Abstract
Breast cancer is a genetically and clinically heterogeneous disease, and the contributions of different target cells and different oncogenic mutations to this heterogeneity are not well understood. Here we report that mammary tumors induced by components of the Wnt signaling pathway contain heterogeneous cell types and express early developmental markers, in contrast to tumors induced by other signaling elements. Expression of the Wnt-1 protooncogene in mammary glands of transgenic mice expands a population of epithelial cells expressing progenitor cell markers, keratin 6 and Sca-1; subsequent tumors express these markers and contain luminal epithelial and myoepithelial tumor cells that share a secondary mutation, loss of Pten, implying that they arose from a common progenitor. Mammary tumors arising in transgenic mice expressing β-catenin and c-Myc, downstream components of the canonical Wnt signaling pathway, also contain a significant proportion of myoepithelial cells and cells expressing keratin 6. Progenitor cell markers and myoepithelial cells, however, are lacking in mammary tumors from transgenic mice expressing Neu, H-Ras, or polyoma middle T antigen. These results suggest that mammary stem cells and/or progenitors to mammary luminal epithelial and myoepithelial cells may be the targets for oncogenesis by Wnt-1 signaling elements. Thus, the developmental heterogeneity of different breast cancers is in part a consequence of differential effects of oncogenes on distinct cell types in the breast.
Transgenic (TG) activation of different oncogenic pathways in mouse mammary glands induces tumors with different gene expression profiles and histopathological features (1–8). The mouse mammary tumor virus (MMTV) promoter usually used to regulate these transgenes is expressed in a diverse population of mammary cells (9, 10). Therefore, cells at different developmental stages may undergo tumorigenesis as a result of the activation of different oncogenic pathways.
It has been difficult to define the precise lineage of target cells of breast cancer because of the lack of animal models expressing oncogenes in specific mammary progenitors. However, studies in the epidermis and the hematopoietic system have demonstrated that cancers that arise from stem or progenitor cells usually express markers of the originating cells (11, 12). Thus, the presence of stem or progenitor cell markers in mammary tumors may suggest that tumors arose from immature cells. Two genes, keratin 6 and stem cell antigen 1 (Sca-1), appear to be preferentially expressed in mammary stem and/or progenitor cells. Keratin 6 is expressed in mammary gland anlage at embryonic day 16.5 (ref. 13 and J.M.R., unpublished observations) and in some of the body cells in the terminal end buds (TEBs); but keratin 6 is not found in the highly proliferative cap cells (14, 15) and rarely in cells in the mature ducts and differentiated alveoli (15), consistent with the distribution of progenitor cells. In addition, keratin 6 is associated with the arrested state of differentiation observed in the mammary ducts from C/EBPβ-null mice (13). Sca-1, encoded by Ly-6A/E, is a GPI-linked protein also found in hematopoietic stem cells (16); it is expressed on the surface of a population of mammary cells enriched for stem cells and present in TEBs (17). Depletion of Sca-1-positive cells results in a loss of functional stem cells in mammary gland reconstitution experiments (17). Although retained in a population of more mature ductal cells, Sca-1 is not observed after cells have further differentiated to express the progesterone receptor (17).
Here we report that keratin 6 and Sca-1 cells are observed in mammary tumors induced by the Wnt-1 signaling pathway, which has been implicated in proliferation and maintenance of undifferentiated cells in several tissue types, including the mammary gland (18–28), but not in tumors induced by oncogenes affecting other pathways. In addition, we provide other evidence to suggest that ectopic activation of the Wnt pathway may target these undifferentiated mammary progenitors for tumorigenesis.
Materials and Methods
Mice. TG mice expressing MMTV-Wnt-1 (29), MMTV-β-catenin (30), MMTV-c-Myc (31), MMTV-Neu (32), MMTV-H-Ras (33), or MMTV-PyMT (34) have been described, as have mice harboring targeted inactivating mutations of Pten (35). MMTV-Wnt-1 TG mice were a mixture of FVB, SJL, and C57BL/6 strains; Pten mutant mice were a mixture of FVB/N, 129, and C57BL/6 strains; all others were in a pure FVB/N background.
Tissue Processing and Immunocytochemistry. Tissues were fixed and processed as described (36). Immunohistochemistry was performed by using Vector ABC and MOM kits (Vector Laboratories) according to the manufacturer's recommendations and as described (35). The following antibodies were used: purified rabbit antibodies against mouse keratin 6 (Covance, Princeton) and Pten (NeoMarkers, Fremont, CA); purified mouse monoclonal antibodies against α-smooth muscle actin (SMA, Dako) and against BrdUrd (BD Biosciences Immunocytometry Systems, product no. 347580); and partially purified rat antibodies against keratin 8 (37, 38), purchased from the Developmental Studies Hybridoma Bank, Iowa City, organized under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa. To label cells in S-phase of cell cycle, BrdUrd (Sigma, product no. B-5002) at 100 μg per gram of body weight in saline was injected i.p. 1 h before mice were killed.
