Abstract
We conducted a pilot randomized controlled trial to determine the feasibility and initial safety and efficacy of omega-3 fatty acids (1.3 g/day) for the treatment of hyperactivity in 27 children ages 3–8 with autism spectrum disorder (ASD). After 12 weeks, hyperactivity, as measured by the Aberrant Behavior Checklist, improved 2.7 (±4.8) points in the omega-3 group compared to 0.3 (±7.2) points in the placebo group (p = 0.40; effect size = 0.38). Correlations were found between decreases in five fatty acid levels and decreases in hyperactivity, and the treatment was well tolerated. Although this pilot study did not find a statistically significant benefit from omega-3 fatty acids, the small sample size does not rule out small to moderate beneficial effects.
Keywords: Autism, Omega-3 fatty acids, Complementary and alternative medicine, Clinical trial
Introduction
Omega-3 fatty acids are a commonly used complementary and alternative medical (CAM) treatment for autism. In a recent survey, 27.8% of families reported treating their affected child with this supplement (Green et al. 2006). A recently published systematic review (Bent et al. 2009) identified only one, small randomized, placebo-controlled trial that examined the efficacy of omega-3 fatty acids for autism. This study randomly assigned 13 children with autism to 6 weeks of treatment with omega-3 fatty acids (1.5 g per day) or an identical placebo, and found no statistically significant improvements in the sub-scales of the Aberrant Behavior Checklist (ABC) (Amminger et al. 2007). However, each of the five subscales (irritability, social withdrawal, stereotypy, hyperactivity, and inappropriate speech) showed a non-significant trend favoring the omega-3 fatty acid treatment, with the largest improvements noted in hyperactivity and stereotypy. Also, four uncontrolled, open-label studies were identified (Bell et al. 2004; Meguid et al. 2008; Patrick and Salik 2005; Politi et al. 2008), and three out of four reported various benefits related to core symptoms of autism, language and learning skills, and general health.
Due to the common use of omega-3 fatty acids and preliminary evidence from one small randomized controlled trial suggesting a possible benefit, we designed a pilot study with a longer duration of treatment and a larger sample size to examine the feasibility and initial safety and efficacy of administering omega-3 fatty acids for the treatment of hyperactivity and the core symptoms of ASD.
Methods
Participants
The study protocol and all procedures were approved by the Committees on Human Research at the University of California, Davis and the University of California, San Francisco. The trial was registered prior to enrolling patients at clinicaltrials.gov (NCT00786799) and took place between November 5, 2008 and June 25, 2009.
Children between the ages of 3 and 8 with a diagnosis of ASD were recruited from the outpatient autism clinic at the MIND Institute (University of California, Davis). The diagnosis of ASD was established using the Autism Diagnostic Observation Scale (ADOS), the Social Communication Questionnaire (SCQ) and by clinical review of the DSM-IV TR criteria by an expert clinician (RLH). Children were required to have a non-verbal IQ of 50 or more, be on a stable medical regimen, and have a clinician rating of at least moderate severity of autistic symptoms (Clinical Global Impression Severity score of ≥4, range 0–7). Children were excluded if they had a history of allergy to fish or nuts, diabetes, a bleeding disorder, a seizure disorder, cancer, perinatal brain injury, other serious medical illness, or current or prior use of omega-3 fatty acids.
Intervention
Children who satisfied all eligibility criteria were randomly assigned to 12 weeks of treatment with omega-3 fatty acids or an identical placebo. Omega-3 fatty acids were provided as orange-flavored pudding packets (Coromega®, Vista, CA) containing 650 mg of omega-3 fatty acids, including 350 mg of eicosapentanoic acid (EPA) and 230 mg of docosahexanoic acid (DHA), given twice daily for a daily dose of 1.3 g of omega-3 fatty acids (and 1.1 g of DHA + EPA). Placebo packets had the same orange-flavored pudding with an identical appearance and taste, but included safflower oil instead of the fish oil (omega-3 fatty acids). Safflower oil was used because it is has a similar texture and is comprised of fatty acids (but not omega-3 fatty acids), and this allowed for an examination of the unique of effects of omega-3 fatty acids compared to “non-omega-3” fatty acids. The orange flavor in both the active and placebo packets masked any fishy taste.
Families were contacted by telephone 2 and 8 weeks after randomization and had a brief clinical evaluation at 6 weeks. The children returned for a final visit at week 12 for outcome assessment and study close-out. Compliance was assessed by asking parents at the final visit how often children were able to take the study medication.
Objectives and Outcomes
The primary objective of this study was to examine the feasibility and initial safety and efficacy of administering omega-3 fatty acids for the treatment of hyperactivity in children with ASD.
Secondary outcomes of the study were designed to examine changes in the core features of autism (communication problems, social difficulties, and repetitive/restricted behavior) as well as biomarkers (serum free fatty acids and cytokines) that might suggest a potential mechanism of action. Communication was assessed with the Peabody Picture Vocabulary Test (which asks individuals to point to a picture after hearing a word and is a standard measure of receptive vocabulary) and the Expressive Vocabulary Test (where the examiner points to a picture and asks the individual to state the name, which is a standard measure of expressive vocabulary). Social interaction was assessed with the Social Responsiveness Scale. Behaviors were assessed with two parent-completed questionnaires: (1) Aberrant Behavior Checklist (ABC), and (2) Behavioral Assessment System for Children (BASC). Global changes in the severity of autistic symptoms were evaluated by a clinician with the Clinical Global Impression-Improvement (CGI-I) scale.
