Abstract
Blockade of epidermal growth factor receptor (EGFR) signaling with specific inhibitors of the EGFR tyrosine kinase retards cellular proliferation and arrests the growth of tumor xenografts. AG1478, an inhibitor of the EGFR tyrosine kinase, is used in laboratory studies; however, its therapeutic potential has not been elucidated. Therefore, we evaluated an aqueous form of AG1478 for its antitumor activity in mice bearing human xenografts expressing the WT EGFR or a naturally occurring ligand-independent truncation of the EGFR [delta2–7 (de2–7) EGFR or EGFRvIII]. Parenteral administration of soluble AG1478 blocked phosphorylation of the EGFR at the tumor site and inhibited the growth of A431 xenografts that overexpress the WT EGFR and glioma xenografts expressing the de2–7 EGFR. Strikingly, even subtherapeutic doses of AG1478 significantly enhanced the efficacy of cytotoxic drugs, with the combination of AG1478 and temozolomide displaying synergistic antitumor activity against human glioma xenografts. AG1478 was also examined in combination with mAb 806, an anti-EGFR antibody that was raised against the de2–7 EGFR but unexpectedly also binds a subset of the EGFR expressed in cells exhibiting amplification of the EGFR gene. The combination of AG1478 and mAb 806 displayed additive, and in some cases synergistic, antitumor activity against tumor xenografts overexpressing the EGFR. Here, we demonstrate that different classes of inhibitors to the EGFR can have synergistic antitumor activity in vivo. These results establish the antitumor efficacy of the EGFR inhibitor AG1478 and provide a rationale for its clinical evaluation in combination with both chemotherapy and other EGFR therapeutics.
The epidermal growth factor receptor (EGFR) is associated with the development and progression of many epithelial cancers (1–3). Consequently, a number of strategies aimed at impeding the function of the EGFR are being developed as potential antitumor therapies (4). These approaches include ligand blocking anti-EGFR antibodies (5) and small molecular weight tyrosine kinase inhibitors (TKIs) specific to the EGFR (6). Two prospective EGFR-specific therapeutics for clinical evaluation include the following: first, the reversible EGFR-specific TKI AG1478 (7) and, second, mAb 806, an antibody directed to the extracellular domain of the EGFR (8–11). We have previously demonstrated that AG1478 has some antitumor activity as a single agent and in one cell line was able to overcome EGFR-induced resistance to cisplatin (12). However, the use of AG1478 as a therapeutic agent alone or in combination with chemotherapy has not been systematically evaluated.
The mAb 806 (8–11) was raised against cells expressing the delta2–7 (de2–7) EGFR, a naturally occurring truncation of the EGFR commonly expressed in glioma (13). This truncation results in the removal of 267 aa from the extracellular domain of the EGFR and renders the receptor unable to bind any known ligand (14). Despite this truncation, the de2–7 EGFR displays low levels of constitutive activation and enhances the tumorgenicity of glioma and breast cells when grown as xenografts in nude mice (15, 16). Unlike other de2–7 EGFR-specific antibodies, which are all specific to the unique de2–7 EGFR junctional peptide, mAb 806 recognizes a different epitope (8, 9). Although mAb 806 recognizes a large fraction of the de2–7 EGFR, it also binds some of the WT EGFR in cells that overexpress the receptor (8, 9). Scatchard analysis has revealed that mAb 806 binds ≈50% of the de2–7 EGFR recognized by mAb DH8.3, an antibody specific for the de2–7 EGFR junctional peptide. In contrast, mAb 806 bound <10% of the WT EGFR overexpressed on A431 cells when compared with the WT EGFR-specific mAb 528. Importantly, mAb 806 does not bind to normal tissue expressing the WT EGFR (8, 9). When used as a single agent, mAb 806 demonstrated significant antitumor activity against human xenografts expressing either the de2–7 or amplified EGFR (10).
In this study, we evaluated the tumor-targeting and antitumor activity of an aqueous formulation of AG1478 against a number of different EGFR-expressing xenografts grown in nude mice. We also assessed the ability of AG1478 to improve the efficacy of several chemotherapy agents including temozolomide, which is often used for the treatment of glioma (17). Finally, we used AG1478 in combination with mAb 806 to see whether disparate inhibitors against the same receptor show increased antitumor potency when used together.
Materials and Methods
Cell Lines and Reagents. The human glioma cell line U87MG.Δ2–7, which expresses recombinant de2–7 EGFR, and the squamous cell carcinoma line A431, which overexpresses the EGFR, have been described (9). HN5 is a head and neck cell line that overexpresses the EGFR (18). mAb 528, which recognizes both de2–7 and WT EGFR, has been described (9). Both mAb 528 and 806 were produced in the Biological Production Facility of the Ludwig Institute, Melbourne, Australia. Production of the tyrphostin AG1478 [4-(3-chloroanilino)-6,7-dimethoxyquinazoline] has been described (7). The AG1478-mesylate (molecular weight 411.1) was manufactured by the Institute of Drug Technology (IDT, Melbourne, Australia). Activity of the AG1478-mesylate was tested in a standard in vitro mitogenic assay by using IL-3-dependent BaF/3 cells transfected with the WT EGFR (19). For animal experiments, the AG1478 was dissolved in 100 mM Captisol (Cydex, Overland Park, KS) at the desired concentration. The concentration of AG1478 in serum and tissues was essentially performed as previously validated (20).
Fluorescence-Activated Cell Sorter Analysis of EGFR Expression. Cells were incubated in serum free media overnight and then incubated with fresh media containing AG1478 or EGF for 10 or 240 min. Cells were then incubated with mAb 806 for 30 min at 4°C with bound antibody detected by using an FITC-coupled goat anti-mouse antibody (Calbiochem). Cells were analyzed on an Epics Elite ESP flow cytometer (Beckman Coulter) and analyzed by using expo for windows.
