Abstract
The chemokine stromal cell-derived factor-1 (SDF-1) plays a critical role in mobilizing precursor cells in the bone marrow and is essential for efficient vascular regeneration and repair. We recently reported that calcium augments the expression of chemokine receptor CXCR4 and enhances the angiogenic potential of bone marrow derived cells (BMCs). Neovascularization is impaired by aging therefore we suggested that aging may cause defects of CXCR4 expression and cellular responses to calcium. Indeed we found that both the basal and calcium-induced surface expression of CXCR4 on BMCs was significantly reduced in 25-month-old mice compared with 2-month-old mice. Reduced Ca-induced CXCR4 expression in BMC from aged mice was associated with defective calcium influx. Diminished CXCR4 surface expression in BMC from aged mice correlated with diminished neovascularization in an ischemic hindlimb model with less accumulation of CD34+ progenitor cells in the ischemic muscle with or without local overexpression of SDF-1. Intravenous injection of BMCs from old mice homed less efficiently to ischemic muscle and stimulated significantly less neovascularization compared with the BMCs from young mice. Transplantation of old BMCs into young mice did not reconstitute CXCR4 functions suggesting that the defects were not reversible by changing the environment. We conclude that defects of basal and calcium-regulated functions of the CXCR4/SDF-1 axis in BMCs contribute significantly to the age-related loss of vasculogenic responses.
Keywords: CXCR4, aging, bone marrow cells, angiogenesis, SDF-1, migration
Introduction
Bone marrow derived cells (BMCs) contribute to angiogenesis by differentiating into endothelial cells, smooth muscle cells and pericytes, and by secreting angiogenic proteins that support vessel growth and maturation [1–3]. The chemotactic cytokine stromal cell-derived factor-1 (SDF-1) plays a key role in mobilizing cells from the bone marrow and promoting homing of the cells to target tissues. SDF-1 acts by binding and activating the cell surface receptor CXCR4 on target cells [4, 5]. CXCR4 is essential for homing and maintenance of haematopoietic stem cells (HSCs) in distinct stromal cell niches within the marrow. CXCR4 surface expression varies between cell types. Most mesenchymal stem cells (MSCs) do not express CXCR4 [6] but endothelial progenitor cells (EPCs) express relative high surface CXCR4 [5, 7]. Only 5% of freshly isolated CD34+ cells from peripheral blood express CXCR4 but the expression can be increased >10-fold by exposing cells to appropriate culture in vitro[8]. Our previous study shows that calcium treatment can increase the surface expression of CXCR4 on BMCs [9].
Accumulating evidence indicates that the level of CXCR4 surface expression on BMCs determines the efficiency of homing and the subsequent angiogenic response within target tissues [9, 10]. Overexpression of CXCR4 in MSCs or CD34+ cells enhances the migration of these cells towards SDF-1 in vitro[6, 11] and increases the efficiency of bone marrow transplant in vivo, probably due to increased cell survival and homing [12]. Infusion of autologous progenitor cells has been shown to promote revascularization of muscle in animal models of peripheral ischemia but this has not been successfully translated into clinical application. One reason may be that bone marrow of aged or diseased individuals contain defective stem/progenitor cells. EPC level and function are reported to be adversely affected by age, diabetes, ischemic heart disease and atherosclerosis in animals and human beings [13–15]. Angiogenesis is also impaired by aging [16–18]. The molecular basis of these defects is not known.
We suggested that defective CXCR4 expression develops during aging, decreasing the number of CXCR4+ progenitor cells in the bone marrow and circulation. This results in reduced progenitor cell presentation to the ischemic tissue and reduces angiogenesis. Here we report that indeed CXCR4 expression was significantly lower in BMCs from old mice (BMCold) versus BMCs from young mice (BMCyoung), and that BMCold were unresponsive to calcium stimulation to enhance CXCR4 surface expression. BMCold displayed impaired responses to SDF-1 in vitro and in vivo and this correlated with a significantly reduced angiogenic response in vivo. These results provide a molecular mechanism for the reduced efficacy of autologous stem cell therapy in aged individuals.
Materials and methods
More detailed information is provided in the Supporting Information.
Flow cytometry analysis of surface CXCR4 on BMCs
All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health), and the procedures were approved by the University of Miami Animal Care and Use Committee. Young male mice (C57BL/6J, age 6 to 8 weeks, from Jackson Laboratory, Bar Harbor, ME, USA) and old male mice (24–26 months old C57BL/6J, from National Institutes of Aging, Bethesda, MD, USA) were used. Bone marrow harvesting, BMC incubation with 1 mM CaCl2 for 4 hrs at 37°C and FACS analysis of surface and intracellular CXCR4 on BMCs were described previously [9].
To examine the CXCR4 expression on the surface of different subpopulations of BMCs, calcium treated or not treated BMCs were suspended in phosphate-buffered saline (PBS) containing 1% bovine serum albumin. In the first group, BMCs (1 × 106 in 100 μl) were incubated with Biotin-conjugated anti-lineage Ab cocktail for 15 min., then with PerCP streptavidin secondary antibody, along with the fluorescein isothiocyanate (FITC)-conjugated anti-Sca1, Allophycocyanin (APC)-conjugated anti-C-kit and Phycoerythrin (PE)-conjugated anti-CXCR4 Abs for 60 min. at 4°C. In the second group, APC-conjugated anti-Flk-1, FITC-conjugated anti-CD34 and PE-conjugated anti-CXCR4 Abs were added together into BMC solution. In the third group, BMCs were incubated with Biotin-conjugated anti-Gr-1 Ab for 15 min., followed by adding PerCP streptavidin secondary antibody, along with FITC-conjugated anti-CD11b, and PE-conjugated anti-CXCR4 Abs for 60 min. at 4°C. Isotype and single colour labelled samples were used as the controls for multi-colour fluorescence-activated cell sorting (FACS) (LSR I; Becton Dickinson, San Jose, CA, USA).
