Abstract
Hepatitis C virus (HCV) RNA replication depends on viral protein association with intracellular membranes, but the influence of membrane composition on viral replication is unclear. We report that HCV RNA replication and assembly of the viral replication complex require geranylgeranylation of one or more host proteins. In cultured hepatoma cells, HCV RNA replication was disrupted by treatment with lovastatin, an inhibitor of 3-hydroxy-3-methyglutaryl CoA reductase, or with an inhibitor of protein geranylgeranyl transferase I, each of which induced the dissolution of the HCV replication complex. Viral replication was not affected by treatment of cells with an inhibitor of farnesyl transferase. When added to lovastatin-treated cells, geranylgeraniol, but not farnesol, restored replication complex assembly and viral replication. Inasmuch as the HCV genome does not encode a canonical geranylgeranylated protein, the data suggest the involvement of a geranylgeranylated host protein in HCV replication. Inhibition of its geranylgeranylation affords a therapeutic strategy for treatment of HCV infection.
Approximately 170 million people worldwide are persistently infected with hepatitis C virus (HCV), and these individuals account for a majority of all cases of chronic liver disease (1). The public health impact of HCV is compounded by the overall low response rate to current IFN-based therapies for treating HCV infection, underscoring the need for new therapeutic strategies to combat the HCV pandemic. HCV is a single-stranded positive sense RNA virus and member of the Flaviviridae (2). The 9.6-kb HCV genome encodes a single polyprotein that is posttranslationally processed into at least 10 individual structural and nonstructural (NS) viral proteins, the latter of which are sufficient to support HCV RNA replication (3). Current studies support a model in which HCV infection results in assembly of the viral RNA and NS proteins into a replication complex that associates with the host cell endoplasmic reticulum (ER). Viral-directed processes convert the ER into a membranous web conducive to virus replication (4–6). The cellular cofactors and membrane constituents that contribute to assembly and maintenance of the HCV replication complex are not known.
Cell membrane composition is subject to modification through the mevalonate pathway, which produces cholesterol and nonsterol isoprenoid products (7). Two of the mevalonate-derived isoprenoids, farnesyl (15 carbons) and geranylgeranyl (20 carbons), are attached to membrane proteins via formation of a cysteine thioether (7, 8). This process, called protein prenylation, targets certain proteins to cell membranes where they regulate many cellular functions, ranging from vesicle budding and fusion to growth. Therapeutic control of the mevalonate pathway has proven effective for the clinical treatment of hypercholesterolemia and is achieved in part through the use of statin compounds (7, 9). Statins block mevalonate production by inhibiting 3-hydroxy-3-methylglutaryl CoA reductase (HMG CoA reductase), resulting in a block in the subsequent steps of cholesterol synthesis (7, 9). At the high concentrations that are attainable in tissue culture cells, statins deplete mevalonate sufficiently to lower the cellular pools of farnesyl and geranylgeranyl pyrophosphates, which are the donors in the protein prenylation reactions (7, 10).
The potential of statins to alter the sterol and protein composition of cellular membranes provides a unique tool to assess the role of these constituents in supporting HCV RNA replication. Because native HCV cannot be efficiently propagated in cultured cells (2), genome-length and subgenomic HCV RNA replicons have been developed to facilitate the study of viral replication. These HCV RNA replicon systems encompass either the entire HCV genome or only the NS3–NS5B protein coding region within a neomycin (G418)-selectable, bicistronic RNA. When introduced into human hepatoma (Huh7) cells, the HCV replicon RNA replicates autonomously on ER membranes (3). Replication is driven by the native 5′ and 3′ UTRs of the virus, thereby approximating normal HCV replication.
In the current study, we used Huh7 cell lines that harbor genome-length replicons (Huh7-C5B3 cells) or subgenomic replicons (Huh7-K2040 and Huh7-HP cells) (11, 12) to examine the influence of lovastatin, the first clinically approved statin inhibitor of HMG CoA reductase, on HCV RNA replication. We show that lovastatin inhibits replicon replication and that this effect is reversed by supplying geranylgeraniol, a donor of prenyl groups. This inhibitory effect is mimicked by an inhibitor of a protein geranylgeranyl transferase, strongly suggesting that HCV replication requires geranylgeranylation of one or more cellular proteins.
Materials and Methods
Reagents used in this study and certain methods (measurement of DNA, mRNA, and protein synthesis and immunofluorescence analysis) may be found in Supporting Methods, which is published as supporting information on the PNAS web site.
HCV RNA Replicons and Cell Culture. The genomic and subgenomic HCV RNA replicons (both are HCV genotype 1b) are comprised of the viral 5′ nontranslated region and internal ribosome entry site (IRES) driving expression of the neo gene, followed by the encephalomyocarditis virus IRES and either the complete HCV-N strain ORF (genome-length RNA) or the Con1 strain NS3–NS5B coding region (subgenomic replicon) and the authentic HCV 3′ nontranslated region (3, 13). Huh7-C5B3 cells and Huh7-HP cells harbor cell culture-adapted genomic and subgenomic HCV replicon RNAs, respectively (11, 12). The K2040 subgenomic HCV replicon RNA was derived from pACYC HCV 1bpt (12) by inserting a K residue within the NS5A coding region of the HCV polyprotein at amino acid position 2040 via the QuikChange XL Site-Directed Mutagenesis kit (Stratagene) as recommended by the manufacturer. Mutagenic primer sequences were 5′-CAC CGG ACA TGT GAA AAA AAA CGG TTC CAT GAG GAT CGT GG-3′ (sense) and 5′-CCA CGA TCC TCA TGG AAC CGT TTT TTT TCA CAT GTC CGG TG-3′ (antisense). The sequence of the resulting plasmid DNA and replicon RNA-derived cDNA was verified by the University of Texas Southwestern Medical Center DNA sequencing core facility.
