Abstract
Na+-independent anion exchangers (AE) mediate electroneutral exchange of Cl- for 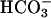 ions across cell membranes, being involved in intracellular pH and cell volume regulation and in transepithelial hydroionic fluxes. Bicarbonate activation of adenylyl cyclase is known to be necessary for sperm motility and sperm capacitation, and a few studies have suggested a possible role of AE carriers in reproduction. Among the four AE genes identified in mammals thus far, only Ae2 (Slc4a2) has been determined to be expressed in the male reproductive system, especially in developing spermatozoa and in epididymal epithelium. Most AE genes drive alternative transcription, which in mouse Ae2 results in several Ae2 isoforms. Here, we generated mice carrying a targeted disruption of Ae2 that prevents the expression of the three AE2 isoforms (Ae2a, Ae2b1, and Ae2b2) normally found in mouse testes. Male Ae2-/- mice (but not female Ae2-/- mice) are infertile. Histopathological analysis of Ae2-/- testes shows an interruption of spermiogenesis, with only a few late spermatids and a complete absence of spermatozoa in the seminiferous tubules. The number of apoptotic bodies is increased in the seminiferous tubules and in the epididymis, which also shows squamous metaplasia of the epididymal epithelium. Our findings reveal an essential role of Ae2 in mouse spermiogenesis and stress the recently postulated involvement of bicarbonate in germ-cell differentiation through the bicarbonate-sensitive soluble-adenylyl-cyclase pathway.
ions across cell membranes, being involved in intracellular pH and cell volume regulation and in transepithelial hydroionic fluxes. Bicarbonate activation of adenylyl cyclase is known to be necessary for sperm motility and sperm capacitation, and a few studies have suggested a possible role of AE carriers in reproduction. Among the four AE genes identified in mammals thus far, only Ae2 (Slc4a2) has been determined to be expressed in the male reproductive system, especially in developing spermatozoa and in epididymal epithelium. Most AE genes drive alternative transcription, which in mouse Ae2 results in several Ae2 isoforms. Here, we generated mice carrying a targeted disruption of Ae2 that prevents the expression of the three AE2 isoforms (Ae2a, Ae2b1, and Ae2b2) normally found in mouse testes. Male Ae2-/- mice (but not female Ae2-/- mice) are infertile. Histopathological analysis of Ae2-/- testes shows an interruption of spermiogenesis, with only a few late spermatids and a complete absence of spermatozoa in the seminiferous tubules. The number of apoptotic bodies is increased in the seminiferous tubules and in the epididymis, which also shows squamous metaplasia of the epididymal epithelium. Our findings reveal an essential role of Ae2 in mouse spermiogenesis and stress the recently postulated involvement of bicarbonate in germ-cell differentiation through the bicarbonate-sensitive soluble-adenylyl-cyclase pathway.
Spermatogenesis is the entire process by which a spermatogonial germ cell is transformed into a uniquely shaped spermatozoon (1). It consists of three sequential phases of cell proliferation and differentiation. Successive mitotic divisions of a spermatogonial stem cell maintain the pool of stem cells and also give rise to primary spermatocytes. Each primary spermatocyte undergoes biphasic meiotic divisions, resulting in four haploid round spermatids. Finally, the nuclear and cellular components of these postmeiotic male germ cells are gradually remodeled into sperm cells through a process involving complex structural and biochemical changes referred to as spermiogenesis. Consecutive spermatogenic cycles evolve as waves, in which several sequential specific cellular associations of germ cells or stages can be distinguished. The number of stages varies between species, and in the mouse each wave can be divided into 12 stages (2). Mature sperm cells are released from the seminiferous tubules and migrate through the epididymis. The concentration of bicarbonate has been reported to be important for sperm motility (3) and for sperm capacitation (4, 5), which is a prerequisite for successful fertilization. These effects of bicarbonate involve activation of a bicarbonate-sensitive adenylyl cyclase (3, 4, 6), recently identified as the soluble adenylyl cyclase (sAC) (7). Recent studies suggested a role for bicarbonate and sAC splice variants in spermatogenesis as well (8).
To trigger cAMP-dependent processes, bicarbonate ions enter germ cells by an anion transporter (4, 9, 10) or are produced by hydration of CO2 through carbonic anhydrase (11). One type of anion transporters is the family of Na+-independent anion exchangers (AE), which mediates electroneutral and reversible exchange of Cl- and 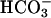 across cell membranes (12). In cooperation with other ion carriers, AE proteins are involved in intracellular pH and cell volume regulation and in transepithelial hydroionic fluxes and acid/base transport. Among the four AE genes identified in mammals thus far (Ae1, Ae2, Ae3, and Ae4) (12, 13), only Ae2 (Slc4a2) has been shown to be expressed in the male reproductive system, especially in developing spermatozoa (14) and in epididymal epithelium (15). Studies carried out in normal male rats demonstrated that the levels of Ae2 mRNA in the seminiferous tubules are higher from the spermiogenic stage VII onward (14).
across cell membranes (12). In cooperation with other ion carriers, AE proteins are involved in intracellular pH and cell volume regulation and in transepithelial hydroionic fluxes and acid/base transport. Among the four AE genes identified in mammals thus far (Ae1, Ae2, Ae3, and Ae4) (12, 13), only Ae2 (Slc4a2) has been shown to be expressed in the male reproductive system, especially in developing spermatozoa (14) and in epididymal epithelium (15). Studies carried out in normal male rats demonstrated that the levels of Ae2 mRNA in the seminiferous tubules are higher from the spermiogenic stage VII onward (14).
