Abstract
Sirolimus is a potent anti-proliferative agent used clinically to prevent renal allograft rejection. However, little is known about the effects of maintenance immunosuppressive agents on the immune response to potentially protective vaccines. Here we show that sirolimus paradoxically increases the magnitude and quality of the CD8+ T cell response to vaccinia vaccination in non-human primates, fostering more robust recall responses compared to untreated and tacrolimus-treated controls. Enhancement of both the central and effector memory compartments of the vaccinia-specific CD8+ T cell response was observed. These data elucidate new mechanistic characteristics of sirolimus and suggest immune applications extending beyond its role as an immunosuppressant.
Keywords: mTOR, CD8+ T-lymphocyte, vaccine
Introduction
More than 150,000 people in the United States are chronically treated with immunosuppressive drugs to prevent transplant rejection, and these individuals are known to be at increased risk for many infectious diseases (1). However, little is known about the effects of maintenance immunosuppressive agents on the immune response to potentially protective vaccines. In particular, although studies have described antibody responses to influenza and pneumococcus vaccination in immunosuppressed patients, information on cell-mediated responses to vaccines is limited (2). Additionally, vaccination of transplant recipients with live-attenuated vaccines is contraindicated due to the potential for progressive viremia and consequent life-threatening complications. Here we examined antigen-specific CD8+ T cell responses following vaccinia vaccination in non-human primates treated with two commonly used clinical immunosuppressive agents: sirolimus, an inhibitor of the serine/threonine kinase mTOR that controls cell cycle progression, and tacrolimus, a calcineurin phosphatase inhibitor that attenuates TCR- dependent T cell activation (3).
Recent studies have demonstrated the role of sirolimus as a modulator of the innate and adaptive immune responses. Sirolimus is used as a potent maintenance immunosuppressant in kidney transplant recipients (4), and has been shown to increase the proportion of CD4+CD25+FoxP3+ Treg cells in these patients (5). Sirolimus suppresses dendritic cell maturation and differentiation (6), interferes with antigen uptake (7), and inhibits type I interferon production by plasmacytoid dendritic cells (8). The mTOR pathway is a major regulator of pro- and anti-inflammatory cytokine production. For example, sirolimus-treated monocytes induce the polarization of TH1 cells by increased expression of IL-12 (9, 10). Furthermore, in vivo treatment with sirolimus has been reported to enhance protection against Listeria monocytogenes infection in BALB/c mice, an effect attributed to increased IL-12 production by macrophages (10).
Data from a pooled analysis of several clinical trials have demonstrated a decreased risk of viral infections in transplant recipients receiving sirolimus as compared to other immunosuppressive agents, such as calcineurin inhibitors and anti-metabolites (11). This effect has been observed in studies of both bone marrow and solid organ transplantation (12, 13). While the mechanisms of this protection are currently unknown, there has been speculation in the literature that sirolimus may exert this effect directly by inhibiting viral replication (11). It has recently been reported that treatment with sirolimus surprisingly also resulted in increased quantity and quality of memory T cell differentiation in a murine model pathogen infection (14, 15), suggesting that the protective effect of rapamycin in attenuating viral infections in transplant recipients may instead be due to its ability to augment protective cellular immunity following viral infection. Here, we further dissected the impact of sirolimus treatment during vaccinia virus vaccination in non-human primates. Our data reveal that sirolimus paradoxically enhances the magnitude, duration and quality of the antigen-specific CD8+ T cell response to vaccination with MVA in a non-human primate model, compared to untreated controls or primates treated with tacrolimus. These findings highlight the complexity of the effects of sirolimus on the immune system, and raise important questions for its application in infectious disease, cancer, transplantation, and as a potential vaccine immune adjuvant.
Materials and Methods
Rhesus macaques
Sixteen colony bred Rhesus macaques (Macaca mulatta) were used in these experiments. All animals were housed at the Yerkes National Primate Research Center (YNPRC) of Emory University and were cared for in conformance with the guidelines of the Committee on the Care and Use of Laboratory Animal Resources, National Research Council and the Health and Human Services guidelines ‘Guide for the Care and Use of Laboratory Animals’.
