Abstract
Medications used to treat rheumatoid arthritis (RA) may confer an increased risk of infection. We conducted a retrospective cohort study of veterans with RA followed in the United States Department of Veterans Affairs health care system from October 1998 through June 2005. Risk of hospitalization for infection associated with tumor necrosis factor (TNF)-α antagonists therapy was measured using an extension of Cox proportional hazards regression, adjusting for demographic characteristics, comorbid illnesses, and other medications used to treat RA.
A total of 20,814 patients met inclusion criteria, including 3796 patients who received infliximab, etanercept, or adalimumab. Among the study cohort, 1465 patients (7.0%) were hospitalized at least once for infection. There were 1889 hospitalizations for infection. The most common hospitalized infections were pneumonia, bronchitis, and cellulitis. Age and several comorbid medical conditions were associated with hospitalization for infection. Prednisone (hazard ratio [HR], 2.14; 95% confidence interval [CI], 1.88–2.43) and TNF-α antagonist use (HR, 1.24; 95% CI, 1.02–1.50) were associated with hospitalization for infection, while the use of disease-modifying antirheumatic drugs (DMARDs) other than TNF-α antagonists was not. Compared to etanercept, infliximab was associated with risk for hospitalization for infection (HR, 1.51; 95% CI, 1.14–2.00), while adalimumab use was not (HR, 0.95; 95% CI, 0.68–1.33). In all treatment groups, rate of hospitalization for infection was highest in the first 8 months of therapy.
We conclude that patients with RA who are treated with TNF-α antagonists are at higher risk for hospitalization for infection than those treated with other DMARDs. Prednisone use is also a risk factor for hospitalization for infection.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disorder affecting approximately 1% of the population, which causes joint destruction, pain, and loss of function. [12] Prior studies indicate that the incidence of infections, particularly pulmonary, soft tissue, and bone and joint infections, is higher in patients with RA compared to the general population. [10,22,31]
Disease-modifying antirheumatic drugs (DMARDs) are the mainstay of RA treatment. Tumor necrosis factor-α (TNF-α) antagonists, a newer class of biologic DMARDs, have previously been associated with an increased risk of several types of infections including tuberculosis, candidiasis, coccidiomycosis, histoplasmosis, listeriosis, and nocardiosis. [33] Prior studies have yielded conflicting evidence about the risk of serious infections requiring hospitalization in patients with RA who are treated with DMARDs. A meta-analysis of clinical trial data showed an increased risk for serious infection with infliximab and adalimumab. [3] Two studies have shown an increased risk of serious infection among patients prescribed TNF-α antagonists, [4,19] while other studies have not found an increased risk for serious infections associated with TNF-α antagonist. [8,28] In prior studies of patients with RA, non-biologic DMARDs were not associated with an increased risk of infection. [17,35]
In the current study, we used the United States Department of Veterans Affairs (VA) national databases to characterize the risk of and risk factors for developing an infection requiring hospitalization in patients with RA initiated on DMARD therapy. Pharmacy and administrative databases were used to identify drug classes that could contribute to the risk for infection.
METHODS
This study was approved by the institutional review boards of the participating institutions.
Databases
All inpatient and outpatient International Classification of Diseases, Version 9, Clinical Modification (ICD-9-CM) diagnosis codes, encounter data, and patient demographic data were obtained from the VA’s Austin Information Technology Center, a repository for VA administrative data. Detailed inpatient and outpatient pharmacy data on the patients were obtained from the VA’s Pharmacy Benefits Management program.
Study sample
The study sample included all veterans who had an inpatient or outpatient ICD-9-CM code diagnosis of RA between October 1, 1998, and September 30, 2005. To capture patients starting on DMARDs who received outpatient medications from the VA, patients were included only if they had been receiving any medication for at least 4 months before first DMARD. Eligible patients were required to have at least 2 separate outpatient or inpatient clinical encounters during the study period, and to have age and sex recorded.
Patients were censored at the date of the last clinical encounter, or receipt of last DMARD prescription plus the number of days of the prescription plus 5 half-lives of the DMARD, whichever was later. Patients who met inclusion criteria but were uncensored were followed until September 30, 2006.
Definitions
RA
Identification of patients with RA was based on a previously validated algorithm. [30] The diagnosis required the occurrence of an ICD-9-CM code for RA on at least 1 occasion in either the inpatient or outpatient record, and the subsequent receipt of at least 1 DMARD prescription. The following codes were defined as coding for RA: RA (714.0), Felty syndrome (714.1), RA with visceral or systemic involvement (714.2), or rheumatoid lung (714.81).
