Abstract
Simian varicella virus (SVV) infection of primates resembles human varicellazoster virus (VZV) infection. After primary infection, SVV becomes latent in ganglia and reactivates after immunosuppression or social and environmental stress. Herein, natural SVV infection was established in 5 cynomolgus macaques (cynos) and 10 African green (AG) monkeys. Four cynos were treated with the immunosuppressant tacrolimus (80 to 300 μg/kg/day) for 4 months and 1 was untreated (group 1). Four AG monkeys were exposed to a single dose (200 cGy) of x-irradiation (group 2), and 4 other AG monkeys were irradiated and treated with tacrolimus for 4 months (group 3); the remaining 2 AG monkeys were untreated. Zoster rash developed 1 to 2 weeks after tacrolimus treatment in 3 of 4 monkeys in group 1, 6 weeks after irradiation in 1 of 4 monkeys in group 2, and 1 to 2 weeks after irradiation in all 4 monkeys in group 3. All monkeys were euthanized 1 to 4 months after immunosuppression. SVV antigens were detected immunohistochemically in skin biopsies as well as in lungs of most monkeys. Low copy number SVV DNA was detected in ganglia from all three groups of monkeys, including controls. RNA specific for SVV ORFs 61, 63, and 9 was detected in ganglia from one immunosuppressed monkey in group 1. SVV antigens were detected in multiple ganglia from all immunosuppressed monkeys in every group, but not in controls. These results indicate that tacrolimus treatment produced reactivation in more monkeys than irradiation and tacrolimus and irradiation increased the frequency of SVV reactivation as compared to either treatment alone.
Keywords: immunosuppression, reactivation, SVV
Introduction
Varicella-zoster virus (VZV) produces chickenpox (varicella) in humans and becomes latent in ganglionic neurons along the entire neuraxis (LaGuardia et al, 1999; Levin et al, 2003). Decades later, VZV reactivation produces shingles (zoster). Zoster develops in the elderly as a result of a natural decline in VZV-specific cell-mediated immunity as well as in cancer patients, organ transplant recipients, and patients with acquired immunodeficiency syndrome (AIDS). Zoster develops in 8% to 18% of renal transplant recipients who receive immunosuppressive therapy (Rifkind, 1966; Fehr et al, 2002). VZV reactivates in bone marrow as well as solid organ transplant recipients treated with tacrolimus (Gourishankar et al, 2004), an immunosuppressive drug that reduces both cell-mediated and humoral immunity (Caproni et al, 2006) and total body irradiation (Koc et al, 2000) or both (Mori et al, 2007). Tacrolimus, a potent immunosuppressant, is used extensively for prophylaxis and to treat organ transplant recipients. The incidence of zoster is greater in patients receiving chemotherapy combined with radiotherapy than in those receiving either alone (Mandal, 1987).
Simian varicella virus (SVV) infection of nonhuman primates produces varicella and reactivates to produce zoster, thus providing a useful model to study latency and reactivation (Mahalingam et al, 2010). Natural SVV infection of both African green and cynomolgus monkeys leads to latent infection (Mahalingam et al, 2002, 2007) of ganglionic neurons (Kennedy et al, 2004) as seen in human ganglia latently infected with VZV (Kennedy et al, 1998). Intrabronchial inoculation of SVV into rhesus macaques also produces latent infection in ganglionic neurons (Messaoudi et al, 2009).
Latent SVV reactivates in rhesus (Schoeb et al, 2008; Kolappaswamy et al, 2007) and pigtailed (Hukkanen et al, 2009) macaques that have undergone organ transplantation and total body irradiation. We recently demonstrated SVV reactivation in latently infected cynomolgus macaques (cynos) subjected to experimental immunosuppression and stress using a combination of x-irradiation, tacrolimus, and prednisone (Mahalingam et al, 2007). To further dissect the role of x-irradiation and tacrolimus, we examined SVV reactivation in latently infected monkeys after natural exposure to virus (Mahalingam et al, 2007) and treatment with x-irradiation or tacrolimus or both.
Results
Establishment of latent SVV infection
Latent SVV infection was established in 5 cynos (group 1: GP02, 04, 06, 07, and 05) and 10 African Green (AG) monkeys (groups 2 and 3: GV71, 72, 73, 74, and 75 and HC01, 06, 07, 08, and 02, respectively) by exposure to monkeys of the same species inoculated intratracheally with 104 plaque-forming units (PFU) of SVV as described (Mahalingam et al, 2002, 2007). All 15 monkeys developed mild to moderate varicella rash 10 to 14 days later (Figure 1). A mild viremia was found in some monkeys, and no elevation of liver enzymes was seen in any monkeys.
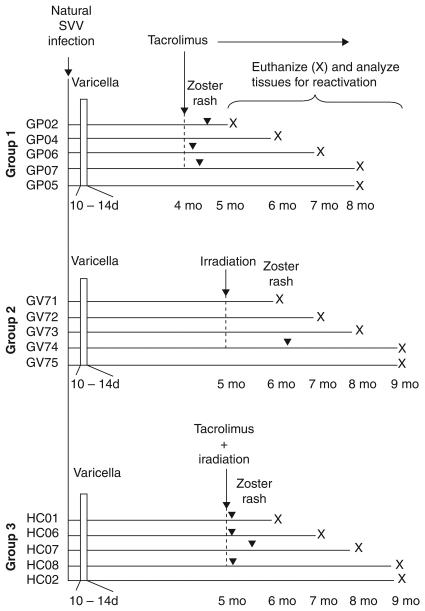
Figure 1.
Experimental design. Five cynos (group 1) and 10 AG monkeys (groups 2 and 3) were exposed to other monkeys previously inoculated intratracheally with SVV (104 PFU). All 15 monkeys developed varicella rash 10 to 14 days after exposure. At 4 months post exposure, 4 monkeys (group 1; GP02, 04, 06, and 07) received tacrolimus. At 5 months post exposure, 4 monkeys (group 2; GV71, 72, 73, and 74) were exposed to x-irradiation and 4 monkeys (group 3: HC01, 06, 07, and 08) received tacrolimus plus x-irradiation. In groups 1 and 3, tacrolimus treatment was continued for the remainder of the time. Zoster rash developed 1 to 2 weeks after tacrolimus treatment in monkeys GP02, 06, and 07 (group 1), 6 weeks after exposure to radiation in monkey GV74 (group 2), and 1 to 2 weeks after treatment with tacrolimus plus radiation in monkeys HC01, 06, 07, and 08 (group 3). Monkeys were euthanized months (X) after the indicated treatments.