Vaccinia immunizations
For experiments described in Figure 1, ten rhesus macaques (RM, Macaca mulatta) were used. Prior to vaccination, 4 animals were treated intramuscularly (IM) with sirolimus, with target blood levels of 5–15 ng/mL (Figure 1A). Two animals were treated with tacrolimus (Prograf) i.m., with target blood levels of 6–8 ng/mL. All animals achieved therapeutic levels of sirolimus or tacrolimus (as indicated) prior to being vacciniated, and maintained levels in the therapeutic range for the entire timecourse of the study. Four animals were kept untreated as healthy controls. Animals were vaccinated and boosted with 108 PFU modified vaccinia Ankara (MVA) i.m. Animals were boosted 28 days after vaccination.
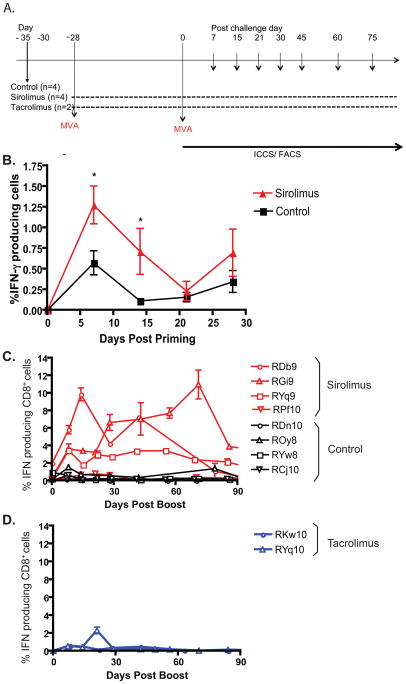
Figure 1. Antigen-specific CD8+ T cell responses to modified vaccinia Ankara (MVA) inrhesus macaques are augmented by sirolimus treatment.
A) Experimental design is depicted. B) Animals were treated with sirolimus or left untreated during primary vaccination with MVA, (n=4 each group). PBMCs isolated at each timepoint were stimulated with vaccinia virus and assessed for IFN-γ. IFN-γ production by CD8+ T cells post priming is shown. Results indicated that sirolimus treatment increased the frequency of antigen-specific CD8+ T cells at weeks 1 and 2 post-priming (p= 0.0216 and p= 0.0322, respectively). C) Animals were treated with sirolimus or left untreated during both vaccination and boost with MVA (n=4 in each group). PBMCs isolated at each timepoint were stimulated with vaccinia virus and assessed for IFN-γ. IFN-γ production by CD8+ T cells post boost is shown. Results indicated that sirolimus treatment increased the frequency of antigen-specific CD8+ T cells at both acute (week 2, p=0.0441) and memory (week 6, p=0.003) timepoints. (D) RM were treated with tacrolimus during both vaccination and boost with MVA (n=2). IFN-γ production post boost is shown; no differences between tacrolimus-treated and untreated animals were observed.
For experiments described in Figure 2, six animals were inoculated with Dryvax (Wyeth, NJ) by scarification in accordance with the US Food and Drug Administration (FDA) guidelines. Briefly, a bifurcated needle was immersed in the vaccine suspension and used to poke the skin 15 consecutive times. At approximately 100 days post vaccination, 3 macaques were treated with sirolimus intramuscularly (IM), with target blood levels of 5–15 ng/mL. 3 macaques were left untreated as controls. At 105 days post vaccination, animals were boosted with 108 PFU Modified Vaccinia Ankara (MVA) IM. Blood was collected at day 0 and weekly post boost to assess viral-specific cytokine responses as described below.
