: Deactivation of lexical candidates in spoken word processing. Brain and Language. 2006;97(1):1–11. doi: 10.1016/j.bandl.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: A case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Lambon Ralph MA. Deficits of knowledge versus executive control in semantic cognition: Insights from cued naming. Neuropsychologia. 2008;46:649–658. doi: 10.1016/j.neuropsychologia.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kello CT. The emergence of a double dissociation in the modulation of a single control parameter in a single dynamic system. Cortex. 2003;39(1):132–134. doi: 10.1016/s0010-9452(08)70083-2. [DOI] [PubMed] [Google Scholar]
- Kello CT, Plaut DC. Strategic control over rate of processing in word reading: A computational investigation. Journal of Memory & Language. 2003;48(1):207–232. [Google Scholar]
- Kello CT, Sibley DE, Plaut DC. Dissociations in performance on novel versus irregular items: Single-route demonstrations with input gain in localist and distributed models. Cognitive Science. 2005;29:627–654. doi: 10.1207/s15516709cog0000_16. [DOI] [PubMed] [Google Scholar]
- Lotto AJ, Hickok GS, Holt LL. Reflections on mirror neurons and speech perception. Trends in Cognitive Sciences. 2009;13(3):110–114. doi: 10.1016/j.tics.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce RD. Individual Choice Behavior. Oxford, England: John Wiley; 1959. [Google Scholar]
- Magnuson JS, Dixon J, Tanenhaus MK, Aslin RN. The dynamics of lexical competition during spoken word recognition. Cognitive Science. 2007;31:133–156. doi: 10.1080/03640210709336987. [DOI] [PubMed] [Google Scholar]
- Magnuson JS, Mirman D, Harris HD. Computational models of spoken word recognition. In: Spivey M, Joanisse M, McRae K, editors. The Cambridge Handbook of Psycholinguistics. Cambridge: Cambridge University Press; (in press) [Google Scholar]
- Magnuson JS, Tanenhaus MK, Aslin RN, Dahan D. The time course of spoken word learning and recognition: Studies with artificial lexicons. Journal of Experimental Psychology: General. 2003;132(2):202–227. doi: 10.1037/0096-3445.132.2.202. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson W, Zwitserlood P. Accessing spoken words: The importance of word onsets. Journal of Experimental Psychology: Human Perception and Performance. 1989;15(3):576–585. [Google Scholar]
- Martin N, Gupta P. Exploring the relationship between word processing and verbal short-term memory: Evidence from associations and dissociations. Cognitive Neuropsychology. 2004;21(2/3/4):213–228. doi: 10.1080/02643290342000447. [DOI] [PubMed] [Google Scholar]
- Martin RC, Breedin SD, Damian MF. The relation of phoneme discrimination, lexical access, and short-term memory: A case study and interactive activation account. Brain and Language. 1999;70(3):437–482. doi: 10.1006/brln.1999.2184. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Elman JL. The TRACE model of speech perception. Cognitive Psychology. 1986;18(1):1–86. doi: 10.1016/0010-0285(86)90015-0. [DOI] [PubMed] [Google Scholar]
- McNellis MG, Blumstein SE. Self-organizing dynamics of lexical access in normals and aphasics. Journal of Cognitive Neuroscience. 2001;13(2):151–170. doi: 10.1162/089892901564216. [DOI] [PubMed] [Google Scholar]
- Milberg W, Blumstein SE. Lexical decision and aphasia: Evidence for semantic processing. Brain and Language. 1981;14(2):371–385. doi: 10.1016/0093-934x(81)90086-9. [DOI] [PubMed] [Google Scholar]
- Milberg WP, Blumstein SE, Dworetzky B. Processing of lexical ambiguities in aphasia. Brain and Language. 1987;31(1):138–150. doi: 10.1016/0093-934x(87)90065-4. [DOI] [PubMed] [Google Scholar]
- Milberg W, Blumstein S, Dworetzky B. Phonological processing and lexical access in aphasia. Brain and Language. 1988;34(2):279–293. doi: 10.1016/0093-934x(88)90139-3. [DOI] [PubMed] [Google Scholar]
- Mirman D, Dixon JA, Magnuson JS. Statistical and computational models of the visual world paradigm: Growth curves and individual differences. Journal of Memory and Language. 2008;59(4):475–494. doi: 10.1016/j.jml.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Magnuson JS. Dynamics of activation of semantically similar concepts during spoken word recognition. Memory & Cognition. 2009;37(7):1026–1039. doi: 10.3758/MC.37.7.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, McClelland JL, Holt LL. An interactive Hebbian account of lexically guided tuning of speech perception. Psychonomic Bulletin & Review. 2006;13(6):958–965. doi: 10.3758/bf03213909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, McClelland JL, Holt LL, Magnuson JS. Effects of attention on the strength of lexical influences on speech perception: Behavioral experiments and computational mechanisms. Cognitive Science. 2008;32(2):398–417. doi: 10.1080/03640210701864063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiurski C, Blumstein SE, Rissman J, Berman D. The role of lexical competition and acoustic-phonetic structure in lexical processing: Evidence from normal subjects and aphasic patients. Brain and Language. 2005;93(1):64–78. doi: 10.1016/j.bandl.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Mitchell MP, Santorini B, Marcinkiewicz MA. Building a large annotated corpus of English: The Penn Treebank. Computational Linguistics. 1993;19:313–330. [Google Scholar]