DMARD
DMARD medications of interest included the following: hydroxycholoroquine, auranofin, injectable gold, penicillamine, sulfasalazine, methotrexate, azathioprine, leflunomide, cyclophosphamide, cyclosporine, anakinra, etanercept, infliximab, and adalimumab.
Medication group
Treatment of RA is highly individualized based on disease activity, duration of disease, and comorbid conditions. RA treatments were subdivided into medication groups based on their use in the treatment of mild, moderate, and severe disease. [18,20,25] Group 1 medications (treatment of mild disease) included hydroxycholoroquine, sulfasalazine, auranofin, injectable gold, and penicillamine. Group 2 medications (treatment of moderate disease) included methotrexate, leflunomide, azathioprine, cyclophosphamide, cyclosporine, and anakinra. Group 3 medications (treatment of severe disease) included the TNF-α antagonists (etanercept, infliximab, and adalimumab). If patients received a medication from more than 1 medication group concurrently, they were assigned to the higher medication group. Prednisone was not included in the DMARD groupings, but use at the time of HFI was assessed.
Patient-time in medication group
Patients were considered to have initiated 1 of the 3 medication groups on the date of the first prescription for a medication in that medication group after a period of 4 months with no prior use of any medication from that group. The patient continued in the assigned medication group until 1) a medication from a higher-numbered medication group was prescribed; 2) the patient switched to an entirely new medication group; or 3) the patient was censored, as described. Thus, a single patient could contribute patient-time from different discrete time periods to more than 1 medication group. A patient could be in only 1 medication group at any point in time. To account for the prolonged immunosuppressive effects of the medications, outcomes were attributed to a medication group after discontinuation for 5 half-lives of the medication or 1 dosing interval plus 1 half-life, whichever was longer.
Hospitalization for infection (HFI)
Data from the 2005 National Hospital Discharge Survey (NHDS)[6] were used to create a composite variable for HFI. All ICD-9-CM codes in the NHDS that code for infection and accounted for at least 10,000 admissions in the survey in patients aged 15 years or greater were included in the HFI variable. Any VA hospitalization with a qualifying ICD-9-CM code in the “primary reason for hospitalization” field was considered a HFI.
Data analyses
Incidence of HFI was calculated as number of events per 100 patient-years. For descriptive and bivariate analysis, dichotomous variables were analyzed using the chi-square test. Continuous variables were analyzed using the Student t-test. A 2-sided p-value of ≤0.05 was considered statistically significant. Risk of outcomes was described using hazard ratios and 95% confidence intervals. Time-to-single-event analysis was performed using Cox proportional hazards regression. Time-to-multiple-event analysis was performed using the Anderson-Gill extension of Cox proportional hazards regression, which allows for multiple events per person. This model assumes that events within an individual patient are independent (for example, patients hospitalized for infection are not at greater risk for a second HFI). This assumption is supported by our analysis using a traditional Cox proportional hazards regression model, which showed no substantial difference in results compared to those obtained using the Anderson-Gill model. When a patient had more than 1 HFI, the start time for the second event began 1 day after hospital discharge for the initial HFI. In regression modeling, membership in each medication group was modeled as a time-dependent dummy variable to account for the change in medication groups over time and adjusted for age, sex, race, and time-dependent comorbid diagnoses. Each drug was also modeled separately and adjusted as above. Our model was adjusted for proximity to the nearest VA hospital based on zip code data. Patients who resided in a zip code where the mean distance to the nearest VA hospital was more than 20 miles at the time of HFI were considered to be distant to a VA hospital. All analyses were performed using SAS software version 6.12 (SAS Institute, Cary, NC). The graph of infection rates per 100 patients was creating using R software version 2.5.1 (R Foundation, Vienna, Austria). A line smoother was employed (spline function) to clarify changes over time.
Validation
Fifty patients with RA who were hospitalized for infection and 100 patients with RA who were not hospitalized for infection at a single center during the study period were randomly identified by ICD-9-CM codes. All medical records during the study period, including discharge summaries, daily notes, and laboratory and radiology data were reviewed by 2 infectious disease specialists (MAL and JRM). Hospitalizations were classified as “for infection” or “not for infection” based on initial reason for hospitalization. Consensus was achieved in all cases. Adjusted sensitivity and specificity were calculated by extrapolating the validation results over the entire study population.
RESULTS
There were 20,814 patients with mean follow-up of 2.7 years in the study cohort, accounting for 56,854 patient-years of observation (Table 1). There were 11,772 patients who contributed patient-time to medication group 1, 13,364 patients to medication group 2, and 3796 patients to medication group 3. Patient demographics, comorbid medical conditions, and medication group are shown in Table 1. RA patients using TNF-α antagonists (group 3) were somewhat younger than medication group 1 or 2 users but had a similar comorbidity profile. Prednisone was used in 12,025 (57.8%) patients at any time during the study. A total of 1465 patients (7.0%) were hospitalized at least once for infection, and there were 1889 HFI. There were 638 HFI in medication group 1 (3.02 per 100 patient-years), 972 HFI in group 2 (3.48 per 100 patient-years), and 279 HFI in group 3 (3.59 per 100 patient-years). The crude rate of HFI in patients on infliximab, adalimumab, and etanercept was 4.90, 3.38, and 3.26 HFI per 100 patient-years, respectively (Table 2).
