Abstract
Introduction
Bone marrow targeted drug delivery systems appear to offer a promising strategy for advancing diagnostic, protective, and/or therapeutic medicine for the hematopoietic system. Liposome technology can provide a drug delivery system with high bone marrow targeting that is mediated by specific phagocytosis in bone marrow.
Area covered
This review focuses on a bone marrow specific liposome formulation labeled with technetium-99m (99mTc). Interspecies differences in bone marrow distribution of the bone marrow targeted formulation are emphasized. This review provides a liposome technology to target bone marrow. In addition, the selection of proper species for the investigation of bone marrow targeting is suggested.
Expert opinion
It can be speculated that the bone marrow macrophages have a role in the delivery of lipids to the bone marrow as a source of energy and for membrane biosynthesis or in the delivery of fat soluble vitamins for hematopoiesis. This homeostatic system offers a potent pathway to deliver drugs selectively into bone marrow tissues from blood. High selectivity of the present BMT-liposome formulation for bone marrow suggests the presence of an active and specific mechanism, but specific factors affecting the uptake of the bone marrow MPS are still unknown. Further investigation of this mechanism will increase our understanding of factors required for effective transport of agents to the bone marrow, and may provide an efficient system for bone marrow delivery for therapeutic purposes.
Keywords: liposomes, bone marrow targeting, drug delivery, biodistribution, scintigraphy, mononuclear phagocyte system
1. Introduction
Bone marrow is an important hematopoietic organ. Conventional delivery of drugs to bone marrow is based on the administration of large doses of drug that are frequently associated with side effects. Development of effective targeted bone marrow drug delivery systems is an important goal for development of diagnostic, protective, and/or therapeutic agents for hematopoietic disorders and infectious diseases in which colonizing pathogens are difficult to eradicate. In addition, bone marrow-targeted carriers may be useful to deliver hematopoiesis-stimulating agents into bone marrow tissues to advance research into hematopoietic functions.
Engineered colloidal particles such as liposomes and other nanoparticles have been advanced as carriers for drug delivery. Numerous reports have indicated that the mononuclear phagocyte system (MPS), consisting of mononuclear cells such as macrophages in liver and spleen, are the primary uptake site for intravenously injected particles [1,2]. It has been demonstrated that several factors such as particle size and surface modification influence the uptake of particles by the MPS [3–5]. Similarly, bioparticles such as aged blood cells and lipoproteins are removed from the blood circulation through the MPS. Macrophages with access to the circulating blood have been reported to reside in the liver, spleen, and bone marrow [6]. These MPS organs have sinusoids with a fenestrated endothelium with associated macrophages that monitor blood circulation through the sinusoidal pores between vascular endothelial cells. Macrophages express several types of receptors on their surface for the uptake of specific bioparticles [7–9]. This specific system is a potent target for tissue-selective drug delivery using nanoparticles.
In comparison with liver and spleen, very little attention has been paid to bone marrow as part of the MPS because its contribution to the MPS is generally much less than that of the liver and spleen in vivo. However, recent studies showed that the bone marrow could be the main organ to have specific uptake of surface modified liposomes [10,11]. These liposomes are expected to serve as drug carriers to deliver drugs specifically into bone marrow. During these bone marrow targeting studies, we found that it is important to understand the biodistribution of the liposomes in multiple species. We have confirmed that the bone marrow has high uptake of our surface modified liposomes in rabbits and rhesus monkeys. On the other hand, surprisingly, in mice and rats, these same liposomes are mainly captured in the liver and spleen, with a lower amount being taken up in bone marrow. This may be one reason why very little attention has been paid to bone marrow as part of the MPS. In this article, we will compare the biodistribution of the bone marrow-targeted liposomes (BMT-liposomes) in several species.
2. Macrophage targeting with nanoparticles
Macrophages, which are produced by the differentiation of monocytes from stem cell precursors located in the bone marrow, are heterogeneous populations existing in various tissues and organs. These macrophages are distributed in liver (Kupffer cells), spleen (splenic macrophages), bone marrow (bone marrow macrophages), blood (monocytes), lung (alveolus macrophages), bone (osteoclasts), brain (microglia), lymph node, thymus, and abdominal cavity to be responsible for numerous metabolic, immunological, and inflammatory processes in physiological and pathological conditions. The therapeutic potential of macrophage targeting includes metabolic diseases [12,13], bacterial and parasitic infection diseases [14–16], viral infectious diseases [17,18], inflammatory diseases [19], and atherosclerosis [20–22].
The phagocytic ability of macrophages contributes to the active targeting of nanoparticulate carriers to macrophages by a simple phagocytic uptake process. Furthermore, the surface modification of the nanoparticles with a targeting ligand that mediates their recognition by specific receptors present on the macrophage membrane allows for specific macrophage targeting. Mannose receptor on macrophages is a pivotal receptor to target macrophages with nanoparticles modified with mannose, in which the nanoparticles modified with mannose are highly captured by the macrophages via mannose receptor mediated phagocytosis [23–30]. Several types of nanoparticles such as liposomes [23–25], polymeric nanoparticles [26,27], and quantum dots [28] that are modified with mannose have been tested to deliver diagnostic or therapeutic agents to macrophages. Other known receptors to target macrophages with nanoparticle systems are galactose receptor [31,32], scavenger receptor A [33,34], scavenger receptor B (CD36) [35], folate receptor [36–38], and nicotinic acetylcholine receptor [19]. Because these receptors are commonly expressed on macrophages in various tissue and organs, it is still a challenge to target macrophages in specific tissues and organs through systemic administration. As specific receptors, folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages [37].
3. Uptake of particles by bone marrow phagocytes
The total blood cell production rate in adult human bone marrow is about 4.9×1011 cells per day or about 88 kg per year [39,40]. From the viewpoint of material balance for cell turnover, it is reasonable to speculate that the bone marrow extensively acquires materials from the blood for hematopoiesis.
Chylomicrons are large lipoprotein particles that consist of triglycerides, phospholipids, cholesterol, and proteins. Hussain and co-workers have reported that rabbit and marmoset bone marrow had significant uptake of chylomicrons labeled with [14C]cholesterol and [3H] retinol [41,42]. Perisinusoidal macrophages protruding through the endothelial cells into the marrow sinuses were responsible for the accumulation of the chylomicrons in the marmoset bone marrow. In contrast to marmosets, chylomicron clearance from the bone marrow of rats, guinea pigs, and dogs was much less, and the spleen in rats and guinea pigs took up a large fraction of chylomicrons. Thus, Hussain et al. concluded that the observed differences in chylomicron metabolism are due to the presence of perisinusoidal macrophages in bone marrow. It was also believed that the differences between bone marrow and spleen uptake of chylomicrons may provide insights into the role of chylomicron catabolism in these organs, both of which are involved in hematopoiesis. It was speculated that the chylomicrons may have a role in the delivery of lipids to the bone marrow and spleen as a source of energy and for membrane biosynthesis or in the delivery of fat soluble vitamins.