ELISA Analysis of EGFR Expression. A431 cells were incubated overnight in serum-free media and then incubated with fresh media containing AG1478 for 10 min. Cells were placed in lysis buffer (1% Triton X-100/30 mM Hepes/150 mM NaCl/500 μM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF)/150 nM aprotinin/1 μM E-64 protease inhibitor/0.5 mM EDTA/1 μM leupeptin, pH 7.4) for 1 h at 4°C. Lysates were clarified by centrifugation and diluted with PBS, and the EGFR was assayed by ELISA as described (21).
Antiproliferative Assays. A431 and U87MG.Δ2–7 cells were set up at a density of 2.5 × 103 cells per well in 96-well plates, allowed to adhere overnight, and then incubated with AG1478 or mAb 806 for 48 h. After 0 and 48 h, viable cell number was determined by using the MTS assay (Promega), and the percentage inhibition was calculated by the following formula: 1 - [A490 (48 h) of treated cells - A490 (0 h)]/[A490 (48 h) of control cells - A490 (0 h)] × 100.
Xenograft Models. A431 or U87MG.Δ2–7 tumor cells were inoculated s.c. into both flanks of female BALB/c nu/nu mice. Because of differences in xenograft growth rate, mice were always inoculated with the same cells on each flank. The therapeutic efficacy of AG1478 alone or in combination was investigated in both preventative and established tumor models as described (10). Differences between treatment groups at given time points were tested for statistical significance by using Student's t test.
Immunohistochemistry of Xenografts. Xenografts were embedded in OCT compound (Sakura Finetek, Torrance, CA) and snap frozen. Sections were cut, fixed in acetone for 10 min, and stained with antibodies to the EGFR (sc-03), phosphorylated EGFR (tyrosine 1173), and phosphorylated Akt (serine 473), all purchased from Santa Cruz Biotechnology.
Results
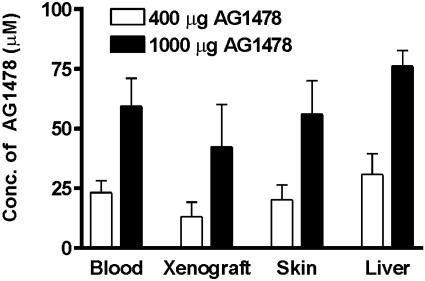
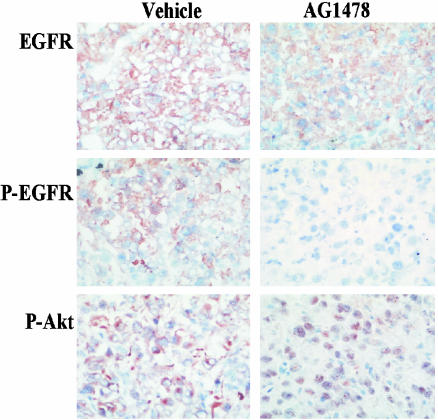
Biodistribution of Soluble AG1478. We have previously demonstrated that serum levels of soluble AG1478 peaked 30 min after s.c. administration (20). Accordingly, the level of AG1478 in normal tissue and U87MG.Δ2–7 xenografts was determined at this time point. Serum AG1478 levels were proportional to dose and consistent between mice, with a mean ± SD concentration of 23 ± 5 μM observed 30 min after a 400-μg i.p. injection and 59 ± 12 μM after a 1-mg injection (Fig. 1). The amount of AG1478 measured in the liver, skin, and xenografts was also proportional to dose 30 min after injection (Fig. 1). Analyses of tissues, including xenografts, at 24 h postinjection showed that AG1478 was no longer detectable (data not shown). The mean concentration seen in the xenografts (13 and 42 μM after a 400- and 1,000-μg dose, respectively) is sufficient to inhibit EGFR phosphorylation at both dose levels (22). Indeed, AG1478 clearly reduced the amount of phosphorylated de2–7 EGFR as assessed by immunohistochemistry (Fig. 2) 30 min after injection. Furthermore, the level of phosphorylated Akt at the plasma membrane, a downstream target of the EGFR, was also clearly reduced (Fig. 2). Interestingly, the remaining phosphorylated Akt had moved in to the nucleus at this time point (Fig. 2), possibly reflecting a survival response to the sudden switching-off of EGFR signaling. Skin samples from AG1478-treated mice stimulated ex vivo with EGF displayed reduced phosphorylation of the murine EGFR in response to ligand, confirming that AG1478 in skin (Fig. 1) is active (data not shown).
Fig. 1.
Biodistribution of soluble AG1478. Mice with U87MG.Δ2–7 xenografts (n = 4) were injected i.p. with AG1478 at the doses indicated and killed 30 min later. Xenografts and tissues were removed and analyzed for levels of AG1478. Data are expressed as mean AG1478 concentration. (Bars = SD.)
Fig. 2.
Immunohistochemistry analysis of U87MG.Δ2–7 xenografts treated with AG1478. U87MG.Δ2–7 xenografts were collected 30 min after injection of vehicle (Left) or AG1478 (1,000 μg) (Right) as described in Fig. 1. Frozen sections were cut and stained for expression of total EGFR, phosphorylated EGFR, and phosphorylated Akt.