Randomization and Blinding
Eligible participants were randomized in equal proportions using a computer-generated randomization list, which was prepared by persons who were not involved with the study. All participants, families, and study personnel (including clinicians and those performing baseline and outcome assessments) were blinded to group assignment for the entire study.
Laboratory Methods
Cytokines were included as a possible biomarker because evidence from prior studies suggests that omega-3 fatty acids may have beneficial effects through anti-inflammatory actions (Simopoulos 2002). Cytokine analysis was performed on plasma samples using multiplex assays (Millipore) according to the manufacturer’s recommendation and read on a Luminex 100™ platform. Lipid profiling was performed at Lipomics Technologies (West Sacramento, CA) using a combination of chromatography methods to quantitatively measure a range of lipid classes and fatty acids within those classes, as previously described (Wiest et al. 2009).
Statistical Methods
Because the study was a pilot study (and limited in size and scope by available resources), the study was not designed with sufficient power to have a high likelihood of detecting small to moderate changes in hyperactivity. Specifically, the study was designed to recruit 24 subjects (12 subjects per group) with a goal of detecting a 25% reduction in the mean baseline hyperactivity subscale of the Aberrant Behavior Checklist (ABC). A 25% reduction in the baseline scores in the ABC has previously been used to define a “positive response” to therapy in autism (McCracken et al. 2002). We increased the target sample size to 27 to account for possible withdrawals. Using an alpha level of 0.05, an estimated standard deviation of the hyperactivity subscale of 7.8 (derived from the prior pilot study), and a one-sided test to detect a beneficial response, the study was estimated to have an 84% power to detect a 25% reduction in hyperactivity. However, this power calculation relied on both a high level of baseline hyperactivity and a large change in hyperactivity (standardized effect size = 1.1), so the study was designed to determine if use of omega-3 fatty acids results in a large change in hyperactivity.
We compared baseline characteristics of the omega-3 and placebo groups using the student’s t-test for continuous variables and chi-square tests for categorical variables. We assessed the significance of the difference in the changes between groups using the Mann–Whitney test. Because this was designed as an exploratory analysis, we did not adjust p-values for multiple testing, which is consistent with prior recommendations (Rothman 1990; Savitz and Olshan 1998; Thompson 1998). All reported p-values are two-sided. If patients discontinued study medication, they were asked to complete all outcome assessments at 12 weeks, and they were analyzed in their assigned treatment group.
The funding organizations and the supplier of the omega-3 and placebo study medication had no role in the design or conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation, review, and approval of the manuscript.
Results
Patient Characteristics
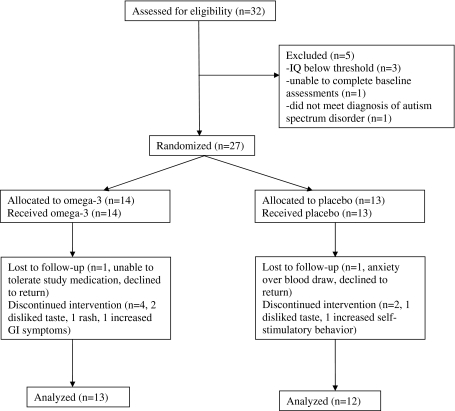
Of 32 children who were screened for eligibility, 27 satisfied all eligibility criteria and underwent randomization, 14 to omega-3 and 13 to placebo. The majority of enrolled patients were male (13/14 in the omega-3 group and 11/13 in the placebo group). Figure 1 shows reasons for exclusion, loss to follow-up, and discontinuation of study medication.
Fig. 1.
Flow diagram showing distribution of participants at each stage
One patient was lost to follow-up in the omega-3 group after being unable to take the study medication (due to disliking taste), and one patient was lost to follow-up in the placebo group (due to anxiety over future blood draws). Both patients declined to complete outcome measures, leaving 13 analyzable patients in the omega-3 group and 12 in the placebo group. Compliance was reported to be perfect or nearly perfect for 9/13 analyzable patients (69%) in the omega-3 group and 9/12 patients (75%) in the placebo group. The baseline characteristics of the two treatment groups were similar (Table 1).
Table 1.