In general, it is believed that aged blood cells are cleared by the liver and spleen. However, recent evidence from several sources indicates that the bone marrow is a significant site for clearance of apoptotic neutrophils [43,44]. The high uptake of white blood cells in bone marrow can also be observed in humans following administration of indium-111 radiolabeled white blood cells that are routinely administered to humans for detection of occult infection (Figure 1). Whole body region-of-interest analysis frequently finds that 60–70% of the administered white blood cells localize to bone marrow while 30–40% localizes to liver and spleen. Interestingly, the uptake of apoptotic neutrophils by bone marrow macrophages has been shown to stimulate the production of granulocyte-colony stimulating factor. Also, bone marrow macrophages, associated with erythroblasts in a hematopoietic environment, participate in erythropoiesis control, and engulfment of nuclei from erythroid precursor cells [45–47]. Recently, Winkler and co-workers reported that the bone marrow macrophages are pivotal to maintain the endosteal hematopoietic stem cell niche and that the loss of such macrophages leads to the egress of hematopoietic stem cells into the blood [48]. They administrated clodronate-loaded liposomes intravenously to deplete the bone marrow macrophages. After depletion of the macrophages, hematopoietic stem cells were found in the blood. These findings provide evidence supporting the critical role that macrophages play in the support of hematopoietic cells in bone marrow. Such specific biology of bone marrow macrophages may offer a therapeutic target for the treatment of hematopoietic disorders.
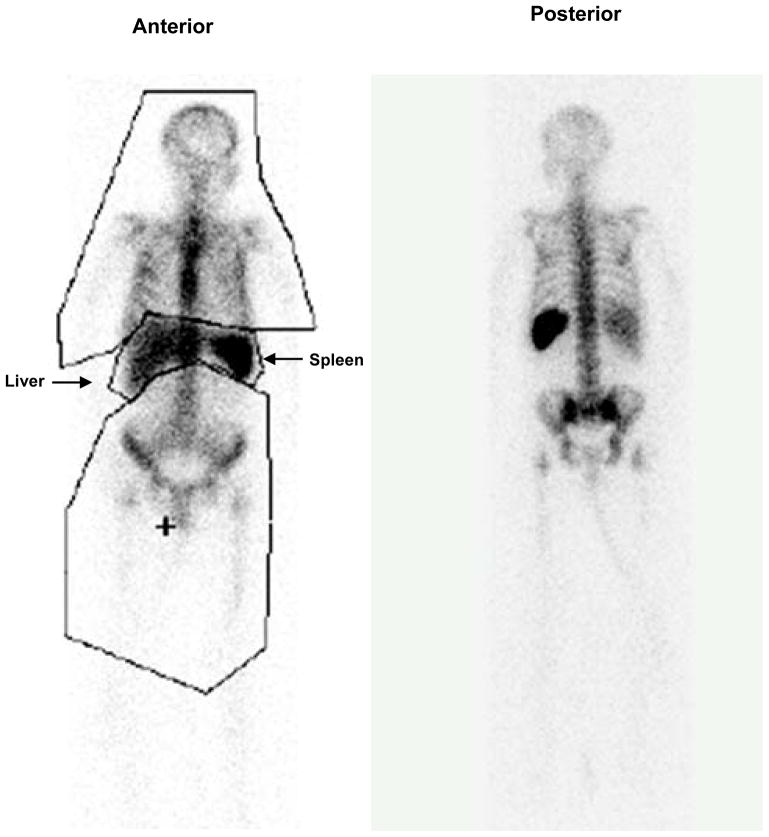
Figure 1.
Distribution of white blood cells labeled with indium-111 in human. White blood cells labeled with indium-111 are commonly administered to humans to detect infection and imaged at 20 hours post-administration. The present anterior and posterior whole body images were acquired at 24 hours post-administration. Region-of-interest analysis of anterior image demonstrated that 72% of the labeled white blood cells were located in the bone marrow with the remainder in the liver and spleen.
As for the engineered colloidal particles, Porter and co-workers observed significant accumulation of small colloidal particulates (150 nm and below, in diameter) that were coated by the block co-polymer poloxamer-407, a non-ionic surfactant, in the bone marrow after intravenous administration in rabbits [49]. In this case, the coated colloids were sequestered by the sinusoidal endothelial cells of the bone marrow instead of macrophages. Importantly, significant uptake could not be achieved with other block co-polymers of similar structure to poloxamer-407, suggesting the participation of a specific interaction mechanism between the particle and the sinusoidal endothelial cell surface.
Schettini and co-workers prepared a novel liposomal formulation of meglumine antimoniate, a drug used for treating leishmaniasis, to deliver the drug to the bone marrow [50]. The liposomes were made from distearoylphosphatidylcholine (DSPC), cholesterol and dicetylphosphate (molar ratio of 5:4:1). The targeting of antimony to the bone marrow was improved (approximately three-fold) with the small liposomal formulation as compared to the large liposome formulation used in dogs with visceral leishmaniasis. These liposomes had no active targeting factor to bone marrow but the passive targeting of the liposomes to the bone marrow of dogs was improved by the reduction of vesicle size from 1200 nm to 400 nm. Recently, other nanoparticles such as guanidinium group-modified nanoparticles and cationic nanoparticles were tested as carriers of oligopeptide and siRNA to deliver the therapeutic agents into bone marrow cells [51,52].
Moghimi and co-workers have reviewed the clearance mechanism of particulate materials from the circulation by bone marrow [49,53]. The endothelium of bone marrow sinusoids is capable of removing particles from the circulation by both transcellular and intercellular routes. The transcellular route occurs through the fenestrate in the endothelial wall, therefore this mechanism is strongly dependent on particle size. The size of the fenestrate was reported to range from 85–150 nm [54]. Liposomes consisting of DSPC, cholesterol, PEG (5000)-DSPE and α-tocopherol and prepared in various sizes (136–318 nm in diameter) have been tested for organ distribution in rabbits, and none of these liposomes show a significant accumulation in bone marrow [4].