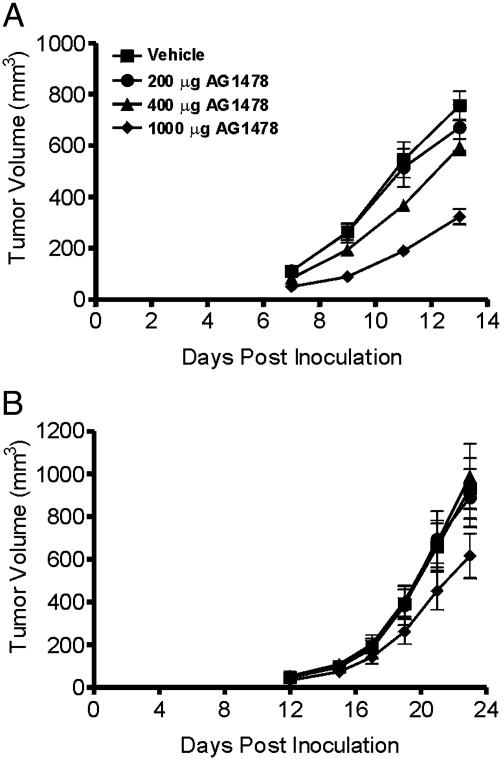
Treatment of Xenografts with AG1478. Mice with either A431 or U87MG.Δ2–7 xenografts were treated i.p. three times per week for 2 weeks with escalating doses of AG1478 in a preventative model to determine its efficacy as a single agent (Fig. 3). AG1478 significantly inhibited the growth of A431 xenografts at the 400-μg (P < 0.01) and the 1,000-μg (P < 0.0001) injection level in a dose-dependent manner (Fig. 3A). Mean tumor volume 13 days after inoculation was 760, 670, 590, and 320 mm3 for the vehicle and 200-, 400-, and 1,000-μg groups, respectively. AG1478 significantly inhibited the growth of U87MG.Δ2–7 xenografts only at the 1,000-μg dose (P < 0.05) (Fig. 3B), suggesting that it may be more difficult to inhibit tumors expressing the constitutively active de2–7 EGFR. Weight loss (≈10%) was observed in mice that received 1,000 μg of AG1478 whereas no weight loss was noted at lower doses.
Fig. 3.
Treatment of A431 (A) and U87MG.Δ2–7(B) xenografts with AG1478 in a preventative model. A431 or U87MG.Δ2–7 cells were injected s.c. into both flanks of nude mice (n = 4) at day 0. Mice were then injected i.p. three times per week for 2 weeks with the indicated dose of AG1478 on days 0, 2, 4, 7, 9, and 11 post tumor inoculation. Data are expressed as mean tumor volume. (Bars = SE.)
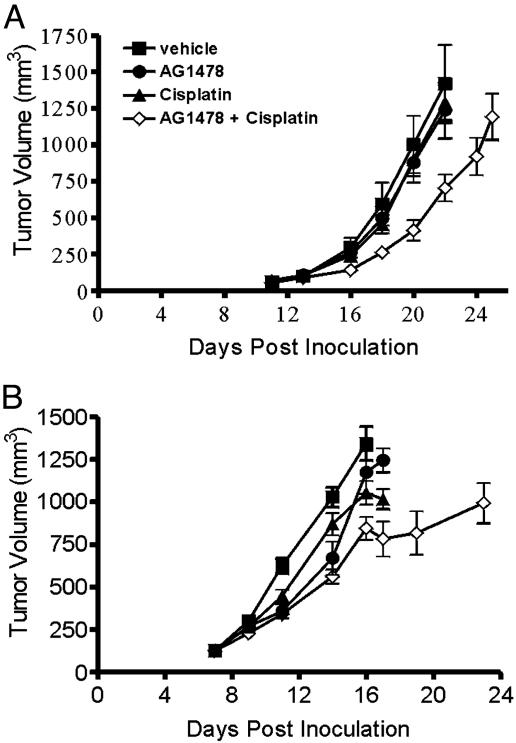
Treatment of Xenografts with AG1478 and Cisplatin in Combination. We examined whether aqueous AG1478 could potentiate the antitumor activity of cytotoxic drugs. To observe genuine enhancement of efficacy, these studies were performed by using doses of AG1478 and cisplatin that were relatively ineffective as a single agent (Fig. 4). Three coinjections of cisplatin and AG1478 given every second day displayed significantly higher antitumor activity against established U87MG.Δ2–7 and A431 xenografts than either agent used alone (Fig. 4). With respect to the U87MG.Δ2–7 xenografts, the combination of AG1478 and cisplatin caused significant inhibition of xenograft growth when compared with the vehicle (P < 0.01), the AG1478 alone (P < 0.01), or cisplatin alone (P < 0.002) treatment group at day 22 post tumor inoculation. At this time, the mean tumor volume was 1,420, 1,240, 1,290, and 700 mm3 for the vehicle, AG1478, cisplatin, and combination groups, respectively (Fig. 4A). The combination of AG1478 and cisplatin also caused significantly higher inhibition of A431 xenografts when compared with vehicle (P < 0.001), the AG1478 alone (P < 0.02), or cisplatin alone (P < 0.02) treatment group at day 16 (Fig. 4B). Some weight loss (10–15%) was observed in the combination treatment groups. Thus, the combination of AG1478 and cisplatin had significant antitumor activity at doses that were ineffective when either agent was used alone.
Fig. 4.
Treatment of established U87MG.Δ2–7 (A) and A431 (B) xenografts with AG1478 and cisplatin in combination. U87MG.Δ2–7 or A431 cells were injected s.c. into both flanks of nude mice (n = 5) at day 0. Once xenografts were established, mice were injected i.p. three times with AG1478 (400 μg) or cisplatin (1.0 and 1.5 mg/kg for U87MG.Δ2–7 and A431 xenografts, respectively) as single agents, or in combination, on days 13, 15, and 17 for U87MG.Δ2–7 xenografts or days 7, 9, and 11 for A431 xenografts. Data are expressed as mean tumor volume. (Bars = SE.)