Baseline characteristics
| Characteristic | Omega-3 group (n = 13) mean (SD) | Placebo (n = 12) mean (SD) | Total (n = 25) mean (SD) | p-value (placebo vs. omega-3) |
|---|---|---|---|---|
| Age (months) | 70.2 (22) | 69.8 (17) | 70.0 (20) | 0.89 |
| CGI-S | 4.6 (0.5) | 4.2 (0.4) | 4.4 (0.5) | 0.03 |
| Stanford Binet—IQ | 77.5 (27) | 77.5 (17) | 77.5 (23) | 0.59 |
| ABC—total | 40.6 (30) | 51.8 (24) | 46.0 (28) | 0.31 |
| ABC—hyperactivity | 16.8 (13) | 20.3 (8) | 18.4 (11) | 0.37 |
| ABC—irritability | 10.1 (10) | 12.0 (7) | 11.0 (8) | 0.35 |
| ABC—stereotypy | 2.8 (3) | 6.0 (5) | 4.3 (4) | 0.12 |
| ABC—lethargy | 7.7 (7) | 8.3 (7) | 8.0 (7) | 0.80 |
| ABC—IS | 3.3 (3) | 5.2 (3) | 4.2 (3) | 0.16 |
| PPVT | 72.2 (28) | 85.5 (12) | 78.6 (23) | 0.11 |
| EVT | 70.8 (33) | 86.4 (14) | 78.3 (26) | 0.33 |
| SRS | 76.9 (11) | 79.0 (8) | 77.9 (10) | 0.76 |
| BASC—externalizing | 53.8 (13) | 66.3 (25) | 59.8 (20) | 0.08 |
| BASC—internalizing | 43.3 (10) | 50.1 (9) | 46.3 (10) | 0.14 |
| BASC—behavioral | 60.9 (14) | 65.4 (3) | 63.0 (11) | 0.31 |
| BASC—adaptive skill | 29.8 (9) | 31.9 (9) | 30.7 (9) | 0.58 |
| BASC-hyperactivity | 61.8 (17) | 64.6 (7) | 63.1 (13) | 0.35 |
| Gastrointestinal symptoms | 2/13 (15%) | 1/12 (8%) | 3/25 (12%) | 1.00 |
| Frequent infections | 2/13 (15%) | 1/12 (8%) | 3/25 (12%) | 1.00 |
| Food allergy | 1/13 (8%) | 2/12 (17%) | 3/25 (12%) | 0.58 |
| Regression | 3/13 (23%) | 2/12 (17%) | 5/25 (20%) | 1.00 |
CGI-S clinical global impression-severity, ABC aberrant behavior checklist, PPVT peabody picture vocabulary test, EVT expressive vocabulary test, SRS social responsiveness scale, BASC behavioral assessment system for children
Participants had a mean age of approximately 6 years and a mean CGI-S score of 4.4, which is between the categories of moderately ill (score of 4) and markedly ill (score of 5).
Clinical Outcomes
Table 2 shows the primary outcome measure and all other clinical outcome measures.
Table 2.
Changes in primary and secondary outcome measures
| Omega-3 change (SD) N = 13 | Placebo change (SD) n = 12 | Difference in change between groups (SD) | 95% confidence interval | Standardized effect sizea | p-value | |
|---|---|---|---|---|---|---|
| Primary outcome measure | ||||||
| ABC hyperactivity | 2.7 (4.8) | 0.3 (7.2) | 2.3 (6.1) | −3.0 to 7.4 | 0.38 | 0.40 |
| Secondary outcome measures | ||||||
| ABC | ||||||
| Irritability | 0.8 (4.7) | 1.1 (5.1) | −0.3 (4.9) | −4.6 to 3.7 | −0.07 | 0.95 |
| Stereotypy | 0.7 (1.2) | 0.3 (2.6) | 0.4 (2.1) | −1.3 to 2.1 | 0.20 | 0.36 |
| Lethargy | 2.7 (4.8) | 1.9 (5.7) | 0.8 (5.3) | −3.7 to 5.2 | 0.16 | 0.54 |
| Inappropriate speech | 1.2 (2.0) | 0.7 (2.3) | 0.5 (2.2) | −1.4 to 2.2 | 0.23 | 0.79 |
| PPVT | 2.7 (11.6) | 1.9 (12.4) | 0.8 (12.0) | −9.1 to 10.7 | 0.06 | 0.60 |
| EVT | 2.2 (7.6) | 5.8 (5.7) | −3.7 (6.7) | −9.3 to 1.9 | −0.55 | 0.18 |
| SRS | −0.9 (6.5) | 1.7 (7.2) | −2.6 (6.8) | −8.7 to 3.5 | −0.39 | 0.38 |
| BASC | ||||||
| Externalizing | 0.1 (6.7) | 6.6 (30.4) | −6.5 (22.0) | −26.3 to 11.1 | −0.30 | 0.45 |
| Internalizing | 0.3 (6.6) | −2.9 (7.6) | 3.2 (7.1) | −4.0 to 8.7 | 0.45 | 0.33 |
| Behavioral | −1.1 (6.1) | −2.0 (4.9) | 0.9 (5.6) | −4.8 to 5.7 | 0.16 | 0.97 |
| Adaptive skill | 1.8 (6.8) | 0.8 (7.1) | 1.0 (6.9) | −5.7 to 6.7 | 0.14 | 0.84 |
| Hyperactivity | 2.1 (6.3) | 1.2 (5.8) | 0.9 (6.1) | −4.5 to 6.0 | 0.15 | 0.83 |
All changes are shown as positive when there was an improvement in the score and negative when there was a worsening of the score
ABC aberrant behavior checklist, PPVT peabody picture vocabulary test, EVT expressive vocabulary test, SRS social responsiveness scale, BASC behavioral assessment system for children
aEffect sizes are positive when the change in omega-3 is greater (more beneficial or less harmful) than the change in placebo
Hyperactivity as measured by the ABC improved 2.7 (±4.8) points in the omega-3 group compared to 0.3 (±7.2) points in the placebo group, but this difference in change scores was not statistically significant (p = 0.40). There was also a small, non-significantly greater improvement in hyperactivity as measured by the BASC (2.1 ± 6.3 in the omega-3 group vs. 1.2 ± 5.8 in the placebo group, p = 0.83). Most of the outcomes showed slightly larger improvements in the omega-3 group compared to the placebo group, as indicated by the positive effect sizes which range from 0.06 to 0.45, but none of these differences were statistically significant. Four outcomes—the irritability subscale of the ABC, the Expressive Vocabulary Test, the Social Responsiveness Scale, and the externalizing subscale of the BASC showed greater improvements in the placebo group (and therefore had negative effect sizes). The CGI-I score, which reflects a clinician’s impression of how the child’s overall symptom burden changed, was also not different between groups. Two out of 13 children (15%) in the omega-3 group and 3 out of 12 children (25%) in the placebo group were rated as much improved or very much improved on the CGI-I (p = 0.64).