TABLE 1.
Demographics and Clinical Characteristics
| Characteristic | Total No. (%) | Medication Group | ||
|---|---|---|---|---|
| Group 1* No. (%) | Group 2† No. (%) | Group 3‡ No. (%) | ||
| N | 20,814 | 11,772 | 13,367 | 3796 |
| Mean age, yr (SD) | 63.0 (12.4) | 62.4 (12.7) | 63.6 (12.0) | 59.4 (11.6) |
| Sex (% male) | 18,882 (90.7) | 10,529 (89.3) | 12,195 (91.2) | 3453 (91.0) |
| Race | ||||
| White | 13,804 (66.3) | 7722 (65.6) | 9021 (67.5) | 2660 (70.1) |
| Black | 2338 (11.2) | 1546 (13.1) | 1354 (10.1) | 335 (8.8) |
| Other | 1066 (5.1) | 647 (5.5) | 678 ( 5.1) | 228 (6.0) |
| Unknown | 3606 (17.3) | 1857 (15.8) | 2311 (17.3) | 573 (15.1) |
| Comorbidities | ||||
| Diabetes mellitus | 6144 (29.5) | 3254 (27.6) | 4001 (29.9) | 1132 (29.8) |
| Hypertension | 15,329 (73.7) | 8405 (71.4) | 9768 (73.1) | 2753 (72.5) |
| Arrhythmia | 4360 (21.0) | 2247 (19.1) | 2769 (20.7) | 725 (19.1) |
| Heart failure | 3012 (14.5) | 1587 (13.5) | 1910 (14.2) | 441 (11.6) |
| Malignancy | 4646 (22.3) | 2464 (20.9) | 2913 (21.8) | 710 (18.7) |
| Chronic lung disease | 8060 (38.7) | 4430 (37.6) | 5048 (37.7) | 1474 (38.8) |
| Renal failure | 1653 (7.9) | 898 (7.6) | 974 (7.3) | 277 (7.3) |
| Liver disease | 1252 (6.0) | 885 (7.5) | 480 (3.6) | 261 (6.9) |
| AIDS§ | 91 (0.4) | 56 (0.5) | 43 (0.3) | 16 (0.4) |
| None of the above | 2295 (11.0) | 1305 (11.1) | 1381 (10.3) | 447 (11.8) |
| Prednisone use | 12,025 (57.8) | 5524 (47.0) | 8071 (60.4) | 2021 (53.2) |
| Mean no. intraarticular procedures (SD) | 0.46 (1.39) | 0.43 (1.29) | 0.44 (1.32) | 0.76 (1.93) |
| Mean no. orthopedic procedures (SD) | 0.06 (0.32) | 0.05 (0.28) | 0.06 (0.31) | 0.11 (0.42) |
Abbreviations: AIDS = acquired immunodeficiency syndrome, SD = standard deviation.
Group 1 medications include hydroxycholoroquine, sulfasalazine, auranofin, injectable gold, and penicillamine.
Group 2 medications include methotrexate, leflunomide, azathioprine, cyclophosphamide, cyclosporine, and anakinra.
Group 3 medications include etanercept, infliximab, and adalimumab.
TABLE 2.
Hospitalization for Infection (HFI) by Medication Group
| Total | Medication Group* | |||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | ||
| No. | 20,814 | 11,772 | 13,367 | 3796 |
| Patient-years | 56,854 | 21,150 | 27,936 | 7768 |
| Patients hospitalized for infection (%) | 1465 (7.0) | 519 (4.4) | 774 (5.8) | 229 (6.0) |
| Total HFI | 1889 | 638 | 972 | 279 |
| HFI per 100 patient-years | 3.32 | 3.02 | 3.48 | 3.59 |
See Table 1 for description of the medication groups.
Pneumonia and bronchitis were the most common infections in all medication groups (Table 3). There was no difference in frequency in these types of infection by medication group. Cellulitis (p = 0.04) occurred more frequently in medication group 3 than in group 1. Urinary tract infections occurred less frequently in medication group 3 than in group 1 (p = 0.02).
TABLE 3.