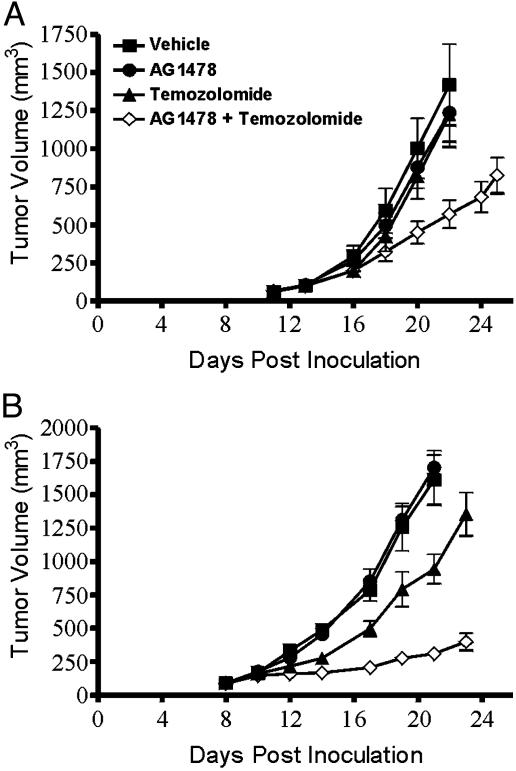
Treatment of U87MG.Δ2–7 Xenografts with AG1478 and Temozolomide in Combination. Because temozolomide has been shown to have efficacy in advanced glioblastoma, we investigated whether AG1478 enhanced its efficacy against U87MG.Δ2–7 human glioma xenografts. The combination of AG1478 and temozolomide caused significant inhibition of tumor growth compared with vehicle control (P < 0.005), AG1478 alone (P < 0.005), and temozolomide alone (P < 0.005) treatment groups at day 22 post tumor inoculation (Fig. 5A) when coinjected three times every second day. At day 22 post tumor inoculation, the mean tumor volume was 1,420, 1,240, 1,230, and 570 mm3 for the vehicle, AG1478, temozolomide, and combination groups, respectively (Fig. 5A). Thus, the combination of AG1478 and temozolomide had significant antitumor activity at doses that were ineffective when either agent was used alone. A second experiment using a higher dose of temozolomide (5 mg/kg vs. 2.5 mg/kg) and a different dose schedule (temozolomide once per week and AG1478 three times per week for 2 weeks) was performed to optimize the antitumor effect of the combination therapy (Fig. 5B). Although AG1478 had no efficacy as a single agent, it clearly displayed synergistic antitumor activity when used in combination with temozolomide (P < 0.0001 for combination therapy compared with temozolomide alone), leading to an 80% reduction in xenograft growth (Fig. 5B). The combination of AG1478 and temozolomide together did not produce any weight loss.
Fig. 5.
Treatment of established U87MG.Δ2–7 xenografts with AG1478 and temozolomide in combination. U87MG.Δ2–7 were injected s.c. into both flanks of nude mice (n = 5) at day 0. Treatment of mice commenced once xenografts were established. In A, mice were injected i.p. three times with AG1478 (400 μg) or temozolomide (2.5 mg/kg) as single agents, or in combination, on days 13, 15, and 17. In B, mice were injected with AG1478 (500 μg) on days 8, 10, 12, 15, 17, and 19 or temozolomide (5 mg/kg) on days 8 and 15 as single agents or in combination. Data are expressed as mean tumor volume. (Bars = SE.)
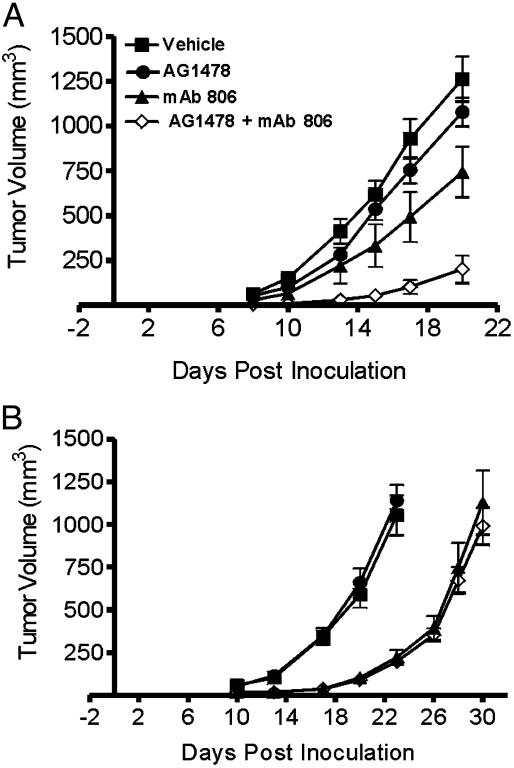
Treatment of Xenografts with a Combination of AG1478 and mAb 806 in Preventative Models. Given the differences in mode and site of action between antibodies and TKIs, we decided to examine whether the combination of AG1478 and EGFR antibody mAb 806 has enhanced antitumor activity. Although suboptimal doses of both AG1478 and mAb 806 inhibited the growth of A431 xenografts in a preventative model when used alone, the combination of both agents displayed synergistic antitumor activity (Fig. 6A). At day 20 post tumor inoculation, the A431 xenografts treated with both agents had a mean volume of 200 mm3, significantly lower than vehicle (1,260 mm3, P < 0.0001), the AG1478 alone (1,080 mm3, P < 0.0001), and mAb 806 alone (740 mm3, P < 0.002) groups. Furthermore, although the take rate of xenografts was 100% in the vehicle and single agent groups, only 60% of tumors successfully established in the group treated with both AG1478 and mAb 806. In contrast to A431 tumors, treatment of U87MG.Δ2–7 xenografts with AG1478 and mAb 806 in combination was no more effective than mAb 806 alone (Fig. 6B).