Most AE genes have been found to drive alternative transcription. In the mouse Ae2, five N-terminal variants may be transcribed: Ae2a from the upstream promoter, Ae2b1 and Ae2b2 from alternate promoter sequences within intron 2, and Ae2c1 and Ae2c2 from sequences within intron 5 (16). To study the specific role of Ae2 in spermatogenesis, we generated mice carrying a targeted disruption of Ae2 that prevents the expression of the three AE2 isoforms (Ae2a, Ae2b1, and Ae2b2) normally expressed in mouse testes.
Methods
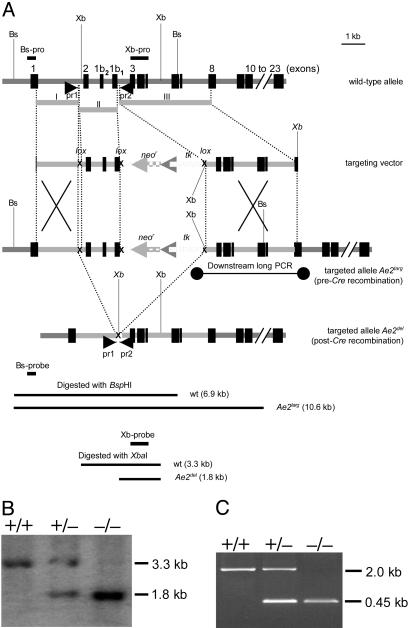
Gene Targeting. The targeting vector for the homologous recombination was prepared in the Cre/loxP plasmid pLox-TK-neo (a gift of P. C. Orban, University of British Columbia, Vancouver). This vector contains the thymidine kinase gene of herpes virus type 2 (tk) and the neomycin-phosphotransferase gene (neor), flanked by loxP sites to allow for excision with Cre recombinase. Vector restriction sites HindIII–XhoI, BamHI, and XbaI were used for sequential subcloning of three consecutive DNA fragments of the mouse AE2 gene regions named fragments I, II, and III. These DNA fragments (Fig. 1) were produced by PCR with an Advantage amplification kit with GC-melt (BD Clontech) on Ae2 DNA obtained from a genomic library of mouse ES-129/Ola cells (16). Subcloning of fragment I (2-kb long) was followed by subcloning of the intermediate fragment II (1.5 kb long) within the excisable region (Fig. 1). An XbaI site present in fragment II was destroyed before subcloning in the targeting vector to allow for the subsequent subcloning of fragment III (3.2 kb long) into the XbaI site of the vector. Fragments I and III are situated outside the vector excisable region (mainly fragment II that includes exons 2, 1b2 and 1b1, each having the ATG start codons for AE2a, AE2b2, and AE2b1 isoforms, respectively; see Fig. 1). Correct fragment orientations were assessed by sequence analysis after each fragment subcloning. Finally, vector-backbone-free targeting DNA suitable for electroporation of mouse embryonic stem (ES) cells was obtained through digestion of the targeting vector with NotI, electrophoresis in agarose gel, and further electroelution from a gel slide as described (17). Electroporated cells were screened by PCR, and resultant positively targeted ES cell clones were confirmed by Southern blot by using ES genomic DNA digested with BspHI (Bs) and the Bs-probe (Fig. 1). After a cytogenetic analysis, we chose two clones with a normal karyotype (1C2 and 3B4) and carried out Cre-catalyzed excisions (18). Coelectroporation with the Crerecombinase plasmid pOG231 and the puromycin-expressing vector pHA262pur (gifts of S. O'Gorman, The Salk Institute for Biological Studies, San Diego, and H. te Riele, The Netherlands Cancer Institute, Amsterdam, respectively) led to enrichment of Cre-expressing ES cells by selection with puromycin, and allowed for an efficient ganciclovir selection of ES cells with Cre excision of the tk-neor box. After PCR screening for positively excised clones and further cytogenetic analysis, two clones with normal karyotype (1C2-11 and 3B4-4, each deriving from the two original clones), were chosen for expansion and further injection into blastocysts. Ten to 15 targeted 129/Ola ES cells (from either clone 1C2-11 or 3B4-4) were injected into C57BL/6 blastocysts isolated at day 3, being further reimplanted into pseudopregnant females as described (19). Male chimeras from both targeted ES clones were mated to FVB females. Offspring were genotyped by PCR and Southern blot analysis, and heterozygotes (Ae2+/-) were interbred to produce Ae2-/- mice. All animals were kept under conventional housing conditions and received humane care according to institutional guidelines.