Immunosuppressive treatment of monkeys and development of zoster rash
Four months post infection, 4 cynos in group 1 (GP02, 04, 06, and 07) were treated daily with tacrolimus; 1 monkey (GP05) was not treated. Zoster rash developed in monkeys GP02, 06, and 07, as evidenced by development of lesions in the right cranioventral thorax, right inguinal region, and left cranioventral thorax at 26, 3, and 10 days, respectively, after starting tacrolimus treatment. No rash was observed in monkey GP04 or control monkey GP05. Five months post infection, 4 AG monkeys in group 2 (GV71, 72, 73, and 74) were irradiated; 1 AG monkey (GV75) was not irradiated. Zoster rash was observed in monkey GV74, as evidenced by development of lesions in the left inguinal region 18 days after irradiation, but not in monkeys GV71, 72, 73, or 75 (control). Five months post infection, 4 AG monkeys in group 3 (HC01, 06, 07, and 08) were irradiated and treated daily with tacrolimus. Zoster rash developed in all 4 AG monkeys as evidenced by lesions in the inguinal region, inguinal region, inguinal region, and ventral abdomen at 6, 6, 19, and 6 days, respectively, after starting treatment with tacrolimus; no rash was observed in the control monkey HC02 (Figure 1).
Effect of immunosuppression on white blood cell (WBC) count and serology of monkeys
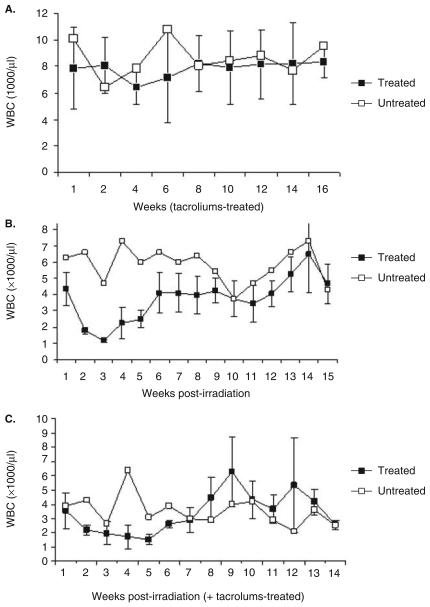
WBC counts as a measure of the effect of immunosuppressive regimens on monkeys latently infected with SVV were obtained biweekly (Figure 2A: group 1)or weekly (Figures 2B and C: groups 2 and 3). Compared to control monkey GP05 in group 1, mean WBC numbers were slightly reduced at 6 weeks post tacrolimus treatment (Figure 2A), whereas average WBC numbers were dramatically decreased in irradiated group 2 monkeys within 3 weeks of treatment and increasing gradually over the next 7 weeks until week 15, although the average WBC levels in the immunosuppressed monkeys were almost always lower than those in the control monkey (Figure 2B). Compared to untreated monkey HC02 in group 3, the average levels of WBCs decreased gradually in irradiated, tacrolimus-treated monkeys in the first 6 weeks after treatment, increasing thereafter over the next 9 weeks to levels comparable to the control monkeys (Figure 2C). WBC numbers in the group 3 control monkeys were much lower (2000 to 3000 cells/μl) than those in control monkeys in groups 1 and 2 (4000 to 11000 cells/μl). Overall, WBC numbers in the treated monkeys in group 3 were slightly lower than in the untreated control monkey.
Figure 2.
White blood cell (WBC) counts in immunosuppressed monkeys latently infected with SVV. WBCs (normal 7–15 × 103/μl) were counted weekly after treatment with tacrolimus or exposure to radiation or both. Four monkeys (group 1: GP02, 04, 06, and 07) received tacrolimus (80 g/kg/day) for the duration of the experiment (A). Four monkeys (group 2: GV71, 72, 73, and 74) received a single dose (200 cGy) of irradiation (B). Four monkeys (group 3: HC01, 06, 07, and 08) received a single dose (200 cGy) of irradiation followed by tacrolimus (80 g/kg/day) for the remainder of the experiment (C). Data are mean (± SE) WBC numbers in latently infected monkeys treated with an immunosuppressive regimen (filled squares) or untreated (open squares). WBC levels were decreased in all monkeys that were irradiated and treated or not treated with tacrolimus (groups 2 and 3).
Table 1 lists the serological data on all monkeys. Serum obtained before varicella revealed no anti-SVV antibody (<1:4 dilution), whereas 30 days after varicella rash, anti-SVV antibody was detected at a 1:4 dilution in all monkeys from groups 1 and 2 and at a 1:6 dilution in all monkeys in group 3. Serum from the positive-control AG monkey inoculated intratracheally with SVV contained antibody at a dilution of 1:320. After immunosuppression, in group 1, SVV antibodies were detected at a dilution of 1:4 in 1 monkey and at a dilution of 1:8 in 3 monkeys. In group 2, SVV antibodies were detected at a 1:6 dilution in 1 monkey, at a 1:8 dilution in 1 monkey, and at a 1:12 dilution in 2 monkeys. In group 3, SVV antibodies were detection at a 1:6 dilution in 1 monkey and at a 1:12 dilution in 3 monkeys. Overall, most monkeys had low SVV antibody titers after acute disease, indicative of a mild primary infection, which may account for difficulty in detection of nucleic acids in tissues (see below).
Table 1.
Antibody response to SVV infection and immunosup-pression in cynomolgus and African green monkeysa
| Group | Monkey | Before varicella |
After varicellab |
After immunosuppressionc |
|---|---|---|---|---|
| 1 | GP02d,e | <1:4 | 1:4 | 1:4 |
| GP04d,e | <1:4 | 1:4 | 1:8 | |
| GP06d,e | <1:4 | 1:4 | 1:8 | |
| GP07d,e | <1:4 | 1:4 | 1:8 | |
| GP05d,f | <1:4 | 1:4 | NAg | |
| 2 | GV71h,i | <1:4 | 1:4 | 1:8 |
| GV72h,i | <1:4 | 1:4 | 1:12 | |
| GV73h,i | <1:4 | 1:4 | 1:6 | |
| GV74h,i | <1:4 | 1:4 | 1:12 | |
| GV75h,e | <1:4 | 1:4 | NAg | |
| 3 | HC01h,j | <1:4 | 1:6 | 1:6 |
| HC06h,j | <1:4 | 1:6 | 1:12 | |
| HC07h,j | <1:4 | 1:6 | 1:12 | |
| HC08h,j | <1:4 | 1:6 | 1:12 | |
| HC02h,e | <1:4 | 1:6 | NAg | |
| Positiveh,k | <1:4 | 1:320 | NAf |
Anti-SVV antibody titers expressed as the serum dilution that neutralized 50% or more of the SVV plaques compared to control cultures.