Calcium influx measurement
Calcium influx was analysed by measuring the increase of intracellular calcium after BMCs were mixed with CaCl2 as described [9]. The mean fluorescence intensity (MFI) of each 15 sec. histogram was used to be normalized against the basal MFI before adding CaCl2 into the BMC suspension, and plotted as a time course.
Real time RT-PCR
The mRNA of CXCR4 was detected by real time-PCR and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels as described [9].
FACS analysis of CXCR4 internalization
After BMCs were mixed with SDF-1α at 37°C for 2 hrs, CXCR4 internalization was analysed by measuring the decrease of surface CXCR4 and increase of intracellular CXCR4 as described [9].
Western blot assay for Akt and ERK phorsphorylation
Western blot analysis was performed to evaluate the phosphorylation of Akt kinase and Extracellular signal-regulated kinases 1 and 2 (ERK1/2). BMCs were homogenized in cell lysis buffer (Cell Signaling Technologies, Danvers, MA, USA), 80 μg protein extracts were separated on 10% SDS-PAGE gels (Bio-Rad) and analysed by Western blot as described [19]. Antibodies against Akt, P-Akt, ERK, p-ERK are from Cell Signaling Technologies.
Migration assay
A modified Boyden chamber assay (n= 3) was performed as previously described [20].
BMC Homing
To study in vivo BMC homing, BMCyoung or BMCold from transgenic GFP-BL6 mice (Jackson Laboratory) were incubated in PBS with or without 1 mM CaCl2 for 4 hrs at 37°C and injected via the tail vein into 10-week-old male C57BL/6J mice that were subjected to limb ischemia as described previously [19]. Mouse SDF-1 (5μg/kg body weight, in PBS) was injected into the ischemic muscles daily for 3 days. Ischemic muscles were recovered 7 days after the cell injection. Cryo-preserved sections were observed by fluorescent microscopy. Cells with green fluorescence were counted under high power of magnification.
Mouse hindlimb ischemic model and LDPI scanning
Surgical creation of mouse hindlimb ischemia, injection of SDF-1 gene transduced NIH 3T3 cells, and laser Doppler perfusion image (LDPI) scanning were performed as described previously [19].
To compare the angiogenic potential of BMCs from young versus old mice, 1 × 106 BMCs from young or old GFP mice were treated with CaCl2, and then injected via tail vein into mice with ischemic hindlimb. At same time, recombinant mouse SDF-1 (5 μg/kg body weight, in PBS) was injected into the ischemic muscles daily for 3 days. LDPI scanning was performed at day 0 and 21.
Analysis of recovered tissues
Capillary endothelium was illustrated by alkaline phosphatase staining on frozen sections [20] and CD31 immunostaining on paraffin section of 7 day muscle samples. Proliferating and CD34+ cells were detected by immunohistochemical staining using anti-Ki67 and anti-CD34 antibodies, respectively, on paraffin sections of 7 day muscle samples as described [20].
Bone marrow transplantation
Bone marrow in femurs and tibias from young and old mice was harvested as described [9]. Recipient mice were subjected to lethal irradiation (950rad) and 1 × 107 whole BMCs were injected into the tail vein 4 hrs later. After a 2-month reconstitution, mice were killed to recover BMCs from femurs and tibias. The BMC surface CXCR4 expression before infusion and after recovery was determined by flow cytometry.
Statistics
Results are expressed as mean ± S.D. Statistically significant differences between groups were compared with one-way anova for multi-groups using GraphPad (San Diego, CA, USA) or two-tailed unpaired Student t-test for two groups only. Significance means P < 0.05.
Results
Aged mice have impaired angiogenic response to ischemia and SDF-1 therapy
We used an ischemic hindlimb model to measure the effect of age on angiogenesis and the response to cell therapy using SDF-1 gene transduced cells (see ‘Materials and methods’). As shown in Figure 1A, limb perfusion of untreated young mice increased significantly from 0.29 ± 0.01 at day 0 to 0.41 ± 0.01 3 weeks later. SDF-1 treatment in the ischemic muscle of young mice significantly enhanced the reperfusion to 0.50 ± 0.05. In contrast, the baseline recovery of old mice was significantly less than that of young mice, and old mice did not show enhanced perfusion in response to SDF-1 therapy (Fig. 1B). The defective response of aged muscles to hindlimb ischemia and SDF-1 therapy were supported by measurements of cell proliferation (Ki67 staining, Fig. 1C) and precursor recruitment (CD34 staining, Fig. 1E). There was significantly less cell proliferation and less CD34+ cells in the ischemic tissue of aged mice compared with young mice (Fig. 1D and F). The difference increased when the mice were treated with SDF-1. These data indicate that old mice may have less progenitor cells being homed at the ischemic site and are less sensitive to SDF-1 treatment than young mice.
Fig 1.