Fig. 1.
(A) Targeting strategy for deletion/inactivation of mouse Ae2. Wild-type Ae2 locus, targeting vector, targeted Ae2 allele after homologous recombination and deleted Ae2 locus by using Cre recombinase, and upstream and downstream recombination of Ae2targ allele were assessed by Southern blotting (ES genomic DNA cut with BspHI and probed with Bs-pro) and by PCR (primers are indicated as connected dots in the Ae2targ diagram), respectively. (B) Initial genotyping to detect targeted mice by Southern blotting of tail genomic DNA cut with XbaI and probed with the Xb-pro. (C) PCR genotyping for routine detection of targeted mice by using primers pr1 and pr2 (represented in A) on tail genomic DNA. Bs, BspHI site; Xb, XbaI site.
Histopathological Examination of Testis and Epididymis. Tissues were fixed in paraformaldehyde and embedded in paraffin. Serial 5-μm sections stained with hematoxylin and eosin allowed us to clearly distinguish nuclei of cells and identify their cell type. Cell populations from 10 seminiferous tubules from four different specimens for each genotype were counted under a light microscope. Stages were evaluated by carefully examining stagerelated spermatid degeneration (2). Apoptosis was determined on 5-μm sections over poly-l-lysine-coated slides through bright field visualization by using two different procedures: Nissel's staining (with toluidine blue) and terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL). For the latter we used an In Situ Cell Death Detection Kit, POD (Roche Molecular Biochemicals), overstained with the peroxidase substrate supplied with the kit and counterstained with eosin.
Analysis of Spermatozoa. Epididymes from five heterozygous and five wild-type mice were cut in small pieces inside a drop of PBS. After centrifuging at low speed (190 × g), we separated the supernatant with remaining spermatozoa and pelleted these germ cells with a new centrifugation at higher speed (2,000 × g). Spermatozoa were counted in a hemocytometer with a light microscope. To evaluate the rate of aberrant structures (microcephalia, bicephalia, two tails, or a small tail) versus normal haploid cells, sperm cells were resuspended in PBS/0.1% polyvinylpyrrolidone (Fluka), stained with 4′,6-diamidino-2-phenylindole (DAKO), and visualized in a hemocytometer under a fluorescent microscope connected to a video camera. Sperm motility was assessed in PBS (with no polyvinylpyrrolidone) through the combined flow and image cytometry procedure (20), which allowed for the identification of haploid cells with propidium iodine and a further evaluation of their mitochondrial activity by using rhodamine 123 (Molecular Probes).
Analysis of mRNAs. Total RNA was isolated from mouse testes according to the guanidinium thiocyanate method by using the TRI Reagent (Sigma). Transcriptional expression of several genes involved in spermatogenesis was analyzed through a semiquantitative RT-PCR-based procedure (16). For each case, bands were evaluated in the linear phase of amplification, i.e., before reaching the plateau effect with more cycles. The sequences and exact positions of the PCR primers are available from the corresponding author on request.
Statistical Analysis. Data are given as mean ± SD. Statistical analyses were carried out with the statistical program SPSS 11.0. Because residuals for each variable had a normal distribution (according to Shapiro–Wilk), we analyzed the data from quantitation of cells in the seminiferous tubules by using parametric tests, i.e., with the one-way ANOVA followed by the Student–Newmann–Keuls post hoc test. Motility values (fluorescence mean intensity for rhodamine) and the rate of aberrant structure in sperm cells were analyzed with the Student t test.
Results
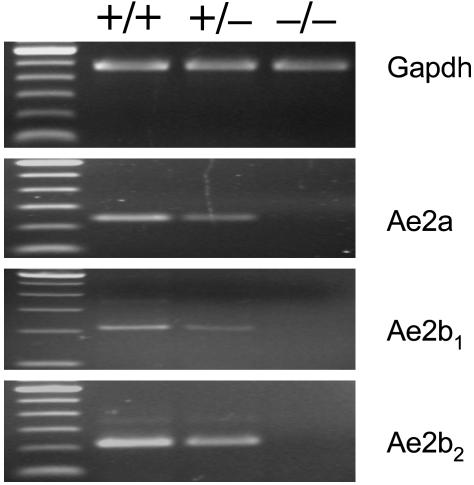
Generation of Ae2-Targeted Mice. A 1.5-kb deletion was produced in mouse Ae2 by gene targeting (Fig. 1). The deleted region is relevant to the three variants Ae2a, Ae2b1, and Ae2b2 normally expressed in mouse testes (Fig. 2); we found that normal mouse testes do not express the intron-5-derived variants Ae2c1 and Ae2c2 (data not shown). Fig. 1 illustrates the strategy used to target one of the alleles of Ae2 in ES cells through homologous recombination. In the first step, the targeted allele incorporated an engineered Ae2 region flanked by loxP sites that allowed for its further excision with Cre recombinase. Removal of the engineered region deprived the allele of exons 2, 1b1, and 1b2, which are the initial coding exons for Ae2a, Ae2b1, and Ae2b2 isoforms (16). Targeted ES clones were identified by PCR and further assessed by Southern blot analysis (not shown). Two independently targeted ES clones tested for a normal karyotype contributed to the germ line of chimeric mice and yielded progeny heterozygous for the Ae2-targeted allele. After setting up crosses between Ae2 heterozygous mice, PCR genotyping of F2 offspring showed the occurrence of Ae2-/- offspring (Fig. 2), although their number was lower than expected according to Mendelian distribution (8% instead of 25%). This was at least partly caused by an increased perinatal lethality (80% of dead animals were Ae2-/-). Ae2-/- mice that survived the perinatal stage exhibited no obvious macroscopic abnormalities.