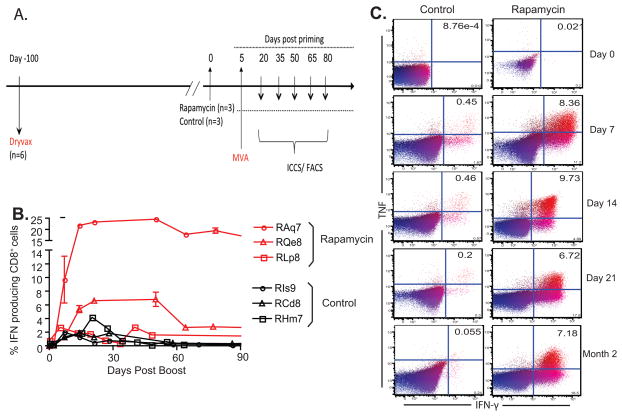
Figure 2. Sirolimus enhances antigen-specific CD8+ T cell responses when administered during secondary rechallenge.
A) Experimental design is depicted. B) RM immunized with Dryvax were boosted with MVA in the presence or absence of sirolimus (n=3 in each group). PBMCs isolated at each timepoint were stimulated with vaccinia virus and assessed for IFN-γ. IFN-γ production by CD8+ T cells post boost is shown. Results indicated that sirolimus treatment increased the frequency of antigen-specific CD8+ T cells at both acute (week 3, p=0.0260) and memory (week 7, p=0.0022) timepoints. c) PBMCs isolated at each timepoint were stimulated with vaccinia virus and assessed for IFN-γ and TNF. Representative examples of TNF and IFN-γ production CD3+ CD8+ cells are shown for time points following MVA booster immunization.
Intracellular Cytokine Staining and Flow Cytometric Analysis
Response to vaccination and boost was determined by intracellular cytokine staining of peripheral blood mononuclear cells (PBMC). PBMC were isolated by density gradient centrifugation. 1.5 million PBMC were incubated at 37° for 15 hours with vaccinia virus (VV) at an MOI of 1 in a volume of 300 ul RPMI containing 10% heat inactivated FBS. Brefeldin A (5 ug/mL) was added for the final 5 hours of incubation. A well of 1.5 million PBMC in 300 ul of media alone was used to assess background cytokine production. Cells were harvested and surface stains added. Cells were washed twice with cold PBS with 2% FBS, and permeabilized with cytofix-cytoperm (BD Biosciences) for 20 minutes on ice. Cells were washed with Perm/wash buffer (BD Biosciences) and intracellular cytokine stains were added for 15 minutes at room temperature. Cells were washed and fixed in 10% paraformaldehyde. 500,000 events were collected on the LSR II (Becton Dickinson).
Statistical Analyses
Frequency of dual producing cells in sirolimus-treated vs control animals showed significant differences between groups using Wilcoxon rank-sum test for variable measure. Frequencies of dual cytokine producing cells within different memory T cell subsets were compared using the Mann-Whitney test for non-parametric data.
Results
Antigen-specific CD8+ T cell responses to modified vaccinia Ankara (MVA) in rhesus macaques are augmented by sirolimus treatment
We evaluated the influence of sirolimus on the resulting antigen-specific CD8+ T cell responses following a modified vaccinia Ankara (MVA) prime-boost strategy. MVA is a live attenuated version of vaccinia virus that lost its ability to effectively replicate in human cells following >500 passages of the virus through chicken embryo fibroblasts (16). In this experiment, all animals were vaccinated with MVA (108 PFU, IM) and subsequently boosted with MVA 28 days later (Figure 1A). Four rhesus macaques were treated with sirolimus (Rapamune, target trough blood levels 5–15 ng/mL) throughout the entire prime-boost course, and four animals were left untreated. Two additional animals were treated with tacrolimus (Prograf, trough levels 5–15 ng/ml), another agent commonly used for the maintenance immunosuppression of transplant recipients. First, the resultant primary vaccinia-specific responses were measured at 1, 2, 3, and 4 weeks post-priming by intracellular cytokine staining. Briefly, PBMC from vaccinated animals were stimulated overnight with vaccinia lysate and subsequently evaluated for cytokine production using multiparameter flow cytometry as described in Materials and Methods. Results indicated that at week 1 post-vaccination, control animals exhibited an average of 0.573 +/− 0.145 % vaccinia-specific IFN-γ-secreting cells in response to ex vivo restimulation (Figure 1B). In contrast, rapamycin-treated animals exhibited a mean of 1.27 +/− 0.229% vaccinia-specific cytokine secreting cells (p=0.0216). A similar trend was observed at week two post-priming, where the mean frequency of IFN-γ-secreting cells in untreated controls was 0.146 +/− 0.037% as compared to 0.712 +/− 0.279 in sirolimus-treated animals (Figure 1B). Thus, sirolimus-treated animals exhibited vaccinia-specific CD8+ T cell responses following priming, indicating that mTOR inhibition is not completely immunosuppressive in this model. On the contrary, vaccinia-specific CD8+ T cell responses measured at weeks 1 and 2 post-priming were paradoxically increased in sirolimus-treated individuals.