Sera obtained >30 days after varicella rash
Sera obtained at necropsy.
Cynomolgus monkey.
Treated with tacrolimus.
No treatment.
Not applicable (no immunosuppression).
African green monkey.
Irradiated.
Irradiated and treated with tacrolimus.
Intratracheally inoculated with SVV.
Detection of SVV DNA in peripheral blood mononuclear cells (MNCs)
During primary infection, SVV DNA was not found in MNCs from any monkeys in group 1 and was detected in MNCs from only 1 monkey (GP06) in this group at 10 days post immunosuppression (d.p.i.). In group 2, SVV DNA was found during primary infection in MNCs from monkeys GV71 (56, 91, and 106 d.p.i.), GV72 (56 d.p.i.), GV74 (56 d.p.i.), and GV75 (20 d.p.i.), but not in MNCs from any monkey, after immunosuppression. In group 3, SVV DNA was detected during primary infection in MNCs from monkeys HC06 (160 d.p.i.) and HC08 (7 and 10 d.p.i.), but not in MNCs from any monkey, after immunosuppression.
Detection of SVV DNA and RNA in ganglionic and nonganglionic tissues
DNA was extracted from lung and a small portion of ganglia from all 15 monkeys and analyzed by real-time DNA polymerase chain reaction (PCR). SVV DNA was not detected in lung from any monkeys, although cellular DNA (glutaraldehyde-3-phosphate dehydrogenase [GAPdH]) sequences were detected in the same samples (Tables 2-4). In group 1, SVV DNA sequences were detected in cervical and sacral ganglia from monkey GP02, in thoracic and lumbar ganglia from monkey GP06, and in cervical and lumbar ganglia from monkey GP05, but not in any ganglia from monkeys GP04 and 07 (Table 2). In group 2, SVV DNA sequences were detected in thoracic ganglia from monkey GV71, in pooled trigeminal and cervical ganglia from monkey GV72, and in pooled trigeminal and cervical ganglia from control monkey GV75 (Table 3). In group 3, SVV DNA sequences were detected only in trigeminal ganglia from monkey HC07 and in lumbar ganglia from control monkey HC02 (Table 4).
Table 2.
Detection of SVV DNA, RNA, and glycoprotein in tissues from cynomolgus monkeys treated with tacrolimus
| Monkey no. | Tissuea | DNA PCRb |
cDNA PCRc |
IHCd (gH + gL) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 21 | GAPdH | 61 | 63 | 40 | 9 | GAPdH | |||
| GP02 | Lung | − | ++e | − | |||||
| Cervical | +f | ++ | − | − | − | − | ++ | NA | |
| TH | − | ++ | + | ||||||
| Lumbar | − | ++ | + | ||||||
| Sacral | + | ++ | − | − | − | − | ++ | + | |
| Skin (thorax) | + | ||||||||
| GP04 | Lung | − | ++ | + | |||||
| Cervical | − | ++ | − | ||||||
| TH | − | ++ | − | ||||||
| Lumbar | − | ++ | + | ||||||
| Sacral | − | ++ | + | ||||||
| GP06 | Lung | − | ++ | + | |||||
| Cervical | − | ++ | + | ||||||
| TH | ++ | ++ | ++ | ++ | − | ++ | ++ | − | |
| Lumbar | + | ++ | ++ | + | − | + | ++ | + | |
| Sacral | − | ++ | − | ||||||
| Skin (lumbar) | + | ||||||||
| GP07 | Lung | − | ++ | + | |||||
| Cervical | − | ++ | − | ||||||
| TH | − | ++ | − | ||||||
| Lumbar | − | ++ | − | ||||||
| Sacral | − | ++ | − | ||||||
| Skin (thorax) | + | ||||||||
| GP05 (untreated control) |
Lung | − | ++ | + | |||||
| Cervical | + | ++ | − | − | − | + | ++ | − | |
| TH | − | ++ | − | ||||||
| Lumbar | ++ | ++ | − | − | − | − | ++ | − | |
| Sacral | − | ++ | − | ||||||
Note. SVV DNA–positive ganglia were analyzed for virus RNA.
TG = trigeminal ganglia; TH = thoracic ganglia; skin = area of skin rash.
Primers specific for SVV ORF 21 or GAPdH were used in PCR.
Primers specific for SVV ORFs 61, 63, 40, and 9 or GAPdH were used in PCR portion of RT-PCR.
Immunohistochemistry.
1–2 copies detected.
<2 copies detected.
Table 4.
Detection of SVV DNA, RNA, and glycoprotein in tissues of irradiated African green monkeys treated with tacrolimus
| Monkey no. | Tissuea | DNA PCRb |
cDNA PCRc |
IHCd (gH + gL) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 63 | GAPdH | 61 | 63 | 40 | 9 | GAPdH | |||
| HC01 | Lung | − | ++e | + | |||||
| TG | − | ++ | + | ||||||
| Cervical | − | ++ | − | ||||||
| TH | − | ++ | + | ||||||
| Lumbar | − | ++ | − | ||||||
| Sacral | − | ++ | + | ||||||
| Skin (lumbar) | NAf | ||||||||
| HC06 | Lung | − | ++ | + | |||||
| Cervical | − | ++ | − | ||||||
| TH | − | ++ | + | ||||||
| Lumbar | − | ++ | − | ||||||
| Sacral | − | ++ | + | ||||||
| Skin (lumbar) | + | ||||||||
| HC07 | Lung | − | ++ | + | |||||
| TG | +g | ++ | − | − | − | − | + | NA | |
| Cervical | − | ++ | + | ||||||
| TH | − | ++ | + | ||||||
| Lumbar | − | ++ | + | ||||||
| Sacral | − | ++ | + | ||||||
| Skin (sacral) | + | ||||||||
| HC08 | Lung | − | ++ | + | |||||
| TG | − | ++ | + | ||||||
| Cervical | − | ++ | + | ||||||
| TH | − | ++ | + | ||||||
| Lumbar | − | ++ | + | ||||||
| Sacral | − | ++ | + | ||||||
| Skin (thorax) | + | ||||||||
| HC02 (untreated control) |
Lung | − | ++ | − | |||||
| Cervical | − | ++ | − | ||||||
| TH | − | ++ | − | ||||||
| Lumbar | ++ | ++ | − | − | − | − | ++ | − | |
| Sacral | − | ++ | − | ||||||
Note. SVV DNA–positive ganglia were analyzed for virus RNA.