Fig. 2.
RT-PCR analysis for the testis expression of Ae2a, Ae2b1 and Ae2b2 mRNA isoforms. Isoform-specific sense primers (in exons 2, 1b1, and 1b2) and a common antisense primer encompassing the exon3/exon4 junction were used. GAPDH mRNA was used as the normalizing control. The thick upper band in the 100-bp ladder is 600 bp long.
Female Ae2-/- mice could become pregnant when crossed with male Ae2+/+ or Ae2+/- mice. Male fertility in heterozygous mice was similar to that in wild-type mice, as denoted by average litter sizes in the respective cross with female Ae2+/+ mice. However, female mice (either Ae2+/+, Ae2+/-,or Ae2-/-) did not become pregnant when crossed with male Ae2-/- mice, indicating male infertility in Ae2-/- mice.
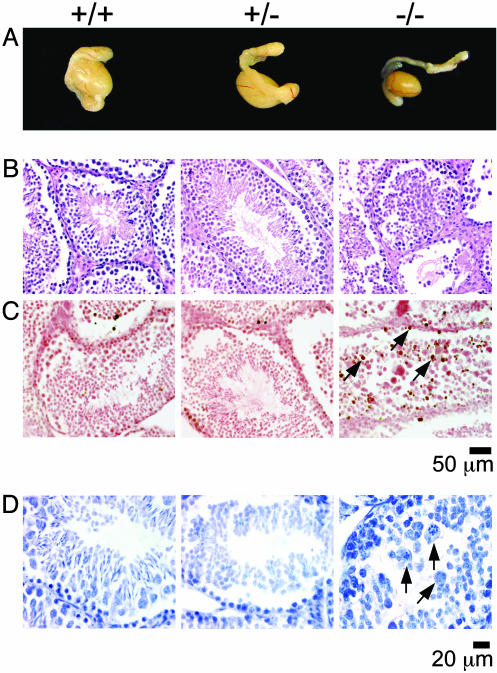
Testis Examinations. The size and weight of the testes in the Ae2-/- mice is reduced by 40–60%, whereas no difference is observed between testes in Ae2+/- mice and wild-type mice (Fig. 3A). Histological examination and quantification of cell populations in the seminiferous tubules showed differences between Ae2-/- mice and both heterozygous and wild-type mice (Table 1 and Fig. 3B). Thus, Ae2-/- mice show reductions in most cell populations, which may account for the reduction in the testicular size and weight. Moreover, Ae2-/- mice have a characteristic disruption of spermiogenesis after stage VII, most cells being in stages IV–VII with no spermatozoa and very few elongating (late) spermatids (Table 1). Also, the number of round (early) spermatids is dramatically reduced in Ae2-/- mice (Table 1). This reduction is more obvious in older mice (8–9 months old), in which spermatid stages become rare (data not shown). The disrupted spermiogenesis in Ae2-/- mice is associated with apoptosis of germ cells, the number of apoptotic bodies within the seminiferous tubules being increased in these targeted animals (Table 1 and Fig. 3 C and D).
Fig. 3.
(A) Macroscopic appearance of testes (with their epididymes) from 20-week-old mice of indicated genotype. (B) Testicular sections from specimens shown in A, stained with hematoxylin and eosin to visualize the seminiferous tubules. (C) TUNEL for in situ detection of apoptotic cells. (D) Nissel's staining showing apoptotic cells during the process of nuclear fragmentation. Some TUNEL- and Nissel-positive cells are identified by arrows.
Table 1. Quantitation of germ cell and somatic cell populations in 10 seminiferous tubules of mouse testis specimens.
|
Ae2 genotype
|
P (ANOVA)
|
|||
|---|---|---|---|---|
| Cell type | -/- (n = 4) | +/- (n = 4) | +/+ (n = 4) | |
| Spermatogonia | 299 ± 90* | 353 ± 31 | 441 ± 41* | 0.024 |
| Spermatocytes | 353 ± 47* | 439 ± 60 | 486 ± 74* | 0.038 |
| Round spermatids | 71 ± 25† | 451 ± 111 | 365 ± 78 | <0.001 |
| Elongated spermatids | 13 ± 11† | 359 ± 27 | 413 ± 122 | <0.001 |
| Spermatozoa | 0† | 632 ± 206 | 536 ± 111 | <0.001 |
| Sertoli cells | 188 ± 27† | 299 ± 15 | 305 ± 26 | <0.001 |
| Apoptotic bodies | 80 ± 8‡ | 12 ± 5‡ | 27 ± 12‡ | <0.001 |
Values are presented as mean ± SD. *, significant differences between labeled groups; †, labeled group is significantly different from the two others; ‡, labeled groups differ from each other (all P values are <0.05 according to the Student–Newmann–Keuls post hoc test).