The intercellular route is triggered by the interaction of particles with endothelium and the importance of this route should be strongly dependent on the surface characteristics of the particles. To study the interaction between liposomes and macrophages, in vitro cultured cell systems have been used. It has been demonstrated that one of the critical components is a negatively charged phospholipid such as phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidic acid (PA), or phosphatidylglycerol (PG), which are recognized by the scavenger receptors on the surface of macrophages [55,56]. Scavenger receptors widely recognize and take up macromolecules having a negative charge as well as modified low-density lipoprotein. Szabó and co-workers investigated the uptake of branched polypeptides by bone marrow culture-derived murine macrophages. They indicated that the succinylation of branched polypeptides significantly enhanced the uptake by macrophages, and the uptake was inhibited by blocking of the class-A scavenger receptors [57]. Also, enhanced uptake of succinylated proteins has been investigated in cultured brain microvessel endothelial cells [58]. Endothelial cells also express the scavenger receptor on their surface. Large succinylated proteins such as catalase (Mw 227 kDa) and bovine serum albumin (Mw 70 kDa) were taken up by the cells via a scavenger receptor-mediated mechanism. These in vitro studies indicate that succinylation of large molecules is involved in the uptake via a scavenger receptor-mediated mechanism. However, the scavenger receptors are present not only on bone marrow macrophages but also on hepatic Kupffer cells, splenic macrophages, and endothelial cells. Further studies, including species specific associations, are needed to clarify the mechanism for the selectivity of bone marrow macrophages in different organs.
Other possible mechanisms of liposome recognition by phagocytic cells may occur via binding plasma proteins. Several types of proteins such as immunoglobulins, complement proteins, apolipoproteins, fetuin, von Willebrand factor, and thrombospondin have been identified as ligands for the macrophage, and these binding proteins are known to accelerate uptake by hepatic Kupffer cells [59]. Hematopoietic factors such as erythropoietin, and iron transporting transferrin have been suggested as possible serum proteins that could provide specificity for bone marrow.
4. Bone marrow-targeted liposomes (BMT-liposomes)
During research carried out by the authors, liposomes with high bone marrow uptake were serendipitously discovered during the development of cell-based artificial oxygen carriers which are phospholipid vesicles (liposomes) encapsulating hemoglobin (Hemoglobin-vesicles, HbV) [60–62]. Because HbV were developed to be infused at tremendously large doses as a red blood cell substitute, their size and lipid components are unique compared with the typical liposome drug carriers which are below 200 nm in diameter and are modified with dense PEG layer (5–10 mol%) for long circulation, so-called stealth liposomes. Though HbV are 250 nm in diameter and modified with only 0.3mol% PEG-lipid, technetium-99m (99mTc) labeled HbV are retained in circulation for fairly long time periods in rats (t1/2; 34.8hr) and rabbits (t1/2; 62.6hr) at large infusion doses (680 mg/kg) [63]. HbV labeled with 125I or 3H-cholesterol also have similar circulation half-lives [64]. During circulation, HbV are gradually taken up by the liver, spleen, and bone marrow without obvious organ selectivity [63–65].
On the other hand, when we injected 99mTc labeled HbV at small dose (15mg/kg) in the rabbit, gamma camera images demonstrated significant uptake of radiolabeled HbV in the bone regions of the rabbit. Because the bone marrow selective distribution was not obvious when administered at higher lipid doses as described above, it appears that the bone marrow selectivity is limited by the injection dosage. We believe that the reason for the dose dependence of bone marrow uptake is as follows. As the vesicle dosage increases, the MPS in the bone marrow becomes saturated; as a result, liver and spleen uptake subsequently increases. Such sequential saturation of the MPS eliminates organ selectivity. Therefore, the bone marrow targeting of the liposomes becomes striking when the dose of liposomes is below the saturation dosage for the bone marrow. We have estimated that the maximum uptake capacity of MPS for the liposomes is around 50 mg/kg body weight in rabbits. When the liposome dosage increases above 50 mg/kg body weight, the bone marrow is the first organ to become saturated, and the accumulation of liposomes then increases in the liver and spleen.
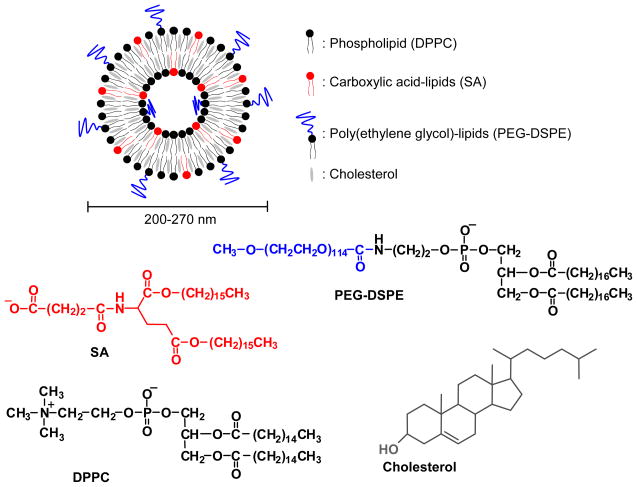
The BMT-liposomes are composed of four kinds of lipid, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), cholesterol, L-glutamic acid, N-(3-carboxy-1-oxopropyl)-, 1,5-dihexadecyl ester (SA-lipid), and poly(ethylene glycol) (PEG) as shown in Figure 2. We have identified that the SA lipid component is the active factor leading to their phagocytosis by bone marrow phagocytes assumed macrophages in rabbits [10]. Furthermore, as little as 0.6 mol% of PEG-DSPE depressed hepatic uptake but did not depress bone marrow uptake. PEG-DSPE can be incorporated into the outer surface of preformed liposomes by using the post incorporation method [10,66,67]. Otherwise PEG-DSPE is mixed with other lipid components before preparation of liposomes. The active targeting factor of SA-lipid and passive targeting factor of PEG-DSPE appear to cooperatively increase the distribution of the liposomes to bone marrow. The size of the liposomes in the range of 200–270 nm is not a significant factor for uptake by bone marrow. The liposomes were designed to have high entrapment capacity with the interfacial electrostatic interaction to form unilamellar membrane [68,69]. These characteristics would facilitate the application of the BMT-liposomes as pharmaceutical carriers to bone marrow.
Figure 2.
Lipid components of bone marrow-targeted liposomes (BMT-liposomes).
5. Species specific biodistribution of BMT-liposomes
Whole body scintigraphic imaging is a powerful tool for noninvasive and quantitative determination of the biodistribution of liposomes in preclinical and clinical evaluations [70–73]. In particular, technetium-99m (99mTc), which has a photopeak of 140 KeV and a half-life of 6 hours, is a commonly used radionuclide for clinical scintigraphic imaging. To label liposomes encapsulating glutathione with 99mTc, Phillips et al has established a remote loading method using a complex of 99mTcO4− and hexamethyl-propyleneamine oxime (HMPAO) [73]. In general, whole body scintigraphic imaging of animals and humans receiving conventional 99mTc-liposomes demonstrates significant uptake of the 99mTc-liposomes in the liver and spleen with minimal uptake in the bone marrow [70–73].