TG = trigeminal ganglia; TH = thoracic ganglia; skin = area of skin rash.
Primers specific for SVV ORF 63 or GAPdH were used in PCR.
Primers specific for SVV ORFs 61, 63, 40, and 9 or GAPdH were used in PCR portion of RT-PCR.
Immunohistochemistry.
1–2 copies detected.
NA = not available.
<2 copies detected.
Table 3.
Detection of SVV DNA, RNA, and glycoprotein in tissues of irradiated African green monkeys
| Monkey no. | Tissuea | DNA PCRb |
cDNA PCRc |
IHCd (gH + gL) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 63 | GAPdH | 61 | 63 | 40 | 9 | GAPdH | |||
| GV71 | Lung | − | ++e | + | |||||
| TG | − | ++ | + | ||||||
| Cervical | − | ++ | − | ||||||
| TH | ++ | ++ | − | − | − | − | ++ | + | |
| Lumbar | − | ++ | − | ||||||
| Sacral | − | ++ | − | ||||||
| GV72 | Lung | − | ++ | + | |||||
| TG + cervicalf | +g | ++ | − | − | − | − | ++ | +h | |
| TH | − | ++ | − | ||||||
| Lumbar | − | ++ | + | ||||||
| Sacral | − | ++ | + | ||||||
| GV73 | Lung | − | ++ | − | |||||
| TG + cervicalf | − | ++ | +h | ||||||
| TH | − | ++ | − | ||||||
| Lumbar | − | ++ | + | ||||||
| Sacral | − | ++ | − | ||||||
| GV74 | Lung | − | ++ | + | |||||
| TG + cervicalf | − | ++ | − h | ||||||
| TH | − | ++ | − | ||||||
| Lumbar | − | ++ | + | ||||||
| Sacral | − | ++ | + | ||||||
| Skin (lumbar) | + | ||||||||
| GV75 (untreated control) |
Lung | − | ++ | + | |||||
| TG + cervicalf | ++ | ++ | − | − | − | − | ++ | h | |
| TH | − | ++ | − | ||||||
| Lumbar | − | ++ | − | ||||||
| Sacral | − | ++ | − | ||||||
Note. SVV DNA–positive ganglia were analyzed for virus RNA.
TG = trigeminal ganglia; TH = thoracic ganglia; skin = area of skin rash.
Primers specific for SVV ORF 63 or GAPdH were used in PCR.
Primers specific for SVV ORFs 61, 63, 40, and 9 or GAPdH were used in PCR portion of RT-PCR.
Immunohistochemistry.
1–2 copies detected.
Pooled trigeminal and cervical ganglia.
<2 copies detected.
Sections of cervical ganglia only.
RNA was extracted from the remaining portion of the SVV DNA–positive ganglia and examined by real-time reverse transcriptase–polymerase chain reaction (RT-PCR) for SVV open reading frame (ORF) 61 (immediate-early), 63 (immediate-early), 40 (late), and 9 (late) transcripts (Tables 2-4). SVV ORF 61 is one of the most abundantly expressed virus genes in latently infected ganglia in rhesus macaques (Messaoudi et al, 2009). VZV ORF 63 is the most frequent and abundantly transcribed VZV gene in latently infected human ganglia (Cohrs and Gilden, 2007). SVV ORF 63 is also transcribed and translated in ganglia of latently infected rhesus macaques (Messaoudi et al, 2009). Because SVV ORFs 40 and 9 encode SVV capsid proteins, their transcription in ganglia would indicate reactivation. Three SVV transcripts (ORFs 61, 63, and 9) were detected in one monkey (GP06 in group 1), and ORF 9–specific transcripts were detected in the group 1 control monkey (Table 2); no SVV transcripts were detected in ganglia from any other monkeys (Tables 2-4).
Detection of SVV glycoproteins in nonganglionic and ganglionic tissues
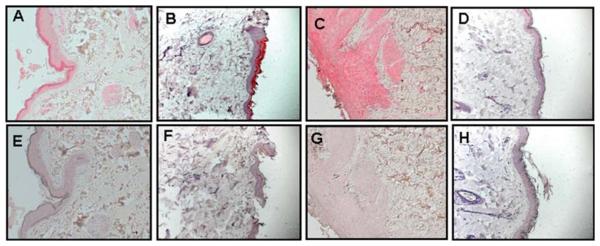
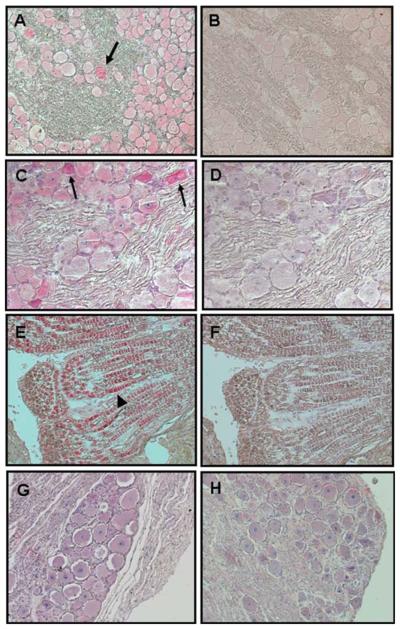
Sections containing punch biopsies from the areas of the skin rash were analyzed immunohistochemically with antibodies specific for SVV glycoproteins H and L. SVV antigen was detected in necrotic skin with zoster rash in monkeys GP07 (group 1) (Figure 3A and Table 2), GV74 (group 2) (Figure 3B and Table 3), and HC06 (group 3) (Figure 3C and Table 4). SVV glycoproteins H and L were not detected in normal skin from a latently infected monkey (Figure 3D). SVV glycoproteins were also detected in skin obtained from monkey HC08 (group 3) during acute varicella (Figure 4A) and during zoster (Figure 4B).