Southern Hybridization and Western Analysis. Tumor DNAs were digested with PstI and processed for Southern blotting by using a [32P]dCTP-labeled probe made from a 0.9-kb template located in intron 5 of Pten as described (36). For Western blotting, tumors were ground to powder in liquid nitrogen and lysed by boiling in the sample-loading buffer for acrylamide gel electrophoresis. Protein lysates were resolved in 10% polyacrylamide gels containing SDS and transferred to nitrocellulose membranes. After incubating with rabbit antibodies against Pten (NeoMarkers, 1:1,000) or mouse monoclonal antibodies against γ-tubulin (Sigma, 1:5,000), the specific reaction was visualized by peroxidase-conjugated secondary antibodies (Roche) and a chemiluminescent substrate (SuperSignal, Pierce).
Flow Cytometry. Mammary cells and tumors were isolated by using collagenase as described (17). After incubating with FITC-conjugated rat IgG against Sca-1 (BD Pharmingen, product no. 553336, 106 cells per μg), fluorescence-activated cell sorting (FACS) was performed as described (17).
Results
Keratin 6 and Sca-1 Are Expressed in Preneoplastic and Neoplastic Mammary Lesions Induced by Components of the Wnt Signaling Pathway but Not by Neu, Ras, and PyMT. While carrying out experiments designed to identify genes differentially regulated in the evolution of mammary tumors in MMTV-Wnt-1 TG mice (S.H., unpublished work), we discovered that both keratin 6 and Sca-1 are more highly expressed in hyperplastic mammary glands and tumors from MMTV-Wnt-1 TG mice than in non-TG virgin mammary glands (Fig. 6, which is published as supporting information on the PNAS web site), suggesting that Wnt-1-induced mammary tumors may originate from progenitor cells. To confirm this observation, we used immunohistochemical staining to identify keratin 6-expressing cells. In non-TG mammary glands, keratin 6 was detected in some body cells within the terminal end buds (TEBs) and occasionally in mature luminal epithelial cells (Fig. 1 B and D), consistent with previous reports (14, 15). However, in MMTV-Wnt-1 TG mice, we found keratin 6 in a greater number of ductal cells, usually in enlarged ducts, in a heterogeneous pattern (Fig. 1 A and C). Many of the stained cells are in the luminal layer, but some of them have invaded into the lumen (Fig. 1 A). Ducts that were not stained were usually smaller in diameter and did not appear to be hyperplastic (Fig. 1C). Because transgenes regulated by MMTV are known to be expressed in heterogeneous patterns in the mammary gland (9, 39–42), the keratin 6-negative ducts may not express the MMTV-Wnt-1 transgene.
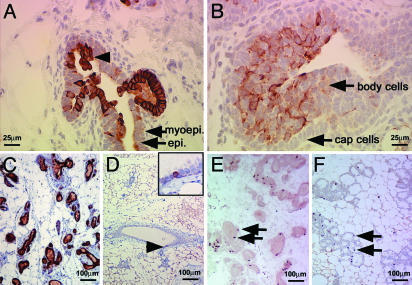
Fig. 1.
Keratin 6-positive cells are expanded in hyperplastic mammary glands from MMTV-Wnt-1 TG mice. Immunohistochemical staining was used to detect keratin 6 (A–D) or BrdUrd (E and F) in mammary gland sections from MMTV-Wnt-1 TG mice that were 3-week-old (A) or 3-month-old (C and E) virgins, from non-TG females that were 3 weeks old (B), 3 months old (D), or in mid-pregnancy (F). The hyperplastic keratin 6-positive cells projecting into the lumen are indicated by an arrowhead (A). The insert in D is a 4-fold higher view of the site denoted with an arrowhead. Staining for BrdUrd, as represented by arrows, demonstrates similar levels of proliferation in mammary glands from MMTV-Wnt-1 TG virgin (E) and wild-type mid-pregnancy (F) mice. Cell types are indicated by arrows in A and B; scale bars are shown in each panel.
The mammary cells in adult MMTV-Wnt-1 TG mice have a greater rate of proliferation than those in non-TG virgin mice, as measured by immunohistochemical staining for cells labeled with BrdUrd and for cells expressing Ki-67, another proliferation marker (data not shown). It is therefore possible that the expansion of keratin 6-positive cells in the mammary glands of MMTV-Wnt-1 TG mice may result from an increased proportion of proliferating cells. Indeed, keratin 6 has been associated with hyperproliferation in the suprabasal layer in the skin (43). To determine whether the increased expression of keratin 6 is an oncogene- or proliferation-induced effect in the mammary gland, we compared mammary glands from adult MMTV-Wnt-1 TG virgins with proliferating mammary glands from midpregnancy non-TG mice. Similar numbers of BrdUrd-stained cells (Fig. 1 E and F) and Ki-67-positive cells (data not shown) were detected in virgin TG and pregnant non-TG mammary glands. However, only a few cells per field were stained with anti-keratin 6 antibodies in the pregnant non-TG mice (data not shown). Taken together, these results suggest that aberrant Wnt-1 expression arrests differentiation of mammary cells at an early phase and stimulate their proliferation and that proliferation per se is not associated with expression of keratin 6.