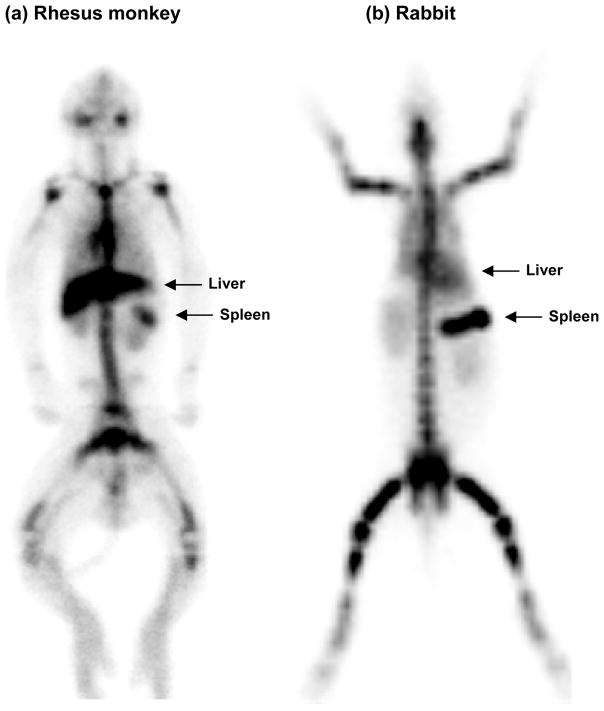
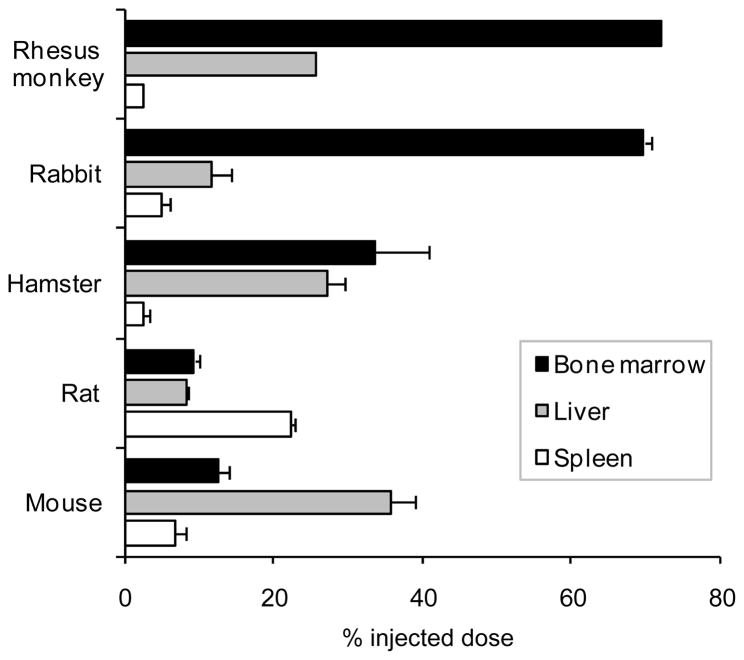
As shown in Figure 3, significant bone marrow targeting properties of the BMT-liposomes have been confirmed in rhesus monkeys and rabbits. Bone marrow had uptake of 72 % injected dose (ID) of BMT-liposomes in rhesus monkeys and 69.7±0.9 %ID in rabbits (Figure 5). In contrast with the distribution of BMT-liposomes in bone marrow throughout the whole body in rabbits, there were less BMT-liposomes distributed in the legs and arms of the monkeys. In adult humans, the myeloid hematopoiesis is distributed in red marrow which is mainly localized in ribs, sternum, spine, pelvis, and proximal shafts of the femora and humeri [74]. It seems that the distribution of the BMT-liposomes in monkeys is similar to the distribution of myeloid hematopoiesis in humans.
Figure 3.
Static gamma camera whole body images of rhesus monkey and rabbit receiving 99mTc-labeled bone marrow targeted-liposomes intravenously. The images were taken in rhesus monkey at 3 hours and in rabbit at 6 hours after injection of the liposomes at 15 mg lipids/kg body weight.
Figure 5.
Biodistribution of bone marrow targeted-liposomes in rhesus monkeys (at 3 h), rabbits (at 6 h), hamsters (at 24 h), rats (at 6 h), and mice (at 6 h) after injection. Bone marrow targeted-liposomes were injected at 15 mg lipids/kg body weight.
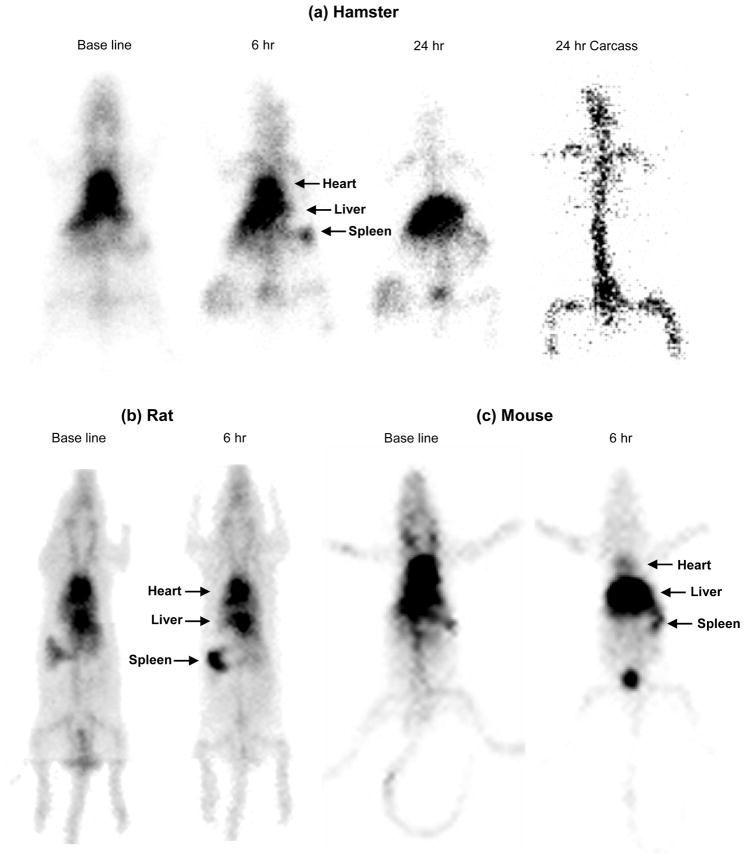
Figure 4 shows whole body images of rodents acquired after intravenous administration of BMT-liposomes. It was found that hamsters still had significant radioactivity in the circulation (as depicted by heart activity visualization) at 6 h after injection, indicating that the distribution of BMT-liposomes to MPS organs in hamster is slower than in rhesus monkeys and rabbits (Figure 4a). In fact, the BMT-liposomes were retained in circulation at 56.9±5.5 %ID in hamster at 6 h after injection. At 24 h, the radioactivity in hamster heart disappeared and most of the radioactivity was distributed in the liver. After removing the liver, we could clearly see the radioactivity in bone marrow of the carcass. Biodistribution calculated from radioactivity of isolated tissues revealed that bone marrow had 33.5±7.2 %ID and liver 27.0±2.5 %ID at 24 h after injection as shown in Figure 5.