To prepare stable cell lines harboring the K2040 HCV RNA replicon, we prepared RNA in vitro from the T7 promoter encoded within the mutated pACYC HCV 1bpt construct as described (12). The purified replicon RNA was transfected into Huh7 cells (obtained from Stephen Polyak, University of Washington, Seattle) by using the TransMessenger reagent (Qiagen, Valencia, CA), and clonal replicon-bearing cell lines were selected by culturing cells in the presence of 400 μg/ml G418. Stable clonal cell lines were isolated exactly as described (12). We confirmed that the selected cells harbored replicon RNA rather than integrated plasmid cDNA, and we characterized the viral RNA abundance, replication efficiency, viral protein expression, and growth rates of the resulting replicon cell lines. The complete nucleotide and deduced amino acid sequence of each respective replicon RNA was determined by sequencing the products of RT-PCR reactions carried out as described (12). We have previously determined that the K2040 mutation provides cell culture adaptation and high replication efficiency to HCV RNA (14, 15). All cells were maintained in DMEM with 4.5 g/liter glucose, 2 mM l-glutamine, 1 mM sodium pyruvate, antibiotic–antimycotic solution (Sigma), and nonessential amino acids supplemented with 10% FCS (DME). Cultures of HCV replicon cells were maintained in DME supplemented with 200 μg/ml G418. Before each experiment, cells were dispensed in the appropriate culture dish and cultured for 24 h in medium A (DMEM with 4.5 g/liter glucose, 2 mM l-glutamine, 1 mM sodium pyruvate, antibiotic–antimycotic solution, nonessential amino acids, and 5% FCS). All culture treatments were conducted in medium A supplemented with various reagents as indicated in each figure legend.
Transfection. Huh7-K2040 cells (4 × 105) were seeded into 60-mm dishes. After 16 h the cells were transfected with plasmids by using the FuGENE 6 transfection reagent exactly as described (12).
Immunoblot Analysis. For protein expression analysis, cells were harvested and suspended in lysis buffer [25 mM Tris·HCl, pH 7.5/150 mM NaCl/1 mM sodium EDTA/1% (vol/vol) Triton X-100/1 mM phenylmethanesulfonyl fluoride/1 mM okadaic acid/10 units/ml aprotinin/10 μl/ml protease inhibitor mixture (Sigma)] exactly as described (14, 15). The protein concentration of each extract was determined by Bio-Rad protein assay reagent according to the manufacturer's instructions. Aliquots of extract (20 μg of protein) were subjected to 12.5% SDS/PAGE and then transferred to polyvinylidene fluoride membranes. The membranes were probed with a previously characterized human HCV patient serum (1:1,000 dilution) that recognizes HCV nonstructural protein NS3, NS4B, and NS5A (15), followed by a 1:5,000 dilution of peroxidase-conjugated goat anti-human IgG, or with a 1:1,000 dilution of monoclonal anti-NS3 antibody followed by a 1:5,000 dilution of peroxidase-conjugated donkey anti-mouse IgG, or with a 1:2,000 dilution of goat anti-human actin serum (Santa Cruz Biotechnology) followed by a 1:5,000 dilution of peroxidase-conjugated donkey anti-goat IgG. Protein bands were visualized by ECL Plus chemiluminescence reagent (Amersham Biosciences) and exposed to Kodak X-Omat Blue XB-1 film.
Quantitative Real-Time PCR. Measurement of HCV RNA levels was carried out exactly as described (16). Total RNA was prepared from cells by using the RNA STAT-60 kit (Tel-Test, Friendswood, TX) and was treated extensively with DNase I (DNA-free, Ambion, Austin, TX). First-strand cDNA was synthesized from the DNA-free RNA by using random hexamer primers and the ABI cDNA synthesis kit (Applied Biosystems). cDNA was mixed with SYBR Green PCR Master Mix (Applied Biosystems) and sets of forward and reverse primers specific for HCV genomic RNA or human 36B4 mRNA and subjected to real-time quantitative PCR with the ABI PRISM 7900HT sequence detection system (Applied Biosystems). All reactions were performed in triplicate. The relative amounts of RNAs were calculated through the comparative cycle threshold (CT) method by using human 36B4 mRNA as the invariant control (16).