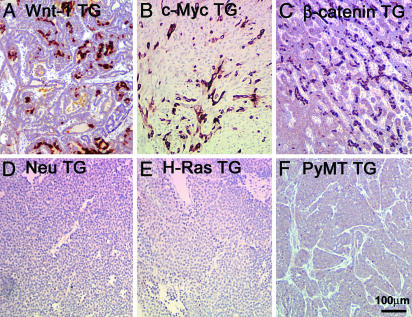
We also used immunohistochemistry to confirm the microarray results suggesting that keratin 6 is also expressed in mammary tumors from MMTV-Wnt-1 TG mice. Strong, although not uniform, staining for keratin 6 was observed in Wnt-1-induced tumors (Fig. 2A). To determine whether the observed expansion of keratin 6-positive cells is unique to the MMTV-Wnt-1 TG model, we stained sections of mammary glands and tumors from a number of other TG models. Interestingly, keratin 6-positive cells were abundant in both mammary gland and tumor sections from MMTV-β-catenin and MMTV-c-Myc TG mice, but keratin 6-positive cells were not detected in premalignant glands or tumors from MMTV-Neu, MMTV-H-Ras, or MMTV-PyMT TG mice (Fig. 2 and Fig. 7, which is published as supporting information on the PNAS web site), implying a transgenespecific effect on the expansion of keratin 6-positive cells with keratin 6 expressed in tumors induced by components of the Wnt signaling pathway. These results confirm that keratin 6 is not a by-product of proliferation, because the frequency of replicating cells is high in mammary tumors from all these TG mice, as evidenced by staining for Ki67 (data not shown).
Fig. 2.
Keratin 6-positive cells in mammary tumors arising in TG mice expressing components of the Wnt signaling pathway. Keratin 6 was detected by immunohistochemical staining of tumors from mice carrying the indicated MMTV TG. The scale is shown in F.
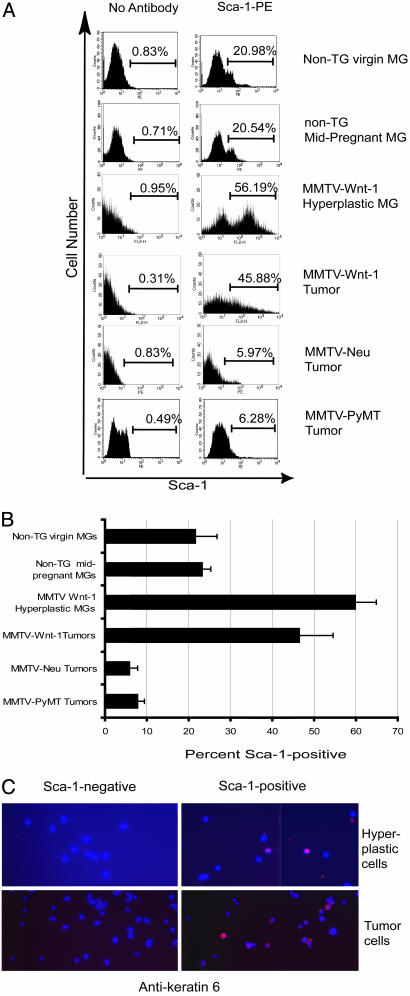
We used FACS to confirm and extend the microarray data in Fig. 6 showing that Sca-1, a second marker for progenitor cells, was expressed in Wnt-1-induced hyperplasias or tumors. About 50% of the mammary cells from the hyperplastic mammary glands and from mammary tumors from virgin MMTV-Wnt-1 TG mice were positive for Sca-1 (Fig. 3 A and B). In contrast, ≈20% of mammary cells from non-TG virgin or pregnant mice were positive, consistent with our earlier report that Sca-1 antibody labels progenitor cells, in addition to a subset of partially differentiated cells (17). Furthermore, <10% of cells from MMTV-Neu or MMTV-PyMT TG mice were positive. To determine whether keratin 6 and Sca-1 are expressed in the same cells, we stained mammary cells prepared from hyperplastic mammary glands and tumors from MMTV-Wnt-1 TG mice with anti-Sca-1 antibodies, separated Sca-1-positive and Sca-1-negative cells by FACS, and stained them with antibodies against keratin 6 (Fig. 3C). Approximately 10% of Sca-1-positive cells were positive for keratin 6 (data not shown), whereas keratin 6 was not detected in Sca-1-negative cells.