Given these unexpected results, the ability of vaccinia-specific CD8+ T cell responses generated under conditions of sirolimus exposure were assessed for their ability to generate secondary responses. Animals were boosted in vivo at day 30 with a second MVA immunization, and the resultant secondary CD8+ T cell responses were assessed by serially analyzing cytokine production in peripheral blood mononuclear cells (PBMCs) post vaccination. Again, all animals treated with rapamycin demonstrated antigen-specific CD8+ responses to vaccinia lysate, indicating that sirolimus administration during both the prime and boost phase of the response did not have an immunosuppressive effect on this virus-specific response (Figure 1C). Furthermore, we again paradoxically observed that the CD8+ T cell compartments in 3 out of 4 monkeys treated with sirolimus instead exhibited sustained, heightened production of IFN-γ as compared to untreated controls (Figure 1C) or tacrolimus treated animals (Figure 1D) following MVA boost. Vaccinia-specific CD8+ T cell responses in tacrolimus-treated animals were not significantly different from those observed in untreated controls (Figures 1C and 1D). In contrast, the increase in the antigen-specific CD8+ T cell response in sirolimus-treated animals was observed during both the peak of the response and during the memory phase (Figure 1C). Specifically, sirolimus-treated animals exhibited an average of 3.533 +/−1.460 % IFN-γ-producing cells at week 2 post-boost as compared to 0.2963 +/−0.09914% IFN-γ-producing cells in control animals (p=0.0441). At week 6 post-boost, sirolimus-treated animals exhibited an average of 4.491 +/−1.171 % IFN-γ-producing cells as compared to 0.3000 +/−0.0.0470% IFN-γ-producing cells in control animals (p=0.003).
Sirolimus also enhances antigen-specific CD8+ T cell responses when administered during secondary rechallenge only
The above results indicated that antigen-specific memory T cell responses to vaccinia epitopes were increased when sirolimus was present during both the priming and boosting phases of the response. We therefore next sought to address the ability of sirolimus to enhance vaccinia-specific responses if administered solely during the boosting phase. Six rhesus macaques were vaccinated with smallpox vaccine (Dryvax) by scarification and left untreated (Figure 2A). At 100 days post vaccination, half of the macaques began treatment with sirolimus, which was continued throughout the course of the study, as was done in a previously published pilot study (14). Animals were then boosted with MVA and the resulting antigen-specific CD8+ T cell recall responses to vaccinia were assessed. We observed that animals treated with sirolimus during the secondary recall response to a vaccine again exhibited a heightened antigen-specific CD8+ T cell response to MVA compared to untreated controls. For example, at week 3 post-boost, the observed frequency of IFN-γ-producing vaccinia-specific T cells in sirolimus-treated animals was 10.66 +/− 4.14% as compared to 2.162 +/− 0.672% for untreated controls (p=0.026) (Figure 2B). Furthermore, treatment with sirolimus dramatically impacted the generation and maintenance of antigen-specific memory T cells; at week 7 post-boost, the observed frequency of IFN-γ-producing vaccinia-specific T cells in sirolimus-treated animals was 11.09 +/−4.43% as compared to 0.325 +/−0.0457% for untreated controls (p=0.0022) (Figure 2B). Taken together, the data presented in Figures 1 and 2 demonstrate that the enhancing effect of sirolimus treatment on vaccinia-specific T cell responses was greater during the memory phase of the response.