- Myers EB. Dissociable effects of phonetic competition and category typicality in a phonetic categorization task: An fMRI investigation. Neuropsychologia. 2007;45(7):1463–1473. doi: 10.1016/j.neuropsychologia.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Phillips J, Price C. The neural areas that control the retrieval and selection of semantics. Neuropsychologia. 2004;42(9):1269–1280. doi: 10.1016/j.neuropsychologia.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: Reexamining the role of Broca's area in sentence comprehension. Cognitive, Affective & Behavioral Neuroscience. 2005;5(3):263–281. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- Patterson K, Plaut DC. “Shallow draughts intoxicate the brain”: Lessons from cognitive science for cognitive neuropsychology. Topics in Cognitive Science. 2009;1:39–58. doi: 10.1111/j.1756-8765.2008.01012.x. [DOI] [PubMed] [Google Scholar]
- Prather PA, Zurif E, Love T, Brownell H. Speed of lexical activation in nonfluent Broca's aphasia and fluent Wernicke's aphasia. Brain and Language. 1997;59(3):391–411. doi: 10.1006/brln.1997.1751. [DOI] [PubMed] [Google Scholar]
- Prather P, Zurif E, Stern C, Rosen TJ. Slowed lexical access in nonfluent aphasia: A case study. Brain and Language. 1992;43(2):336–348. doi: 10.1016/0093-934x(92)90134-z. [DOI] [PubMed] [Google Scholar]
- Righi G, Blumstein SE, Mertus J, Worden MS. Neural systems underlying lexical competition: An eye tracking and fMRI study. Journal of Cognitive Neuroscience. 2010;22(2):213–224. doi: 10.1162/jocn.2009.21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Dell GS, Martin N, Gahl S, Sobel P. A case-series test of the interactive two-step model of lexical access: Evidence from picture naming. Journal of Memory and Language. 2006;54(2):228–264. doi: 10.1016/j.jml.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Slowiaczek LM, Nusbaum HC, Pisoni DB. Phonological priming in auditory word recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13(1):64–75. doi: 10.1037//0278-7393.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Feigenson K, Thompson-Schill SL. Prefrontal cortical response to conflict during semantic and phonological tasks. Journal of Cognitive Neuroscience. 2007;19(5):761–775. doi: 10.1162/jocn.2007.19.5.761. [DOI] [PubMed] [Google Scholar]
- Strauss TJ, Harris HD, Magnuson JS. jTRACE: A reimplementation and extension of the TRACE model of speech perception and spoken word recognition. Behavior Research Methods. 2007;39(1):19–30. doi: 10.3758/bf03192840. [DOI] [PubMed] [Google Scholar]
- Swinney D, Prather P, Love T. The time-course of lexical access and the role of context: Converging evidence from normal and aphasic processing. In: Grodzinsky Y, Shapiro LP, Swinney D, editors. Language and the brain: Representation and processing. Foundations of neuropsychology series. San Diego, CA, US: Academic Press; 2000. pp. 273–292. [Google Scholar]
- Swinney D, Zurif E, Nicol J. The effects of focal brain damage on sentence processing: An examination of the neurological organization of a mental module. Journal of Cognitive Neuroscience. 1989;1(1):25–37. doi: 10.1162/jocn.1989.1.1.25. [DOI] [PubMed] [Google Scholar]
- Tanenhaus MK, Magnuson JS, Dahan D, Chambers C. Eye movements and lexical access in spoken-language comprehension: Evaluating a linking hypothesis between fixations and linguistic processing. Journal of Psycholinguistic Research. 2000;29(6):557–580. doi: 10.1023/a:1026464108329. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utman JA, Blumstein SE, Sullivan K. Mapping from sound to meaning: Reduced lexical activation in Broca's aphasics. Brain and Language. 2001;79(3):444–472. doi: 10.1006/brln.2001.2500. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nature Neuroscience. 2004;7(7):701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- Yee E, Blumstein SE, Sedivy JC. The time course of lexical activation in Broca's aphasia: Evidence from eye movements; La Jolla, CA. Poster presented at the 13th Annual CUNY Conference on Human Sentence Processing.2000. [Google Scholar]
- Yee E, Blumstein S, Sedivy JC. Lexical-semantic activation in Broca’s and Wernicke’s aphasia: Evidence from eye movements. Journal of Cognitive Neuroscience. 2008;20(4):1–21. doi: 10.1162/jocn.2008.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee E, Sedivy JC. Eye movements to pictures reveal transient semantic activation during spoken word recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32(1):1–14. doi: 10.1037/0278-7393.32.1.1. [DOI] [PubMed] [Google Scholar]
- Yuen I, Davis MH, Brysbaert M, Rastle K. Activation of articulatory information in speech perception. Proceedings of the National Academy of Sciences. 2010;107(2):592–597. doi: 10.1073/pnas.0904774107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: Music and speech. Trends in Cognitive Sciences. 2002;6(1):37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]