Fig. 3.
Increased proportions of Sca-1-positive cells in hyperplastic mammary glands and mammary tumors from MMTV-Wnt-1 TG mice. Representative FACS histograms (A) and a summary bar graph (B) representing data from three to five independent FACS experiments are shown for mammary glands from wild-type virgin and mid-pregnancy mice, hyperplastic mammary glands from MMTV-Wnt-1 TG mice, and mammary tumors from TG mice carrying MMTV-Wnt-1, MMTV-Neu, or MMTV-PyMT transgenes. Hyperplastic cells or tumor cells prepared from MMTV-Wnt-1 TG mice were stained with anti-Sca-1-FITC, sorted by FACS, and stained for keratin 6 (C). Red indicates positive staining for keratin 6. Images were captured by using a ×40 objective.
Myoepithelial and Luminal Epithelial Tumor Cells Coexist in MMTV-Wnt-1 TG Mice but Not in Neu, H-Ras, or PyMT TG Mice. Tumors arising from stem or progenitor cells may show mixed lineage differentiation (11). To investigate whether tumors from MMTV-Wnt-1 TG mice may contain transformed myoepithelial cells (the normal type of which constitutes the outer layer of normal mammary ducts) in addition to transformed luminal epithelial cells (which comprise the majority of human and transgene-induced murine mammary carcinomas), we stained their sections for both keratin 8, a marker for luminal epithelial cells (44), and α-SMA, which is expressed in myoepithelial cells and pericytes in non-TG mammary glands (ref. 45 and data not shown). The number of α-SMA-positive cells was high, approximately equal to the number of keratin 8-positive cells (Fig. 4). Keratin 14, another marker for myoepithelial cells (46), was also found in the α-SMA-positive subset of cells (data not shown). These results suggest that the Wnt-1-induced tumors are composed of two predominant cellular components, luminal epithelial and myoepithelial cells, consistent with the findings of Rosner et al. (2) and Cui and Donehower (49).
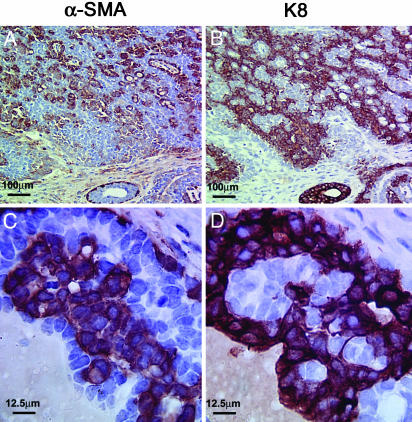
Fig. 4.
Abnormal myoepithelial cells in mammary tumors from MMTV-Wnt-1 TG mice. Consecutive paraffin sections of a mammary tumor from an MMTV-Wnt-1 TG mouse were stained for α-SMA and keratin 8 as indicated. Note the large nuclei and disorganization of the α-SMA-positive cells in the tumor (C). The scales are shown in each panel.
Because the α-SMA-positive cells are pleomorphic, hyperproliferative, disorganized, and present in large quantities (Fig. 4 A and C), they are probably neoplastic cells rather then benign cells recruited to the tumor mass. The presence of myoepithelial cells was also observed in mammary tumors from MMTV-β-catenin or MMTV-c-Myc TG mice, but not in those from MMTV-Neu, MMTV-H-ras, or MMTV-PyMT TG mice (Fig. 8, which is published as supporting information on the PNAS web site), similar to the report by Rosner et al. (2). However, in contrast to the plump abnormal myoepithelial cells detected in tumors from MMTV-Wnt-1 TG mice, the myoepithelial cells in β-catenin- and c-Myc-induced tumors were more elongated and well organized, forming a single layer around the keratin 8-positive tumor cells, as in normal ducts.
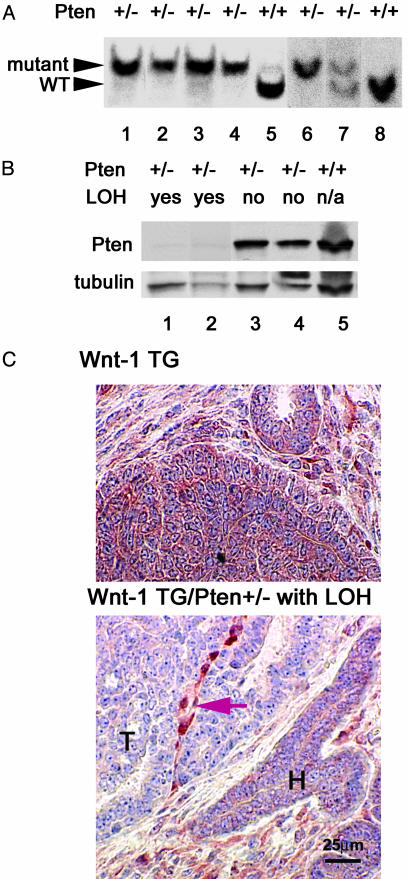
Loss of Pten Occurs in Both Luminal Epithelial and Myoepithelial Cells in Wnt-1-Induced Mammary Tumors. In previous studies, we found that the wild-type allele of Pten was undetectable in the majority of Wnt-1-induced mammary tumors arising in a Pten heterozygous background (36); an expanded set of samples is shown in Fig. 5A. Because such tumors, like Wnt-1-induced tumors in a wild-type Pten background, are composed approximately equally of luminal epithelial and myoepithelial cells (Fig. 8), loss of heterozygosity (LOH) must have occurred in both cell types.