Laboratory Outcomes
Changes in serum free fatty acids are shown in Table 3.
Table 3.
Free fatty acid changes
| Free fatty acid | Baseline | SD | Mean change omega-3 | SD | Mean change Placebo | SD | p-value |
|---|---|---|---|---|---|---|---|
| 14:0 | 1.27 | 0.8 | −0.10 | 0.8 | −0.18 | 0.8 | 0.82 |
| 15:0 | 0.23 | 0.1 | 0.01 | 0.1 | −0.03 | 0.1 | 0.02 |
| 16:0 | 20.64 | 2.6 | −1.20 | 2.4 | 0.08 | 2.2 | 0.22 |
| 18:0 | 8.07 | 0.7 | 0.15 | 0.6 | −0.17 | 0.6 | 0.22 |
| 20:0 | 0.18 | 0.0 | 0.02 | 0.0 | −0.02 | 0.1 | 0.10 |
| 22:0 | 0.41 | 0.2 | 0.06 | 0.1 | −0.10 | 0.2 | 0.02 |
| 24:0 | 0.37 | 0.1 | 0.06 | 0.1 | −0.09 | 0.1 | 0.01 |
| 14:1n5 | 0.09 | 0.1 | −0.03 | 0.1 | 0.00 | 0.1 | 0.45 |
| 16:1n7 | 1.29 | 0.7 | −0.37 | 0.8 | 0.03 | 0.5 | 0.22 |
| 18:1n7 | 1.19 | 0.2 | −0.05 | 0.1 | 0.07 | 0.2 | 0.20 |
| 18:1n9 | 18.90 | 3.6 | −2.43 | 4.6 | 1.44 | 1.9 | 0.01 |
| 20:1n9 | 0.17 | 0.1 | −0.03 | 0.1 | 0.00 | 0.1 | 0.32 |
| 20:3n9 | 0.09 | 0.0 | −0.02 | 0.0 | −0.01 | 0.0 | 0.42 |
| 22:1n9 | 0.03 | 0.0 | 0.00 | 0.0 | 0.00 | 0.0 | 0.35 |
| 24:1n9 | 0.36 | 0.1 | 0.06 | 0.1 | −0.02 | 0.1 | 0.15 |
| 18:2n6 | 33.72 | 4.8 | 2.04 | 5.5 | −0.66 | 4.4 | 0.15 |
| 18:3n6 | 0.57 | 0.3 | −0.15 | 0.4 | −0.06 | 0.3 | 0.64 |
| 20:2n6 | 0.32 | 0.1 | −0.03 | 0.1 | −0.03 | 0.1 | 0.56 |
| 20:3n6 | 1.54 | 0.4 | −0.30 | 0.6 | −0.04 | 0.3 | 0.30 |
| 20:4n6 | 6.30 | 1.5 | −0.28 | 1.5 | −0.10 | 0.9 | 0.38 |
| 22:2n6 | 0.01 | 0.0 | 0.00 | 0.0 | 0.00 | 0.0 | 0.49 |
| 22:4n6 | 0.25 | 0.1 | −0.06 | 0.1 | 0.00 | 0.0 | 0.03 |
| 22:5n6 | 0.22 | 0.1 | −0.08 | 0.1 | −0.01 | 0.0 | 0.01 |
| 18:3n3 | 0.78 | 0.4 | −0.02 | 0.3 | −0.02 | 0.3 | 0.95 |
| 18:4n3 | 0.04 | 0.0 | −0.01 | 0.0 | −0.01 | 0.0 | 0.81 |
| 20:4n3 | 0.07 | 0.0 | −0.01 | 0.0 | −0.02 | 0.0 | 0.82 |
| 20:5n3 (EPA) | 0.43 | 0.6 | 0.99 | 1.3 | −0.04 | 0.1 | 0.03 |
| 22:5n3 | 0.43 | 0.1 | 0.23 | 0.2 | −0.01 | 0.1 | 0.003 |
| 22:6n3 (DHA) | 1.34 | 0.8 | 1.36 | 1.4 | −0.01 | 0.2 | 0.02 |
| dm16:0 | 0.35 | 0.1 | 0.07 | 0.1 | −0.01 | 0.0 | 0.18 |
| dm18:0 | 0.23 | 0.1 | 0.08 | 0.1 | 0.00 | 0.1 | 0.10 |
| dm18:1n7 | 0.02 | 0.0 | 0.01 | 0.0 | 0.00 | 0.0 | 0.45 |
| dm18:1n9 | 0.11 | 0.0 | 0.00 | 0.1 | 0.01 | 0.0 | 0.95 |
| % Saturated | 31.75 | 3.3 | −0.84 | 3.0 | −0.51 | 2.8 | 0.60 |
| % Monounsaturated | 22.15 | 4.0 | −2.83 | 5.0 | 1.5 | 2.3 | 0.007 |
| % Polyunsaturated | 46.09 | 5.4 | 3.67 | 6.6 | −1.01 | 3.7 | 0.04 |
| % Omega-3 | 3.08 | 1.4 | 2.54 | 2.8 | −0.10 | 0.3 | 0.01 |
| % Omega-6 | 42.92 | 5.2 | 1.15 | 5.9 | −0.90 | 3.7 | 0.30 |
| % Omega-7 | 2.47 | 0.7 | −0.42 | 0.9 | 0.10 | 0.6 | 0.15 |
| % Omega-9 | 19.55 | 3.6 | −2.42 | 4.5 | 1.41 | 2.0 | 0.01 |
| % Dimethyl | 0.70 | 0.2 | 0.15 | 0.3 | 0.0 | 0.1 | 0.09 |
Fatty acids are described by their lipid number, which takes the form C:D, where C is the number of carbon atoms in the fatty acid and D is the number of double bonds in the fatty acid. All units are expressed as moles of fatty acid as a percentage of total moles fatty acid in the lipid class
dm dimethyl, EPA eicosapentanoic acid, DHA docosahexanoic acid
As expected, the percentage of serum omega-3 fatty acids increased in the omega-3 group and decreased slightly in the placebo group (+2.5% vs. −0.1%, p = 0.01). Both DHA and EPA also showed statistically significant increases in the treatment group compared to the placebo group. Nine individual fatty acid levels and four of the eight class percentages (% monounsaturated, % polyunsaturated, % omega-3, and % omega-9) also showed statistically significant differences in changes over the course of the study, suggesting that omega-3 supplementation for 12 weeks led to marked differences in fatty acid profiles.