Fig. 6.
Combination treatment of A431 (A) and U87MG.Δ2–7 (B) xenografts with AG1478 and mAb 806 in a preventative model. A431 or U87MG.Δ2–7 cells were injected s.c. into both flanks of nude mice (n = 5) at day 0. Mice were then injected i.p. three times per week for 2 weeks with mAb 806 (0.1 mg) on days -1, 1, 3, 6, 8, and 10 or AG1478 (400 μg) on days 0, 2, 4, 7, 9, and 11 as single agents or in combination. Data are expressed as mean tumor volume. (Bars = SE.)
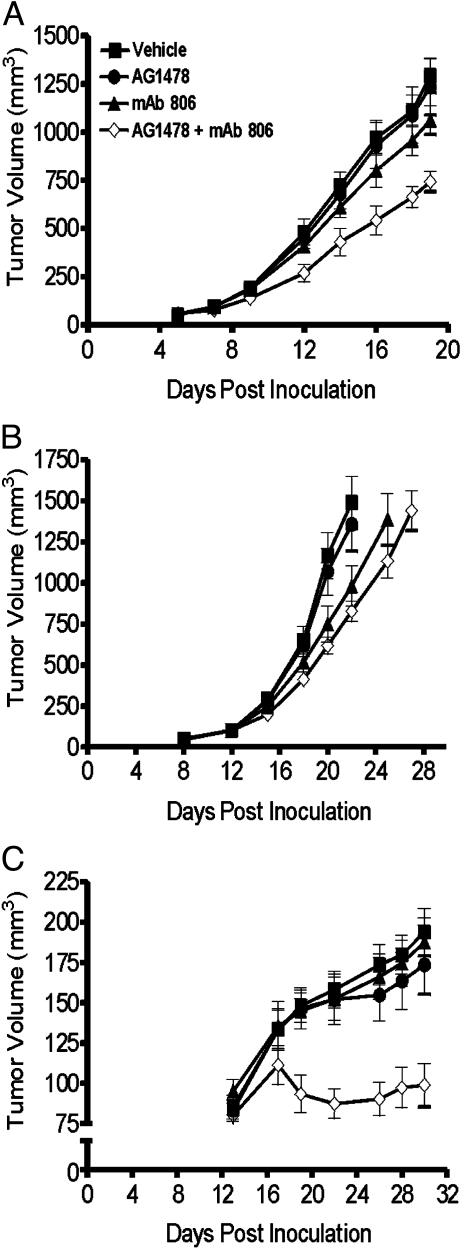
Treatment of Established Xenografts with a Combination of AG1478 and mAb 806. The efficacy of the combination EGFR therapy was also tested against established A431 or U87MG.Δ2–7 xenografts. When used as a single agent at this dose, AG1478 had no effect on the growth of A431 xenografts whereas mAb 806 caused slight inhibition (P < 0.03) (Fig. 7A). In contrast, the combination of AG1478 and mAb 806 produced significant growth inhibition that was greater than additive. At day 19 post tumor inoculation, the A431 xenografts treated with both agents had a mean volume of 740 mm3, which was significantly smaller than vehicle control (1,290 mm3, P < 0.0001), AG1478 alone (1,240 mm3, P < 0.002), and mAb 806 alone (1,060 mm3, P < 0.001) treatment groups (Fig. 7A). As in the preventative model, treatment of U87MG.Δ2–7 xenografts with AG1478 and mAb 806 in combination was no more effective than mAb 806 alone (Fig. 7B). We extended our studies by treating HN5 xenograft bearing mice with a combination of AG1478 and mAb 806. Treatment of established HN5 xenografts with AG1478 and mAb 806 as single agents had no effect on tumor growth at the doses used (Fig. 7C). In comparison, the combination of these agents virtually abrogated the growth of HN5 xenografts (P < 0.0001, 0.002, and 0.0001 compared with vehicle, AG1478 alone, and mAb 806 alone, respectively) and actually induced tumor regression between day 17 and 22 post inoculation, indicative of a synergistic antitumor effect (Fig. 7C). No weight loss was observed in any of the treatment groups.
Fig. 7.
Combination treatment of A431 (A), U87MG.Δ2–7 (B), and HN5 (C) xenografts with AG1478 and mAb 806 in an established model. A431, U87MG.Δ2–7, or HN5 cells were injected s.c. into both flanks of nude mice (n = 6) at day 0. Treatment of mice commenced when xenografts were established. (A) Mice with A431 xenografts were then injected i.p. three times per week for 2 weeks with mAb 806 (0.1 mg) on days 5, 7, 9, 12, 14, and 16 or AG1478 (400 μg) on days 6, 8, 10, 13, 15, and 17 as single agents or in combination. (B) Mice with U87MG.Δ2–7 xenografts were injected (as for A) with mAb 806 on days 12, 14, 16, 19, 21, and 23 or AG1478 on days 13, 15, 17, 20, 22, and 24. (C) Mice with HN5 xenografts were injected (as for A) with mAb 806 on days 13, 15, 17, 20, 22, and 24 or AG1478 on days 14, 16, 18, 21, 23, and 25. Data are expressed as mean tumor volume. (Bars = SE.)