Results
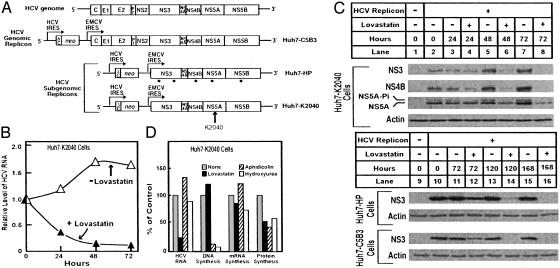
Fig. 1A shows a schematic representation of the HCV genome and the three replicons (genomic and subgenomic) used in this study. Replication of all three of these replicons is driven by the native NS proteins and the 5′ and 3′ UTRs of the virus. The viral polyprotein is translated from an encephalomyocarditis virus IRES inserted into the RNA sequence. Subgenomic replicon Huh7-HP contains at least five adaptive (point) mutations within the viral NS coding region (R.S. and M.G., unpublished observations). Subgenomic replicon Huh7-K2040 contains a lysine (K) residue inserted at amino acid position 2040 in the NS5A coding region. These mutations allow efficient replication of the HCV genome in Huh7 cells (12, 15).
Fig. 1.
Effects of lovastatin on HCV RNA and protein abundance. (A) The HCV genome and its genomic and subgenomic HCV RNA replicons. The polyprotein cleavage products and 5′ and 3′ nontranslated regions are indicated. The bicistronic replicon genomes encode a fusion protein consisting of the first 12 aa of the HCV core protein (ΔC) fused to the amino terminus of the neomycin phosphotransferase gene (neo), the translation of which is directed by the HCV IRES. Translation of the HCV polyprotein is directed from the second cistron by the encephalomyocarditis virus (EMCV) IRES. Representative Huh7 cell lines harboring the genomic and subgenomic HCV RNA replicons are shown at the right. Sites of adaptive (point) mutations in the Huh7-HP replicon are denoted by dots; the site of the lysine (K) insertion in the Huh7-K2040 replicon is denoted by the vertical arrow. (B and C) Huh7-K2040 (4 × 105), Huh7-HP (2 × 105), or Huh7-C5B3 (2 × 105) cells were cultured in 60-mm dishes. At time 0, the culture medium was replaced with medium containing 5% FCS (medium A) or medium A containing 50 μM lovastatin, and cultures were further incubated for the indicated time at which point cells were harvested for analysis of RNA and protein. (B) Total RNA from Huh7-K2040 cells cultured without (▵) or with (▴) lovastatin for the indicated time were subjected to real-time RT-PCR for quantification of HCV RNA. The relative level of HCV RNA at each time point is presented as a proportion of the time 0 level, which was assigned a value of 1.0. (C) Immunoblot analysis of HCV NS3, NS4B, and NS5A protein abundance in Huh7-K2040 cells (lanes 1–8) or HCV NS3 protein abundance in Huh7-C5B3 and Huh7-HP cells (lanes 9–16). Lanes 1 and 9 show protein levels in control Huh7 cells; the rest of the lanes show protein levels in cells harboring the indicated HCV replicon RNA cultured in the absence (-) or presence (+) of lovastatin for the indicated time. NS3, NS4B, and NS5A in Huh7-K2040 cells, NS3 in Huh7-HP and Huh7-C5B3 cells, and actin were detected by immunoblot analysis with a human HCV patient serum (1:1,000 dilution), monoclonal anti-NS3 antibody (1:1,000 dilution), and anti-human actin serum (1:2,000 dilution), respectively. The positions of the basal phosphorylated (NS5A) and hyperphosphorylated (NS5A-Pi) isoforms (2) are indicated. (D) Huh7-K2040 cells (2 × 105) were cultured in 37-mm culture dishes. At time 0, the medium was replaced with medium A alone (gray bars) or with medium A containing 50 μM lovastatin (filled bars), 100 μM aphidicolin (hatched bars), or 10 mM hydroxyurea (open bars). After 24 h, one culture set was harvested and the relative level of HCV RNA was analyzed by quantitative real-time RT-PCR. Cells in the remaining cultures were pulse labeled with [3H]thymidine, [3H]uridine, or [35S]methionine for the respective analysis of DNA, mRNA, or protein synthesis as described in Material and Methods. For each analysis, the results are normalized to the value obtained from the medium A control culture, which was set as 100%. The 100% control values for DNA, RNA, and protein synthesis were 174, 482, and 21,100 cpm/mg protein, respectively. Each value is the mean of three replicate incubations.
In the absence of lovastatin, HCV RNA replication was robust, and viral RNA and protein levels increased concomitantly with the culture density of Huh7-K2040 cells (Figs. 1 B and C). Lovastatin treatment of Huh7-K2040 cell cultures reduced RNA levels >70% after a 24-h treatment and >95% after 72 h (Fig. 1B). The lovastatin-induced decline in HCV RNA abundance was first apparent between 12 and 24 h posttreatment (data not shown). The drop in viral RNA abundance was accompanied by a reduction in viral protein levels over a 72-h culture period (Fig. 1C). A similar reduction in viral RNA was observed in lovastatin-treated Huh7-C5B3 and Huh7-HP cells (data not shown), indicating that the reduction was not dependent on any single viral adaptive mutation. Lovastatin treatment also reduced the levels of viral proteins within Huh7-C5B3 and Huh7-HP cells (Fig. 1C). Thus, lovastatin suppressed HCV RNA and protein abundance irrespective of viral genome variation and potential phenotypic differences among cell lines. We note that the lovastatin-induced reduction in viral RNA was not complete, as indicated by the observation that some Huh7-K2040 cells survived selection with G418 after treatment with lovastatin for 1 week. This survival indicates that a low level of the HCV replicon, which contains the G418 resistance gene (neo), remained within the cells.