Fig. 5.
Loss of Pten heterozygosity in both luminal epithelial and myoepithelial cells of mammary tumors from Pten+/-, MMTV-Wnt-1 TG mice. (A) Southern blotting analysis for LOH at the Pten locus in mammary tumors from MMTV-Wnt-1 TG mice; the Pten genotypes are indicated above the panel. Fragments of the wild-type (WT) and target mutant alleles of Pten are indicated by arrowheads. The image is a composite of two independent blots (lanes 1–5 and lanes 6–8). (B) Western blotting analysis for Pten in MMTV-Wnt-1-derived mammary tumors; the Pten genotype and LOH status are indicated above the panel. The image of the same blot after a subsequent incubation with antibodies against γ-tubulin is also shown to indicate variations in amounts of protein loaded. The faint signal in the samples with evidence of LOH is most likely due to protein from the stroma. (C) Immunohistochemical staining for Pten in mammary tumors; the genotype is indicated above each panel. Areas of tumor (T) and hyperplastic (H) ducts are indicated. The string of positively stained cells (indicated by an arrow) probably represents stroma recruited into the tumor. The scale is shown in Lower.
We confirmed the absence of Pten protein by both Western blotting and immunohistochemical staining, using a purified rabbit antibody specific for Pten (Fig. 5 B and C). By Western blotting, Pten was detected in tumors from Pten-wild-type, MMTV-Wnt-1 TG mice and in tumors from MMTV-Wnt-1 TG/Pten+/- mice without LOH; but Pten was almost undetectable in mammary tumors that were MMTV-Wnt-1 TG/Pten+/- with LOH at the Pten locus (Fig. 5B). In accord with the results of the Western blotting, by immunohistochemical staining, Pten was undetectable in both luminal epithelial and myoepithelial tumor cells in MMTV-Wnt-1 TG/Pten+/- mammary glands that had undergone LOH (Fig. 5C Lower and ref. 36). On the other hand, Pten was readily detected in stromal cells within and outside of the tumor and in hyperplastic ducts surrounding the tumor. Pten was also detected in most or all cells in tumors from Wnt-1 TG/Pten+/+ mice (Fig. 5C Upper). Together, these results strongly suggest that the loss of Pten occurs in both luminal epithelial and myoepithelial cells of tumors arising in MMTV-Wnt-1 TG/Pten+/- mice. Because it is unlikely that the two cell types sustained independent mutations, a single event most likely occurred in a common progenitor to these cells.
Discussion
In this study, we present several lines of evidence to support a role for mammary progenitor cells in mammary tumorigenesis induced by Wnt signaling. First, many keratin 6-positve cells are present in both preneoplasias (Fig. 1) and tumors (Fig. 2) from MMTV-Wnt-1 TG mice. Second, keratin 6-positive hyperplastic and tumor cells in these mice also express Sca-1 (Fig. 3C). Third, luminal epithelial and myoepithelial cells coexist in these tumors, suggesting transformation of a common precursor. Finally, in some mammary tumors arising in Pten heterozygous, MMTV-Wnt-1 TG mice, the wild-type Pten locus is missing in both luminal epithelial and myoepithelial cells (Fig. 5), suggesting that loss of Pten occurred in a common precursor to these two populations of tumor cells. Efforts are underway to attempt to define a subset of tumor cells from MMTV-Wnt-1 TG mice capable of cancer regeneration, in light of a recent report (47) that only a small number of human breast cancer cells have the capacity to regenerate tumors in immunodeficient mice.
Mammary tumors from MMTV-β-catenin and MMTV-c-Myc mice, which express components of the Wnt signaling pathway, are similar to tumors from MMTV-Wnt-1 TG mice. Collectively, our data suggest that deregulated Wnt signaling causes excess proliferation of mammary progenitor cells and predisposes them to cancer. This interpretation is consistent with reports that loss of Wnt signaling blocks early mammary development (25, 26), and it is also consistent with the role of Wnt signaling in other tissues. Wnt signaling is now known to play a critical role in the proliferation of hematopoietic stem cells and those in the skin, colon, and other organs (18–28), and deregulated activation of this pathway is a cancer-predisposing factor in several tissues including the colon, skin, and liver (19, 48).