The absolute number of the other populations of germ cells (spermatogonia and spermatocytes) and Sertoli cells are similar in Ae2+/+ mice and Ae2+/- mice (Table 1). Although these cell populations are significantly reduced in Ae2-/- mice, their reduction (30–40%) is less dramatic than that observed for round spermatids and further maturing cells (Table 1). On the other hand, the appearance of Sertoli cells and that of Leydig cells is normal in Ae2-/- mice. Moreover, these mice show a normal pattern of expression of stem cell factor (Scf or Kit ligand) mRNA in their testis (see below), which suggests a normal functionality of the remaining Sertoli cells (21, 22). Also, Leydig cell function appeared to be correct in Ae2-/- mice, as indicated by normal serum levels of testosterone in these animals (data not shown).
The testicular alterations observed in Ae2-/- mice are virtually absent in heterozygous mice. Thus, the seminiferous tubules in Ae2+/- mice display a normal spermatogenic wave and cellular composition (Table 1 and Fig. 3B), suggesting that spermiogenesis may progress correctly for both Ae2+ and Ae2- haploid germ cells. In fact, the analysis of mature sperm cell characteristics such as the rate of aberrant structure and motility estimation showed no differences between Ae2+/- mice and wild-type littermates (Table 2). Moreover, after setting up eight crosses between male Ae2+/- mice and female Ae2+/+ mice, the proportion of heterozygous and wild-type animals were similar (45% and 55%, respectively).
Table 2. Sperm characteristics and motility evaluation.
|
Ae2 genotype
|
||
|---|---|---|
| Parameter | +/– (n = 5) | +/+ (n = 5) |
| No. of spermatozoa, × 105 | 80.1 ± 29 | 77.5 ± 34 |
| Haploid spermatozoa, % | 78.2 ± 4 | 82.4 ± 3.3 |
| Aberrant structure, % | 21.8 ± 4 | 17.6 ± 3.3 |
| Fluorescence mean intensity for rhodamine 123 as a motility estimation | 144.7 ± 18 | 150 ± 12.4 |
Values are presented as mean ± SD. No significant differences occurred between wild-type and heterozygous mice (all P values are >0.05 according to the Student t test).
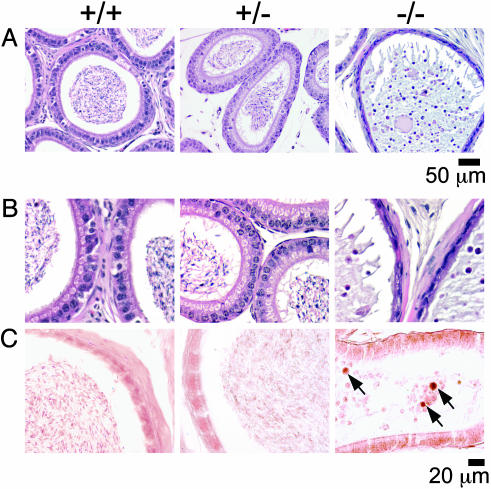
Epididymis Examinations. The epididymal epithelium in Ae2-/- mice shows abnormalities indicative of squamous metaplasia, with a flattened appearance in corpus (Fig. 4A) and cauda, and a cubic distribution in the caput epididymis (not shown). Most of the content in the epididymis of these targeted animals consists of apoptotic bodies together with a few spermatocytes and early spermatids released from the seminiferous tubules and with no mature sperm cells (Fig. 4 B and C). The appearance of the epididymal wall and the frequency of apoptotic cells within the epididymis in heterozygous mice are similar to those in wild-type animals (Fig. 4).
Fig. 4.
(A) Sections of the corpus epididymis from specimens shown in Fig. 3A, stained with hematoxylin and eosin. (B) Two-fold magnification of sections in A; a squamous metaplasia of the epididymal epithelium with a flattened appearance, and a loss of cilia are observed in Ae2-/- mice (Right). (C) TUNEL for in situ detection of apoptotic cells within epididymis. TUNEL-positive cells are identified by arrows.