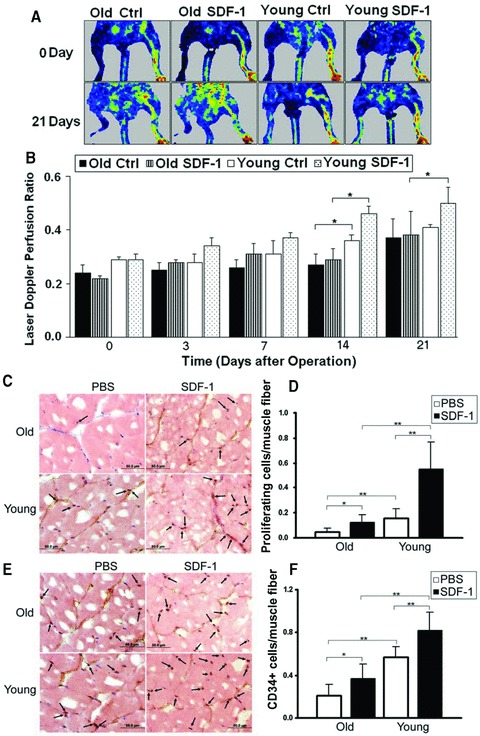
Angiogenesis in ischemic hindlimb of young and old mice. PBS (Ctrl) or NIH 3T3 cells retrovirally transduced with the SDF-1 gene (SDF-1) were injected into ischemic muscles immediately after the ischemia surgery. (A) Blood flow was measured using an LDPI analyser at the indicated time-points. The colour changes from blue to red represent the increase in perfusion. NIH 3T3 cells that had been transduced with lacZ gene had been used as a control to demonstrate that NIH 3T3 cells did not have effect on angiogenesis [19]. (B) Quantitative measurement of perfusion from LDPI at limb. Results are expressed as a ratio of ischemic leg (right) to normal leg (left). *P < 0.05, n= 6. (C) Ki67 immunostaining of paraffin sections of the ischemic muscle fibres obtained from the mice at day 7 after ischemic surgery. (E) CD34 immunostaining of paraffin sections of the ischemic muscle fibres obtained at day 7. Arrows indicate Ki67+ or CD34+ cells, respectively. (D) and (F) Quantification of Ki67+ and CD34+ cells per muscle fibre, respectively (n= 6). *P < 0.05; **P < 0.01.
BMCs of old mice have lower surface expression of CXCR4
To explore the mechanism of the impaired angiogenesis in aged mice, CXCR4 expression on the surface of BMColdversus BMCyoung was analysed by flow cytometry (Fig. 2A and B). BMCold expressed significant less CXCR4 (7.4 ± 2.6%) as compared to BMCyoung (13.4 ± 2.0%; n= 13, P < 0.01) (Fig. 2C). The corresponding MFI was 7.2 ± 1.9 versus 14.2 ± 6.2 (Fig. 2D). These data indicate BMCold have less number of cells expressing CXCR4 and less CXCR4 on the surface of each cell (Fig. S1).
Fig 2.
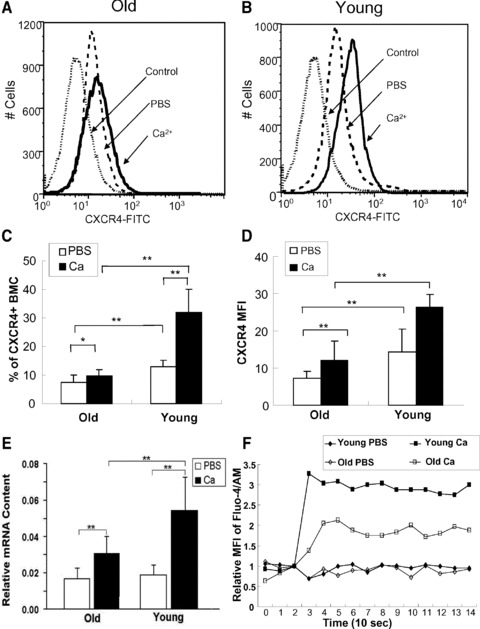
Comparison of CXCR4 expression in BMCs from young versus old mice. BMCs were incubated at 37°C for 4 hrs in PBS (white bar) or PBS plus CaCl2 (1 mM) (black bar) and labelled with FITC-conjugated anti-CXCR4 mAb. BMCs from young (A) and old mice (B) were analysed for surface CXCR4 expression by FACS. CXCR4+ BMCs from old and young mice were quantified as percentage of total BMCs (C) or as MFI (D). (E) Quantification of CXCR4 mRNA by RT-PCR. The CXCR4 mRNA levels were normalized to the mRNA levels of housekeeper gene GAPDH. *P < 0.05; **P < 0.01. (F) Time course of calcium influx into BMCs from young and old mice. Each set point represents the MFI of a 15 sec. histogram. Values were normalized against the basal MFI before adding CaCl2 into the BMC suspension.
Furthermore, the inducibility of CXCR4 surface expression in response to environmental change was also compared between BMCold and BMCyoung. As we reported previously, CXCR4 expression on BMCyoung could be enhanced by calcium [9]. To determine whether BMCold has the similar inducibility, BMCold and BMCyoung were subjected to 1 mM CaCl2 treatment for 4 hrs. CXCR4 surface expression in BMCold increased significantly less (9.6 ± 2.4%) in comparison with BMCyoung whose CXCR4 expression was more than doubled after calcium treatment (30.0 ± 8.0%, n= 13, P < 0.01) (Fig. 2A and C).
To determine whether these differences were due to transcription of the CXCR4 gene, CXCR4 mRNA was quantified by RT-PCR (Fig. 2E). Basal level of CXCR4 mRNA was similar between BMCyoung and BMCold. However, calcium treatment of the BMCs increased CXCR4 mRNA in BMCyoung by 2.89 ± 0.48-fold (n= 3, P < 0.05) whereas the increase in BMCold (1.82 ± 0.2-fold) was significantly lower (n= 3; P < 0.05). To examine the mechanism of defective calcium-induced CXCR4 surface expression in BMCold, calcium influx into BMCs was measured by flow cytometry. BMCold had significant lower calcium influx than BMCyoung (Figs 2F and S2). The data indicate that cytoplasmic Ca++ concentration is an important factor that regulates CXCR4 transcription. Defective calcium influx in BMCold is one of the direct reasons for the impaired Ca-induced CXCR4 expression.