Although progenitor cells are the likely precursors to cancer in mammary glands that have activated the Wnt signaling pathway, keratin 6 and/or Sca-1 are not present in mammary hyperplasias and tumors from several other TG lines, including MMTV-Neu, MMTV-H-Ras, and MMTV-PyMT TG mice (Figs. 2 and 7). In addition, a detectable second tumor cell type, such as myoepithelial tumor cells, is absent in these tumors (Fig. 8). These results are consistent with previous reports that initiating genetic lesions exert a significant influence on both gene expression patterns and histological features of mammary tumors from both humans and TG animals (1–8). However, our findings also suggest that the differentiation status of putative target cells may contribute to the heterogeneity of breast cancer. There are several possible mechanisms by which target cells may influence the histopathology and molecular features of tumors resulting from different oncogenes. Transgenes encoding Neu, H-Ras, and PyMT may transform progenitor cells, but either fail to arrest them at the progenitor stage or actively induce differentiation of the transformed cells. Alternatively, Neu, H-Ras, and PyMT may be able to transform only more differentiated cells that no longer express keratin 6 or Sca-1. In addition, there is a remote possibility that different oncogene RNAs expressed from MMTV are differentially translated in progenitor and differentiated cells. Finally, we cannot rule out the possibility that all of these oncogenes transform differentiated cells, but Wnt-1 signaling leads to dedifferentiation; however, the loss of Pten in both myoepithelial and luminal epithelial cells argues strongly against this possibility.
Although it remains to be determined whether the Wnt-1 signaling pathway and the oncogenic pathways mediated by Neu, H-Ras, and PyMT induce tumors in mammary cells at precisely the same stage of differentiation, these transgenes are presumably expressed in similar populations of mammary cells, because they are all under the control of the MMTV LTR. In accord with this assumption, interbreeding of MMTV-c-Myc and MMTV-H-Ras led to more rapid formation of mammary tumors (33); but differentiation markers have not been studied in these tumors to ask whether the H-Ras- or c-Myc-induced phenotype predominates. To ask this question in a different context, we have recently bred MMTV-Wnt-1 with MMTV-Neu TG mice. The resulting bi-TG females develop mammary tumors as early as 12 weeks of age, sooner than in mice carrying either transgene alone, implying that both transgenes were expressed in these tumors (K.P. and Y.L., unpublished work). Interestingly, neoplastic cells in these tumors are positive for keratin 6, Sca-1, and myoepithelial markers, and they are histopathologically more similar to Wnt-1-induced tumors than to Neu-induced tumors (K.P. and Y.L., unpublished work). Therefore, it appears that Neu does not relieve the arrest of differentiation that may be imposed by Wnt-1 signaling in mammary progenitor cells.
In conclusion, we provide several lines of evidence to suggest that components of the Wnt signaling pathway transform mammary progenitors, and that these cells develop into heterogeneous tumors containing different histological cell types expressing markers of both mature and immature epithelial cells. Thus, breast cancer heterogeneity may result from transformation of distinct cell types by different oncogenes.
Supplementary Material
Acknowledgments
We thank Jennifer Doherty and Mary Barrett for excellent technical assistance, Jose Vargas for animal care, Eric Holland and Gilbert Smith for stimulating discussions, and William Pao, Brian Lewis, and Gary Chamness for critical reading of the manuscript. Y.L. and S.H. were supported by Department of Defense Breast Cancer Research Program awards, K.P. was supported by a Cancer Research Institute award, B.W. was supported by funds from the National Institutes of Health (Grants CA057621 and ES07106) to Z.W., and M.E. was supported by the Danish Cancer Society. This work was supported in part by National Institutes of Health Grants U01-CA842243 (to J.M.R.) and P01 CA94060-02 (to H.E.V.) and funds from the Martell Foundation (to H.E.V.).
Abbreviations: FACS, fluorescence-activated cell sorting; LOH, loss of heterozygosity; MMTV, mouse mammary tumor virus; SMA, smooth muscle actin; TEB, terminal end bud; TG, transgenic.