Figure 3.
Detection of SVV antigens in zoster rash of immunosuppressed monkeys. Immunohistochemistry using rabbit polyclonal antibodies against SVV glycoproteins H and L revealed SVV antigen in sections of skin from the area of zoster in monkeys GP07 (group 1) (A), GV74 (group 2) (B), and HC06 (group 3) (C), whereas staining of the respective adjacent sections with normal rabbit serum revealed no signal (E, F, and G). Rabbit polyclonal antibodies against SVV glycoproteins H and L (D) and normal rabbit serum (H) did not detect SVV antigens in normal skin from a latently infected monkey. (Magnification, ×200.)
Figure 4.
Detection of SVV antigens during varicella and zoster rash in the same monkey. Immunohistochemistry using rabbit polyclonal antibodies against SVV glycoproteins H and L revealed SVV antigen in a section of skin rash (varicella) (A) obtained 20 days post infection and from another section of skin (zoster) from monkey HC08 (group 3) (B). Adjacent sections stained with normal rabbit serum (D and E) and normal skin from a latently infected monkey stained with anti-SVV glycoprotein antibody were negative (C and F). (Magnification, ×200.)
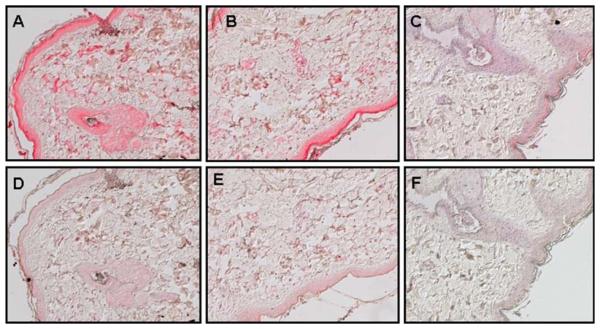
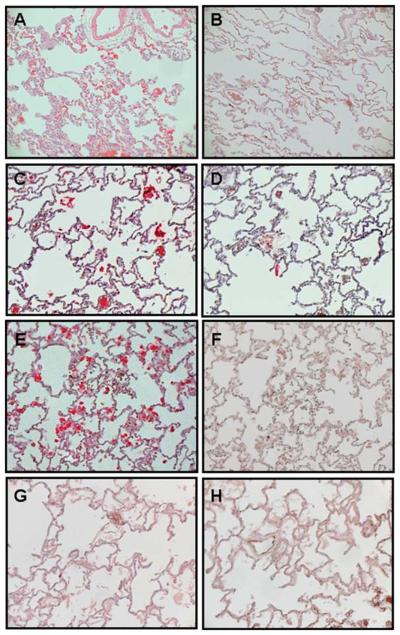
In group 1, SVV glycoproteins were detected in lung from monkeys GP04, GP06, GP07, and control monkey GP05 but not in lung from monkey GP02 (Figure 5A, B and Table 2), wereas lung from all 5 monkeys in group 2 expressed SVV glycoproteins (Figure 5C, D and Table 3). In group 3, SVV glycoproteins were detected in lung from all 4 immunosuppressed monkeys but not in the control monkey (Figure 5E–H and Table 4).
Figure 5.
Detection of SVV antigens in lung from immunosuppressed monkeys. Immunohistochemistry using rabbit polyclonal antibodies against SVV glycoproteins H and L revealed viral antigens in alveoli from immunosuppressed monkeys GP07 (group 1) (A), GV74 (group 2) (C), and HC06 (group 3) (E). The respective adjacent sections stained with normal rabbit serum were negative (B, D, and F). Lung from control monkey HC02 (group 3) stained with rabbit polyclonal antibodies against SVV glycoproteins H and L (G) or normal rabbit serum (H) was negative for SVV antigen. (Magnification, ×200.)
Multiple ganglia located on the side of the neuraxis opposite to ganglia used for DNA and RNA analysis were examined in all three groups by immunohistochemistry using antibodies specific for SVV glycoproteins H and L. In group 1, these SVV antigens were detected in neuronal cytoplasm in all of 3 ganglia from monkey GP02, in 2 of 4 ganglia from monkey GP04, in 2 of 4 ganglia from monkey GP06, but not in any ganglia from monkey GP07 or from control monkey GP05 (Figure 6A, B, H and Table 2). In group 2, SVV antigen was found in the neuronal cytoplasm in 2 of 5 ganglia from monkey GV71, 3 of 4 ganglia from monkey GV72, in 2 of 4 ganglia from monkey GV73, in 2 of 4 ganglia from monkey GV74, but not in any ganglia of untreated monkey GV75 (Figure 6C, D, G and Table 3). In group 3, SVV antigen was detected in the neuronal cytoplasm in 3 of 5 ganglia from monkey HC01 and in 2 of 4 ganglia from monkey HC06. In monkey HC07, SVV glycoproteins were detected in the neuronal cytoplasm in 3 of 4 ganglia and in the axons of 1 of 4 cervical ganglia (Figure 6E, F and Table 4). In monkey HC08, SVV glycoproteins were detected in the neuronal cytoplasm in 3 of 4 ganglia (Table 4) and in nonneuronal cells in 1 of 4 lumbar ganglia (data not shown). SVV glycoproteins were not detected in any ganglia of untreated monkey HC02.
Figure 6.
Detection of SVV antigens in ganglia from immunosuppressed monkeys. Immunohistochemistry using rabbit polyclonal antibodies against SVV glycoproteins H and L shows viral antigens in neuronal cytoplasm in cervical ganglia from monkey GP06 (group 1) (A), in lumbar ganglia from monkey GV74 (group 2) (C), and in axons in cervical ganglia from monkey HC07 (group 3) (E). Normal rabbit serum showed no such staining in the respective adjacent sections (B, D, and F). Arrows in A and C indicate SVV antigen-positive neurons. Arrowhead in E indicates SVV antigen-positive axons. No virus antigen was detected using rabbit polyclonal antibodies against SVV glycoproteins H and L in cervical ganglia from monkey GV75 (group 2) (G) or cervical ganglia from monkey GP07 (group 1) (H). (Magnification, ×200.)