Increased frequency of high-quality dual-cytokine producing effector cells in sirolimus-treated macaques
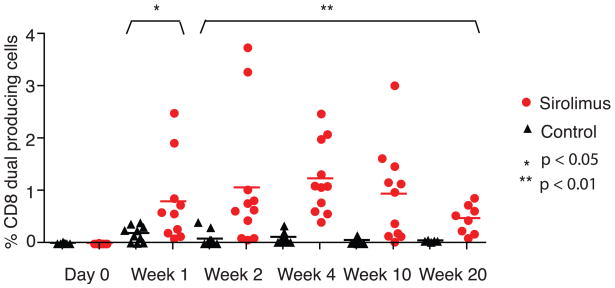
Because we observed an augmentation in the quantity of the antigen-specific T cell response with sirolimus treatment following MVA boost, we next sought to determine the quality of this response. Several studies have highlighted the importance of T cell quality in controlling infections (reviewed in (17)). CD8+ T cells which secrete both TNF and IFN-γ, or dual-producing cells, are more efficient at killing, have enhanced cytolytic activity, and secrete more IFN-γ on a per-cell basis than single cytokine producing cells. We found that both animals that were treated with sirolimus during the boosting phase alone (Figure 2C) and those that were treated with sirolimus during both the priming and boosting phases (Figure 3) exhibited increased frequencies of IFN-γ+ TNF+ double-producing cells as compared to untreated controls in the weeks following MVA challenge (p<0.05 at week 1, p<0.01 for weeks 2–20). In animals treated with sirolimus during both the prime and boost phases that were followed out long-term, this effect lasted up to 20 weeks post boost (Figure 3).
Figure 3. Increased frequency of high-quality dual-cytokine producing effector cells in sirolimus-treated macaques.
RM were treated with sirolimus or left untreated during both vaccination and boost with MVA (n=4 in each group). PBMCs isolated at each timepoint were stimulated with vaccinia virus and assessed for IFN-γ and TNF by ICCS. Percentage of CD3+ CD8+ T cells which produce both TNF and IFN-γ at day 0 of vaccination with MVA and in the weeks following MVA are shown; up to three replicates for each RM at each timepoint are depicted. (* p<0.05, ** p<0.01).
Sirolimus treatment leads to prolonged enhancement of antigen-specific CD8+ TCM cells
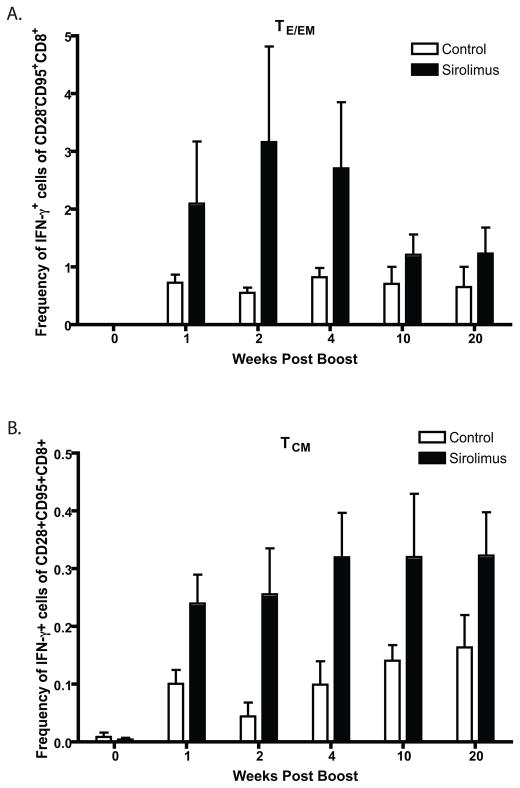
In order to further dissect the impact of sirolimus treatment on the generation of vaccine-specific T cell responses, we examined the relative enhancement of individual memory T cell subsets following sirolimus administration. CD8+ T cells from control or sirolimus-treated macaques were gated on CD95hi CD28neg cells (18) (Teff/EM compartment) or CD95hiCD28pos cells (TCM compartment) and analyzed for IFN-γ-secreting cells within those compartments. We observed that sirolimus augmented the frequency of IFN-γ-secreting cells in both the Teff/EM and TCM compartments as compared to untreated controls (Figure 4, p<0.05 for weeks 1–20). However, while the sirolimus-induced augmentation was most evident in the Teff/EM cells during early timepoints post-boost (Figure 4A), the sirolimus-induced augmentation of TCM was more protracted (Figure 4B). Thus, these results suggest that sirolimus impacts antigen-specific T cell responses to increase long-lived memory precursors and TCM.
