Abstract
To elucidate a genetic predisposition to major depressive disorder, we investigated two polymorphisms (−31T/C and −511C/T) in the interleukin-1beta promoter region in patients who suffered from major recurrent depression. The aim of the current work was to compare alleles and genotype layout between patients with major recurrent depression and healthy people. We would like to indicate such combination of genotypes which corresponds with major recurrent depression. Correlations between genotypes for analyzed polymorphisms and number of episodes, number of points in Hamilton Depression Rating Scale, and age of onset were investigated as well. The study group consisted of 94 patients diagnosed with major recurrent depression. The control group included 206 healthy individuals. Both groups involved representatives of Caucasian population. Genotyping of polymorphisms was performed by using PCR-RFLP technique. A specific haplotype, composed of the C allele at −31 and the T allele at −511, has a tendency to have a statistically significant difference (p = 0.064) between patients and control group. Correspondence analysis revealed that genotype T/T at −31 and genotype C/C at −511 are associated with major recurrent depression. No association was found between genotypes for studied polymorphic sites and number of episodes, number of points in Hamilton Depression Rating Scale, and age of onset.
Keywords: Polymorphism, Major recurrent depression, Interleukin-1beta, Genetic association studies
Introduction
In developed countries, major depressive disorder (MDD) is one of the most important psychiatric problems of both genders (Ustun et al. 2004; Andrews et al. 2005). In patients with MDD, increase in peripheral inflammatory biomarkers including inflammatory cytokines, which have access to brain and affect brain function, was observed. These observations pointed a possible correlation of MDD with the activation of the inflammatory response (Sluzewska 1999; Kubera et al. 2000; Khairova et al. 2009; Miller et al. 2009). Systemic administration of interleukin-1beta (IL-1beta) to rats leads to arising of depression-like symptoms including anhedonia, anorexia, and disturbed sleep pattern (Connor and Leonard 1998). The same symptoms are also typical for cytokine immunotherapy treatment (Capuron et al. 2000).
Several studies have found that the IL-1beta level increases in the cerebrospinal and blood (Anisman et al. 1999; Owen et al. 2001). Furthermore, elevated cerebrospinal fluid levels correspond with the duration of illness and age of onset (Dantzer 2004). However, in the data that have been published so far, no significant increase was found in IL-1beta serum level in MDD patients (Capuron et al. 2000; Kagaya et al. 2001).
Taking into account the close correlation between IL-1beta and MDD, two polymorphisms in IL-1beta gene in patients with major recurrent depression (MRD) have been investigated in this study. Both polymorphisms are in the promoter region of the IL-1beta gene, at position −31T/C (rs1143627) and −511C/T (rs 16944), respectively. The aim of the current work was to compare alleles and genotype layout between patients group and controls. The polymorphism −511C/T was selected for analysis because in vitro level of IL-1beta secretion correlates with genotype in −511 position (Pociot et al. 1992). To our knowledge, the polymorphism −31T/C and haplotype analysis of two IL-1beta polymorphisms have been investigated in MRD for the first time. We would like to select such combination of genotypes for both polymorphic sites, which corresponds with MRD. We would also like to investigate linkage disequilibrium between the two analyzed polymorphisms as well correlations between these polymorphisms and number of episodes, number of points in Hamilton Depression Rating Scale (HDRS; Hamilton 1967), and age of onset.
Materials and Methods
Subjects
The patient group consisted of 94 individuals [67 (71.3%) women and 27 (28.7%) men, mean age 53.8 ± 9.4 (median = 54.0; 24–81)] meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for major recurrent depression. The diagnosis has been assigned based on the Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (First et al. 1997) by two independent experienced psychiatrists. Additionally, bipolar spectrum was excluded according to Ghaemi et al. (2001) criteria as well as by the application of the Mood Disorder Questionnaire (Hirschfeld et al. 2000).
The control group consisted of 206 healthy individuals [75 (36.4%) females and 131 (63.6%) males age- and gender-matched somatically healthy blood donors (age range, 20–65 years, mean age 42.3 ± 8.8 (median = 43.0; 20–61)]. Before sampling, it was verified by direct interview if they had current psychiatric problems or family history of psychiatric or neurological disorders. Both patients and controls were biologically unrelated individuals of Caucasian Polish origin living in Silesia region.
Genotyping
Genomic DNA was isolated from the blood samples, and the quality of extracts was checked electrophoretically.
For −31T/C polymorphic site, a 420-bp PCR fragment was amplified using the primers as described in Kimura et al. (2004). PCR conditions were as follows: 96°C for 1 min, followed by 35 cycles of 94°C for 50 s, 50°C for 1 min, 72°C for 40 s, and final elongation at 72°C for 10 min. The PCR products were digested with AluI. The allele T has three cut sites resulting in four fragments of the following sizes: 247, 97, 57, and 20 bp. The C allele has two cut sites, resulting in fragments of sizes 344, 57, and 20 bp. In each stage of the experiment, two control samples with C/C and T/T genotypes were used.