As summarized in Table 5, zoster rash developed in 3 of 4 monkeys treated with tacrolimus, in 1 of 4 irradiated monkeys, and in all 4 monkeys exposed to irradiation and treated with tacrolimus; rash did not develop in any of the 3 monkeys that were not immunosuppressed. SVV glycoproteins were detected in lung (3 of 4) and in ganglia (3 of 4) of monkeys treated with tacrolimus. In untreated monkeys, SVV antigen was detected in lung from 2 of 3 monkeys, but not in ganglia from any of the 3 monkeys.
Table 5.
Summary of results of immunosuppression in cynomolgus and African green monkeys
| Treatment |
||||||||
|---|---|---|---|---|---|---|---|---|
| Tacrolimus (cynomolgus) |
Irradiation (African green) |
Tacrolimus + irradiation (African green) |
Control | |||||
| Rasha | 3/4 | 1/4 | 4/4 | 0/3 | ||||
| SVV antigenb | Lung | Ganglia | Lung | Ganglia | Lung | Ganglia | Lung | Ganglia |
| 3/4 | 3/4 | 4/4 | 4/4 | 4/4 | 4/4 | 2/3 | 0/3 | |
Number of monkeys with zoster/number of monkeys treated.
Number of monkeys in which antigen was detected/number of monkeys treated.
Discussion
The objective of this study was to determine the respective roles of x-irradiation and the immunosuppressive drug tacrolimus in inducing reactivation of latent SVV in monkeys. Zoster rash developed in 3 of 4 latently infected cynomolgus monkeys treated with tacrolimus, 1 of 4 latently infected irradiated AG monkeys, and all 4 latently infected AG monkeys treated with tacrolimus and irradiated (Figure 1). Zoster rash was confirmed by immunohistochemical detection of SVV glycoproteins in sections of punch biopsies of skin rash, as well as in nonganglionic and ganglionic tissues. Overall, despite a small sample size necessitated by cost considerations, tacrolimus treatment produced reactivation in more monkeys than irradiation alone; furthermore, irradiation and tacrolimus increased the frequency of SVV reactivation as compared to either treatment alone, although each was capable of inducing SVV reactivation.
In humans, tacrolimus interferes with cell-mediated immunity by altering antigen presentation by dendritic cells and the function of regulatory T cells (Caproni et al, 2006). Irradiation in mice reduces interferon (IFN)-γ expression and STAT1 signals (Han et al, 2002). In our studies, treatment with tacrolimus alone resulted in a transient reduction of WBCs, whereas irradiation alone or in combination with tacrolimus led to a substantial decrease in WBCs. Similar to earlier findings in monkeys irradiated and treated with tacrolimus and prednisone (Mahalingam et al, 2007), the number of MNCs showing SVV DNA did not increase after varicella or immunosuppression. Although minimal, an SVVspecific antibody response was seen after varicella in monkeys from all three groups. Higher titers of anti-SVV antibody detected after immunosuppression, particularly in monkeys in groups 2 (irradiation) and 3 (tacrolimus and irradiation), is probably a result of the response to the reactivated virus.
We demonstrated, for the first time, the presence of SVV glycoproteins in sections of skin during varicella and zoster in the same monkey, an analysis that can be used for future comparisons of virus-specific immune responses associated with skin rash in both varicella and zoster. Although the major clinical feature during zoster is skin rash, reactivated virus probably spreads to multiple visceral organs. However, analysis of snap-frozen pieces of lung from immunosuppressed monkeys revealed no virus DNA, unlike our earlier observation in monkeys irradiated and treated with tacrolimus and prednisone (Mahalingam et al, 2007). The lack of detection of SVV DNA in lung was not surprising, since SVV glycoproteins, which were detected in sections of paraformaldehyde-fixed lung samples from some immunosuppressed monkeys and in 2 control monkeys (GP05 and GV75) that had been subjected to the stress of transportation and isolation, were found only in a small (<5%) isolated area of lung sections. Furthermore, DNA and RNA from lung and ganglia were examined 1 to 3 months after reactivation as compared to antigen-positive skin sections that were examined at the time of zoster.
The presence of SVV glycoproteins in lungs from nonimmunosuppressed monkeys is likely due to subclinical reactivation, which has been observed in monkeys and humans (Mahalingam et al, 2007; Kolappaswamy et al, 2007; Kronenberg et al, 2005; Mehta et al, 2004; Ljungman et al, 1986).
Low levels of SVV DNA were found in ganglia in a few monkeys from all three groups. The sensitivity of detection of SVV DNA sequences was 1 copy per 500 ng of total tissue DNA. Cellular DNA (GAPdH) was detected in all samples, suggesting that the limited detection of virus DNA in tissue samples rests on its low prevalence during natural exposure. Analysis of the remaining portion of SVV DNA–positive ganglia for SVV-specific transcripts revealed RNA specific for SVV ORFs 61, 63, and 9, the latter indicating reactivation, in thoracic and lumbar ganglia from one monkey in group 1 that developed zoster after treatment with tacrolimus (GPO6). SVV-specific transcripts were not detected in ganglia from any other monkeys, whereas SVV glycoprotein was detected by immunohistochemistry in at least one ganglion from 11 of 12 immunosuppressed monkeys but not in any of the 3 control monkeys. These results suggest that SVV glycoprotein is a better marker of SVV subclinical reactivation than virus nucleic acids. SVV glycoproteins were detected mostly in neurons and occasionally in non-neuronal cells (data not shown) and axons. SVV antigens are sometimes also found in the nucleus of neurons, further indicating productive infection. Although unlikely, we cannot rule out the effect of biological differences between cynos and AG monkeys in the patterns of SVV reactivation.
Because most if not all transplant recipients are treated with either tacrolimus or irradiation to prevent graft-versus-host disease and more than 30% of these patients are likely to develop zoster within 3 months, an understanding of the role of immunosuppression in varicella reactivation is important. Although a decline in cell-mediated immunity correlates with the incidence of VZV reactivation in humans, virus-specific T cells are not detected in human ganglia latently infected with VZV (Verjans et al, 2007). Thus, although this makes it difficult to examine the role of virus-specific T cells during reactivation, the nonhuman primate model described here can be used to determine the role of cell-mediated immunity in maintenance of VZV latency as well as in reactivation.
Materials and methods
Monkeys
SVV-seronegative cynos and AG monkeys (1 to 5 years old, both male and female) housed in the Tulane National Primate Research Center in Covington, LA, were used in all experiments.