Figure 4.
Static gamma camera images of rodents receiving 99mTc-labeled bone marrow targeted-liposomes at 15 mg lipids/kg body weight intravenously. The base line image was taken just after injection of the liposomes. (a) Hamster: Images were taken at 6 and 24 hours after injection. The carcass image was taken after removing the internal organs at 24 hours. (b) Rat: Images were taken at 6 hours after injection. (c) Mouse: Images were taken at 6 hours after injection.
In contrast with rhesus monkey, rabbit, and hamster, bone marrow is not a primary organ for uptake of the BMT-liposomes in rats and mice. As shown in Figure 4b, the rat still has significant radioactivity in heart at 6 h after injection of BMT-liposomes. BMT-liposomes were retained in circulation at 44.6±0.5 %ID at this time point. Although bone marrow still had significantly higher uptake of BMT-liposomes than standard liposome formulations which generally have no more than 1–2% uptake in the bone marrow, the level of 9.3±0.7 %ID in rats and 12.5±1.5 %ID in mice is much lower than those in rhesus monkeys and rabbits. In addition, to our surprise, these most frequently used rodent species showed different uptake patterns of organ activity of the BMT-liposomes. As shown in Figure 4b and 5, the spleen of rat had significant uptake of BMT-liposomes. On the other hand, the liver of mice had the greatest uptake (Figures 4c and 5). These observations might suggest that small rodents such as mouse and rat are specialized in their handling of circulating particles in ways that differ from large animals. Imaging in multiple species may be particularly important in studies of nanoparticles because studies have shown that there are significant differences in the circulation times and distribution of nanoparticles in larger animals and humans as compared with mice and rats. Species-dependent organ specificity for BMT-liposome uptake is fairly similar with the species specificity of chylomicron metabolism by bone marrow as described in Section 3 above. This might be one explanation for the differences observed in the uptake of BMT-liposomes between different species. However, the bone marrow of rabbits does not have high uptake of standard liposome formulations but only of BMT-liposomes. Therefore, the presence of perisinusoidal macrophages in bone marrow is not a sufficient condition for the targeting of the bone marrow even if it is a necessary condition. It can be speculated that perisinusoidal macrophages have specific receptors that can recognize the surface of liposomes containing SA-lipid.
One reason for this species-dependence is likely related to the difference of mass balance of MPS organs between species in competitive uptake. In a previous study of a large infusion dose of liposomes, we have found that the balance between organ weight and body weight is a crucial factor determining the difference in biodistribution of liposomes between rats and rabbits [63]. The spleen-to-body weight ratio of the rat is 3 times greater than that of the primate and 9 times greater than the spleen-to-body weight ratio of the rabbit. If the BMT-liposomes are being taken up in relationship to total cell turnover, it may be that rats have a very high rate of lymphocyte production in their large spleen as compared with other species which could be related to their high uptake of BMT-liposomes in the spleen and relatively lower uptake in the bone marrow.
6. Conclusion
Bone marrow, a hematopoietic organ, is an important, albeit challenging target for drug delivery [50–53,75,76]. Surface-modified liposomes provide a promising methodology to deliver liposomal drugs into bone marrow via specific bone marrow phagocytosis. This bone marrow delivery system has potential as a drug delivery carrier for chemotherapy of hematopoietic malignancies such as myelocytic leukemia and multiple myeloma. Potential clinical uses of this bone marrow delivery system include the delivery of agents that protect the marrow from the toxic effects of chemotherapy and radiation, and the delivery of agents to effectively and safely ablate bone marrow prior to bone marrow transplant. Also, the ability to specifically deliver stimulants of hematopoietic cell proliferation may open novel therapeutic system for increasing hematopoiesis [51,77]. Development of an effective bone marrow delivery system in humans could prove valuable due to the vital importance of the bone marrow as a crucial hematopoiesis site.
7. Expert Opinion
Nanoparticle-based drug delivery is a promising approach to increase the local drug level in target tissues. In particular, liposomes are convenient nanoparticles for drug delivery applications because they encapsulate various agents such as anticancer drugs, anti-infective (bacteria, fungi, protozoan) drugs, bioactive proteins and peptides, nucleic acid drugs, and radionuclides. The presently described BMT-liposomes may offer a strategy to control the hematopoietic cells by the stimulation of macrophages that are associated with those cells. This specific bone marrow phagocytosis was present in rabbits and rhesus monkeys, while was significantly less prominent in hamsters, mice and rats. These interspecies differences demonstrate that the biodistribution of liposomes in small animals do not necessarily reflect liposome distribution in large animals. Based on our observations with BMT-liposome nanoparticles, we believe it is important to study nanoparticle distributions in a variety of animal species, particularly larger experimental animals such as rabbits and non-human primates.
High selectivity of the present BMT-liposome formulation for bone marrow suggests the presence of an active and specific mechanism, but specific factors affecting the uptake of the bone marrow MPS are still unknown. Further investigation of this mechanism will increase our understanding of factors required for effective transport of agents to the bone marrow, and may provide an efficient system for bone marrow delivery for therapeutic purposes. We have identified an anionic lipid having succinic acid (SA-lipid) as the targeting molecule to increase the bone marrow uptake. This molecular structure might be effective for targeting other types of nanoparticles to bone marrow for diagnostic and therapeutic applications.