Lovastatin slows the proliferation of some malignant cell types by indirectly suppressing DNA synthesis (17), which may negatively affect HCV RNA replication (18). We therefore examined the influence of lovastatin on the synthesis of DNA, mRNA, and protein in Huh7-K2040 cells. As shown in Fig. 1D, treatment of Huh7-K2040 cultures with lovastatin for 24 h reduced HCV RNA levels to ≈25% of untreated control cells but did not significantly affect cellular DNA or mRNA synthesis. The incubation of parallel cultures with the DNA polymerase inhibitors aphidicolin or hydroxyurea blocked DNA synthesis but did not affect viral RNA or cellular mRNA levels during a 24-h culture period. Each compound moderately suppressed the global rate of cellular protein synthesis. At the concentrations used, lovastatin had no significant effects on the morphological appearance of the cells in the light microscope when cultures were treated for 14 days (data not shown). Our results demonstrate an antiviral action of lovastatin that is not attributable to global effects on cellular DNA, mRNA, or protein synthesis. Rather, the data suggest that HCV RNA replication requires one or more products derived from mevalonate, the product of the HMG CoA reductase reaction (7).
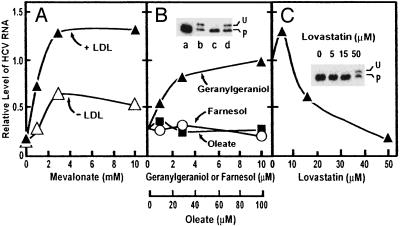
To define the mevalonate-derived products required for HCV RNA replication, Huh7-K2040 cells were cultured with or without lovastatin in the absence or presence of various metabolites whose synthesis requires HMG CoA reductase. During a 24-h culture period, lovastatin treatment reduced HCV RNA levels to 10–20% of untreated control cultures (Fig. 2A), and this was unaffected when the culture medium was supplemented with exogenous cholesterol in the form of low-density lipoprotein (LDL) (Fig. 2 A). On the other hand, mevalonate produced a dose-dependent rescue of HCV RNA levels, and the effect was enhanced by the presence of exogenous LDL (Fig. 2 A). Mevalonate serves as precursor to cholesterol and nonsterol isoprenoids (7). When cholesterol is not available, most mevalonate is directed into cholesterol. When LDL is available, the cholesterol demand is satisfied and more of the mevalonate is directed to nonsterol isoprenoids (19).
Fig. 2.
Mevalonate and geranylgeraniol restore HCV RNA replication in lovastatin-treated cells. Huh7-K2040 cells (4 × 105) were cultured for 16 h in 60-mm dishes. The cultures were then subjected to the following treatments. (A) Cultures were changed to medium A with (▴) or without (▵) 100 μg of protein per ml of LDL. Except for the nontreated control (not plotted), all other cultures received 50 μM lovastatin and increasing amounts of sodium mevalonate as indicated. (B) Cultures were changed to medium A containing 100 μg of protein per ml of LDL. Except for the nontreated control (not plotted), cells were treated with 50 μM lovastatin and increasing amounts of geranylgeraniol (▴), farnesol (○), or oleate (▪) as indicated. (C) Cultures were changed to medium A containing increasing amounts of lovastatin as indicated. In all treatments, cultures were continued for a further 24 h, after which the cells were harvested and total RNA was extracted. The HCV RNA level was determined by real-time quantitative RT-PCR analysis. Values are presented as a proportion of the nontreated control, which was set at 1.0. (B Inset and C Inset) Cultures in B Inset were changed to medium A containing no addition (lane a), 50 μM lovastatin (lanes b–d), 10 μM geranylgeraniol (lane c), or 10 μM farnesol (lane d). Cultures in C Inset were changed to medium A containing the indicated amount of lovastatin. Forty-eight hours later, cells were harvested and 20-μg aliquots of cell lysate were subjected to SDS/PAGE and immunoblot analysis with anti-Rap1a antibody (0.4 μg/ml). U and P denote unprenylated and prenylated forms of Rap1a, respectively.
The results with LDL suggest that lovastatin treatment depletes the cells of one or more nonsterol end products of mevalonate metabolism that are required to support HCV RNA replication. To identify this end-product, we supplemented the lovastatin-treated cultures of Huh7-K2040 cells with the mevalonate-derived isoprenoids, geranylgeraniol or farnesol. In parallel control experiments, we supplemented the cultures with oleate, a long-chain fatty acid that is not derived from mevalonate. Exogenous geranylgeraniol, but neither farnesol nor oleate, mediated a dose-dependent rescue of HCV RNA levels from the suppressive actions of lovastatin (Fig. 2B).