To further define the cause of defective CXCR4 surface expression in old BMCs, both intracellular and surface CXCR4 were measured using a differential labelling system with two- colour-conjugated Abs (‘Materials and methods’). The intracellular CXCR4 in old BMCs was not significantly different from young BMCs (Fig. 3A). These data indicate the lower CXCR4 surface expression in old cells is not caused at the levels of transcription or translation.
Fig 3.
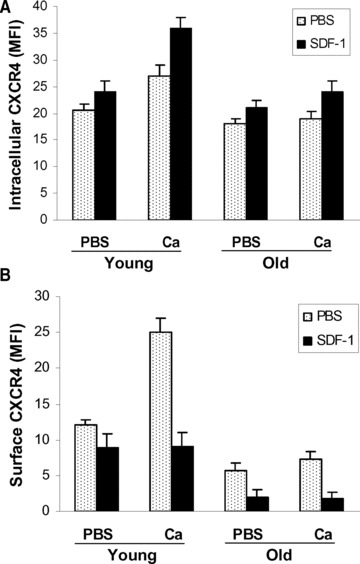
Internalization of CXCR4. Intracellular (A) and surface (B) CXCR4 was differentially measured by FACS after BMCs, treated with (black) or without (dots) calcium, were mixed with SDF-1 for 1 hr. Disappearance of surface CXCR4 and increase of intracellular CXCR4 represent the internalization of CXCR4.
Ligand-induced CXCR4 internalization
SDF-1 binding causes internalization of the CXCR4 receptor. To determine whether this activity is normal in BMCold, we measured the decrease of surface CXCR4 and increase of intracellular CXCR4 after BMCs were mixed with SDF-1 to allow internalization of SDF-1/CXCR4 complex. As shown in Figure 3, surface CXCR4 declined after SDF-1 treatment of BMCyoung (Fig. 3B) and there was a reciprocal increase of intracellular CXCR4 (Fig. 3A). These changes were enhanced by calcium treatment. In BMCold, SDF-1 also caused significant CXCR4 receptor internalization. But unlike the BMCyoung this was not amplified by calcium. These results suggest that CXCR4 responds normally to SDF-1 in cells from old mice and that the main difference is lower basal CXCR4 expression and absence of a response to calcium.
CXCR4 expression in subgroups
To further examine CXCR4 expression in the subpopulation of BMCs and to determine whether a subset of cells was selectively impaired during aging, we compared CXCR4 expression in subpopulations of BMC from young and old mice. As shown in Figure 4 and Table 1, we found that cell composition in BMCs was similar between old and young mice in the examined subsets except the lineage negative subpopulation which is significantly higher in young mice than old mice (Fig. 4A). The surface CXCR4 expression of Lin–/Sca1+ cells from young mice was significantly higher than that of old mice, whereas CXCR4 expression on CD34+/Flk1+ subset was not different (Fig. 4B and C). We note that Gr1+/CD11b+ subpopulation is significantly higher in old mice than young mice, while the CXCR4 surface expression on this subpopulation is higher in young mice (Fig. 4).
Fig 4.
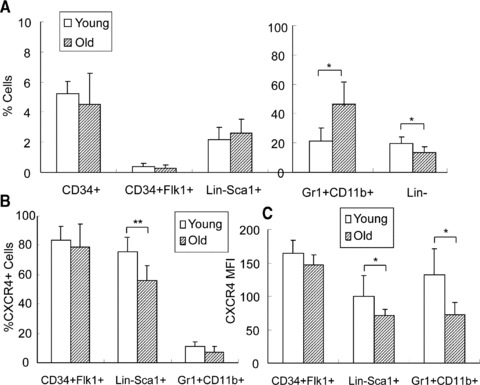
CXCR4 expression in BMC subpopulations. (A) Subpopulations of BMCs were analysed by FACS and compared between young and old mice. (B) Quantification of CXCR4+ cells in the subpopulation of BMCs from young and old mice by FACS. (C) Quantification of CXCR4 surface expression using MFI. n= 6. *P < 0.05, **P < 0.01.
Table 1.
CXCR4 expression in the subpopulations of BMCs from young and old mice
| Subpopulations | % Cells* | P-value (n= 4) | % CXCR41 Cells† | P-value‡ (n= 4) | ||
|---|---|---|---|---|---|---|
| Young | Old | Young | Old | |||
| CD34+ | 5.2 ± 0.8 | 4.5 ± 2.1 | 0.52 | 59.3 ± 7.6 | 62.7 ± 20.5 | 0.67 |
| CD34+/Flk1+ | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.56 | 83.4 ± 9.7 | 79.0 ± 15.4 | 0.46 |
| Lin− | 19.4 ± 4.7 | 13.6 ± 3.5 | 0.045 | 26.9 ± 12.6 | 26.9 ± 12.6 | 0.99 |
| Lin−/Sca1+ | 2.18 ± 0.81 | 2.62 ± 0.93 | 0.31 | 75.5 ± 9.6 | 55.6 ± 10.7 | 0.01 |
| Lin−/Sca1+/cKit+ | 0.18 ± 0.09 | 0.19 ± 0.09 | 0.8 | 45.9 ± 15.4 | 32.4 ± 14.3 | 0.08 |
| Gr1+/CD11b+ | 21.2 ± 8.1 | 46.7 ± 17.2 | 0.045 | 11.1 ± 3.0 | 7.0 ± 3.1 | 0.06 |
Percentage of subpopulation cells in total BMCs were analysed by FACS.