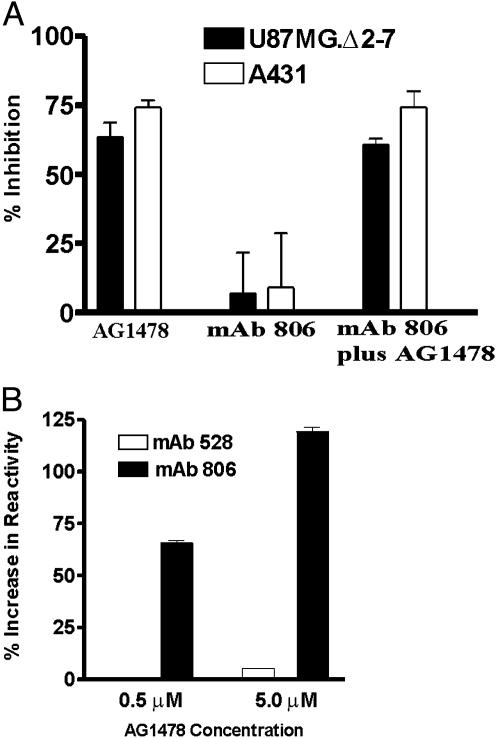
In Vitro Studies with AG1478 and mAb 806. We compared the in vitro anti-proliferative effects of AG1478 and mAb 806 on A431 and U87MG.Δ2–7 cells. AG1478 was able to inhibit the proliferation of both cells lines even in the presence of 10% FCS (P < 0.0001 in both cases) (Fig. 8A). In contrast, mAb 806 had minimal effect on the proliferation of A431 and U87MG.Δ2–7 cells grown in 10% FCS (data not shown) or 0.5% FCS (Fig. 8A), even though the concentration used exceeded that which can be achieved at the tumor site in vivo. Furthermore, the combination of AG1478 and mAb 806 had no greater in vitro efficacy than AG1478 alone (Fig. 8A). This result indicates that these EGFR therapeutics have different modes of action and that the antitumor activity of mAb 806 is observed only in vivo.
Fig. 8.
In vitro effects of AG1478 and mAb 806. (A) Antiproliferative activity of AG1478 and mAb 806. A431 and U87MG.Δ2–7 cells were treated in vitro with AG1478 (6 μM) or mAb 806 (10 μg/ml) alone or in combination, and the data were expressed as percentage inhibition of cell growth. (Bars = SD.) (B) Effect of AG1478 treatment on antibody reactivity. A431 cells were treated for 10 min with AG1478 at the doses indicated, after which cells were lysed and the amount of EGFR was recognized by mAb 806 or 528 quantified by ELISA. Data are expressed as the percentage increase in antibody reactivity. (Bars = SD.)
Because mAb 806 binds only 5–10% of the EGFR on the surface of A431 cells, we examined whether AG1478 increased the level of mAb 806 reactivity. By using a quantitative ELISA to measure total cellular EGFR, it was apparent that even a 10-min exposure to 0.5 or 5 μM AG1478 increases the level of mAb 806 reactivity in a dose-dependent manner (Fig. 8B). The level of mAb 528 reactivity also increased but was more modest in percentage terms. We sought to confirm that mAb 806 reactivity was also increased on the cell surface. To achieve this result, we compared the binding of mAb 806 to U87MG.Δ2–7 and U87MG.wtEGFR cells after AG1478 treatment. The U87MG.wtEGFR cells overexpress the EGFR and were used because they could be directly compared with the U87MG.Δ2–7 cells. Treatment with 2 μM AG1478 modestly, but reproducibly, increased the surface binding of mAb 806 to U87MG.wtEGFR cells at 10 and 240 min (Table 1). In comparison, AG1478 treatment under identical conditions actually reduced binding of mAb 806 to the surface of U87MG.Δ2–7 (Table 1). Treatment of cells with 100 ng/ml of EGF, which induces EGFR down-regulation, caused a reduction of mAb 806 reactivity as expected.
Table 1. Surface binding of mAb 806 after treatment with 2 μM AG1478.
| Relative mean fluorescence, %
|
||||
|---|---|---|---|---|
| Cell line | Control | AG1478 (10 min) | AG1478 (240 min) | EGF (10 min) |
| U87MG.wtEGFR | 100 | 115 | 117 | 46 |
| U87MG.Δ2–7 | 100 | 91 | 72 | 93 |
Discussion
The quinazoline derivative AG1478 is a specific reversible inhibitor of the EGFR (7). We now demonstrate that a AG1478-mesylate salt, which is soluble in sulfobutylether β-cyclodextrin (Captisol), has significant antitumor activity. Currently, all TKIs undergoing clinical evaluation have been administered via the oral route (4). The oral route was chosen for its ease of administration and capacity to generate sustained serum levels, an outcome considered desirable because it results in the extended inhibition of the EGFR. Despite the advantages of oral administration, the potential benefits of i.v. delivery, which is how our soluble AG1478 would be administered, should be considered. One outcome of i.v. administration would be the ability to obtain higher local concentrations of AG1478 at the tumor site compared with the oral route. Because EGFR signaling can be initiated from a relatively small number of receptors (23), it may be necessary to inhibit a large proportion of the receptors to obtain a complete abrogation of signaling, something more achievable with the i.v route because of the higher concentration of TKI at the tumor site. Furthermore, the brief but repetitive inactivation of the EGFR that would result from i.v. administration may have greater and/or different antitumor effects. Indeed, it has been shown that the brief inactivation of MYC in osteogenic sarcoma cells causes tumor regression, with the subsequent reactivation of MYC inducing apoptosis in the surviving tumor cells (24). Given these considerations and the antitumor activity seen in our animal models, we intend to explore the clinical efficacy of AG1478 after i.v. administration.