To demonstrate that lovastatin depletes cells of geranylgeranylated proteins, we subjected the proteins of Huh7-K2040 cells to SDS/PAGE and blotted with an antibody against Rap1a, a small GTP-binding protein that is known to be geranylgeranylated (8). Lovastatin caused the appearance of a slow-migrating form of Rap1a, which represents the unprenylated protein (Fig. 2B Inset, lane b) (20). Addition of geranylgeraniol, but not farnesol, eliminated the upper band, indicating restoration of geranylgeranylation (Fig. 2B Inset, lanes c and d). Fig. 2C shows that lovastatin reduced HCV RNA at the same concentrations in which it prevented geranylgeranylation of Rap1a (Fig. 2C Inset). Considered together, these results suggest that one or more geranylgeranylated proteins is required for HCV RNA replication and that lovastatin blocks HCV replication by depleting endogenous geranylgeranyl pyrophosphate, thereby preventing geranylgeranylation of the critical protein(s). Although Rap1a was used as an indicator of protein geranylgeranylation in this study, there is no evidence that it is the protein required for HCV RNA replication.
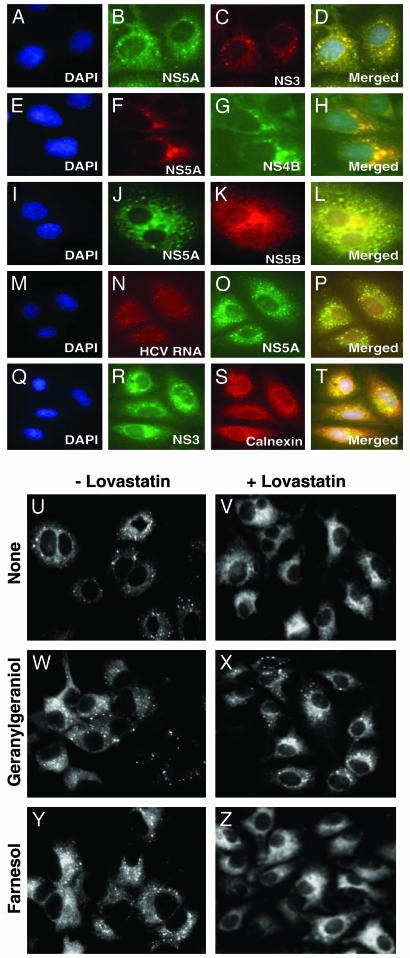
Among other functions, geranylgeranylated proteins mediate the interaction of membranes with cytoskeletal proteins (8). Such interactions are likely to be required for the formation of the ER-associated HCV replication complex, which contains the viral RNA as well as the nonstructural viral proteins (1, 2). Using immunocytochemical techniques, we show in Fig. 3 A–T that HCV nonstructural proteins (NS3–NS5B) colocalize with viral RNA and appear in a punctate staining pattern associated with ER membranes. To test whether lovastatin affects the replication complex, we stained cells with an antibody to NS5A and an FITC-labeled second antibody (Fig. 3 U–Z). In the absence of lovastatin treatment, much of the NS5A was localized in a punctate pattern consistent with the ER localization of the HCV replication complex (Fig. 3U). After treatment with lovastatin for 48 h, the punctate NS5A staining pattern largely disappeared, and the protein was diffuse throughout the cytoplasm (Fig. 3V). Incubation of the cells with geranylgeraniol (Fig. 3X), but not farnesol (Fig. 3Z), prevented the loss of the punctate distribution. These data suggest that one or more geranylgeranylated proteins is required for the maintenance of the HCV replication complex.
Fig. 3.
Geranylgeraniol, but not farnesol, restores the HCV replication complex in lovastatin-treated cells as visualized by immunofluorescence. (A–T) Huh7-K2040 cells (5 × 104) were cultured for 16 h in the wells of a 1.8 × 0.9-cm chamber slide. Cells were fixed, and multicolor immunofluorescence microscopy analysis was performed as described in Materials and Methods. (A–C, E–G, I–K, M–O, and Q–S) Cells were counterstained with the nucleic acid probe 4′,6′-diamidino-2-phenylindole hydrochloride (DAPI) or the indicated antibody. (M–O) Cells were transfected with 5-bromouridine 5′-triphosphate by using FuGENE 6 reagent as described (6) and cultured in the presence of actinomycin D for 2 h to facilitate the specific incorporation of bromouridine into HCV RNA. HCV RNA was visualized by immunostaining of bromouridine. (D, H, L, P, and T) These images are merged fluorescent composite images of panels A–C, E–G, I–K, M–O, and Q–S, respectively. Within each merged composite image, yellow denotes protein colocalization and the position of the HCV replication complex. (U–Z) Huh7-K2040 cells (105) were cultured for 16 h in the wells of a 1.8 × 0.9-cm chamber slide, after which the culture medium was switched to medium A containing the indicated additions and cultures were continued for a further 48 h. Cells were then fixed and immunostained with monoclonal anti-NS5A and FITC-labeled secondary antibody. Shown is NS5A protein localization in cells that were cultured with medium A with no addition (U), 50 μM lovastatin (V), 10 μM geranylgeraniol (W), 50 μM lovastatin plus 10 μM geranylgeraniol (X), 10 μM farnesol (Y), or 50 μM lovastatin plus 10 μM farnesol (Z). No fluorescence signal was detected in parallel cultures of control Huh7 cells (data not shown).