Infections Causing Hospitalization
| Medication Group* | P (1 vs 2) | P (1 vs 3) | |||
|---|---|---|---|---|---|
| Group 1 No. (%) | Group 2 No. (%) | Group 3 No. (%) | |||
| Most Common HFI | |||||
| Pneumonia | 192 (30.1) | 294 (30.3) | 79 (28.3) | 0.94 | 0.53 |
| Bronchitis | 110 (17.2) | 155 (16.0) | 40 (14.3) | 0.49 | 0.51 |
| Cellulitis | 99 (15.5) | 139 (14.3) | 54 (19.4) | 0.50 | 0.04 |
| Urinary/kidney infection | 56 (8.7) | 101 (10.4) | 16 (5.7) | 0.29 | 0.02 |
| Infection due to implant | 23 (3.6) | 38 (3.9) | 10 (3.6) | 0.63 | 0.80 |
| Postoperative infection | 22 (3.5) | 31 (3.2) | 14 (5.0) | 0.65 | 0.15 |
| Osteomyelitis | 21 (3.3) | 33 (3.4) | 10 (3.6) | 0.91 | 0.88 |
| Diverticulitis | 18 (2.8) | 24 (2.5) | 9 (3.2) | 0.66 | 0.49 |
| Septicemia | 17 (2.7) | 30 (3.2) | 4 (1.4) | 0.62 | 0.13 |
| Intestinal infection | 12 (1.9) | 29 (3.0) | 6 (2.2) | 0.17 | 0.46 |
See Table 1 for description of the medication groups.
In multivariate analysis, age and several comorbidities were predictive of HFI (Table 4). A higher number of orthopedic procedures was predictive of HFI (hazard ratio [HR], 1.22; 95% confidence interval [CI], 1.09–1.36), though a higher number of intraarticular procedures was not predictive (HR, 0.99; 95% CI, 0.95–1.03). Among medications, prednisone use was associated with HFI (HR, 2.14; 95% CI, 1.88–2.43), as was use of DMARDs in medication group 3 (HR, 1.24; 95% CI, 1.02–1.50). In multivariate analysis comparing the TNF-α antagonists to each another, infliximab use was associated with HFI (HR, 1.51; 95% CI, 1.14–2.00), while adalimumab use was not (HR, 0.95; 95% CI, 0.68–1.33) when compared to etanercept. There was no substantial difference in results when time-to-single-event analysis was performed (data not shown).
TABLE 4.
Multivariable-Adjusted, Independent Predictors of Hospitalization for Infection*
| Never Hospitalized for Infection | Hospitalized ≥1 for Infection | Hazard Ratio | 95% CI | |
|---|---|---|---|---|
| No. | 19,349 | 1465 | - | - |
| Mean age, yr (SD) | 63.0 (12.5) | 63.8 (12.0) | 1.01 | 1.00–1.01 |
| Sex (% male) | 17,530 (90.6) | 1352 (92.3) | 1.19 | 0.93–1.52 |
| Comorbidities (%) | ||||
| Diabetes mellitus | 5513 (28.5) | 631 (43.1) | 1.26 | 1.11–1.43 |
| Hypertension | 14,081 (72.8) | 1248 (85.2) | 1.18 | 1.01–1.38 |
| Arrhythmia | 3780 (19.5) | 580 (39.6) | 1.33 | 1.16–1.53 |
| Heart failure | 2488 (12.9) | 524 (35.8) | 1.71 | 1.47–1.98 |
| Malignancy | 4148 (21.4) | 499 (34.1) | 1.30 | 1.13–1.49 |
| Chronic lung disease | 7040 (36.4) | 1020 (69.6) | 2.29 | 2.02–2.60 |
| Renal failure | 1388 (7.2) | 265 (18.1) | 1.46 | 1.21–1.77 |
| Liver disease | 1103 (5.7) | 149 (10.2) | 1.36 | 1.07–1.72 |
| AIDS | 75 (0.4) | 16 (1.1) | 0.74 | 0.31–1.75 |
| Mean no. intraarticular procedures (SD) | 0.44 (1.35) | 0.73 (1.81) | 0.99 | 0.95–1.03 |
| Mean no. orthopedic procedures (SD) | 0.06 (0.30) | 0.13 (0.54) | 1.22 | 1.09–1.36 |
| Medications† | ||||
| Prednisone use (%) | 10,826 (56.0) | 1199 (81.8) | 2.14 | 1.88–2.43 |
| Group 1 | 11,253 (58.2) | 519 (35.4) | 1.0 | Referent |
| Group 2 | 12,590 (65.1) | 774 (52.8) | 1.08 | 0.95–1.24 |
| Group 3 | 3567 (18.4) | 229 (15.6) | 1.24 | 1.02–1.50 |
| Within TNF-α antagonist use comparisons | ||||
| Etanercept | 2464 (69.1) | 135 (58.9) | 1.0 | Referent |
| Infliximab | 771 (21.6) | 60 (26.2) | 1.51 | 1.14–2.00 |
| Adalimumab | 1217 (34.1) | 39 (17.0) | 0.95 | 0.68–1.33 |
Model adjusted for proximity to nearest VA hospital.