High levels of AG1478-mesylate were detected in tumors 30 min after injection, resulting in complete abrogation of EGFR phosphorylation and growth inhibition of A431 xenografts. The antitumor effect against A431 xenografts is smaller than previous TKIs because we used less frequent administration to identify a dose schedule suitable for use with chemotherapy. In contrast to A431 xenografts, AG1478 only slightly inhibited the growth of U87MG.Δ2–7 xenografts at the highest dose. Although the relative resistance of U87MG.Δ2–7 xenografts to AG1478 may simply reflect intrinsic differences in sensitivity between cell lines, we have previously shown that de2–7 EGFR expressing U87MG xenografts are more resistant to AG1478 than U87MG cells overexpressing the WT EGFR (25). This comparative resistance of de2–7 EGFR expressing xenografts to AG1478 was not seen in vitro (Fig. 8), suggesting that some aspects of the enhanced in vivo tumorgenicity mediated by the de2–7 EGFR is not inhibited by AG1478. Indeed, a recent report (26) demonstrated that, in contrast to cells expressing the WT EGFR, the EGFR-specific TKI Iressa was ineffective against intracranial xenografts expressing the de2–7 EGFR.
Two recent reports demonstrated that Iressa enhances the antitumor activity of several cytotoxic agents against a range of tumors (27, 28). These experiments were done using doses of Iressa and the cytotoxic drug that produced significant tumor inhibition when used as single agents. In contrast, we treated established A431 and U87MG.Δ2–7 xenografts with doses of AG1478 and cisplatin that were essentially ineffective as single agents but in combination had a significant antitumor effect. This result demonstrates that, even in the absence of direct antitumor activity, AG1478 can sensitize cells to subsequent treatment with cytotoxic drugs. The combination of AG1478 and temozolomide also significantly inhibited growth of human glioma xenografts, even when used at doses that were refractory as single agents. This report demonstrates that EGFR inhibition enhances the efficacy of temozolomide and provides a strong basis for using AG1478 and temozolomide in combination for the treatment of glioma. Indeed, a recent abstract¶ showed that patients receiving the EGFR inhibitor OSI-774 in combination with temozolomide displayed a low level of clinical response.
A further significant finding in this paper is the observation that AG1478 and mAb 806 in combination had additive and, in the case of HN5, synergistic antitumor activity against xenografts expressing the WT EGFR. Here, we demonstrate that different classes of inhibitor directed to the EGFR can lead to enhanced antitumor activity in vivo in multiple cell lines. A previous study (29) demonstrated that the combination of an EGFR-specific TKI and antibody (i.e., PD153035 and C225) did have an additive antiproliferative effect, but this finding was performed only in vitro against a single cell line. The mAb 806 antibody recognizes only a proportion of the WT EGFR expressed on the surface of tumor cell lines such as A431 and HN5 (8–10). Treatment of cells with AG1478 increased the level of mAb 806 reactivity on the surface of these cell lines. This result is consistent with previous studies (30, 31) that have demonstrated that AG1478 and other TKIs increase the total level of EGFR on the cell surface by reducing receptor internalization. Addition of AG1478 also induces the EGFR to assume an inactive dimer configuration (30); thus, it is possible that mAb 806 preferentially recognizes this conformation of the EGFR. AG1478 did not improve the efficacy of mAb 806 against a de2–7 EGFR-expressing xenograft, further emphasizing that the de2–7 EGFR responds to EGFR inhibitors in a manner different from the WT EGFR. Improved binding of mAb 806 to tumor cells may not be the only mechanism leading to enhanced antitumor activity when AG1478 and mAb 806 are used in combination. It is likely that these two EGFR inhibitors mediate their antitumor action through independent mechanisms although they target the same receptor. Thus, when used in combination, each inhibitor would suppress unique aspects of EGFR signaling and function, leading to the additive or synergistic antitumor activity. The mechanism by which mAb 806 inhibits xenograft growth has only been partially elucidated, largely because it has no antiproliferative or proapoptotic activity in any in vitro system. However, the in vivo antitumor activity of mAb 806 is not mediated by immune effector function (T.G.J, unpublished data) although it does have some antiangiogenic properties, which may partially explain its in vivo activity (9–11).
In conclusion, we have demonstrated that parenteral administration of soluble AG1478 has significant antitumor activity as a single agent, even though this approach only transiently exposes the xenograft to AG1478. Furthermore, nontherapeutic doses of AG1478 significantly enhanced the cytotoxicity of both cisplatin and temozolomide, providing a rationale for the use of AG1478 in glioma patients being treated with temozolomide. Finally, we ascertained that disparate inhibitors to the EGFR can produce synergistic antitumor activities. Overall, these studies provide an impetus for using soluble AG1478 in combination with chemotherapy or mAb 806 in the treatment of patients with EGFR-positive tumors.
Acknowledgments
This work was supported in part by the National Health and Medical Research Council of Australia and the Garnett Passe and Rodney Williams Foundation.
Abbreviations: de2–7, delta2–7; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Footnotes
Prados, M., Chang, S., Burton, E., Kapadia, A., Rabbitt, J., Page, M., Federoff, A., Kelly, S. & Fyfe, G. (2003) Proc. Am. Soc. Clin. Oncol. 22, 99, abstr. 394.