Percentage of CXCR4+ cells in the gated subpopulations of the previous column ‘% Cells’.
P-values were obtained from t-test analysis of data between young versus old.
A muted signalling response to SDF-1 in BMCold
To quantify downstream SDF-1/CXCR4 signalling in BMCold, we measured the SDF-1-mediated phosphorylation of protein kinases Akt (Fig. 5A) and ERK1/2 (Fig. 5B). SDF-1 treatment induced 2–3-fold increase in the phosphorylation of Akt in BMCs from young mice, which was enhanced by calcium treatment (Fig. 5C). In contrast there was no significant change in AKT phosphorylation in response to SDF-1 or calcium treatment of BMCs from old mice. The data indicate that BMCold have reduced sensitivity to calcium stimulation and depressed intracellular signalling by SDF-1/CXCR4/Akt as a consequence. In contrast, the ERK1/2 phosphorylation was similar between old and young BMCs and was not changed by calcium treatment (Fig. 5D). These results indicate that the ERK1/2 signalling pathway is not altered in BMCold. The fact that SDF-1 increased Akt phosphorylation only in young BMCs correlated with cell protection. Young, but not old, BMCs had reduced apoptosis and necrosis after exposed to hypoxia and nutrition depression for 20 hrs (Fig. S3).
Fig 5.
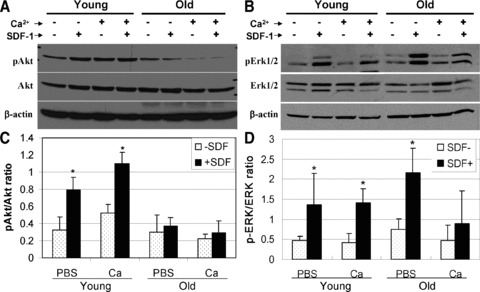
Effect of SDF-1 and calcium treatment on phosphorylation of AKT and ERK1/2. BMCs from young and old mice were incubated in PBS with or without CaCl2 for 4 hrs, then mixed with SDF-1 for 5 min. at 37°C before they were analysed by Western blot using specific antibodies against Akt (A) and ERK1/2 (B), and their phosphorylated forms (p-Akt and p-ERK). The relative p-Akt (C) and p-ERK (D) are presented as a ratio of phosphorylated kinase over total kinase after measuring the density of each band. *P < 0.05; n= 3.
Defective SDF-1 mobility of aged BMCs in vitro
Boyden chamber assays were use to quantify the migration of BMCs from young and old mice in response to a gradient of SDF-1. As indicated in Figure 6A, the mobility of BMCyoung was enhanced by calcium treatment. In contrast, BMCold showed lower mobility and this was not enhanced by calcium pre-treatment.
Fig 6.
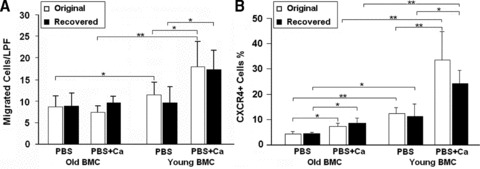
The effects of environment on CXCR4 expression and BMC migration. BMCs of young and old mice were eliminated by lethal irradiation and reciprocally replaced by transplanting old into young and vice versa. After 2 months reconstitution, BMCs were recovered and subjected to calcium treatment. (A) BMC migration was performed by a modified Boyden chamber assay. Migrated cells were quantified microscopically (LPF) 16 hrs after culture. (B) CXCR4+ BMCs was determined by FACS as described above. *P < 0.05; **P < 0.01, n= 5. ‘Original’ and ‘Recovered’ represent BMCs from mice before and after transplantation, respectively.
To determine whether the effects of aging on BMCs were reversible, we carried out reciprocal old→young and young→old bone marrow transplants as described in ‘Materials and methods’. Two months after transplantation, CXCR4 surface expression of the recovered BMCs was measured and migration to SDF-1 was determined. CXCR4 expression of aged BMCs remained low after transplanted into young mice while that of young BMCs remained high after transplanted into old mice (Fig. 6B). The changes due to transplantation were not significantly different in either case. Similarly transplantation did not change basal or calcium-stimulated mobility toward SDF-1 (Fig. 6A).
In vivo homing of BMCs from old mice is impaired
To determine whether impaired CXCR4 expression affects the ability of cells to home to ischemic muscle, BMCs were isolated from young and old GFP mice and pre-treated with PBS alone or PBS + CaCl2. Equal cell aliquots were delivered by tail vein injection into wild-type young mice with ischemic hindlimbs which received SDF-1 injections to increase chemo-attraction for CXCR4+ cells. One week later, significantly more GFP+ cells presented in the ischemic muscle from the young versus old donors (P < 0.05, n= 3) (Fig. 7A). Homing of GFP cells to ischemic muscle was further enhanced by calcium treatment of young but not old GFP BMCs (Fig. 7B). These results confirm the defective SDF-1-mediated homing capacity and calcium response of aged BMCs.
Fig 7.