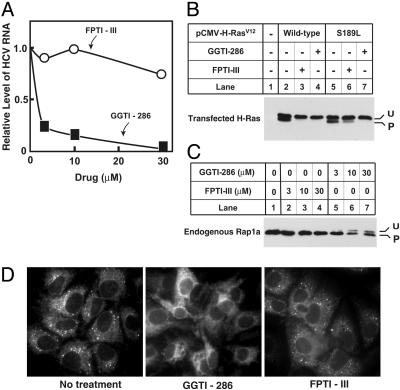
A large class of geranylgeranylated proteins is synthesized by protein geranylgeranyl transferase 1 (GGTase I), which recognizes carboxyl terminal Cys-A-A-X sequences (CAAX boxes), where A is an aliphatic amino acid and X is typically leucine (8). The enzyme is closely related to protein farnesyl transferase, which recognizes CAAX boxes terminating in serine or methionine and transfers farnesyl instead of geranylgeranyl to the cysteine of the CAAX box (8). A second class of GGTases called Rab GGTase (or GGTase II) attaches geranylgeranyl only to Rab proteins, which lack CAAX boxes but contain COOH-terminal CXC or XXCC sequences (8). To determine whether GGTase I is required for HCV RNA replication, we treated Huh7-K2040 cells with increasing concentrations of two inhibitors based on CAAX boxes: the GGTase I inhibitor GGTI-286 (21) or the farnesyl transferase inhibitor FPTI-III (22).
As shown in Fig. 4A, treatment with GGTI-286 resulted in a dose-dependent decrease in the level of HCV RNA. In contrast, FPTI-III had no effect on viral RNA levels. To confirm the specificity of GGTI-286 and FPTI-III action, we evaluated the effect of each compound on the prenylation of wild-type H-Ras, which is normally farnesylated, and a mutant H-Ras(S189L) in which the carboxyl-terminal amino acid has been changed to leucine, making it a substrate for GGTase 1 (20). In the experiment of Fig. 4B, both Ras proteins showed an upper and lower band in the absence of inhibitors, indicating that prenylation was not complete (lanes 2 and 5), perhaps owing to overexpression. FPTI-III at 10 μM eliminated the lower band in the H-Ras blot (lane 3) but had only a partial effect on the prenylation of H-Ras(S189L) (lane 6), consistent with preferential specificity for farnesyl transferase. GGTI-286 at 10 μM showed no such specificity, inhibiting farnesylation of H-Ras (lane 4) as well as geranylgeranylation of H-Ras(S189L) (lane 7). To compare the effects of the two inhibitors on geranylgeranylation of an endogenous protein, we again studied Rap1a. As shown in Fig. 4C, FPTI-III did not inhibit geranylgeranylation of Rap1a, even at doses up to 30 μM (lane 4), whereas GGTI-286 showed clear-cut inhibitory effects at 10 μM (lane 6). Inasmuch as FPT-III eliminated farnesylation but failed to inhibit HCV RNA replication (Fig. 4A), we conclude that farnesylation is not required for this process. Thus, the inhibitory activity of GGTI-286 on HCV RNA replication must be due to its action against GGTase I, and not farnesyl transferase. Consistent with this conclusion, GGTI-286, but not FPTI-III, produced a redistribution of the HCV NS5A protein that was similar to that observed in lovastatin-treated cells; namely, a change from a punctate pattern to a diffuse pattern (Fig. 4D). Taken together, these results demonstrate a specific dependence on protein geranylgeranylation for the localization and assembly of the HCV RNA replication complex.
Fig. 4.
Disruption of HCV replication through specific inhibition of geranylgeranyl transferase I. (A–C) Huh7-K2040 cells (4 × 105) were seeded in 60-mm dishes on day 0 and cultured for 16 h. (A) Cells were then switched to medium A containing 100 μM DTT. Except for the nontreated control, all dishes received increasing amounts of GGTI-286 (▪) or FPTI-III (○) as indicated. After 24 h the cells were harvested and total RNA was extracted. HCV RNA level was determined by quantitative real-time RT-PCR analysis. Values are presented as a proportion of the nontreated control, which was set at 1.0. (B) Cells were transfected on day 1 with 0.5 μg of the indicated plasmid (2 μgof total DNA per dish, using pcDNA3.1 empty vector). Six hours later the cells were treated with 10 μM FPTI-III or GGTI-286. On day 2, the cells were harvested, and 20 μg of cell lysate was subjected to SDS/PAGE and immunoblot analysis with anti-H-Ras antibody (0.2 μg/ml) as described (20). U and P denote unprenylated and prenylated form of H-Ras, respectively. (C) Cells were treated with the indicated concentrations of FPTI-III (lanes 2–4) or GGTI-286 (lanes 5–7). On day 2, the cells were processed for immunoblot analysis with anti-Rap1a antibody (0.4 μg/ml) as described in B. (D) NS5A protein localization was analyzed in Huh7-K2040 cells. Cells (105) were cultured for 16 h in the wells of a 1.8 × 0.9-cm chamber slide, after which the culture medium was replaced with medium A containing one of the following additions: none (Left), 20 μM GGTI-286 (Center), or 20 μM FPTI-III (Right). Cultures were continued for a further 24 h, after which the cells were fixed and immunostained with monoclonal anti-NS5A and a FITC-conjugated secondary antibody. No fluorescent signal was detected in control Huh7 cells (data not shown).