For −511C/T polymorphic site, a 304-bp PCR fragment was amplified, and products were digested as described in Katila et al. (1999).
Statistical Analysis
The distribution of variables was evaluated by the Shapiro–Wilk test. Homogeneity of variance was assessed by the Levene test. The significance of the differences in alleles as well as genotype frequencies in control and patient groups were compared either by the χ 2 test or with the maximum likelihood χ 2 test. The odds ratio was used. One-way multivariate analysis ANOVA or Kruskal–Wallis ANOVA, according to distributions, was used. Associations of co-distribution of genotypes for −31T/C and −511C/T polymorphisms in the IL-1beta gene between control group and patients with MRD were assessed with the correspondence analysis with hierarchical clustering (Ward’s method and Euclidean distance). Statistical calculations were performed by using the Statistica 8.0 software (www.statsoft.com), R software (cran.r-project.org/), and SNPStats (bioinfo.inconcologia.net).All p values were two-tailed, and significance level was set at p < 0.05.
Results and Discussion
Previous studies indicated that −31T/C polymorphic site is a part of TATA box, and T allele enhanced binding of transcription factors. In consequence, the presence of T allele is associated with increase in the production of IL-1beta (El-Omar et al. 2000; Chen et al. 2006). On the other hand, some reports not only did not show any significant association between −31T/C polymorphisms and IL-1beta protein production in vitro (Santtila et al. 1998) but also pointed at allele C to be connected with higher IL-1beta expression in vivo (Hwang et al. 2002). Several studies indicated that −511C/T polymorphic site is implicated in differential expression of IL-1beta protein as well (Hall et al. 2004). Other authors did not record the positive correlation between allelic differences and transcriptional activity of IL-1beta gene, but observed the influence of −511 polymorphic site on transcriptional activity in the context of −31 polymorphic site more through interactions than direct contact with trans-activating proteins, which bind to regulatory elements within this region (Chen et al. 2006).
Quite strong linkage disequilibrium between analyzed polymorphic sites (LD analysis, D′ = 0.6294; r = 0.4994; p < 0.0001) was observed. Previous published reports showed that polymorphism −511C/T is in almost complete linkage disequilibrium with the functional polymorphism −31T/C (El-Omar et al. 2000, 2001; Hall et al. 2004; Misener et al. 2008).
We have not found an association in alleles and genotype layout for both polymorphisms between control and patient group (Table 1). Our results support some previous studies which postulated the lack of association between −511C/T polymorphism with MRD and bipolar disorder, respectively (Yu et al. 2003; Papiol et al. 2004). Similarly, studies concentrated on −31T/C polymorphisms revealed absence of association with MRD (Misener et al. 2008). On the other hand, an association of the −511C/T polymorphism with Alzheimer’s disease (Grimaldi et al. 2000; McCulley et al. 2004) and depressive symptoms in schizophrenia has been found (Rosa et al. 2004). It should be emphasized here that current results may be different with reference to some other similar experiments because our patient group is homogeneous and includes individuals only with major recurrent depression.
Table 1.
Genotypes and allele frequencies in the patient and control groups
| Case n = 94 (%) | Controls n = 206 (%) | χ 2 | p | |||
|---|---|---|---|---|---|---|
| −31T/C | Genotypes | C/C | 8 (8.5) | 29 (14) | 3.11 | 0.2111 |
| T/C | 58 (61.7) | 131 (63.7) | ||||
| T/T | 28 (29.8) | 46 (22.3) | ||||
| Alleles | T | 114 (60.7) | 223 (54) | 2.22 | 0.1362 | |
| C | 74 (39.3) | 189 (46) | ||||
| −511C/T | Genotypes | C/C | 42 (44.7) | 76 (36.9) | 1.84 | 0.3985 |
| C/T | 49 (52) | 120 (58.3) | ||||
| T/T | 3 (3.3) | 10 (4.8) | ||||
| Alleles | T | 55 (293) | 140 (34) | 1.31 | 0.2524 | |
| C | 133 (70.7) | 272 (66) |
Haplotype analysis has been also provided, but haplotype predispose to MRD was not identified (Table 2). However, a tendency to have a statistically significant difference was observed in patients carrying T allele at position −511 and C allele at position −31 in IL-1beta gene promoter. In a single SNP analysis, allele C at −31 polymorphic site reduced promoter activity in relation to allele T, but substantially increased its activity in the context of allele T at −511 polymorphic site (Chen et al. 2006). Some authors suggest that this specific haplotype is associated with increase in IL-1beta protein secretion (Hall et al. 2004). In the present experiment, significant differences may appear when analysis has been conducted on a larger group of patients.