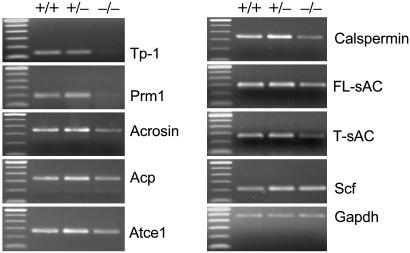
Differential Expression of Testis Genes. Testis steady-state levels of messengers for a few well characterized markers involved in spermatogenesis were analyzed by a semiquantitative RT-PCR procedure (Fig. 5). Expression of the genes for the transition protein 1 (Tp-1) and protamine 1 (Prm1) was each virtually absent in Ae2-/- testis. By contrast, the levels of transcripts from other postmeiotic genes, which are normally activated at earlier stages of spermiogenesis such as those for acrosin, for a testisspecific form of an actin-capping protein (Acp), and for an Atf/Creb-like peptide specifically expressed in mid/late round spermatids (Atce1), were only slightly reduced in Ae2-/- testis. Similarly, testis levels of the calspermin message, which normally appear in pachytene primary spermatocytes and continue to increase as cells differentiate, were slightly reduced in targeted mice. Analysis of the two splice variants of the germ cell sAC, i.e., the full-length sAC (FL-sAC) and the truncated sAC (T-sAC) showed minor reductions in Ae2-/- testis, mainly the T-sAC variant (Fig. 5). No specific alterations were found in testis expression of the cAMP-sensitive transcription factor members Creb and Crem, in analyzed Ae2-/- animals (data not shown). Finally, the expression of Scf, which in testis is specifically activated in Sertoli cells (22), is normal in targeted mice (Fig. 5).
Fig. 5.
Expression of indicated mRNAs were analyzed by semiquantitative RT-PCR. All shown bands correspond to amplicons that are in the linear phase of amplification at the following number of cycles: 20 cycles for Tp-1 and Prm1; 30 cycles for acrosin, Acp, Atce1, calspermin, and sAC (both splice variants FL-sAC and T-sAC); 35 cycles for Scf. RT-PCR for Gapdh mRNA (25 cycles) was used as a normalizing control. Each sAC isoform was amplified separately by using a common sense primer (in exon 9) and isoform-specific antisense primers. The sequence of the antisense primer specific for the FL-sAC isoform is within exon 11 (the spliced out exon in the T-sAC isoform), whereas that for the T-sAC isoform encompasses the junction of exons 10 (8 nt) and 12 (12 nt). In the 100-bp ladder, the thick upper band is 600 bp long.
Discussion
As indicated in the introduction, bicarbonate stimulation of adenylyl cyclase and subsequent increase of intracellular cAMP levels play an important role for male reproductive processes such as sperm motility and capacitation (3, 4, 6), the involved bicarbonate-sensitive adenylyl cyclase being identified as the sAC (7). Recently, a role for bicarbonate and sAC splice variants in spermatogenesis was suggested as well (8). For initiating these processes bicarbonate ions need to enter germ cells by way of an anion transporter (4, 9, 10). Ae2 carrier may mediate reversible exchange of Cl- and 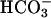 across cell membranes and, therefore, a bicarbonate influx in a gradient-dependent manner. Studies in male rats have shown that Ae2 is expressed in developing spermatozoa and that Ae2 mRNA levels are increased in the seminiferous tubules from the spermiogenic stage VII onward (14), thus suggesting an involvement of the Ae2 gene in spermatogenesis.
across cell membranes and, therefore, a bicarbonate influx in a gradient-dependent manner. Studies in male rats have shown that Ae2 is expressed in developing spermatozoa and that Ae2 mRNA levels are increased in the seminiferous tubules from the spermiogenic stage VII onward (14), thus suggesting an involvement of the Ae2 gene in spermatogenesis.
Here we have demonstrated by gene targeting in mice the critical role for Ae2 in spermiogenesis and male fertility. Male mice with a 1.5-kb deletion of Ae2 that prevents the expression of the three testis Ae2 variants, i.e., Ae2a, Ae2b1, and Ae2b2, are infertile. Histopathological analysis of Ae2-/- testes revealed that spermiogenesis is interrupted after stage VII, with a complete absence of mature spermatozoa and occasional late spermatids. Interrupted spermiogenesis is associated with an increased number of apoptotic bodies. Overall, these findings indicate that expression of Ae2 is crucial for normal progression of spermiogenesis, especially when spermatids start elongation in stage VIII, and suggest that, in the absence of Ae2, germ cells reaching middle–late stages (IV–VII) fall in apoptosis. In fact, most of the content in the epididymis of Ae2-/- animals consists of apoptotic bodies together with a few spermatocytes and early spermatids released from the seminiferous tubules. Moreover, the epididymal epithelium in these animals shows abnormalities indicative of squamous metaplasia, suggesting that Ae2 may also have a relevant homeostatic function in epididymal cells in which it is expressed (15).
In addition to arrested spermiogenesis at middle–late stages, Ae2-/- testes show a reduction in all cell populations within the seminiferous tubules (Table 1). Thus, the dramatic reduction in the number of round and elongated spermatids (and the absence of spermatozoa) is associated with a moderate but significant decrease (30–40%) in the remaining early germ cell and Sertoli cell populations, and small testis size. Although Sertoli cells seem to have a normal phenotype, their reduced number may contribute to the trophic testicular alterations observed in Ae2-/- mice.