Figure 4. Sirolimus treatment leads to prolonged enhancement of antigen-specific CD8+ TCM cells.
RM were treated with sirolimus or left untreated during both vaccination and boost with MVA (n=4 in each group). PBMCs isolated at each timepoint were stimulated with vaccinia virus and assessed for IFN-γ and TNF. Percentage of dual TNF and IFN-γ producing CD8+ T cells within the TEFF/EM (A) vs TCM (B) subsets are shown. TCM cells were defined as CD3+ CD8+ CD28+CD95+; TEM cells are CD3+ CD8+ CD28−CD95+. p<0.05 for comparisons between sirolimus-treated and untreated controls for each timepoint within both the TEFF/EM and TCM subsets.
Discussion
In this study we investigated the impact of sirolimus on the primary and recall CD8+ T cell responses to smallpox vaccination in rhesus macaques, and found that this therapy increased both the quantity and quality of antigen-specific T cell responses. Given the fact that MVA is a replication-deficient vaccine (16), in this model there is no possibility of alterations in the T cell response due to a secondary effect of mTOR inhibition on viral replication. Instead, our results support the hypothesis that sirolimus functions to increase the magnitude of the antigen-specific T cell responses that mediate protection against the virus. These findings are consistent with recently published results demonstrating in a mouse model that sirolimus acts in a T cell-intrinsic manner to enhance antigen-specific memory T cell generation and maintenance following viral infection (14). Specifically, experiments using RNAi knockdown technology to abrogate mTOR signaling only in antigen-specific T cells demonstrated that sirolimus-mediated enhancement of cellular immunity is likely a T cell-intrinsic effect (14). However, in the murine study, sirolimus was administered for only 10–30 days, while in the current study it was administered for > 90 days. Thus, the current results also extend those findings by demonstrating that even long-term treatment with sirolimus does not impair, and in fact still enhances, virus-specific CD8+ T cell responses following vaccination. This finding is relevant to the potential clinical application of sirolimus to boost T cell responses to viruses and vaccines in transplant recipients.
The data presented here demonstrate that sirolimus enhanced viral-specific CD8+ T cell quality as well as quantity. Specifically, we showed that sirolimus treatment resulted in a greater frequency of multi-cytokine producing virus-specific T cells (Figure 3). Given the known function of pathogen-specific multi-cytokine producing cells in vivo (17), these results suggest that treatment of transplant recipients with sirolimus during either de novo vaccination or boosting may result in augmentation of high-quality dual cytokine producing cells that are likely to provide potent protective immunity against infectious challenge.