References
- 1.Mendelsohn, J. (2002) J. Clin. Oncol. 20, 1S-13S. [PubMed] [Google Scholar]
- 2.Arteaga, C. L. (2002) Semin. Oncol. 29, 3-9. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson, R. I., Gee, J. M. & Harper, M. E. (2001) Eur. J. Cancer 37, Suppl. 4, S9-S15. [DOI] [PubMed] [Google Scholar]
- 4.de Bono, J. S. & Rowinsky, E. K. (2002) Trends Mol. Med. 8, S19-S26. [DOI] [PubMed] [Google Scholar]
- 5.Herbst, R. S. & Shin, D. M. (2002) Cancer 94, 1593-1611. [DOI] [PubMed] [Google Scholar]
- 6.Wakeling, A. E. (2002) Curr. Opin. Pharmacol. 2, 382-387. [DOI] [PubMed] [Google Scholar]
- 7.Yaish, P., Gazit, A., Gilon, C. & Levitzki, A. (1988) Science 242, 933-935. [DOI] [PubMed] [Google Scholar]
- 8.Jungbluth, A. A., Stockert, E., Huang, H. J., Collins, V. P., Coplan, K., Iversen, K., Kolb, D., Johns, T. J., Scott, A. M., Gullick, W. J., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johns, T. G., Stockert, E., Ritter, G., Jungbluth, A. A., Huang, H. J., Cavenee, W. K., Smyth, F. E., Hall, C. M., Watson, N., Nice, E. C., et al. (2002) Int. J. Cancer 98, 398-408. [DOI] [PubMed] [Google Scholar]
- 10.Luwor, R. B., Johns, T. G., Murone, C., Huang, H. J., Cavenee, W. K., Ritter, G., Old, L. J., Burgess, A. W. & Scott, A. M. (2001) Cancer Res. 61, 5355-5361. [PubMed] [Google Scholar]
- 11.Mishima, K., Johns, T. G., Luwor, R. B., Scott, A. M., Stockert, E., Jungbluth, A. A., Ji, X. D., Suvarna, P., Voland, J. R., Old, L. J., et al. (2001) Cancer Res. 61, 5349-5354. [PubMed] [Google Scholar]
- 12.Han, Y., Caday, C. G., Nanda, A., Cavenee, W. K. & Huang, H. J. (1996) Cancer Res. 56, 3859-3861. [PubMed] [Google Scholar]
- 13.Wikstrand, C. J., Reist, C. J., Archer, G. E., Zalutsky, M. R. & Bigner, D. D. (1998) J. Neurovirol. 4, 148-158. [DOI] [PubMed] [Google Scholar]
- 14.Sugawa, N., Ekstrand, A. J., James, C. D. & Collins, V. P. (1990) Proc. Natl. Acad. Sci. USA 87, 8602-8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishikawa, R., Ji, X. D., Harmon, R. C., Lazar, C. S., Gill, G. N., Cavenee, W. K. & Huang, H. J. (1994) Proc. Natl. Acad. Sci. USA 91, 7727-7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang, C. K., Gong, X. Q., Moscatello, D. K., Wong, A. J. & Lippman, M. E. (2000) Cancer Res. 60, 3081-3087. [PubMed] [Google Scholar]
- 17.Friedman, H. S., Kerby, T. & Calvert, H. (2000) Clin. Cancer Res. 6, 2585-2597. [PubMed] [Google Scholar]
- 18.Kwok, T. T. & Sutherland, R. M. (1991) Br. J. Cancer 64, 251-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker, F., Hibbs, M. L., Zhang, H. H., Gonez, L. J. & Burgess, A. W. (1998) Growth Factors 16, 53-67. [DOI] [PubMed] [Google Scholar]
- 20.Ellis, A. G., Nice, E. C., Weinstock, J., Levitzki, A., Burgess, A. W. & Webster, L. K. (2001) J. Chromatogr. B Biomed. Sci. Appl. 754, 193-199. [DOI] [PubMed] [Google Scholar]
- 21.Schooler, K. & Wiley, H. S. (2000) Anal. Biochem. 277, 135-142. [DOI] [PubMed] [Google Scholar]
- 22.Bishop, P. C., Myers, T., Robey, R., Fry, D. W., Liu, E. T., Blagosklonny, M. V. & Bates, S. E. (2002) Oncogene 21, 119-127. [DOI] [PubMed] [Google Scholar]
- 23.DeWitt, A. E., Dong, J. Y., Wiley, H. S. & Lauffenburger, D. A. (2001) J. Cell Sci. 114, 2301-2313. [DOI] [PubMed] [Google Scholar]
- 24.Jain, M., Arvanitis, C., Chu, K., Dewey, W., Leonhardt, E., Trinh, M., Sundberg, C. D., Bishop, J. M. & Felsher, D. W. (2002) Science 297, 102-104. [DOI] [PubMed] [Google Scholar]
- 25.Nagane, M., Narita, Y., Mishima, K., Levitzki, A., Burgess, A. W., Cavenee, W. K. & Huang, H. J. (2001) J. Neurosurg. 95, 472-479. [DOI] [PubMed] [Google Scholar]
- 26.Heimberger, A. B., Learn, C. A., Archer, G. E., McLendon, R. E., Chewning, T. A., Tuck, F. L., Pracyk, J. B., Friedman, A. H., Friedman, H. S., Bigner, D. D. & Sampson, J. H. (2002) Clin. Cancer Res. 8, 3496-3502. [PubMed] [Google Scholar]
- 27.Sirotnak, F. M., Zakowski, M. F., Miller, V. A., Scher, H. I. & Kris, M. G. (2000) Clin. Cancer Res. 6, 4885-4892. [PubMed] [Google Scholar]
- 28.Ciardiello, F., Caputo, R., Bianco, R., Damiano, V., Pomatico, G., De Placido, S., Bianco, A. R. & Tortora, G. (2000) Clin Cancer Res. 6, 2053-2063. [PubMed] [Google Scholar]
- 29.Bos, M., Mendelsohn, J., Kim, Y. M., Albanell, J., Fry, D. W. & Baselga, J. (1997) Clin. Cancer Res. 3, 2099-2106. [PubMed] [Google Scholar]
- 30.Arteaga, C. L., Ramsey, T. T., Shawver, L. K. & Guyer, C. A. (1997) J. Biol. Chem. 272, 23247-23254. [DOI] [PubMed] [Google Scholar]
- 31.Lichtner, R. B., Menrad, A., Sommer, A., Klar, U. & Schneider, M. R. (2001) Cancer Res. 61, 5790-5795. [PubMed] [Google Scholar]