Male fertility in Ae2+/- mice is similar to that in wild-type mice, and heterozygous mice show none of the testicular and epididymal alterations observed in Ae2-/- mice. Normal spermatogenic waves and cellular composition in the seminiferous tubules in Ae2+/- mice (Table 1) and sperm characteristics (Tables 2) suggest that spermiogenesis may progress correctly for both Ae2+ and Ae2- haploid germ cells. Most probably this progression is facilitated by the occurrence of intercellular bridges between germinal cells derived from each single spermatogonial cell (2, 23). Intercellular bridges typically result from incomplete cytokinesis at each of the mitotic and meiotic cell divisions, and they remain until the very end of the differentiation of spermatozoa, when these germ cells are released to the lumen of the seminiferous tubules. Such bridges allow developing germinal haploid cells to share a common cytoplasm with their neighbors and become phenotypically diploid (24).
We investigated the effect of Ae2 deficiency on testis steady-state mRNA levels for a few well characterized markers involved in spermatogenesis. Expression of Tp-1 (the gene for the transition protein 1, an intermediate nucleoprotein in the histone-to-protamine exchange), was virtually absent in Ae2-/- testis. Similar results were obtained for protamine 1 (Prm1). Most probably, the undetectable expression of these two haploid germ-cell genes is due to the absence of late spermatids. On the other hand, expression from other postmeiotic genes that are normally activated at early stages of spermiogenesis was only slightly reduced in Ae2-/- testis. For instance, this was the case of the genes for acrosin (25), Acp (a testis-specific form of an actin-capping protein) (26, 27), and Atce1 (a Creb-like peptide specifically expressed in mid/late round spermatids) (28). And similar results were obtained for the testis levels of the calspermin message, which normally appear in pachytene primary spermatocytes and continue to increase as cells complete meiosis and undergo terminal differentiation (29). When we analyzed the levels of the two splice variants for the germ cell soluble adenylyl cyclase FL-sAC and T-sAC (8), a minor reduction was found in Ae2-/- testis for both variants, mainly the T-sAC variant. T-sAC variant seems to be expressed in late spermatogenesis and it has been estimated to be responsible for 70% of the adenylyl cyclase activity in the adult testis cytosol (8). The expression of sAC in Ae2-/- germ cells, albeit at reduced level, suggests that it is already expressed at a stage before the arrest of differentiation.
The spermiogenesis disruption found in the Ae2-/- mice resembles that observed in the Crem-/- mice (30, 31), including the absence of expression of Tp-1 and Prm1. Crem is a transcriptional factor that is phosphorylated and activated by the cAMP-regulated protein kinase A (32, 33). In germ cells of sexually mature male rodents, cAMP levels are largely dependent on the activity of the two splice variants of sAC (8), which is directly regulated by bicarbonate ions (7, 8). Our present data suggest that Ae2 is the postulated anion transporter required for the entry of bicarbonate in sperm cells (10). The possibility that the lack of Ae2 prevents the bicarbonate-controlled cAMP signaling and renders germ cells like a Crem-deficient state despite their normal Crem expression warrants further studies.
Acknowledgments
We thank P. Garcés for excellent technical assistance cutting tissue slides, F. Arenas for support with illustrations, and Drs. M. GarcíaGranero and M. Zaratiegui for help with statistics. This work was supported by grants from the Spanish Fondo de Investigaciones Sanitarias (project 01/0777) and from the Instituto de Salud Carlos III (C03/02) (to J.F.M.) and from an agreement between Fundación para la Investigación Médica Aplicada and the Spanish “UTE for CIMA project” and by a grant from the Netherlands Organization for Scientific Research (program 912-02-073) (to R.P.J.O.E.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AE, anion exchanger; ES, mouse embryonic stem cell; sAC, soluble adenylyl cyclase; FL-sAC, full length sAC; T-sAC, truncated sAC; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1.Clermont, Y. (1972) Physiol. Rev. 52, 198-236. [DOI] [PubMed] [Google Scholar]