Changes in cytokine levels (pg/ml) are shown in Table 4.
Table 4.
Laboratory values: cytokines
| Cytokines | Baseline mean | SD | Omega-3 mean change | SD | Placebo mean change | SD | p-value |
|---|---|---|---|---|---|---|---|
| TGF-β-1 | 12242.07 | 7673.9 | −3608.93 | 10725.9 | 4606.84 | 10992.2 | 0.11 |
| TGF-β-2 | 12674.20 | 7594.9 | −3354.03 | 11186.9 | 4202.95 | 9947.3 | 0.11 |
| BDNF | 864.75 | 535.3 | −120.00 | 768.9 | −176.64 | 434.8 | 0.77 |
| IL-1α | 218.75 | 288.1 | −35.34 | 557.0 | 174.92 | 528.7 | 0.72 |
| IL-1β | 2.27 | 2.6 | −0.15 | 1.0 | 0.11 | 1.1 | 0.59 |
| IL-2 | 2.44 | 3.0 | 6.24 | 16.3 | 0.28 | 2.9 | 0.25 |
| IL-3 | 4.90 | 0.0 | 0.72 | 2.5 | 0.00 | 0.0 | 0.41 |
| IL-4 | 7.20 | 11.9 | −2.19 | 7.7 | −0.16 | 3.1 | 0.57 |
| IL-5 | 2.04 | 3.6 | −1.14 | 5.8 | 0.57 | 1.5 | 0.52 |
| IL-6 | 6.83 | 11.1 | 3.19 | 25.7 | 7.68 | 24.8 | 0.97 |
| IL-7 | 9.06 | 19.3 | 1.82 | 14.3 | 1.53 | 13.5 | 0.49 |
| IL-8 | 5.90 | 7.8 | 6.45 | 14.1 | −0.57 | 10.2 | 0.12 |
| IL-10 | 6.20 | 2.8 | 2.23 | 15.4 | 6.78 | 16.4 | 0.28 |
| IL-12(p40) | 83.76 | 104.5 | 14.32 | 80.5 | −6.85 | 62.1 | 0.72 |
| IL-12(p70) | 4.73 | 4.6 | 24.61 | 74.3 | −0.50 | 9.4 | 0.19 |
| IL-13 | 1.55 | 1.5 | 33.67 | 116.8 | −0.15 | 2.0 | 0.57 |
| IL-15 | 2.73 | 1.3 | 8.82 | 31.4 | −0.28 | 0.9 | 0.57 |
| IL-17 | 8.64 | 17.7 | 13.91 | 37.9 | −5.22 | 26.0 | 0.20 |
| IP-10 | 346.73 | 313.2 | 63.52 | 865.6 | 125.25 | 913.6 | 0.92 |
| MCP-1 | 337.82 | 76.6 | −30.84 | 123.8 | −27.76 | 179.2 | 0.87 |
| MIP-1α | 46.92 | 56.1 | 16.75 | 68.9 | −16.18 | 51.0 | 0.45 |
| MIP-1β | 27.06 | 14.7 | 8.39 | 24.9 | 1.03 | 17.5 | 0.34 |
| TNF-α | 5.21 | 1.2 | 1.34 | 2.5 | −0.89 | 2.1 | 0.023 |
| TNF-β | 3.51 | 2.9 | 5.25 | 19.7 | 0.34 | 3.2 | 0.73 |
| INF-γ | 11.90 | 19.1 | 11.92 | 32.1 | −0.83 | 19.9 | 1.00 |
| INF-α2 | 218.75 | 288.1 | −22.66 | 557.9 | 174.92 | 528.7 | 0.97 |
| GM-CSF | 54.16 | 63.5 | 20.29 | 66.4 | 15.18 | 89.7 | 0.92 |
| G-CSF | 58.67 | 40.6 | −18.40 | 53.2 | 25.52 | 102.0 | 0.31 |
| Eotaxin | 83.73 | 36.0 | 0.92 | 17.7 | −33.43 | 47.8 | 0.060 |
All units are expressed in picograms/milliliter
TGF tumor growth factor, BDNF brain-derived neurotrophic factor, IL interleukin, IP-10 10 kDa interferon-gamma-induced protein, MCP-1 monocyte chemotactic protein-1, MIP macrophage inflammatory protein, TNF tumor necrosis factor, INF interferon, GM-CSF granulocyte-macrophage colony-stimulating factor, G-CSF granulocyte colony-stimulating factor
Of 29 cytokines measured, only one (TNFα) revealed a statistically significant difference in the mean change over the course of the study between groups. Interestingly, this cytokine increased in the omega-3 group (by 1.3 ± 2.5 pg/ml) and decreased in the placebo group (by −0.9 ± 2.1 pg/ml, p = 0.023).