See Table 1 for description of the medication groups.
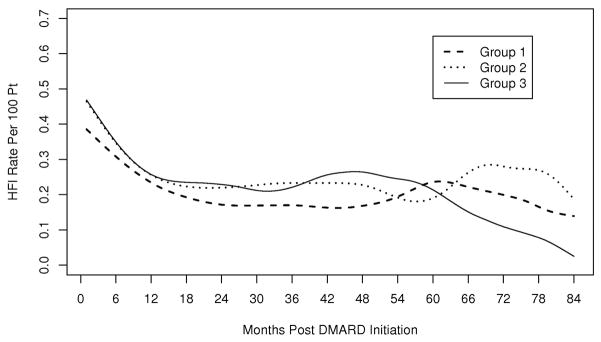
The mean length of hospitalization and duration of intravenous antibiotics was not significantly different between medication groups. The rate of HFI per 100 patients was highest in the first 9 months after entry into a new medication group, for all medication groups (Figure 1).
Fig 1.
Time to hospitalization for infection (HFI). Group 1 medications include hydroxycholoroquine, sulfasalazine, auranofin, injectable gold, and penicillamine. Group 2 medications include methotrexate, leflunomide, azathioprine, cyclophosphamide, cyclosporine, and anakinra. Group 3 medications include etanercept, infliximab and adalimumab.
The medical records of 50 hospitalizations that were identified as HFI were reviewed, and 46 of the 50 were confirmed HFI, yielding a positive predictive value of the administrative data to validly identify HFI of 92%. Medical records of 100 patients who were coded in the administrative data as being hospitalized during the study period for reasons other than infection were also reviewed. Of 162 hospitalizations in this group, there were 12 HFI during the study period. The adjusted sensitivity of our coding algorithm was 83%, adjusted specificity was 97%, and kappa was 0.80 (95% CI, 0.71–0.89), indicating good agreement between the code and gold standard.
DISCUSSION
In this large cohort of patients with RA in the national United States VA health care system, we identified hospitalizations for infection and types of infection utilizing ICD-9-CM codes and showed the validity of our outcome using electronic medical records. We demonstrated that use of prednisone and TNF-α antagonists was associated with HFI when compared to use of non-cytotoxic DMARDs. We also showed that infliximab was associated with an increased risk of infection compared to etanercept, with an absolute difference in risk of approximately 1–2 hospitalized infections per 100 patient-years.
The overall incidence of HFI in our cohort, 3.32 per 100 patient-years, is similar to that described in prior studies. In a study utilizing Medicare data, the rate of serious bacterial infections in elderly patients was 2.2 per 100 patient-years. [28] A prospective observational study utilizing a British biologics registry reported infection rates of 4.14 and 5.32 infections per 100 patient-years in those treated with traditional DMARDs and with TNF-α antagonists, respectively. [8] In a Swedish registry of patients with RA, the rate of HFI was 5.4 per 100 patient-years in patients prescribed TNF-α antagonists. [2]
Pneumonia, bronchitis, and cellulitis were the most common infections causing hospitalization in our cohort. These infections were among the most common in previous studies. [4,10] Compared to NHDS data, sepsis was seen less frequently in our study population, which may be due to variability in the accuracy of ICD-9-CM codes to identify patients with sepsis. [23,27] Additionally, patients aged ≥75 years accounted for over two-thirds of the discharges for sepsis in the NHDS. [6] It is possible that clinicians prescribed DMARDs to only the healthiest of elderly RA patients, creating a cohort less at risk for developing sepsis. The crude incidence of implant-related infection, postoperative infection, and osteomyelitis was higher in the current study than in NHDS, possibly due to the older age and high burden of comorbid illness in the VA population.
We found that age and several medical comorbidities were independent risk factors for HFI. While some comorbidities (diabetes, malignancy) may contribute directly to infection risk, others (heart failure, arrhythmia) probably contribute to the likelihood that an infected patient will be hospitalized because of perceived frailty and thus risk of poor outcome.