Table 2.
Haplotype frequencies in the patient and control groups
| −31T/C | −511C/T | Frequency | Depression | Control | Odds ratio (95% CI) | p |
|---|---|---|---|---|---|---|
| T | C | 0.4940 | 0.5428 | 0.4712 | 1.00 | |
| C | T | 0.2573 | 0.2290 | 0.2697 | 1.66 (0.97–2.85) | 0.0640 |
| C | C | 0.1810 | 0.1646 | 0.1890 | 1.45 (0.85–2.45) | 0.1700 |
| T | T | 0.0677 | 0.0636 | 0.0701 | 1.41 (0.60–3.31) | 0.4300 |
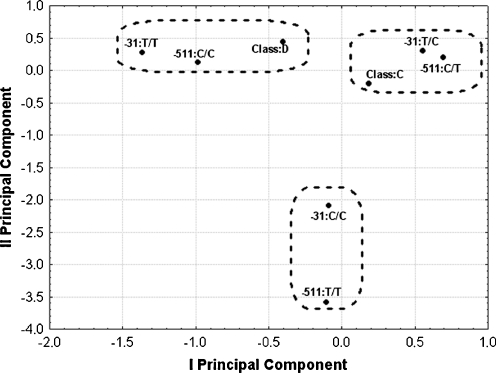
Correspondence analysis revealed that combination of genotype T/T for −31 polymorphic site and genotype C/C at −511 position is connected with MRD, while heterozygous combination at both polymorphic sites is connected with controls (Fig. 1). These results are in agreement with previous reports which described the same differences in relation to allele distribution. Co-presence of allele T at −31 polymorphic site and allele C at −511 polymorphic site influenced the binding of transcription factors to the IL-1beta gene promoter region (El-Omar et al. 2000; Chen et al. 2006).
Fig. 1.
Results of correspondence analysis. Dashed regions show clusters yielded with hierarchical agglomeration with the Ward’s method and the Euclidean distance. D major recurrent depression, C control
We did not found an association between genotypes of both studied polymorphic sites and number of episodes, number of points in HDRS, and age of onset. Previously, Levine et al. (1999) demonstrated that IL-1beta level was positively correlated with the severity of depression. Similarly, Yu et al. (2003) suggest that polymorphism −511C/T may be related to the severity of MDD and early insomnia in MDD patients.
There are several factors that may limit the power to detect strong association by some statistical analysis for polymorphisms investigated in this work. Our group of patients is rather small, but it is worth to remark that the group is homogeneous, and only patients diagnosed with MRD were included in the experiment. It is conceivable that some statistical significance might be detected in an experiment of a large study group, especially in the case of some parameter analyses, a tendency to have a statistical significance has been observed.
In conclusion, this study was carried out to investigate two polymorphisms in IL-1beta gene. Obtained data provide evidence suggesting that genotypes T/T at −31 polymorphic site and C/C in −511 polymorphic site correspond with MRD. We also investigated that polymorphisms are in quite strong linkage disequilibrium. Current results support the role of polymorphisms in IL-1beta gene as a genetic risk factor for MRD.
Acknowledgments
A part of this work was supported by a grant from Polish Genetic Society for Student’s Scientific Circles. Some needed reagents were transferred through supporting corporations: Sigma-Aldrich, Bio-Rad, Roche Diagnostic, ABO.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Andrews G, Poulton R, Skoog I. Lifetime risk of depression: restricted to a minority or waiting for most? Br J Psychiatry. 2005;187:495–496. doi: 10.1192/bjp.187.6.495. [DOI] [PubMed] [Google Scholar]
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Mol Psychiatry. 1999;4:182–188. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- Chen H, Wilkins LM, Nazneen A, Cannings Ch, Wyllie DH, Bingle C, et al. Single nucleotide polymorphisms in the human interleukin-1B gene affect transcription according to haplotype context. Hum Mol Genet. 2006;4:519–529. doi: 10.1093/hmg/ddi469. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Leonard BE. Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sci. 1998;62:583–606. doi: 10.1016/S0024-3205(97)00990-9. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412:99. doi: 10.1038/35083631. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington: American Psychiatric; 1997. [Google Scholar]