Furthermore, we found that the enhancing effect of sirolimus treatment on vaccinia-specific T cell responses was greater during the memory phase of the response (Figures 1 and 2). This is consistent with observations made in murine models of viral infection, in which sirolimus markedly reduced the contraction phase of the response and resulted in an increased frequency of antigen-specific memory T cells (14). Our data also suggest that sirolimus impacts antigen-specific T cell responses to increase long-lived memory precursors and TCM, perhaps more permanently than TEM (Figure 4). These findings are consistent with recently published observations in murine models, in which sirolimus increased the frequency of CD62Lhi KLRG-1lo CD127hi T cells at memory timepoints (14). Furthermore, they suggest that administration of sirolimus at the time of vaccination may result in the enhanced generation of memory T cells which are uniquely poised, by virtue of their increased proliferative potential, to mount an effective secondary recall response upon encounter with antigen (19).
Understanding the impact of immunosuppressive agents on protective immunity is critical in order to maximize patient health following transplantation. Our results, along with those of recently published studies, suggest that sirolimus may act to increase the protective immune response to a vaccine or live virus challenge. Interestingly, in a murine model where the effects of sirolimus on the immune response directed against a pathogen versus an allograft were directly compared, sirolimus was found to augment the CD8+ T cell response to the pathogen but not the graft (20). While the exact mechanisms of sirolimus-mediated enhancement in cellular immunity remain to be elucidated, these data strongly support the further investigation of sirolimus in both transplant patients as well as non-transplant recipients as a means of augmenting immunological memory and protective immunity. First, whether or not human T cells behave in a similar manner remains to be determined, and second, since human transplant recipients are commonly simultaneously treated with other immunosuppressive agents such as tacrolimus or mycophenolate mofetil, future studies are needed in order to determine how the presence of these other immunosuppressive agents may impact the enhancing effects of rapamycin on viral-specific T cell responses in human patients following transplantation.
Acknowledgments
The authors would like to thank Vicki Hertzberg for help with statistical analyses and Aneesh K. Mehta for critical reading of the manuscript. This work was supported by N01 AI050025 to C.P.L.
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1998–2007. U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: 2008. [Google Scholar]
- 2.Gangappa S, Kapadia SB, Speck SH, Virgin HWt. Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell-deficient mice. J Virol. 2002;76(22):11460–11468. doi: 10.1128/JVI.76.22.11460-11468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 4.Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67(3):369–391. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- 5.Segundo DS, Ruiz JC, Izquierdo M, Fernandez-Fresnedo G, Gomez-Alamillo C, Merino R, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation. 2006;82(4):550–557. doi: 10.1097/01.tp.0000229473.95202.50. [DOI] [PubMed] [Google Scholar]
- 6.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9(5):324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood. 2002;100(3):1084–1087. doi: 10.1182/blood.v100.3.1084. [DOI] [PubMed] [Google Scholar]
- 8.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9(10):1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112(3):635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29(4):565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Demopoulos L, Polinsky M, Steele G, Mines D, Blum M, Caulfield M, et al. Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplant Proc. 2008;40(5):1407–1410. doi: 10.1016/j.transproceed.2008.03.084. [DOI] [PubMed] [Google Scholar]
- 12.Haririan A, Morawski K, West MS, El-Amm JM, Doshi MD, Cincotta E, et al. Sirolimus exposure during the early post-transplant period reduces the risk of CMV infection relative to tacrolimus in renal allograft recipients. Clin Transplant. 2007;21(4):466–471. doi: 10.1111/j.1399-0012.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 13.Marty FM, Bryar J, Browne SK, Schwarzberg T, Ho VT, Bassett IV, et al. Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood. 2007;110(2):490–500. doi: 10.1182/blood-2007-01-069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karkhanis LU, Ross TM. Mucosal vaccine vectors: replication-competent versus replication-deficient poxviruses. Curr Pharm Des. 2007;13(19):2015–2023. doi: 10.2174/138161207781039832. [DOI] [PubMed] [Google Scholar]
- 17.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8(4):247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 18.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and Homeostasis of T Cell Memory in Rhesus Macaque. J Immunol. 2002;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 20.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, et al. Cutting Edge: Rapamycin Augments Pathogen-Specific but Not Graft-Reactive CD8+ T Cell Responses. J Immunol. 2010;185(4):2004–2008. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]