The effect of calcium treatment on BMC homing and promoting angiogenesis. BMCyoung and BMCold from GFP mice, treated with/without calcium, were intravenously injected via the tail vein into young recipients with ischemic hindlimb. SDF-1 was injected into ischemic muscle once a day for three days after femoral artery resection. (A) Ischemic muscles were harvested 1 week after the surgery. The injected GFP cells were detected under fluorescent microscopy. Bar = 100 μm. (B) Quantitative measurement of injected BMC homing at the ischemic tissues. *P < 0.05; n= 3. (C) LDPI showing blood flow before and 3 weeks after the treatment. (D) Quantitative measurement of reperfusion. (E) CD31 immunofluorescence staining to show the capillaries in ischemic muscles. Bar = 50 μm. (F) Quantification of capillary density per high power field. (G) CD34 (green fluorescence) and Ki67 (red fluorescence) double immunofluorescence staining to illustrate the proliferating CD34+ cells. Bar = 50 μm. (H) Quantification of CD34 and Ki67double positive cells indicating proliferating CD34+ cells per high power field. *P < 0.05, **P < 0.01; n= 6.
The angiogenic ability of BMCold was impaired
BMCold or BMCyoung were intravenously injected into young recipient mice with ischemic hindlimb. Blood reperfusion was measured by LDPI. BMCyoung promoted significantly more angiogenesis than BMCold (Fig. 7C and D). Calcium treatment of BMCyoung but not BMCold further augmented the recovery of blood flow. The perfusion index increased from 0.67 ± 0.07 of PBS-treated BMCyoung to 0.80 ± 0.12 in Ca-treated BMCyoung while there was no significant difference between groups injected with BMCold (P > 0.05, n= 6) (Fig. 7D). Injection of BMCyoung resulted in significantly increased capillary density (Fig. 7E and F) and proliferating CD34+ cells (Fig. 7G and H) in the ischemic muscles relative to that injected with BMCold. These effects were enhanced by calcium pre-treatment of young, but not old, BMCs.
Discussion
Aging in animals and human beings is associated with impaired angiogenesis and arteriogenesis [16, 18, 21–23]. Here we confirm that old mice have impaired vessel regeneration in response to ischemia and fewer proliferating cells and less CD34+ progenitor cells in the ischemic muscle (Fig. 1). We propose that this is a consequence of impaired CXCR4 surface expression on BMCs and desensitization of CXCR4 expression to calcium.
Compared to BMCs from young mice, those from aged mice had: (1) reduced surface expression of CXCR4; (2) diminished stimulation of CXCR4 surface expression by calcium; (3) significant lower Ca-influx and loss of calcium-stimulated CXCR4 mRNA accumulation; (4) dramatic reduction of SDF-1-mediated CXCR4 internalization both in the presence and absence of calcium stimulation and (5) marked abrogation of Akt phosphorylation in response to SDF-1 treatment. These effects of aging on CXCR4 expression in BMCs translated into the following functional impairments: (1) BMCs failed to migrate in an SDF-1 gradient; (2) Calcium failed to enhance migration; (3) SDF-1 failed to enhance BMC viability; (4) BMCs failed to home in vivo to ischemic tissue in response to SDF-1 in ischemic hindlimbs and (5) Angiogenesis was markedly impaired despite injected SDF-1 in the ischemic muscle. Fewer capillaries and proliferating precursor cells were present at the lesion site. The impact of aging on CXCR4 expression and SDF-1-mediated migration were retained after reciprocal transplant of cells from young to old and old to young mice (Fig. 6), indicating that the change in CXCR4 expression is irreversible.
Our results provide the first evidence that defective CXCR4 amount and function of bone marrow progenitors contributes to impaired neovascularization in aged animals. This is consistent with reports that age-related dysfunctions can be reversed by gene-mediated overexpression of CXCR4 [24].
There was a selective effect of aging on BMC subpopulations (Table 1). The number of lineage negative BMCs were significantly reduced in older mice while other subpopulations were not significantly altered (Fig. 4A). CXCR4 surface expression was significantly lower in the Lin–/Sca1+ subpopulation of old mice (Fig. 4B and C). Because Lin–/Sca1+ cells are major precursors of haematopoietic cells [25], our data suggest that HSCs are important for initiating angiogenesis and lower CXCR4 surface expression on HSC impairs the angiogenesis in older mice. Less homing could also be due to different composition of different cell populations between young and old mice. We also showed that other cell types such as CD11b+ and Gr1+ cells loose CXCR4 expression during aging (Table 1 and Fig. 4) and this may contribute to the global loss of CXCR4 that we observed; these cells also contribute to angiogenesis in addition to CD34+ cells.
It is interesting to note that BMCold contain more neutrophils (Gr1+/CD11b+) than BMCyoung, while the neutrophils in BMCold have less CXCR4 than those in BMCyoung (Fig. 4 and Table 1). These results agree with the reports [26, 27] that there are more neutrophils in circulation of older as neutrophils have less CXCR4 and are easier to be mobilized into circulation. However, there are discrepancies in the literatures regarding the CXCR4 expression on neutrophils. Several reports have shown that neutrophils have relative low CXCR4 expression (∼4–10%) [26, 28–30] as we observed, while other studies [26, 27] reported that ∼70% neutrophils are CXCR4+. The difference may reflect different methods of cell gating, cell treatments and/or immunostaining.
Mechanistically, we found that transcription of the CXCR4 gene in BMCs was not affected by aging because the basal mRNA levels of CXCR4 were similar between BMCyoung and BMCold (Fig. 2D). Furthermore, we also found that the intracellular CXCR4 in old BMCs was not significantly different from young BMCs (Fig. 3A). These results indicate that the defective surface expression in old cells is most likely a post-translational consequence that may occur at the step of exocytosis. After calcium stimulation, CXCR4 mRNA accumulation was significantly lower in old relative to young BMCs, an effect that we attribute to defective calcium influx in old BMCs. Less calcium influx results in less calcium-induced transcription and less calcium-induced CXCR4 surface presentation. These results suggest that loss of a transcriptional response to extracellular stimulators along with repressed receptor externalization all contribute to the defective CXCR4 surface expression in aged mice.