The specific targeting of drugs and other biologically active agents to macrophages could be useful for therapeutic applications in both infectious and non-infectious diseases. After trapping in the macrophage, liposomes would be gradually digested by resident enzymes in macrophage lysosomes [65]. Therefore, the delivery of therapeutic agents to bone marrow tissues surrounding macrophages might be facilitated by their controlled release from macrophages. Liposomes, which are molecularly assembled by secondary weak interactions, have clear advantages for the controlled release of encapsulated agents at specifically targeted points, because their assembling properties can be critically changed by the environmental conditions. This controlled release technology has been reported in a variety of applications such as gene delivery into the nucleus [78–82]. A controlled release gene delivery methodology is based on the collapse of vesicular structures at low environmental pH following fusion with endosomes where the pH is below 5.0, i.e. pH-sensitive liposomes [79,80]. Other approaches for the controlled release of agents from vesicular nanoparticles have utilized temperature-sensitive [81] or chemically degradable liposomes [82]. Macrophages produce a wide range of biologically active molecules related to the development of various disorders [83,84]. Their intercellular control systems may widen the target to not only the targeted macrophages, themselves, but also to neighboring cells such as hematopoietic cells and tumor cells [85–88]. Further biological investigations will increase the clinical potential of this targeting system to bone marrow.
Article highlights.
Bone marrow macrophages have uptake of bioparticles such as chylomicrons and apoptotic neutrophils. It can be speculated that the bone marrow macrophages have a role in the delivery of lipids to the bone marrow as a source of energy and for membrane biosynthesis or in the delivery of fat soluble vitamins for hematopoiesis. This homeostatic system offers a potent pathway to deliver drugs selectively into bone marrow tissues from blood.
Liposomal carriers with high targeting to bone marrow have been discovered. Bone marrow macrophages offer a specific mechanism for uptake of liposomes. An anionic lipid having succinic acid is a critical component to interact with bone marrow cells. Furthermore, PEG-modification of the liposomes passively increases the bone marrow selectivity by inhibiting the hepatic uptake. This information would be useful for designing bone marrow-targeted nanoparticles.
The bone marrow of rabbits and rhesus monkeys has high uptake of BMT-liposomes. On the other hand, in hamsters, mice and rats the bone marrow-targeted liposomes are mainly captured in liver and spleen instead of the bone marrow. This fact suggests the importance of studying nanoparticle distribution in multiple animal species.
The liposomal carriers can be used as a bone marrow-targeted drug delivery system for anticancer drugs, antifungal drugs, bioactive proteins and peptides, nucleic acid drugs, and radionuclides.
Acknowledgments
The authors gratefully acknowledge Dr. Eishun Tsuchida, Dr. Shinji Takeoka, and Dr. Hiromi Sakai (Waseda University) for their valuable suggestions and discussion on this research, Dr. Anuradha Soundararajan (UTHSCSA) for technical help in acquiring the mouse and rat images and Anjana Gupta (UTHSCSA) for technical expertise with the mouse experiment. This work was partly supported by the Japan Society for the Promotion of Science (JSPS) Bilateral Joint Project between Japan-US, NIH/NCI grants (K01CA104180 and P01CA04335) and Department of Defense-Air Force (Contract No. FA7014-07-C-0034).
Footnotes
Declaration of interest
This paper has been funded by the Japan Scoiety for the Promotion of Science (JSPS), a bilateral joint project between Japan-US NIH/NCI grants (KA1CA104180 and P01CA04335) and Department of Defense-Air Force (Contract No. FA7014-07-C-0034).
The authors declare no conflicts of interest.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Janknegt R, de Marie S, Bakker-Woudenberg IA, Crommelin DJ. Liposomal and lipid formulations of amphotericin B. Clinical pharmacokinetics. Clin Pharmacokinet. 1992;23:279–91. doi: 10.2165/00003088-199223040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specificity nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 3.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–7. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 4.Awasthi VD, Garcia D, Goins BA, Phillips WT. Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int J Pharm. 2003;253:121–32. doi: 10.1016/s0378-5173(02)00703-2. [DOI] [PubMed] [Google Scholar]
- 5.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 6.Van Furth R. Inflammation: Basic Principles and Clinical Correlates. Raven Press; New York: 1992. Development and distribution of mononuclear phagocytes. [Google Scholar]
- 7.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–61. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 8.Fadok VA, Voelker DR, Campbell PA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16. [PubMed] [Google Scholar]
- 9.Taylor PR, Martinez-Pomares L, Stacey M, et al. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–44. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 10•.Sou K, Goins B, Takeoka S, et al. Selective uptake of surface-modified phospholipid vesicles by bone marrow macrophages in vivo. Biomaterials. 2007;28:2655–66. doi: 10.1016/j.biomaterials.2007.01.041. This paper provides detail biodistribution of bone marrow-targeted liposomal carriers. [DOI] [PubMed] [Google Scholar]
- 11.Sou K, Goins B, Leland MM, et al. Bone marrow-targeted liposomal carriers: a feasibility study in nonhuman primates. Nanomedicine. 2010;5:41–9. doi: 10.2217/nnm.09.78. [DOI] [PubMed] [Google Scholar]
- 12.Mistry PK, Wraight EP, Cox TM. Therapeutic delivery of proteins to macrophages: implications for treatment of Gaucher’s disease. Lancet. 1996;348:1555–9. doi: 10.1016/S0140-6736(96)04451-0. [DOI] [PubMed] [Google Scholar]
- 13.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci USA. 2009;106:1261–6. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamazo C, Prior S, Concepción Lecároz M, et al. Biodegradable gentamicin delivery systems for parenteral use for the treatment of intracellular bacterial infections. Expert Opin Drug Deliv. 2007;4:677–88. doi: 10.1517/17425247.4.6.677. [DOI] [PubMed] [Google Scholar]