Studies examining the impact of TNF-α antagonists on the risk of serious infections have yielded mixed results. The current cohort, with 20,814 patients on DMARDs and 1889 HFI, is one of the largest such studies to date. In our study, TNF-α antagonist use was independently associated with an increased risk of HFI (HR, 1.24; 95% CI, 1.02–1.50). A meta-analysis of clinical trial data showed an increased risk for serious infection with infliximab and adalimumab use when compared to comparator arms of those trials (pooled odds ratio, 2.0, 95% CI, 1.3–3.1), but follow-up was limited to the study period of the trials, and there were only 26 serious infections. [3] Among 1529 German RA patients on DMARDs, including 858 treated with TNF-α antagonists, there was a 2-fold increased risk for serious infections associated with TNF-α antagonist use compared to conventional DMARDs (rate ratio = 2.1). [19] Covariates used for multivariate modeling in this study included multiple markers of RA severity, but a limited number of comorbid conditions that predispose to infection (chronic lung disease, diabetes mellitus, and psoriasis). Administrative claims data from 2393 patients with RA treated with TNF-α antagonists showed a nearly 2-fold increase in risk for serious bacterial infections among patients treated with TNF-α antagonists when compared to methotrexate alone (HR, 1.9; 95% CI, 1.3–2.8). [4] Our study used a broader outcome measure (including bacterial, viral, and fungal infections), and a different comparison group. Compared to these prior studies, our study had a larger number of observed patient-years.
In contrast, several studies have not found an association between TNF-α antagonists use and serious infections. An observational study of 7664 patients with severe RA from the British Society for Rheumatology Biologics Register did not show an increased risk of infection with TNF-α antagonist use compared to conventional DMARD use after adjustment for disease severity, comorbidity, baseline steroid use, and smoking (incidence risk ratio, 1.03; 95% CI, 0.68–1.57). [8] However, re-analysis of the study data using alternate “at risk” windows did show an increased risk for infection among patients treated with TNF-α antagonists. [7] In a study of 15,597 older, low income United States patients treated with DMARDs, there was no increased risk for infections due to TNF-α antagonists compared to methotrexate. [28] That study included only a select group of bacterial and opportunistic infections as outcomes, while the current study included the most common infections causing hospitalization in United States hospitals. Thus, the discordant results between our study and prior studies may be due to differences in study power, comparison groups, and outcome definitions.
Hospitalization for infection was most common within the first 9 months of therapy across all medication groups. This initial period of increased risk for infection is consistent with the period of increased infection risk reported in previous studies, [2,4,5,7] and may in part be due to the depletion of susceptible patients as those who develop infections discontinue treatment, leaving a healthier cohort that remains on treatment. Further research on patterns of DMARD discontinuation following HFI is warranted to better understand the risk associated with continued DMARD therapy at the time of HFI.
The current study has several strengths. The use of large, national administrative databases allowed us to identify relatively rare events. The comprehensive nature of our data sources allowed inclusion of and adjustment for relevant covariates that affect the risk of HFI. Our definition of HFI was novel compared to previous studies, and was based on the most common infection-related hospitalizations from the NHDS, so our definition systematically captured the most commonly-used ICD-9-CM codes for HFI. The validation results demonstrate that this definition captured our desired endpoint accurately.
This study has several limitations. ICD-9-CM codes have varying degrees of validity, causing bias due to misclassification. [16,24] However, our validation of the study outcome demonstrated very good agreement between our ICD-9-CM definition and electronic medical records (kappa = 0.80). Our RA and covariate definitions were developed using VA data [11,30] and have been widely applied in other epidemiologic studies using administrative data. [15,21] There is insufficient current evidence to rank DMARDs by their immunosuppressive qualities. Therefore we chose to group them according to their roles in the treatment of RA based on disease severity, supported by current practice guidelines. [18,20,25] While our grouping methodology did not allow evaluation of risk associated with most individual medications, it provided sufficient statistical power to reach meaningful conclusions about infection risk for TNF-α antagonists, a key focus of our study.
TNF-α antagonists have been associated with an increased risk of tuberculosis, coccidiomycosis, and histoplasmosis. [33] The current study was not designed to examine these endpoints. We included only those infections that caused at least 10,000 hospitalizations among patients aged 15 years or more according to the 2005 NHDS. [6] These infections did not meet this minimum frequency threshold and were not included in our analysis. We believe that our study highlights the significant impact TNF-α antagonists have beyond granulomatous infections.
RA severity is correlated with increased risk for infection, [10] causing confounding by indication in the attribution of outcomes to medications. Although there is no widely accepted tool to control for RA severity using administrative data, surgical procedures have been identified as a marker of RA disease severity, [32] and have been used in previous studies to control for it. [2,4,28] We used intraarticular steroid injection and orthopedic procedures to adjust for RA disease severity, and these procedures were correlated with higher medication group in our study. A study evaluating the relative impact of RA treatment and severity on infection risk estimated that approximately one-third of the excess infection risk was related to RA severity, while two-thirds was related to treatment. [9] Thus, the excess infection seen in higher medication groups was likely caused in part by medication, and in part by RA severity.