The molecular mechanism for age-related down-regulation of CXCR4 is unknown. In lymphocytes CXCR4 expression is regulated by cytokines, interleukin 2 [31], transforming growth factor β and interferon-γ[32, 33]. CXCR4 mRNA, protein and cell surface expression in endothelial cells has been shown to be up-regulated by β–FGF and down-regulated by TNFα[34–36]. Aging causes deficient expression and defective function of angiogenic growth factors [16]. Reduced SDF-1α levels in aged wounds was also reported [37].
Cellular responses to stimulation on CXCR4 expression are cell specific. For example proliferation stimuli decrease CXCR4 expression in lymphocytes [38], but increase CXCR4 expression in MSC and possibly in glial progenitors [39, 40]. Our observation of differential effects of CXCR4 expression in bone marrow (BM) subpopulations may help reconcile apparently conflicting reports on the effects of aging on progenitor cells. Chang et al. reported that age-associated decline in vasculogenesis results from failure of peripheral tissues to generate a suitable signal for EPC recruitment rather than from primary EPC depletion or dysfunction [41]. They showed that intrinsic EPC function and number remained intact both in human beings and in a mouse ischemic flap model. Our data also show that the number of CD34+/Flk1+ cells (considered as EPCs) in BM was not altered by aging (Fig. 4A). Dimmeler’s group reported that the expression of CXCR4 on BM-MNC was not significantly different between healthy young adults and aged patients with chronic ischemic heart disease [42]. It seems possible that marked effects of aging on CXCR4 expression on subpopulations of BMCs is responsible at least in part for defective angiogenesis and vessel repair.
Acknowledgments
This study was supported by grants from America Heart Association #0855311 (H.Y.); NIH RO1 # HL072924 (K.A.W.) and State of Florida James & Esther King Biomedical Research Program, Team Science Project # 07KT-02 (H.Y., K.A.W.), Walter G. Ross Distinguished Chair of Vascular Biology (K.A.W.) and China Scholarship #2008632079 (Q.X.).
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1 FACS analysis of surface CXCR4 on BMC. Aftercalcium treatment, both the number of cells expressing surfaceCXCR4 and the amount of CXCR4 on the surface are increased. TheFACS pictures show that more cells above the base line indicatingmore CXCR4+ cells, and that higher intensity of fluorescence (higher above the line) indicating increased amount of CXCR4 on surface.
Fig. S2 Calcium influx into BMC. Calcium influx intocells was measured by flow cytometry with Fluo-4/AM staining.Arrows indicate the time when PBS or CaCl2 were added. The FACS data were converted into line graphs in Figure 1F.
Fig. S3 Viability and apoptosis of BMCs. BMCs from young(white bar) and old (black bar) mice were cultured under hypoxia(0.5% O2) in serum-free DMEM supplemented with orwithout SDF-1α for 20 hrs. The apoptosis and necrosis cellswere detected by annexin V and PI staining and thereafter flowcytometry analysis. The unhealthy cells represent the cells thatwere positive for either staining. *P < 0.05; n = 3.
References
- 1.Majka SM, Jackson KA, Kienstra KA, et al. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin. Invest. 2003;111:71–9. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aranguren XL, McCue JD, Hendrickx B, et al. Multipotent adult progenitor cells sustain function of ischemic limbs in mice. J Clin Invest. 2008;118:505–14. doi: 10.1172/JCI31153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purhonen S, Palm J, Rossi D, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumour growth. Proc Natl Acad Sci USA. 2008;105:6620–5. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 5.Mohle R, Bautz F, Rafii S, et al. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–30. [PubMed] [Google Scholar]
- 6.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7:19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q, Shao H, Eton D, et al. Extracellular calcium increases CXCR4 expression on bone marrow-derived cells and enhances pro-angiogenesis therapy. J Cell Mol Med. 2009;13:3764–73. doi: 10.1111/j.1582-4934.2009.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, G-C Fan, Zhou X, et al. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardio. 2008;44:281–92. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn J, Byk T, Jansson-Sjostrand L, et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–9. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 12.Porecha NK, English K, Hangoc G, et al. Enhanced functional response to CXCL12/SDF-1 through retroviral overexpression of CXCR4 on M07e cells: implications for hematopoietic stem cell transplantation. Stem Cells Dev. 2006;15:325–33. doi: 10.1089/scd.2006.15.325. [DOI] [PubMed] [Google Scholar]
- 13.Heiss C, Keymel S, Niesler U, et al. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–8. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 14.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–9. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 15.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–6. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 16.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–20. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 17.Bach MH, Sadoun E, Reed MJ. Defects in activation of nitric oxide synthases occur during delayed angiogenesis in aging. Mech Ageing Dev. 2005;126:467–73. doi: 10.1016/j.mad.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Westvik TS, Fitzgerald TN, Muto A, et al. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J Vasc Surg. 2009;49:464–73. doi: 10.1016/j.jvs.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan Y, Shao H, Eton D, et al. Stromal Cell-Derived Factor-1 Enhances Pro-Angiogenic Effect of Granulocyte Colony Stimulating Factor. Cardiovasc Res. 2007;73:823–32. doi: 10.1016/j.cardiores.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao H, Tan Y, Eton D, et al. Statin and stromal cell derived factor-1 additively promote angiogenesis by enhancement of progenitor cells incorporation into new vessels. Stem Cells. 2008;26:1376–84. doi: 10.1634/stemcells.2007-0785. [DOI] [PubMed] [Google Scholar]