- Ghaemi SN, Ko JY, Goodwin FK. The bipolar spectrum and the antidepressant view of the world. J Psychiatr Pract. 2001;7:287–297. doi: 10.1097/00131746-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Grimaldi L, Casadei VM, Ferri C, Veglia F, Licastro F, Annoni G, et al. Association of early-onset Alzheimer’s disease with an interleukin-1α gene polymorphism. Ann Neurol. 2000;47:361–368. doi: 10.1002/1531-8249(200003)47:3<361::AID-ANA12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Hall SK, Perregaux DG, Gabel CA, Woodworth T, Durham LK, Huizinga TW, et al. Correlation of polymorphic variation in the promoter region of the interleukin-1 beta gene with secretion of interleukin-1 beta protein. Arthritis Rheum. 2004;50:1976–1983. doi: 10.1002/art.20310. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RMA, Williams JBW, Spitzer RL, Calabrese JR, Flynn L, Keck PE, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the mood disorder questionnaire. Am J Psychiatry. 2000;157:1873–1874. doi: 10.1176/appi.ajp.157.11.1873. [DOI] [PubMed] [Google Scholar]
- Hwang IR, Kodama T, Kikuchi S, Sakai K, Peterson LE, Graham DY, et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793–1803. doi: 10.1053/gast.2002.37043. [DOI] [PubMed] [Google Scholar]
- Kagaya A, Kugaya A, Takebayashi M, Fukue-Saeki M, Seaki T, Yamawaki S. Plasma concentrations of interleukin-1beta, interleukin-6, soluble interleukin-2 receptor and tumor necrosis factor Ralpha of depressed patients in Japan. Neuropsychobiology. 2001;43:59–62. doi: 10.1159/000054867. [DOI] [PubMed] [Google Scholar]
- Katila H, Hanninen K, Hurme M. Polymorphisms of the interleukin-1 gene complex in schizophrenia. Mol Psychiatry. 1999;4:179–181. doi: 10.1038/sj.mp.4000483. [DOI] [PubMed] [Google Scholar]
- Kimura R, Nishioka T, Soemantri A, Ishida T. Cis-acting effects of the IL1B C-31T polymorphism on IL-1β mRNA expression. Genes Immun. 2004;5:572–575. doi: 10.1038/sj.gene.6364128. [DOI] [PubMed] [Google Scholar]
- Khairova RA, Machado-Vieira R, Du J, Manji HK. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2009;12:561–578. doi: 10.1017/S1461145709009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M, Kenis G, Bosmans E, Zieba A, Dudek D, Nowak G, Maes M. Plasma levels of interleukin-6, interleukin-10, and interleukin-1 receptor antagonist in depression: comparison between the acute state and after remission. Pol J Pharmacol. 2000;52:237–241. [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- McCulley MC, Day IN, Holmes C. Association between interleukin 1-β promoter (−511) polymorphism and depressive symptoms in Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2004;124:50–53. doi: 10.1002/ajmg.b.20086. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misener VL, Gomez L, Wigg KG, Luca P, King N, Kiss E, et al. Cytokine genes TNF, IL1A, IL1B, IL6, IL1RN and IL10, and childhood-onset mood disorders. Neuropsychobiology. 2008;58:71–80. doi: 10.1159/000159775. [DOI] [PubMed] [Google Scholar]
- Owen BM, Eccleston D, Ferrier IN, Young AH. Raised levels of plasma interleukin-1beta in major and postviral depression. Acta Psychiatr Scand. 2001;103:226–228. doi: 10.1034/j.1600-0447.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- Papiol S, Rosa A, Gutierrez B, Martin B, Salgado P, Catalan R, et al. Interleukin-1 cluster is associated with genetic risk for schizophrenia and bipolar disorder. J Med Genet. 2004;41:219–223. doi: 10.1136/jmg.2003.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pociot F, Molving J, Wogensen L, Worsaae H, Nerup J. A TaqI polymorphism in the human interleukin-1beta (IL-1beta) gene correlates with IL-1 beta secretion in vitro. Eur J Clin Investig. 1992;22:396–402. doi: 10.1111/j.1365-2362.1992.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Rosa A, Peralta V, Papiol S, Cuesta MJ, Serrano F, Martinez-Larrea A, Fananas L. Interleukin-1β (IL-1β) gene and increased risk for the depressive symptom-dimension in schizophrenia spectrum disorders. Am J Med Genet B Neuropsychiatr Genet. 2004;124:10–14. doi: 10.1002/ajmg.b.20074. [DOI] [PubMed] [Google Scholar]
- Santtila S, Savinainen K, Hurme M. Presence of the IL-1RA allele 2 (IL1RN*2) is associated with enhanced IL-1beta production in vitro. Scand J Immunol. 1998;47:195–198. doi: 10.1046/j.1365-3083.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- Sluzewska A. Indicators of immune activation in depressed patients. Adv Exp Med Biol. 1999;461:59–73. doi: 10.1007/978-0-585-37970-8_4. [DOI] [PubMed] [Google Scholar]
- Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- Yu YW, Chen TJ, Hong CJ, Chen HM, Tsai SJ. Association study of the interleukin-1β(C-511T) genetic polymorphisms with major depressive disorder, associated symtomatology, and antidepressant response. Neuropsychopharmacology. 2003;28:1182–1185. doi: 10.1038/sj.npp.1300172. [DOI] [PubMed] [Google Scholar]