We examined correlations between changes in each of the measured laboratory values (free fatty acids and cytokines) and changes in hyperactivity among all study participants. A change in one cytokine level (IL-2) showed a correlation with change in hyperactivity (as IL-2 increased, hyperactivity decreased; Spearman rank −0.44, p = 0.045). Five free fatty acids showed strong correlations such that decreases in 18:1n9, 22:1n9, dm18:1n9, % monounsatured, and % omega-9 fatty acids were associated with decreases in hyperactivity (Spearman coefficients are respectively 0.48, 0.59, 0.47, 0.48, and 0.46 with p-values 0.02, 0.003, 0.02, 0.02, and 0.03).
We also examined whether baseline levels of free fatty acids were correlated with changes in hyperactivity among patients in the omega-3 group, with the goal of identifying predictors of response to therapy. The baseline level of five fatty acids showed statistically significant correlations with changes in hyperactivity among patients in the omega-3 group. For two of these fatty acids, 22:0 and 24:0, lower baseline levels were correlated with reductions in hyperactivity (Spearman coefficients of 0.73 and 0.72 and p-values of 0.007 and 0.009 respectively). For three of these fatty acids (20:2n6, 22:2n6, and 18:3n3), higher baseline levels were associated with reductions in hyperactivity (Spearman coefficients of −0.79, −0.65, and −0.64 with p-values of 0.002, 0.03, and 0.03, respectively).
Side Effects
No serious adverse events were reported during the study, and there was no difference in the number of reported non-serious adverse events in the two treatment groups. Five of fourteen patients reported adverse events in the omega-3 group (2 rashes, 1 upper respiratory infection, 1 nose bleed, 1 increased GI symptoms); four of thirteen patients reported adverse events in the placebo group (3 increased hyperactivity, 1 increased self-stimulatory behavior).
Adequacy of Blinding
Among families who used at least some study medication and responded to the question, “do you think your child was taking omega-3 fatty acids or placebo?” at the end of the study, there was no statistical difference in the percentage of families who believed they had been given omega-3 (7/11 or 64% in the placebo group vs. 4/10 or 40% in the omega-3 group, p = 0.39).
Discussion
In this pilot study, we found that treatment with omega-3 fatty acids did not lead to a statistically significant improvement in hyperactivity in children with ASD. Although non-significant, there was a greater reduction in hyperactivity in the treatment vs. the placebo group in this study, which corresponds to a standardized effect size of 0.38 and is considered to be a small treatment effect (McDowell and Newell 1996). The interpretation of these findings is difficult and highlights the limitations of pilot studies and the inherent challenges of studying the efficacy of CAM therapies for ASD. The most conservative interpretation of this study is that we found no statistically significant treatment effect, and since the study was designed with enough power to find a large treatment effect (84% power to identify a treatment effect size = 1.1), we might conclude that omega-3 fatty acids do not produce a large beneficial effect for reducing hyperactivity, at least among children with ASD who have mild-to-moderate baseline levels of hyperactivity.
However, because pilot studies generally have small sample sizes, the resulting effect estimates are unreliable and therefore decisions regarding the need for future, larger studies should not be based on the results of pilot studies alone (Kraemer et al. 2006). Small pilot studies are not designed to provide definitive scientific evidence regarding efficacy; their primary purpose is to prepare for larger studies by examining issues such as the availability of eligible subjects, the recruitment methods, the procedures for staff training, the tolerability of treatment, and the collection and storage of data (Kraemer et al. 2006). This presents a difficult dilemma for the scientific investigation of the efficacy of CAM therapies for ASD. There is little or no high-quality scientific evidence evaluating the safety and efficacy of most CAM therapies for ASD even though these therapies are used by as much as 95% of affected children. Because there is limited scientific evidence for the efficacy of most of these CAM therapies, it is difficult to justify or obtain funding to conduct a large, randomized controlled trial that would provide definitive scientific evidence regarding efficacy. Therefore, most clinical investigations of CAM therapies can only attract small amounts of funding to conduct small pilot studies, and these studies are often (incorrectly) used as the basis for “triage” or decision making regarding the need for future, larger studies. It is a difficult cycle: there is no evidence, so only funding for small studies can be obtained, and these studies do not provide enough evidence to make decisions regarding future studies. So, the question remains, how does one decide whether to conduct larger studies if effect estimates from pilot studies are inaccurate and insufficient?
The answer to this question is complex and involves considerations of other evidence of efficacy of the agent in question, examination of a plausible mechanism of action, the severity of the clinical problem and the availability of other effective treatments. In the case of omega-3 fatty acids for the treatment of hyperactivity for ASD, only one prior, small, pilot randomized controlled trial has been conducted. This study found a non-significant trend of a greater reduction in hyperactivity in patients in the omega-3 group compared to the placebo group (p = 0.098) (Amminger et al. 2007). In that study, the standardized effect size for the improvement in hyperactivity was 0.71, about twice the size of the effect estimate observed in the current study (0.38), and this difference is possibly due to the fact that patients in the prior study had higher baseline levels of hyperactivity (baseline ABC hyperactivity score of 33.3 in the omega-3 group of the prior study compared to a baseline score of 16.8 in the current study). It is also possible that the observed effect size in the prior study was not due to treatment with omega-3 fatty acids, but merely due to the fact that patients in the omega-3 group had higher baseline hyperactivity scores than patients in the placebo group (Gilbert 2008). However, the observation that both the current study and the one prior randomized controlled trial found an effect estimate in the same direction may provide some limited evidence that omega-3 fatty acids could have a small beneficial effect.