- 2.Oakberg, E. F. (1956) Am. J. Anat. 99, 391-413. [DOI] [PubMed] [Google Scholar]
- 3.Okamura, N., Tajima, Y., Soejima, A., Masuda, H. & Sugita, Y. (1985) J. Biol. Chem. 260, 9699-9705. [PubMed] [Google Scholar]
- 4.Visconti, P. E., Stewart-Savage, J., Blasco, A., Battaglia, L., Miranda, P., Kopf, G. S. & Tezon, J. G. (1999) Biol. Reprod. 61, 76-84. [DOI] [PubMed] [Google Scholar]
- 5.Gadella, B. M. & Harrison, R. A. (2000) Development (Cambridge, U.K.) 127, 2407-2420. [DOI] [PubMed] [Google Scholar]
- 6.Garty, N. B. & Salomon, Y. (1987) FEBS Lett. 218, 148-152. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Y., Cann, M. J., Litvin, T. N., Iourgenko, V., Sinclair, M. L., Levin, L. R. & Buck, J. (2000) Science 289, 625-628. [DOI] [PubMed] [Google Scholar]
- 8.Jaiswal, B. S. & Conti, M. (2001) J. Biol. Chem. 276, 31698-31708. [DOI] [PubMed] [Google Scholar]
- 9.Spira, B. & Breitbart, H. (1992) Biochim. Biophys. Acta 1109, 65-73. [DOI] [PubMed] [Google Scholar]
- 10.Kaupp, U. B. & Weyand, I. (2000) Science 289, 559-560. [DOI] [PubMed] [Google Scholar]
- 11.Parkkila, S., Kaunisto, K., Kellokumpu, S. & Rajaniemi, H. (1991) Histochemistry 95, 477-482. [DOI] [PubMed] [Google Scholar]
- 12.Alper, S. L., Darman, R. B., Chernova, M. N. & Dahl, N. K. (2002) J. Nephrol. 15, Suppl. 5, S41-S53. [PubMed] [Google Scholar]
- 13.Tsuganezawa, H., Kobayashi, K., Iyori, M., Araki, T., Koizumi, A., Watanabe, S., Kaneko, A., Fukao, T., Monkawa, T., Yoshida, T., et al. (2001) J. Biol. Chem. 276, 8180-8189. [DOI] [PubMed] [Google Scholar]
- 14.Holappa, K., Mustonen, M., Parvinen, M., Vihko, P., Rajaniemi, H. & Kellokumpu, S. (1999) Biol. Reprod. 61, 981-986. [DOI] [PubMed] [Google Scholar]
- 15.Jensen, L. J., Stuart-Tilley, A. K., Peters, L. L., Lux, S. E., Alper, S. L. & Breton, S. (1999) Biol. Reprod. 61, 973-980. [DOI] [PubMed] [Google Scholar]
- 16.Lecanda, J., Urtasun, R. & Medina, J. F. (2000) Biochem. Biophys. Res. Commun. 276, 117-124. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook, J. & Russell, D. W. (2001) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 18.Gu, H., Zou, Y. R. & Rajewsky, K. (1993) Cell 73, 1155-1164. [DOI] [PubMed] [Google Scholar]
- 19.Hooper, M., Hardy, K., Handyside, A., Hunter, S. & Monk, M. (1987) Nature 326, 292-295. [DOI] [PubMed] [Google Scholar]
- 20.Auger, J., Ronot, X. & Dadoune, J. P. (1989) J. Androl. 10, 439-448. [DOI] [PubMed] [Google Scholar]
- 21.Tajima, Y., Onoue, H., Kitamura, Y. & Nishimune, Y. (1991) Development (Cambridge, U.K.) 113, 1031-1035. [DOI] [PubMed] [Google Scholar]
- 22.Vincent, S., Segretain, D., Nishikawa, S., Nishikawa, S. I., Sage, J., Cuzin, F. & Rassoulzadegan, M. (1998) Development (Cambridge, U.K.) 125, 4585-4593. [DOI] [PubMed] [Google Scholar]
- 23.Fawcett, D. W., Ito, S. & Slautter-Back, D. (1959) J. Biophys. Biochem. Cytol. 5, 453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun, R. E., Behringer, R. R., Peschon, J. J., Brinster, R. L. & Palmiter, R. D. (1989) Nature 337, 373-376. [DOI] [PubMed] [Google Scholar]
- 25.Florke, S., Phi-van, L., Muller-Esterl, W., Scheuber, H. P. & Engel, W. (1983) Differentiation (Berlin) 24, 250-256. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura, Y., Tanaka, H., Nozaki, M., Yomogida, K., Shimamura, K., Yasunaga, T. & Nishimune, Y. (1999) Gene 237, 193-199. [DOI] [PubMed] [Google Scholar]
- 27.Hurst, S., Howes, E. A., Coadwell, J. & Jones, R. (1998) Mol. Reprod. Dev. 49, 81-91. [DOI] [PubMed] [Google Scholar]
- 28.Stelzer, G. & Don, J. (2002) Endocrinology 143, 1578-1588. [DOI] [PubMed] [Google Scholar]
- 29.Means, A. R., Cruzalegui, F., LeMagueresse, B., Needleman, D. S., Slaughter, G. R. & Ono, T. (1991) Mol. Cell. Biol. 11, 3960-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nantel, F., Monaco, L., Foulkes, N. S., Masquilier, D., LeMeur, M., Henriksen, K., Dierich, A., Parvinen, M. & Sassone-Corsi, P. (1996) Nature 380, 159-162. [DOI] [PubMed] [Google Scholar]
- 31.Blendy, J. A., Kaestner, K. H., Weinbauer, G. F., Nieschlag, E. & Schutz, G. (1996) Nature 380, 162-165. [DOI] [PubMed] [Google Scholar]
- 32.Don, J. & Stelzer, G. (2002) Mol. Cell. Endocrinol. 187, 115-124. [DOI] [PubMed] [Google Scholar]
- 33.De Cesare, D., Fimia, G. M. & Sassone-Corsi, P. (2000) J. Endocrinol. Invest. 23, 592-596. [DOI] [PubMed] [Google Scholar]