- 15.Chono S, Tanino T, Seki T, Morimoto K. Efficient drug targeting to rat alveolar macrophages by pulmonary administration of ciprofloxacin incorporated into mannosylated liposomes for treatment of respiratory intracellular parasitic infections. J Control Release. 2008;127:50–8. doi: 10.1016/j.jconrel.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Romero EL, Morilla MJ. Drug delivery systems against leishmaniasis? Still an open question. Expert Opin Drug Deliv. 2008;5:805–23. doi: 10.1517/17425247.5.7.805. [DOI] [PubMed] [Google Scholar]
- 17.Schäfer V, von Briesen H, Andreesen R, et al. Phagocytosis of nanoparticles by human immunodeficiency virus (HIV)-infected macrophages: a possibility for antiviral drug targeting. Pharm Res. 1992;9:541–6. doi: 10.1023/a:1015852732512. [DOI] [PubMed] [Google Scholar]
- 18•.Chellat F, Merhi Y, Moreau A, Yahia L. Therapeutic potential of nanoparticulate systems for macrophage targeting. Biomaterials. 2005;26:7260–75. doi: 10.1016/j.biomaterials.2005.05.044. A review covering the therapeutic potential of macrophage targeting by using nanoparticles. [DOI] [PubMed] [Google Scholar]
- 19.Kim SS, Ye C, Kumar P, et al. Targeted delivery of siRNA to macrophages for anti- inflammatory treatment. Mol Ther. 2010;18:993–1001. doi: 10.1038/mt.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno PR, Falk E, Palacios IF, et al. Macrophage infiltration in acute coronary syndromes: implications for plaque rupture. Circulation. 1994;90:775–8. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 21.Wilson HM, Barker RN, Erwig LP. Macrophages: promising targets for the treatment of atherosclerosis. Curr Vasc Pharmacol. 2009;7:234–43. doi: 10.2174/157016109787455635. [DOI] [PubMed] [Google Scholar]
- 22.Antoniades C, Psarros C, Tousoulis D, et al. Nanoparticles: a promising therapeutic approach in atherosclerosis. Curr Drug Deliv. 2010;7:303–11. doi: 10.2174/156720110793360586. [DOI] [PubMed] [Google Scholar]
- 23•.Kawakami S, Sato A, Nishikawa M, et al. Mannose receptor-mediated gene transfer into macrophages using novel mannosylated cationic liposomes. Gene Ther. 2000;7:292–9. doi: 10.1038/sj.gt.3301089. This paper demonstrated mannose receptor-mediated macrophage targeting by liposomes. [DOI] [PubMed] [Google Scholar]
- 24.Wijagkanalan W, Kawakami S, Takenaga M, et al. Efficient targeting to alveolar macrophages by intratracheal administration of mannosylated liposomes in rats. J Control Release. 2008;125:121–30. doi: 10.1016/j.jconrel.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Matsui M, Shimizu Y, Kodera Y, et al. Targeted delivery of oligomannose-coated liposome to the omental micrometastasis by peritoneal macrophages from patients with gastric cancer. Cancer Sci. 2010;101:1670–7. doi: 10.1111/j.1349-7006.2010.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nahar M, Jain NK. Preparation, characterization and evaluation of targeting potential of amphotericin B-loaded engineered PLGA nanoparticles. Pharm Res. 2009;26:2588–98. doi: 10.1007/s11095-009-9973-4. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan S, Prashant CK, Koul V, et al. Receptor specific macrophage targeting by mannose-conjugated gelatin nanoparticles- an in vitro and in vivo study. Current Nanoscience. 2010;6:413–21. [Google Scholar]
- 28.Higuchi Y, Oka M, Kawakami S, Hashida M. Mannosylated semiconductor quantum dots for the labeling of macrophages. J Control Release. 2008;125:131–6. doi: 10.1016/j.jconrel.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Kumar PV, Asthana A, Dutta T, Jain NK. Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J Drug Target. 2006;14:546–56. doi: 10.1080/10611860600825159. [DOI] [PubMed] [Google Scholar]
- 30.Irache JM, Salman HH, Gamazo C, Espuelas S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin Drug Deliv. 2008;5:703–24. doi: 10.1517/17425247.5.6.703. [DOI] [PubMed] [Google Scholar]
- 31.Wu F, Wuensch SA, Azadniv M, et al. Galactosylated LDL nanoparticles: a novel targeting delivery system to deliver antigen to macrophages and enhance antigen specific T cell responses. Mol Pharm. 2009;6:1506–17. doi: 10.1021/mp900081y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haensler J, Schuber F. Preparation of neo-galactosylated liposomes and their interaction with mouse peritoneal macrophages. Biochim Biophys Acta. 1988;946:95–105. doi: 10.1016/0005-2736(88)90461-0. [DOI] [PubMed] [Google Scholar]
- 33.Fujiwara M, Baldeschwieler JD, Grubbs RH. Receptor-mediated endocytosis of poly(acrylic acid)-conjugated liposomes by macrophages. Biochim Biophys Acta. 1996;1278:59–67. doi: 10.1016/0005-2736(95)00183-2. [DOI] [PubMed] [Google Scholar]
- 34.Rensen PC, Gras JC, Lindfors EK, et al. Selective targeting of liposomes to macrophages using a ligand with high affinity for the macrophage scavenger receptor class A. Curr Drug Discov Technol. 2006;3:135–44. doi: 10.2174/157016306778108893. [DOI] [PubMed] [Google Scholar]
- 35.Lipinski MJ, Frias JC, Amirbekian V, et al. Macrophage-specific lipid-based nanoparticles improve cardiac magnetic resonance detection and characterization of human atherosclerosis. JACC Cardiovasc Imaging. 2009;2:637–47. doi: 10.1016/j.jcmg.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turk MJ, Waters DJ, Low PS. Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer Lett. 2004;213:165–72. doi: 10.1016/j.canlet.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 37•.Xia W, Hilgenbrink AR, Matteson EL, et al. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113:438–46. doi: 10.1182/blood-2008-04-150789. This paper demonstrated activated macrophages targeting via folate receptor. [DOI] [PubMed] [Google Scholar]
- 38.Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Expert Opin Drug Deliv. 2008;5:309–19. doi: 10.1517/17425247.5.3.309. [DOI] [PubMed] [Google Scholar]
- 39.Fliedner TM, Steinbach KH, Hoelzer D. Adaptation to environmental change: the role of cell-renewal systems. In: Finckh ES, editor. The Effects of Environment on Cells and Tissues. Amsterdam: Excerpta Medica; 1976. [Google Scholar]
- 40.Fliedner TM, Graessle D, Paulsen C, Reimers K. Structure and function of bone marrow hemopoiesis: mechanisms of response to ionizing radiation exposure. Cancer Biother Radiopharm. 2002;17:405–26. doi: 10.1089/108497802760363204. [DOI] [PubMed] [Google Scholar]
- 41.Hussain MM, Mahley RW, Boyles JK, et al. Chylomicron-chylomicron remnant clearance by liver and bone marrow in rabbits. Factors that modify tissue-specific uptake. J Biol Chem. 1989;264:9571–9582. [PubMed] [Google Scholar]