References
- 1.Morrison, B. W. & Leder, P. (1994) Oncogene 9, 3417-3426. [PubMed] [Google Scholar]
- 2.Rosner, A., Miyoshi, K., Landesman-Bollag, E., Xu, X., Seldin, D. C., Moser, A. R., MacLeod, C. L., Shyamala, G., Gillgrass, A. E. & Cardiff, R. D. (2002) Am. J. Pathol. 161, 1087-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardiff, R. D., Anver, M. R., Gusterson, B. A., Hennighausen, L., Jensen, R. A., Merino, M. J., Rehm, S., Russo, J., Tavassoli, F. A., Wakefield, L. M., et al. (2000) Oncogene 19, 968-988. [DOI] [PubMed] [Google Scholar]
- 4.Cardiff, R. D., Sinn, E., Muller, W. & Leder, P. (1991) Am. J. Pathol. 139, 495-501. [PMC free article] [PubMed] [Google Scholar]
- 5.Hedenfalk, I., Duggan, D., Chen, Y., Radmacher, M., Bittner, M., Simon, R., Meltzer, P., Gusterson, B., Esteller, M., Kallioniemi, O. P., et al. (2001) N. Engl. J. Med. 344, 539-548. [DOI] [PubMed] [Google Scholar]
- 6.Desai, K. V., Xiao, N., Wang, W., Gangi, L., Greene, J., Powell, J. I., Dickson, R., Furth, P., Hunter, K., Kucherlapati, R., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 6967-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perou, C. M., Sorlie, T., Eisen, M. B., van de Rijn, M., Jeffrey, S. S., Rees, C. A., Pollack, J. R., Ross, D. T., Johnsen, H., Akslen, L. A., et al. (2000) Nature 406, 747-752. [DOI] [PubMed] [Google Scholar]
- 8.Sorlie, T., Perou, C. M., Tibshirani, R., Aas, T., Geisler, S., Johnsen, H., Hastie, T., Eisen, M. B., van de Rijn, M., Jeffrey, S. S., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 10869-10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner, K. U., McAllister, K., Ward, T., Davis, B., Wiseman, R. & Hennighausen, L. (2001) Transgenic Res. 10, 545-553. [DOI] [PubMed] [Google Scholar]
- 10.Gunther, E. J., Belka, G. K., Wertheim, G. B., Wang, J., Hartman, J. L., Boxer, R. B. & Chodosh, L. A. (2002) FASEB J. 16, 283-292. [DOI] [PubMed] [Google Scholar]
- 11.Owens, D. M. & Watt, F. M. (2003) Nat. Rev. Cancer 3, 444-451. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Losada, J. & Balmain, A. (2003) Nat. Rev. Cancer 3, 434-443. [DOI] [PubMed] [Google Scholar]
- 13.Grimm, S. L., Seagroves, T. N., Kabotyanski, E. B., Hovey, R. C., Vonderhaar, B. K., Lydon, P., Miyoshi, K., Hennighausen, L., Ormandy, C. J., Lee, A. V., et al. (2002) Mol. Endocrinol. 16, 2675-2691. [DOI] [PubMed] [Google Scholar]
- 14.Sapino, A., Macri, L., Gugliotta, P., Pacchioni, D., Liu, Y. J., Medina, D. & Bussolati, G. (1993) Differentiation 55, 13-18. [DOI] [PubMed] [Google Scholar]
- 15.Smith, G. H., Mehrel, T. & Roop, D. R. (1990) Cell Growth Differ. 1, 161-170. [PubMed] [Google Scholar]
- 16.Spangrude, G. J., Heimfeld, S. & Weissman, I. L. (1988) Science 241, 58-62. [DOI] [PubMed] [Google Scholar]
- 17.Welm, B. E., Tepera, S. B., Venezia, T., Graubert, T. A., Rosen, J. M. & Goodell, M. A. (2002) Dev. Biol. 245, 42-56. [DOI] [PubMed] [Google Scholar]
- 18.Dontu, G., Abdallah, W. M., Foley, J. M., Jackson, K. W., Clarke, M. F., Kawamura, M. J. & Wicha, M. S. (2003) Genes Dev. 17, 1253-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso, L. & Fuchs, E. (2003) Genes Dev. 17, 1189-1200. [DOI] [PubMed] [Google Scholar]
- 20.Reya, T., Duncan, A. W., Ailles, L., Domen, J., Scherer, D. C., Willert, K., Hintz, L., Nusse, R. & Weissman, I. L. (2003) Nature 423, 409-414. [DOI] [PubMed] [Google Scholar]
- 21.van de Wetering, M., Sancho, E., Verweij, C., de Lau, W., Oving, I., Hurlstone, A., van der Horn, K., Batlle, E., Coudreuse, D., Haramis, A. P., et al. (2002) Cell 111, 241-250. [DOI] [PubMed] [Google Scholar]
- 22.Kielman, M. F., Rindapaa, M., Gaspar, C., van Poppel, N., Breukel, C., van Leeuwen, S., Taketo, M. M., Roberts, S., Smits, R. & Fodde, R. (2002) Nat. Genet. 32, 594-605. [DOI] [PubMed] [Google Scholar]