Establishment of latent SVV infection
SVV (Delta herpesvirus strain) isolated from a naturally infected monkey (Erythrocebus patas) was propagated in Vero (African green monkey kidney) cells, and a virus stock was prepared as described (Mahalingam et al, 1992). For each group, 5 SVV-seronegative cynos or AG monkeys were exposed to other monkeys of the same species previously inoculated intratracheally with 104 plaque-forming units (PFU) of SVV as described (Mahalingam et al, 2001, 2007). All monkeys were examined daily and blood was obtained either weekly or biweekly. In all monkeys in all three groups, a mild varicella rash developed 10 to 14 days later. All procedures were performed following guidelines and protocols approved by the Institutional Animal Care and Use Committee of the Tulane National Primate Research Center.
Immunosuppressive regimens
All immunosuppressive treatments have been described (Mahalingam et al, 2007). At 4 months after natural exposure to SVV, group 1 monkeys GP02, 04, 06, and 07 were treated orally with 500 g daily (80 g/kg/day) of tacrolimus (Prograf) for 4 months until euthanized; monkey GP05 was not treated with tacrolimus (Figure 1). In groups 2 and 3, all monkeys, at 5 months after natural SVV infection, were transported approximately 40 miles across Lake Pontchartrain in a van (a 1-h ride) to the Tulane University Cancer Center in New Orleans, LA, for anesthesia followed by irradiation and then transported back to the research facility. Monkeys GV75 and HC02 were not irradiated, but were subjected to the same stress of transportation and anesthesia. All monkeys were anesthetized intramuscularly (IM) with Telazol (tiletamine/zolazepam) (8 mg/kg). Before and after irradiation, all animals were also treated IM with Zofran (ondansetron, 0.1 mg/kg) to prevent nausea and vomiting that often develops after irradiation. Monkeys GV71–74 and HC01, 06, 07, and 08 received total body irradiation consisting of 200 cGy delivered at a dose-rate of <20 cGy/min using a 6-MV x-ray beam for 10 to 20 min: half the dose was delivered with the animal supine and prone, respectively. After irradiation, monkeys in group 3 (HC01, 06, 07, and 08) were treated with tacrolimus as described above; monkey HC02 was not treated.
Harvesting and processing of tissue samples
Areas of varicella or zoster skin rash were punch-biopsied from monkeys under anesthesia and fixed in 4% paraformaldehyde and paraffin-embedded. Lung samples from each monkey were harvested and divided into two portions. One portion was snap-frozen for DNA extraction and the other portion was fixed in 4% paraformaldehyde and paraffin-embedded. Ganglia on the two sides of the neuraxis were kept separately. Ganglia from each dermatome were pooled. Pooled ganglia from specific dermatomes and from one side of the neuraxis were snap-frozen in liquid nitrogen, whereas pooled ganglia from the same dermatomes of the other side of the neuraxis were fixed in 4% paraformaldehyde and paraffin-embedded.
Determination of anti-SVV antibody titers
Titers of anti-SVV antibody in serum obtained from all monkeys, before and after varicella and at necropsy, were determined using a plaque reduction assay (Soike et al, 1991). Serum obtained from an AG monkey inoculated intratracheally with SVV served as a positive control.
DNA and RNA extraction
DNA extraction from blood MNCs and tissue samples, and RNA extraction from lungs and ganglia were performed as described (White et al, 2002a, 2002b; Mahalingam et al, 2007).
Real-time DNA and RT-PCR
Real-time DNA and RT-PCR were conducted as described (Messaoudi et al, 2009). Primers specific for either SVV ORF 21 or 63 were used for real-time DNA PCR, and primers specific for SVV ORFs 9, 40, 61, and 63 were used for real-time RT-PCR. DNA and RNA analysis was performed three times on each sample.
Immunohistochemistry
Immunohistochemical analysis of sections (5 mm) of lung, liver, or ganglia for the presence of SVV glycoproteins H and L was performed as described (Mahalingam et al, 1996, 2007; Messaoudi et al, 2009) using normal rabbit serum (1:2000 dilution) or rabbit polyclonal antibodies against SVV glycoproteins H and L (1:2000 dilution) (Ashburn and Gray, 2002). Each experiment was repeated at least three times.
Acknowledgments
This work was supported in part by Public Health Service grants NS32623 (D.G. and R.M.), AG032958, and AG06127 (D.G.) from the National Institutes of Health, and grants 2M01RR005096, 1G20RR016930, 1G20RR018397, 1G20RR019628, 1G20RR013466, 1G20RR012112, 1G20RR015169, and 2P51RR000164 (V.T.-D.) from the National Center for Research and Resource.
The authors thank Dr. Wayne Gray for providing the SVV gH and gL antibodies, Drs. Jeffrey Bennett and Mark Burgoon for excellent photography, Marina Hoffman for editorial assistance, and Cathy Allen for manuscript preparation.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing the paper.