Discussion
The current results add to a small but growing body of evidence implicating protein prenylation in viral replication. Previous studies showed that inhibitors of farnesyl transferase block the replication of hepatitis delta virus, which encodes a protein that terminates in a farnesyl-type CAAX box (23). Here, we show that geranylgeranylation as opposed to farnesylation is required for HCV replication, and we propose that one or more cellular proteins, rather than a viral protein, is the target. Although HCV does not encode a protein that terminates in a CAAX box, the virus does encode a protein, NS5A, that terminates in CC. Such a protein would not be a substrate for GGTase I, which shows an absolute requirement for a CAAX box (8). It would also not be a substrate for Rab GGTase (GGTase II) because this enzyme only prenylates Rab proteins (8). Thus, inasmuch as the HCV genome does not encode a canonical geranylgeranylated protein, HCV replication appears to be dependent on the geranylgeranylation of a cellular rather than a virus-encoded protein. To our knowledge, we are unaware of previous data implicating geranylgeranylation in the replication of any virus.
Recent studies of HCV showed that treatment with 25-hydroxycholesterol reduces the level of HCV replicon RNA within hepatoma cells, but the mechanism of this effect is unknown (24). The current data suggest an explanation for this finding. Inasmuch as 25-hydroxycholesterol lowers transcription of the HMG CoA reductase gene (25), 25-hydroxycholesterol may inhibit HCV replication by depleting cells of geranylgeranyl pyrophosphate, the mevalonate-derived donor of protein geranylgeranylation (7, 10). A more general role for host cell geranylgeranylation in viral replication may be inferred from the finding that African swine fever virus, an icosahedral DNA virus, encodes a membrane-bound prenyltransferase that synthesizes geranylgeranyl pyrophosphate (26).
A unique observation in the current study is that both lovastatin and GGTI-286 acutely and markedly suppress HCV RNA levels. The three HCV replicon-containing cell lines that we examined all contained genotype 1 HCV RNA, which is associated with the most severe clinical disease and has the poorest response rate to IFN-based therapy (27, 28). HCV replicates as populations of genetically distinct variants or quasispecies that are continuously generated, presumably owing to lack of proofreading by the viral NS5B RNA-dependent RNA polymerase (2, 29). Because viral genetic complexity provides a pool from which therapy-resistant variants can emerge (30, 31), the quasispecies nature of HCV has proved troublesome for contemporary antiviral therapeutic strategies and is certain to limit the benefits of future therapies that are directed against virus-specific targets (31). On the other hand, therapeutic approaches that target host-specific proteins required for HCV RNA replication, such as the putative geranylgeranylated protein(s) postulated in this study, are much less likely to be affected by viral genetic variation.
The Huh7 human hepatoma cells used in these studies survived long-term exposure to concentrations of lovastatin that would have been lethal for other cell lines. Whether this resistance is a property of this particular cell line (obtained from Stephen Polyak, University of Washington, Seattle), or whether it is a general property of other hepatoma-derived cells, remains to be discovered.
In animal models, administration of statins in high doses inhibits the geranylgeranylation of Rap1a in certain tissues, such as bone (32), suggesting that lovastatin might be effective in treating HCV-infected patients. In patients, however, in order for statins to inhibit HCV RNA replication, the drug would have to be delivered to the liver in concentrations that are much higher than those obtained at current therapeutic doses, and this will likely cause toxicity in liver and other organs. The action of protein GGTase I inhibitors may provide a new avenue of antiviral therapy that avoids such toxicity. In this regard, the identification of the crucial geranylgeranylated cellular protein(s) required for HCV replication may yield an even more specific target for treatment of HCV infection.
Supplementary Material
Acknowledgments
We thank Lorena Avila for her excellent technical assistance; Jeff Cormier and Erin Friedman for real-time PCR analysis; and Drs. S. Lemon, D. Moradpour, R. Bartenschlager, T. Imagawa, I. Julkunen, and P. Harran for reagents. This work was supported by National Institutes of Health Grants HL-20948 and AI48235, the Perot Family Foundation, and the Ellison Medical Foundation. M.G. is the Nancy C. and Jeffrey A. Marcus Scholar in Medical Research.
Abbreviations: ER, endoplasmic reticulum; GGTase, geranylgeranyl transferase; HCV, hepatitis C virus; HMG CoA reductase, 3-hydroxy-3-methylglutaryl CoA reductase; IRES, internal ribosome entry site; LDL, low-density lipoprotein; NS, nonstructural.
References
- 1.Wasley, A. & Alter, M. J. (2000) Semin. Liver Dis. 20, 1-16. [DOI] [PubMed] [Google Scholar]
- 2.Reed, K. E. & Rice, C. M. (1998) in Hepatitis C Virus, ed. Reesink, H. W. (Karger, Basel), pp. 55-84.