Other relevant evidence regarding the potential efficacy of omega-3 fatty acids comes from studies of this supplement in other psychiatric disorders. A recent systematic review examining the evidence for efficacy of omega-3 fatty acids for mental health disorders (conducted by the Agency for Healthcare Research and Quality) noted many potential mechanisms of action of omega-3 fatty acids which might contribute to normal brain development and function (Schachter et al. 2005). However, there were very few high-quality randomized controlled trials examining efficacy, leading the authors to conclude that there was insufficient evidence to determine whether omega-3 fatty acids are effective for depression, and only limited evidence to suggest that they might be effective for the treatment of schizophrenia. A separate systematic review identified 11 small randomized controlled trials of omega-3 fatty acids in patients with attention-deficit-hyperactivity disorder (ADHD) and found that the overall quality of evidence was poor, preventing definitive conclusions regarding efficacy (Raz and Gabis 2009). A recent randomized, placebo-controlled trial in 81 young adults who were high-risk for developing psychosis found that patients treated with 12 weeks of omega-3 fatty acids had a much lower risk of progression to a psychotic disorder (4.9% progressed in the omega-3 group vs. 27.5% in the placebo group) (Amminger et al. 2010). Overall, the prior evidence for efficacy of omega-3 fatty acids in other mental disorders is limited, though there are clearly several plausible biological mechanisms of action and some clinical trial evidence to support a possible beneficial effect in certain conditions.
If omega-3 fatty acids are effective for reducing hyperactivity in ASD, the mechanism of action is not clear, but the laboratory measurements in this study suggest some possible lines of inquiry. As expected, there were differences in the changes of fatty acid profiles in treated vs. placebo patients, most notably with an increase in omega-3 fatty acids among treated patients. Also, five fatty acid measurements showed correlations with changes in hyperactivity, suggesting that, at least for some individuals, a change in fatty acid profile may be necessary to elicit a positive behavioral response. Two of three previous studies reported lower levels of omega-3 fatty acids in children with autism compared to controls (Bell et al. 2004; Bu et al. 2006; Vancassel et al. 2001), but this is the first study to show that changes in specific fatty acid levels correlate with changes in a behavioral outcome. Similarly, baseline levels of five of the measured fatty acids were correlated with a reduction in hyperactivity among treated patients. Future research is needed to determine if these specific fatty acids might be useful for predicting response to therapy.
The primary limitation of this study is the small sample size, which (as noted above) results in a limited power to detect small to moderate treatment effects. The study was also limited by the relatively mild level of hyperactivity in both the placebo and omega-3 treatment groups. The goal of the study was to examine the effect of omega-3 fatty acids in unselected children with ASD, where the prevalence of hyperactivity is already quite high. For example, in a survey of 487 children with ASD, the prevalence of symptoms related to hyperactivity was very high: difficulty concentrating (49%); easily distracted (60%); fidgets, wiggles, and squirms (42%); overactive (41%); and short attention span (54%) (Lecavalier 2006). However, because our enrolled population had only mildly elevated levels of hyperactivity, future studies should consider setting a threshold level of hyperactivity as an inclusion criterion to ensure a greater level of severity and therefore more opportunity to detect improvement in hyperactivity with treatment. A suitable goal might be to select children with at least a one standard deviation increase in a baseline measure of hyperactivity such as the Aberrant Behavior Checklist hyperactivity subscale.
In summary, this pilot study revealed no statistically significant effects of omega-3 fatty acids on hyperactivity or the core symptoms of autism after 12 weeks of treatment. However, because the study was a pilot study with a relatively small sample size, the estimate of treatment effect is unreliable, and this study alone does not provide definitive evidence regarding the efficacy of omega-3 fatty acids. Based on this study and the scientific literature to date, we believe it is not possible to determine whether omega-3 fatty acids are effective for treating hyperactivity in ASD. However, there are a number of compelling reasons to suggest that a larger study is indicated. Both the current study and one prior study did find small, non-significant improvements in hyperactivity in the omega-3 group compared to the placebo group. Also, in the current study, changes in hyperactivity were correlated with changes in certain fatty acids levels, suggesting a possible mechanism of action. The treatment was well-tolerated and resulted in no serious side effects. Omega-3 fatty acids are widely used, relatively inexpensive, and have substantial evidence establishing safety in children. Further, hyperactivity is a very common problem among children with ASD. A recent survey of 479 parents of children with ASD found that 46% of children had used stimulant medications by adolescence, suggesting that almost half of children with ASD have hyperactivity that is troubling enough to require a trial of stimulant medication (Goin-Kochel et al. 2007). Current drug treatment of hyperactivity in children with ASD has limited efficacy and is associated with significant side effects (Aman and Langworthy 2000). In order to definitively determine if omega-3 fatty acids are a useful treatment option for hyperactivity in children with ASD, a larger randomized controlled trial with sufficient power to identify a clinically relevant benefit is needed.