References
- Ashburn CV, Gray WL. Expression of the simian varicella virus glycoprotein L and H. Arch Virol. 2002;147:335–348. doi: 10.1007/s705-002-8323-6. [DOI] [PubMed] [Google Scholar]
- Caproni M, Torchia D, Antiga E, Volpi W, Bianco ED, Fabbri P. The effects of tacrolimus ointment on regulatory T lymphocytes in atopic dermatitis. J Clin Immunol. 2006;26:370–375. doi: 10.1007/s10875-006-9034-2. [DOI] [PubMed] [Google Scholar]
- Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J Virol. 2007;81:2950–2956. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr T, Bossart W, Wahl C, Binswanger U. Disseminated varicella infection in adult renal allograft recipients: four cases and a review of the literature. Transplantation. 2002;73:608–611. doi: 10.1097/00007890-200202270-00023. [DOI] [PubMed] [Google Scholar]
- Gourishankar S, McDermid JC, Jhangri GS, Preiksaitis JK. Herpes zoster infection following solid organ transplantation: incidence, risk factors and outcomes in the current immunosuppressive era. Am J Transplant. 2004;4:108–115. doi: 10.1046/j.1600-6143.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- Han SK, Song JY, Yun YS, Yi SY. Gamma irradiation-reduced IFN-gamma expression, STAT1 signals, and cell-mediated immunity. J Biochem Mol Biol. 2002;35:583–589. doi: 10.5483/bmbrep.2002.35.6.583. [DOI] [PubMed] [Google Scholar]
- Hukkanen RR, Gillen M, Grant R, Liggitt HD, Kiem HP, Kelley ST. Simian varicella virus in pigtailed macaques (Macaca nemestrina): clinical, pathologic, and virologic features. Comp Med. 2009;59:482–487. [PMC free article] [PubMed] [Google Scholar]
- Kennedy PGE, Grinfeld E, Gow JW. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc Natl Acad Sci U S A. 1998;95:4658–4662. doi: 10.1073/pnas.95.8.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PGE, Grinfeld E, Traina-Dorge V, Gilden DH, Mahalingam R. Neuronal localization of simian varicella virus DNA in ganglia of naturally infected African green monkeys. Virus Genes. 2004;28:273–276. doi: 10.1023/b:viru.0000025774.19557.39. [DOI] [PubMed] [Google Scholar]
- Koc Y, Miller KB, Schenkein DP, Griffith J, Akhtar M, Jardin DJ, Snydman DR. Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant. 2000;6:44–49. doi: 10.1016/s1083-8791(00)70051-6. [DOI] [PubMed] [Google Scholar]
- Kolappaswamy K, Mahalingam R, Traina-Dorge V, Shipley ST, Gilden DH, Kleinschmidt-DeMasters BK, McLeod CG, Jr, Hungerford LL, DeTolla LJ. Disseminated simian varicella virus infection in an irradiated rhesus macaque (Macaca mulatta) J Virol. 2007;81:411–415. doi: 10.1128/JVI.01825-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg A, Bossart W, Wuthrich RP, Cao C, Lautenschlager S, Wiegand ND, Mullhaupt B, Noll G, Mueller NJ, Speck RF. Retrospective analysis of varicella zoster virus (VZV) copy DNA numbers in plasma of immunocompetent patients with herpes zoster, of immunocompromised patients with disseminated VZV disease, and of asymptomatic solid organ transplant recipients. Transpl Infect Dis. 2005;7:116–121. doi: 10.1111/j.1399-3062.2005.00106.x. [DOI] [PubMed] [Google Scholar]
- LaGuardia JJ, Cohrs RJ, Gilden DH. Prevalence of varicella-zoster virus DNA in dissociated human trigeminal ganglion neurons and nonneuronal cells. J Virol. 1999;73:8571–8577. doi: 10.1128/jvi.73.10.8571-8577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MJ, Cai GY, Manchak MD, Pizer LI. Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J Virol. 2003;77:6979–6987. doi: 10.1128/JVI.77.12.6979-6987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman P, Lönnqvist B, Gahrton G, Gahrton G, Ringdén O, Sundqvist VA, Wahren B. Clinical and subclinical reactivations of varicella-zoster virus in immunocompromised patients. J Infect Dis. 1986;153:840–847. doi: 10.1093/infdis/153.5.840. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Clarke P, Wellish M, Dueland AN, Soike KF, Gilden DH, Cohrs R. Prevalence and distribution of latent simian varicella virus DNA in monkey ganglia. Virology. 1992;188:193–197. doi: 10.1016/0042-6822(92)90749-f. [DOI] [PubMed] [Google Scholar]
- Mahalingam R. Simian varicella virus pathogenesis. In: Abendroth A, editor. Current Topics in Microbiology and Immunology. Vol. 342. Springer; Heidelberg: 2010. pp. 309–321. Varicella-zoster virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam R, Traina-Dorge V, Wellish M, Lorino R, Sanford R, Ribka EP, Alleman SJ, Brazeau E, Gilden DH. Simian varicella virus reactivation in cynomolgus monkeys. Virology. 2007;368:50–59. doi: 10.1016/j.virol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Traina-Dorge V, Wellish M, Smith J, Gilden DH. Naturally acquired simian varicella virus infection in African green monkeys. J Virol. 2002;76:8548–8550. doi: 10.1128/JVI.76.17.8548-8550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Cohrs R, Debrus S, Piette J, Rentier B, Gilden DH. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci U S A. 1996;93:2122–2124. doi: 10.1073/pnas.93.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Soike K, White T, Kleinschmidt-DeMasters BK, Gilden DH. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology. 2001;279:339–342. doi: 10.1006/viro.2000.0700. [DOI] [PubMed] [Google Scholar]
- Mandal BK. Herpes zoster and the immunocompromised. J Infect. 1987;14:1–5. doi: 10.1016/s0163-4453(87)90652-9. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–179. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Barron A, Wellish M, Engelmann F, Legasse A, Planer S, Gilden D, Nikolich-Zugich J, Mahalingam R. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog. 2009;5:e1000657. doi: 10.1371/journal.ppat.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Aisa Y, Nakazato T, Yamazaki R, Shimizu T, Mihara A, Yamane A, Ikeda Y, Okamoto A. Tacrolimus and methotrexate for the prophylaxis of graft-versus-host disease after unrelated donor cord blood transplantation for adult patients with hematologic malignancies. Transplant Proc. 2007;39:1615–1619. doi: 10.1016/j.transproceed.2006.12.042. [DOI] [PubMed] [Google Scholar]
- Rifkind D. The activation of varicella-zoster virus infections by immunosuppressive therapy. J Lab Clin Med. 1966;68:463–474. [PubMed] [Google Scholar]
- Schoeb TR, Eberle R, Black DH, Parker RF, Cartner SC. Diagnostic exercise: papulovesicular dermatitis in rhesus macaques (Macaca mulatta) Vet Pathol. 2008;45:592–594. doi: 10.1354/vp.45-4-592. [DOI] [PubMed] [Google Scholar]
- Soike KF, Huang JL, Zhang JY, Bohm R, Hitchcock MJ, Martin JC. Evaluation of infrequent dosing regimens with (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]-cytosine (S-HPMPC) on simian varicella infection in monkeys. Antiviral Res. 1991;16:17–28. doi: 10.1016/0166-3542(91)90055-v. [DOI] [PubMed] [Google Scholar]
- Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TM, Mahalingam R, Traina-Dorge V, Gilden DH. Persistence of simian varicella virus DNA in CD4(+) and CD8(+) blood mononuclear cells for years after intratracheal inoculation of African green monkeys. Virology. 2002a;303:192–198. doi: 10.1006/viro.2002.1664. [DOI] [PubMed] [Google Scholar]
- White TM, Mahalingam R, Traina-Dorge V, Gilden DH. Simian varicella virus DNA is present and transcribed months after experimental infection of adult African green monkeys. J NeuroVirol. 2002b;8:191–203. doi: 10.1080/13550280290049705. [DOI] [PubMed] [Google Scholar]