- 23.Polesskaya, A., Seale, P. & Rudnicki, M. A. (2003) Cell 113, 841-852. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita, Y. M., Jones, D. L. & Fuller, M. T. (2003) Science 301, 1547-1550. [DOI] [PubMed] [Google Scholar]
- 25.Andl, T., Reddy, S. T., Gaddapara, T. & Millar, S. E. (2002) Dev. Cell 2, 643-653. [DOI] [PubMed] [Google Scholar]
- 26.van Genderen, C., Okamura, R. M., Farinas, I., Quo, R. G., Parslow, T. G., Bruhn, L. & Grosschedl, R. (1994) Genes Dev. 8, 2691-2703. [DOI] [PubMed] [Google Scholar]
- 27.Willert, K., Brown, J. D., Danenberg, E., Duncan, A. W., Weissman, I. L., Reya, T., Yates, J. R., III, & Nusse, R. (2003) Nature 423, 448-452. [DOI] [PubMed] [Google Scholar]
- 28.Murdoch, B., Chadwick, K., Martin, M., Shojaei, F., Shah, K. V., Gallacher, L., Moon, R. T. & Bhatia, M. (2003) Proc. Natl. Acad. Sci. USA 100, 3422-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsukamoto, A. S., Grosschedl, R., Guzman, R. C., Parslow, T. & Varmus, H. E. (1988) Cell 55, 619-625. [DOI] [PubMed] [Google Scholar]
- 30.Imbert, A., Eelkema, R., Jordan, S., Feiner, H. & Cowin, P. (2001) J. Cell Biol. 153, 555-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart, T. A., Pattengale, P. K. & Leder, P. (1984) Cell 38, 627-637. [DOI] [PubMed] [Google Scholar]
- 32.Guy, C. T., Webster, M. A., Schaller, M., Parsons, T. J., Cardiff, R. D. & Muller, W. J. (1992) Proc. Natl. Acad. Sci. USA 89, 10578-10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinn, E., Muller, W., Pattengale, P., Tepler, I., Wallace, R. & Leder, P. (1987) Cell 49, 465-475. [DOI] [PubMed] [Google Scholar]
- 34.Guy, C. T., Cardiff, R. D. & Muller, W. J. (1992) Mol. Cell. Biol. 12, 954-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Podsypanina, K., Ellenson, L. H., Nemes, A., Gu, J., Tamura, M., Yamada, K. M., Cordon-Cardo, C., Catoretti, G., Fisher, P. E. & Parsons, R. (1999) Proc. Natl. Acad. Sci. USA 96, 1563-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, Y., Podsypanina, K., Liu, X., Crane, A., Tan, L. K., Parsons, R. & Varmus, H. E. (2001) BMC Mol. Biol. 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brulet, P., Babinet, C., Kemler, R. & Jacob, F. (1980) Proc. Natl. Acad. Sci. USA 77, 4113-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemler, R., Brulet, P., Schnebelen, M. T., Gaillard, J. & Jacob, F. (1981) J. Embryol. Exp. Morphol. 64, 45-60. [PubMed] [Google Scholar]
- 39.Hennighausen, L., Wall, R. J., Tillmann, U., Li, M. & Furth, P. A. (1995) J. Cell. Biochem. 59, 463-472. [DOI] [PubMed] [Google Scholar]
- 40.Hennighausen, L., McKnight, R., Burdon, T., Baik, M., Wall, R. J. & Smith, G. H. (1994) Cell Growth Differ. 5, 607-613. [PubMed] [Google Scholar]
- 41.Stocklin, E., Botteri, F. & Groner, B. (1993) J. Cell Biol. 122, 199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller, W. J., Sinn, E., Pattengale, P. K., Wallace, R. & Leder, P. (1988) Cell 54, 105-115. [DOI] [PubMed] [Google Scholar]
- 43.Moll, R., Franke, W. W., Schiller, D. L., Geiger, B. & Krepler, R. (1982) Cell 31, 11-24. [DOI] [PubMed] [Google Scholar]
- 44.Guelstein, V. I., Tchypysheva, T. A., Ermilova, V. D., Litvinova, L. V., Troyanovsky, S. M. & Bannikov, G. A. (1988) Int. J. Cancer 42, 147-153. [DOI] [PubMed] [Google Scholar]
- 45.Lazard, D., Sastre, X., Frid, M. G., Glukhova, M. A., Thiery, J. P. & Koteliansky, V. E. (1993) Proc. Natl. Acad. Sci. USA 90, 999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wetzels, R. H., Holland, R., van Haelst, U. J., Lane, E. B., Leigh, I. M. & Ramaekers, F. C. (1989) Am. J. Pathol. 134, 571-579. [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. (2003) Proc. Natl. Acad. Sci. USA 100, 3983-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bienz, M. & Clevers, H. (2000) Cell 103, 311-320. [DOI] [PubMed] [Google Scholar]
- 49.Cui, X. S. & Donehower, L. A. (2000) Oncogene 19, 5988-5996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.