Acknowledgments
We gratefully acknowledge the families and children who participated in this study to help advance scientific research in autism. This work was supported by grants from Autism Speaks, the Higgins Family Foundation, The Emch Foundation, The Taube Foundation, NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131 (Dr. Bent) and the MIND Institute (Dr. Hendren).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Clinical Trial Registry Omega-3 Fatty Acids for Autism Treatment; NCT00786799; http://www.clinicaltrials.gov/ct2/show/NCT00786799.
References
- Aman MG, Langworthy KS. Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. Journal of Autism and Developmental Disorders. 2000;30(5):451–459. doi: 10.1023/A:1005559725475. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Berger GE, Schafer MR, Klier C, Friedrich MH, Feucht M. Omega-3 fatty acids supplementation in children with autism: A double-blind randomized, placebo-controlled pilot study. Biological Psychiatry. 2007;61(4):551–553. doi: 10.1016/j.biopsych.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: A randomized, placebo-controlled trial. Archives of General Psychiatry. 2010;67(2):146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- Bell JG, MacKinlay EE, Dick JR, MacDonald DJ, Boyle RM, Glen AC. Essential fatty acids and phospholipase A2 in autistic spectrum disorders. Prostaglandins Leukotrienes and Essential Fatty Acids. 2004;71(4):201–204. doi: 10.1016/j.plefa.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Bent S, Bertoglio K, Hendren RL. Omega-3 fatty acids for autistic spectrum disorder: A systematic review. Journal of Autism and Developmental Disorders. 2009;39(8):1135–1154. doi: 10.1007/s10803-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu B, Ashwood P, Harvey D, King IB, Water JVD, Jin LW. Fatty acid compositions of red blood cell phospholipids in children with autism. Prostaglandins Leukotrienes and Essential Fatty Acids. 2006;74(4):215–221. doi: 10.1016/j.plefa.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Gilbert DL. Regarding “omega-3 fatty acids supplementation in children with autism: A double-blind randomized, placebo-controlled pilot study”. Biological Psychiatry. 2008;63(2):e13–e15. doi: 10.1016/j.biopsych.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Goin-Kochel RP, Myers BJ, Mackintosh VH. Parental reports on the use of treatments and therapies for children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2007;1(3):195–209. doi: 10.1016/j.rasd.2006.08.006. [DOI] [Google Scholar]
- Green VA, Pituch KA, Itchon J, Choi A, O’Reilly M, Sigafoos J. Internet survey of treatments used by parents of children with autism. Research in Developmental Disabilities. 2006;27(1):70–84. doi: 10.1016/j.ridd.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Archives of General Psychiatry. 2006;63(5):484–489. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism and Developmental Disorders. 2006;36(8):1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, et al. Risperidone in children with autism and serious behavioral problems. New England Journal of Medicine. 2002;347(5):314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- McDowell I, Newell C. Measuring health: A guide to rating scales and questionnaires. New York, NY: Oxford University Press; 1996. [Google Scholar]
- Meguid NA, Atta HM, Gouda AS, Khalil RO. Role of polyunsaturated fatty acids in the management of Egyptian children with autism. Clinical Biochemistry. 2008;41(13):1044–1048. doi: 10.1016/j.clinbiochem.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Patrick, L., & Salik, R. (2005). The effect of essential fatty acid supplementation on language development and learning skills in Autism and Asperger’s Syndrome. Autism Asperger’s Digest, 36–37.
- Politi P, Cena H, Comelli M, Marrone G, Allegri C, Emanuele E, et al. Behavioral effects of omega-3 fatty acid supplementation in young adults with severe autism: An open label study. Archives of Medical Research. 2008;39(7):682–685. doi: 10.1016/j.arcmed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Raz R, Gabis L. Essential fatty acids and attention-deficit-hyperactivity disorder: A systematic review. Developmental Medicine and Child Neurology. 2009;51(8):580–592. doi: 10.1111/j.1469-8749.2009.03351.x. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. doi: 10.1097/00001648-199001000-00010. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Olshan AF. Describing data requires no adjustment for multiple comparisons: A reply from Savitz and Olshan. American Journal of Epidemiology. 1998;147(9):813–814. doi: 10.1093/oxfordjournals.aje.a009532. [DOI] [PubMed] [Google Scholar]
- Schachter HM, Kourad K, Merali Z, Lumb A, Tran K, Miguelez M. Effects of omega-3 fatty acids on mental health. Evidence Report/Technology Assessment (Summary) 2005;116:1–11. [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. Journal of the American College of Nutrition. 2002;21(6):495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- Thompson JR. Invited commentary: Re: “Multiple comparisons and related issues in the interpretation of epidemiologic data”. American Journal of Epidemiology. 1998;147(9):801–806. doi: 10.1093/oxfordjournals.aje.a009530. [DOI] [PubMed] [Google Scholar]
- Vancassel S, Durand G, Barthelemy C, Lejeune B, Martineau J, Guilloteau D, et al. Plasma fatty acid levels in autistic children. Prostaglandins Leukotrienes and Essential Fatty Acids. 2001;65(1):1–7. doi: 10.1054/plef.2001.0281. [DOI] [PubMed] [Google Scholar]
- Wiest MM, German JB, Harvey DJ, Watkins SM, Hertz-Picciotto I. Plasma fatty acid profiles in autism: A case-control study. Prostaglandins Leukotrienes and Essential Fatty Acids. 2009;80(4):221–227. doi: 10.1016/j.plefa.2009.01.007. [DOI] [PubMed] [Google Scholar]