Finally, patients who receive health benefits through the Veterans Health Administration may receive medical care from non-VA hospitals as well as VA hospitals (“dual use”). Dual use will limit the ability of a study to determine accurate incidence rates. However, as long as the dual use is nondifferential in content and evenly distributed among relevant covariates, it will not affect the accuracy of studies examining risk factors for outcomes occurring within the VA healthcare system. Prior studies suggest that dual use is more common among patients aged ≥65 years. [29,34] This practice may potentially introduce bias in our study population. First, if dual users are more likely to be hospitalized for infection at non-VA hospitals, the HFI rate will be decreased in the current study. One might hypothesize that patients with infection may be more likely to seek care at a hospital closer to their home. To account for this possibility, our multivariate model was adjusted for proximity to the nearest VA hospital. Our estimates of the impact of age and comorbid conditions on HFI may be blunted if older patients, who may have a higher comorbidity burden, are more likely to be hospitalized for infection at non-VA hospitals. Prior studies have shown that older age has little effect on differential DMARD selection for treatment of RA. [1,13,14,26] Based on these factors, we do not believe that dual use will substantially affect our estimates of impact of specific DMARDs on HFI risk.
This study demonstrates that patients with RA treated with TNF-α antagonists or prednisone are at increased risk for infections requiring hospitalization. HFIs are associated with significant morbidity and mortality, as well as cost. The overall effectiveness of a medication is determined not only by its therapeutic efficacy, but also by the magnitude and impact of associated adverse events. Data from the current study contribute to the understanding of the overall safety of TNF-α antagonists, and may guide clinical decision-making in patient selection for treatment.
Acknowledgments
Financial support: The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service project number IAF 06-026 (PI: Dr. McDonald). Dr. Lane has received career development support from the Goldfarb Patient Safety & Quality Fellowship program and the Barnes-Jewish Hospital Foundation. The career development of Dr. McDonald has been supported by NIH K12RR023249 and KL2RR024994. Dr. Caplan is supported by VA HSR&D Career Development Award 07-221.
Abbreviations
- CI
confidence interval
- DMARD
disease-modifying antirheumatic drug
- HFI
hospitalization for infection
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, Version 9, Clinical Modification
- NHDS
National Hospital Discharge Survey
- RA
rheumatoid arthritis
- TNF-α
tumor necrosis factor-α
- VA
Veterans Affairs
Footnotes
Conflict of interest: The authors have no conflicts of interest to report.
The views expressed in this article do not necessarily represent the views of the Department of Veterans Affairs.
References
- 1.Aletaha D, Smolen JS. The rheumatoid arthritis patient in the clinic: comparing more than 1300 consecutive DMARD courses. Rheumatology. 2002;41:1367–1374. doi: 10.1093/rheumatology/41.12.1367. [DOI] [PubMed] [Google Scholar]
- 2.Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Feltelius N, Coster L, Geborek P, Jacobsson LT, Lindblad S, Lysholm J, Rantapaa-Dahlqvist S, Saxne T, van Vollenhoven RF, Klareskog L. Time-dependent increase in risk of hospitalisation with infection among Swedish RA patients treated with TNF antagonists. Ann Rheum Dis. 2007;66:1339–1344. doi: 10.1136/ard.2006.062760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 4.Curtis JR, Patkar N, Xie A, Martin C, Allison JJ, Saag M, Shatin D, Saag KG. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125–1133. doi: 10.1002/art.22504. [DOI] [PubMed] [Google Scholar]
- 5.Curtis JR, Xi J, Patkar N, Xie A, Saag KG, Martin C. Drug-specific and time-dependent risks of bacterial infection among patients with rheumatoid arthritis who were exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:4226–4227. doi: 10.1002/art.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007;13:1–209. [PubMed] [Google Scholar]
- 7.Dixon WG, Symmons DP, Lunt M, Watson KD, Hyrich KL, Silman AJ. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum. 2007;56:2896–2904. doi: 10.1002/art.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:2368–2376. doi: 10.1002/art.21978. [DOI] [PubMed] [Google Scholar]
- 9.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–1609. [PubMed] [Google Scholar]
- 10.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum. 1999;42:415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Gibofsky A, Palmer WR, Goldman JA, Lautzenheiser RL, Markenson JA, Weaver A, Schiff MH, Keystone EC, Paulus HE, Harrison MJ, Whitmore JB, Leff JA. Real-world utilization of DMARDs and biologics in rheumatoid arthritis: the RADIUS (Rheumatoid Arthritis Disease-Modifying Anti-Rheumatic Drug Intervention and Utilization Study) study. Curr Med Res Opin. 2006;22:169–183. doi: 10.1185/030079906X80341. [DOI] [PubMed] [Google Scholar]