- 3.Lohmann, V., Korner, F., Koch, J.-O., Herian, U., Theilmann, L. & Bartenschlager, R. (1999) Science 285, 110-113. [DOI] [PubMed] [Google Scholar]
- 4.Konan, K. V., Giddings, T. H., Jr., Ikeda, M., Li, K., Lemon, S. M. & Kirkegaard, K. (2003) J. Virol. 77, 7843-7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi, S. T., Lee, K.-J., Aizaki, H., Hwang, S. B. & Lai, M. M. C. (2003) J. Virol. 77, 4160-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Hage, N. & Luo, G. (2003) J. Gen. Virol. 84, 2761-2769. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein, J. L. & Brown, M. S. (1990) Nature 343, 425-430. [DOI] [PubMed] [Google Scholar]
- 8.Zhang, F. L. & Casey, P. J. (1996) Annu. Rev. Biochem. 65, 241-269. [DOI] [PubMed] [Google Scholar]
- 9.Mahley, R. W. & Bersot, T. P. (2001) in Goodman and Gilman's The Pharmacological Basis of Therapeutics, eds. Hardman, J. G., Limbird, L. E. & Gilman, A. G. (McGraw–Hill, New York), pp. 971-1002.
- 10.Muntz, K. H., Sternweis, P. C., Gilman, A. G. & Mumby, S. M. (1992) Mol. Biol. Cell 3, 49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda, M., Yi, M.-K., Li, K. & Lemon, S. M. (2002) J. Virol. 76, 2997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foy, E., Li, K., Wang, C., Sumpter, R., Jr., Ikeda, M., Lemon, S. M. & Gale, M., Jr. (2003) Science 300, 1145-1148. [DOI] [PubMed] [Google Scholar]
- 13.Beard, M. R., Abell, G., Honda, M., Carroll, A., Gartland, M., Clarke, B., Suzuki, K., Lanford, R., Sangar, D. V. & Lemon, S. M. (1999) Hepatology 30, 316-324. [DOI] [PubMed] [Google Scholar]
- 14.Pflugheber, J., Frederickson, B., Sumpter, R., Jr., Wang, C., Ware, F., Sodora, D. L. & Gale, M., Jr. (2002) Proc. Natl. Acad. Sci. USA 99, 4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, C., Pflugheber, J., Sumpter, R., Jr., Sodora, D. L., Hui, D., Sen, G. C. & Gale, M., Jr. (2003) J. Virol. 77, 3898-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang, G., Yang, J., Horton, J. D., Hammer, R. E., Goldstein, J. L. & Brown, M. S. (2002) J. Biol. Chem. 277, 9520-9528. [DOI] [PubMed] [Google Scholar]
- 17.Chan, K. K. W., Oza, A. M. & Siu, L. L. (2003) Clin. Cancer Res. 9, 10-19. [PubMed] [Google Scholar]
- 18.Lohmann, V., Hoffmann, S., Herian, U., Penin, F. & Bartenschlager, R. (2003) J. Virol. 77, 3007-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown, M. S. & Goldstein, J. L. (1980) J. Lipid Res. 21, 505-517. [PubMed] [Google Scholar]
- 20.James, G. L., Brown, M. S., Cobb, M. H. & Goldstein, J. L. (1994) J. Biol. Chem. 269, 27705-27714. [PubMed] [Google Scholar]
- 21.Lerner, E. C., Qian, Y., Hamilton, A. D. & Sebti, S. M. (1995) J. Biol. Chem. 270, 26770-26773. [DOI] [PubMed] [Google Scholar]
- 22.Wang, D., Yu, X. & Brecher, P. (1998) J. Biol. Chem. 273, 33027-33034. [DOI] [PubMed] [Google Scholar]
- 23.Glenn, J. S., Marsters, J. C., Jr., & Greenberg, H. B. (1998) J. Virol. 72, 9303-9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su, A. I., Pezacki, J. P., Wodicka, L., Brideau, A. D., Supekova, L., Thimme, R., Wieland, S., Bukh, J., Purcell, R. H., Schultz, P. G., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 15669-15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown, M. S. & Goldstein, J. L. (1997) Cell 89, 331-340. [DOI] [PubMed] [Google Scholar]
- 26.Alejo, A., Andres, G., Vinuela, E. & Salas, M. L. (1999) J. Biol. Chem. 274, 18033-18039. [DOI] [PubMed] [Google Scholar]
- 27.Enomoto, N., Sakuma, I., Asahina, Y., Kurosaki, M., Murakami, T., Yamamoto, C., Ogura, Y., Izumi, N., Marumo, F. & Sato, C. (1996) N. Engl. J. Med. 334, 77-82. [DOI] [PubMed] [Google Scholar]
- 28.McHutchison, J. G. & Patel, K. (2002) Hepatology 36, S245-S252. [DOI] [PubMed] [Google Scholar]
- 29.Forns, X. & Bukh, J. (1999) Clin. Liver Dis. 3, 693-716. [DOI] [PubMed] [Google Scholar]
- 30.Farci, P., Strazzera, R., Alter, H. J., Farci, S., Degioannis, D., Coiana, A., Peddis, G., Usai, F., Serra, G., Chessa, L., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 3081-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan, S. L., Pause, A., Shi, Y. & Sonenberg, N. (2002) Nat. Rev. Drug Discov. 1, 867-881. [DOI] [PubMed] [Google Scholar]
- 32.Staal, A., Frith, J., French, M. H., Swartz, J., Gungor, T., Harrity, T. W., Tamasi, J., Rogers, M. J. & Feyen, J. H. M. (2003) J. Bone Miner. Res. 18, 88-96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.