- 21.Reed MJ, Corsa A, Pendergrass W, et al. Neovascularization in aged mice: delayed angiogenesis is coincident with decreased levels of transforming growth factor beta1 and type I collagen. Am J Pathol. 1998;152:113–23. [PMC free article] [PubMed] [Google Scholar]
- 22.Reed MJ, Edelberg JM. Impaired angiogenesis in the aged. Sci Aging Knowledge Environ. 2004:pe7. doi: 10.1126/sageke.2004.7.pe7. 2004. [DOI] [PubMed] [Google Scholar]
- 23.Shimada T, Takeshita Y, Murohara T, et al. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148–55. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 24.Kyriakou C, Rabin N, Pizzey A, et al. Factors that influence short-term homing of human bone marrow-derived mesenchymal stem cells in a xenogeneic animal model. Haematologica. 2008;93:1457–65. doi: 10.3324/haematol.12553. [DOI] [PubMed] [Google Scholar]
- 25.Ratajczak MZ, Zuba-Surma EK, Shin D-M, et al. Very small embryonic-like (VSEL) stem cells in adult organs and their potential role in rejuvenation of tissues and longevity. Exp Gerontol. 2008;43:1009–17. doi: 10.1016/j.exger.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suratt BT, Petty JM, Young SK, et al. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–71. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- 27.Eash KJ, Means JM, White DW, et al. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113:4711–9. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin C, Burdon PC, Bridger G, et al. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–93. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 29.Weisel KC, Bautz F, Seitz G, et al. Modulation of CXC chemokine receptor expression and function in human neutrophils during aging in vitro suggests a role in their clearance from circulation. Mediat Inflamm. 2009 doi: 10.1155/2009/790174. ; 2009: 790174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jablonska J, Leschner S, Westphal K, et al. Neutrophils responsive to endogenous IFN-beta regulate tumour angiogenesis and growth in a mouse tumour model. J Clin Invest. 2010;120:1151–64. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loetscher P, Seitz M, Baggiolini M, et al. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–77. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burke F, Relf M, Negus R, et al. A cytokine profile of normal and malignant ovary. Cytokine. 1996;8:578–85. doi: 10.1006/cyto.1996.0077. [DOI] [PubMed] [Google Scholar]
- 33.Franitza S, Kollet O, Brill A, et al. TGF-beta1 enhances SDF-1alpha-induced chemotaxis and homing of naive T cells by up-regulating CXCR4 expression and downstream cytoskeletal effector molecules. Eur J Immunol. 2002;32:193–202. doi: 10.1002/1521-4141(200201)32:1<193::AID-IMMU193>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 34.Volin MV, Joseph L, Shockley MS, et al. Chemokine receptor CXCR4 expression in endothelium. Biochem Biophys Res Commun. 1998;242:46–53. doi: 10.1006/bbrc.1997.7890. [DOI] [PubMed] [Google Scholar]
- 35.Gupta SK, Lysko PG, Pillarisetti K, et al. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273:4282–7. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 36.Feil C, Augustin HG. Endothelial cells differentially express functional CXC-chemokine receptor-4 (CXCR-4/fusin) under the control of autocrine activity and exogenous cytokines. Biochem Biophys Res Commun. 1998;247:38–45. doi: 10.1006/bbrc.1998.8499. [DOI] [PubMed] [Google Scholar]
- 37.Loh SA, Chang EI, Galvez MG, et al. SDF-1[alpha] expression during wound healing in the aged is HIF dependent. Plast Reconstr Surg. 2009;123:65S–75S. doi: 10.1097/PRS.0b013e318191bdf4. [DOI] [PubMed] [Google Scholar]
- 38.Cristillo AD, Bierer BE. Regulation of CXCR4 expression in human T lymphocytes by calcium and calcineurin. Mol Immunol. 2003;40:539–53. doi: 10.1016/s0161-5890(03)00169-x. [DOI] [PubMed] [Google Scholar]
- 39.Helbig G, Christopherson KW, II, Bhat-Nakshatri P, et al. NF-kB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–8. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Yu X, Lin S, et al. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780–4. doi: 10.1016/j.bbrc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 41.Chang EI, Loh SA, Ceradini DJ, et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116:2818–29. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 42.Heeschen C, Lehmann R, Honold J, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–22. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 FACS analysis of surface CXCR4 on BMC. Aftercalcium treatment, both the number of cells expressing surfaceCXCR4 and the amount of CXCR4 on the surface are increased. TheFACS pictures show that more cells above the base line indicatingmore CXCR4+ cells, and that higher intensity of fluorescence (higher above the line) indicating increased amount of CXCR4 on surface.
Fig. S2 Calcium influx into BMC. Calcium influx intocells was measured by flow cytometry with Fluo-4/AM staining.Arrows indicate the time when PBS or CaCl2 were added. The FACS data were converted into line graphs in Figure 1F.
Fig. S3 Viability and apoptosis of BMCs. BMCs from young(white bar) and old (black bar) mice were cultured under hypoxia(0.5% O2) in serum-free DMEM supplemented with orwithout SDF-1α for 20 hrs. The apoptosis and necrosis cellswere detected by annexin V and PI staining and thereafter flowcytometry analysis. The unhealthy cells represent the cells thatwere positive for either staining. *P < 0.05; n = 3.