- 14.Harrison MJ, Kim CA, Silverberg M, Paget SA. Does age bias the aggressive treatment of elderly patients with rheumatoid arthritis? J Rheumatol. 2005;32:1243–1248. [PubMed] [Google Scholar]
- 15.Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. 2009;301:2120–2128. doi: 10.1001/jama.2009.722. [DOI] [PubMed] [Google Scholar]
- 16.Kashner TM. Agreement between administrative files and written medical records: a case of the Department of Veterans Affairs. Med Care. 1998;36:1324–1336. doi: 10.1097/00005650-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Lacaille D, Guh DP, Abrahamowicz M, Anis AH, Esdaile JM. Use of nonbiologic disease-modifying antirheumatic drugs and risk of infection in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59:1074–1081. doi: 10.1002/art.23913. [DOI] [PubMed] [Google Scholar]
- 18.Ledingham J, Deighton C. Update on the British Society for Rheumatology guidelines for prescribing TNFalpha blockers in adults with rheumatoid arthritis (update of previous guidelines of April 2001) Rheumatology (Oxford) 2005;44:157–163. doi: 10.1093/rheumatology/keh464. [DOI] [PubMed] [Google Scholar]
- 19.Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova-Scholz M, Gromnica-Ihle E, Antoni C, Herzer P, Kekow J, Schneider M, Zink A. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52:3403–3412. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 20.Luqmani R, Hennell S, Estrach C, Basher D, Birrell F, Bosworth A, Burke F, Callaghan C, Candal-Couto J, Fokke C, Goodson N, Homer D, Jackman J, Jeffreson P, Oliver S, Reed M, Sanz L, Stableford Z, Taylor P, Todd N, Warburton L, Washbrook C, Wilkinson M. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (after the first 2 years) Rheumatology (Oxford) 2009;48:436–439. doi: 10.1093/rheumatology/ken450a. [DOI] [PubMed] [Google Scholar]
- 21.McDonald JR, Zeringue AL, Caplan L, Ranganathan P, Xian H, Burroughs TE, Fraser VJ, Cunningham F, Eisen SA. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis. 2009;48:1364–1371. doi: 10.1086/598331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutru O, Laakso M, Isomaki H, Koota K. Ten year mortality and causes of death in patients with rheumatoid arthritis. Br Med J (Clin Res Ed) 1985;290:1797–1799. doi: 10.1136/bmj.290.6484.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ollendorf DA, Fendrick AM, Massey K, Williams GR, Oster G. Is sepsis accurately coded on hospital bills? Value Health. 2002;5:79–81. doi: 10.1046/j.1524-4733.2002.52013.x. [DOI] [PubMed] [Google Scholar]
- 24.O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40:1620–1639. doi: 10.1111/j.1475-6773.2005.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M, Outman R, Allison JJ, Suarez Almazor M, Bridges SL, Jr, Chatham WW, Hochberg M, MacLean C, Mikuls T, Moreland LW, O’Dell J, Turkiewicz AM, Furst DE. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 26.Schmajuk G, Schneeweiss S, Katz JN, Weinblatt ME, Setoguchi S, Avorn J, Levin R, Solomon DH. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 2007;57:928–934. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 27.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran’s affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60:397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Schneeweiss S, Setoguchi S, Weinblatt ME, Katz JN, Avorn J, Sax PE, Levin R, Solomon DH. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:1754–1764. doi: 10.1002/art.22600. [DOI] [PubMed] [Google Scholar]
- 29.Shen Y, Findley PA, Maney M, Pogach L, Crystal S, Rajan M, Findley TW. Department of Veterans Affairs-Medicare dual beneficiaries with stroke: where do they get care? J Rehabil Res Dev. 2008;45:43–51. doi: 10.1682/jrrd.2006.07.0081. [DOI] [PubMed] [Google Scholar]
- 30.Singh JA, Holmgren AR, Noorbaloochi S. Accuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum. 2004;51:952–957. doi: 10.1002/art.20827. [DOI] [PubMed] [Google Scholar]
- 31.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26(Suppl 51):S35–S61. [PubMed] [Google Scholar]
- 32.Ting G, Schneeweiss S, Katz JN, Weinblatt ME, Cabral D, Scranton RE, Solomon DH. Performance of a rheumatoid arthritis records-based index of severity. J Rheumatol. 2005;32:1679–1687. [PubMed] [Google Scholar]
- 33.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–1265. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 34.West AN, Weeks WB. Who pays when VA users are hospitalized in the private sector? Evidence from three data sources. Med Care. 2007;45:1003–1007. doi: 10.1097/MLR.0b013e318070c6e2. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 2006;54:628–634. doi: 10.1002/art.21568. [DOI] [PubMed] [Google Scholar]