- 42•.Hussain MM, Mahley RW, Boyles JK, et al. Chylomicron metabolism. Chylomicron uptake by bone marrow in different animal species. J Biol Chem. 1989;264:17931–8. This paper describes the species specific uptake of chylomicron by bone marrow. [PubMed] [Google Scholar]
- 43.Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008;22:3111–9. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology. 2008;125:281–8. doi: 10.1111/j.1365-2567.2008.02950.x. This review demonstrated the importance of bone marrow for neutrophil clearance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadahira Y, Mori M. Role of the macrophage in erythropoiesis. Pathol Int. 1999;49:841–8. doi: 10.1046/j.1440-1827.1999.00954.x. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida H, Kawane K, Koike M, et al. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. 2005;437:754–8. doi: 10.1038/nature03964. [DOI] [PubMed] [Google Scholar]
- 47.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–8. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSC. Blood. 2010 doi: 10.1182/blood-2009-11-253534. (in press) [DOI] [PubMed] [Google Scholar]
- 49•.Porter CJ, Moghimi SM, Illum L, Davis SS. The polyoxyethylene/polyoxypropylene block co-polymer poloxamer-407 selectively redirects intravenously injected microspheres to sinusoidal endothelial cells of rabbit bone marrow. FEBS Lett. 1992;305:62–6. doi: 10.1016/0014-5793(92)80655-z. This paper describes the significant uptake of nanoparticles by sinusoidal endothelial cells of rabbit bone marrow. [DOI] [PubMed] [Google Scholar]
- 50.Schettini DA, Ribeiro RR, Demicheli C, et al. Improved targeting of antimony to the bone marrow of dogs using liposomes of reduced size. Int J Pharm. 2006;315:140–7. doi: 10.1016/j.ijpharm.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 51.Chi B, Park SJ, Park MH, et al. Oligopeptide delivery carrier for osteoclast precursors. Bioconjug Chem. 2010;21:1473–8. doi: 10.1021/bc100066k. [DOI] [PubMed] [Google Scholar]
- 52.Harris TJ, Green JJ, Fung PW, et al. Tissue-specific gene delivery via nanoparticle coating. Biomaterials. 2010;31:998–1006. doi: 10.1016/j.biomaterials.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Moghimi SM. Exploiting bone marrow microvascular structure for drug delivery and future therapies. Adv Drug Deliv Rev. 1995;17:61–73. A review suggesting the mechanism of particle uptake by bone marrow and describing perspective of the therapeutic potential of bone marrow targeting drug delivery. [Google Scholar]
- 54.Huang TS. Passage of foreign particles through the sinusoidal wall of the rabbit bone marrow-an electron microscopic study. Acta Pathol Jpn. 1971;21:349–67. doi: 10.1111/j.1440-1827.1971.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 55.Allen TM, Austin GA, Chonn A, et al. Uptake of liposomes by cultured mouse bone marrow macrophages: influence of liposome composition and size. Biochim Biophys Acta. 1991;1061:56–64. doi: 10.1016/0005-2736(91)90268-d. [DOI] [PubMed] [Google Scholar]
- 56.Nishikawa K, Arai H, Inoue K. Scavenger receptor-mediated uptake and metabolism of lipid vesicles containing acidic phospholipids by mouse peritoneal macrophages. J Biol Chem. 1990;265:5226–31. [PubMed] [Google Scholar]
- 57•.Szabó R, Peiser L, Plüddemann A, et al. Uptake of branched polypeptides with poly[L-lys] backbone by bone-marrow culture-derived murine macrophages: the role of the class A scavenger receptor. Bioconjug Chem. 2005;16:1442–50. doi: 10.1021/bc050168f. This paper provides information on class A scavenger receptor targeting on bone marrow macrophage in vitro. [DOI] [PubMed] [Google Scholar]
- 58.Tokuda H, Masuda S, Takakura Y, et al. Specific uptake of succinylated proteins via a scavenger receptor-mediated mechanism in cultured brain microvessel endothelial cells. Biochem Biophys Res Commun. 1993;196:18–24. doi: 10.1006/bbrc.1993.2210. [DOI] [PubMed] [Google Scholar]
- 59.Allen TM. The use of glycolipids and hydrophilic polymers in avoiding rapid uptake of liposomes by the mononuclear phagocyte system. Adv Drug Deliv Rev. 1994;13:285–309. [Google Scholar]
- 60.Tsuchida E. Blood Substitute: Present and Future Perspective. Elsevier Science; Amsterdam: 1998. [Google Scholar]
- 61.Tsuchida E, Sou K, Nakagawa A, Sakai H, Komatsu T, Kobayashi K. Artificial oxygen carriers, hemoglobin vesicles and albumin-hemes, based on bioconjugate chemistry. Bioconjugate Chem. 2009;20:1419–40. doi: 10.1021/bc800431d. [DOI] [PubMed] [Google Scholar]
- 62.Sakai H, Sou K, Horinouchi H, et al. Haemoglobin-vesicles as artificial oxygen carriers: present situation and future visions. J Intern Med. 2008;263:4–15. doi: 10.1111/j.1365-2796.2007.01893.x. [DOI] [PubMed] [Google Scholar]
- 63.Sou K, Klipper R, Goins B, et al. Circulation kinetics and organ distribution of Hb-vesicles developed as a red blood cell substitute. J Pham Exp Ther. 2005;312:702–9. doi: 10.1124/jpet.104.074534. [DOI] [PubMed] [Google Scholar]
- 64.Taguchi K, Urata Y, Anraku M, et al. Pharmacokinetic study of enclosed hemoglobin and outer lipid component after the administration of hemoglobin vesicles as an artificial oxygen carrier. Drug Metab Dispos. 2009;37:1456–63. doi: 10.1124/dmd.109.027094. [DOI] [PubMed] [Google Scholar]
- 65.Sakai H, Horinouchi H, Tomiyama K, et al. Hemoglobin-vesicles as oxygen carriers: influence on phagocytic activity and histopathological changes in reticuloendothelial system. Am J Pathol. 2001;159:1079–88. doi: 10.1016/S0002-9440(10)61783-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uster PS, Allen TM, Daniel BE, et al. Insertion of poly-(ethylene glycol) derivatized phospholipid into pre-formed liposomes results in prolonged in vivo circulation time. FEBS Lett. 1996;386:243–6. doi: 10.1016/0014-5793(96)00452-8. [DOI] [PubMed] [Google Scholar]
- 67.Sou K, Endo T, Takeoka S, Tsuchida E. Poly(ethylene glycol)-modification of the phospholipid vesicles by using the spontaneous incorporation of poly(ethylene glycol)-lipid into the vesicles. Bioconjug Chem. 2000;11:372–9. doi: 10.1021/bc990135y. [DOI] [PubMed] [Google Scholar]
- 68.Sou K, Naito Y, Endo T, et al. Effective encapsulation of proteins into size-controlled phospholipid vesicles using freeze-thawing and extrusion. Biotechnol Prog. 2003;19:1547–52. doi: 10.1021/bp0201004. [DOI] [PubMed] [Google Scholar]
- 69.Sato T, Sakai H, Sou K, et al. Static structures and dynamics of hemoglobin vesicle (HBV) developed as a transfusion alternative. J Phys Chem B. 2009;113:8418–28. doi: 10.1021/jp9002142. [DOI] [PubMed] [Google Scholar]
- 70.Goins B, Phillips WT. The use of scintigraphic imaging as a tool in the development of liposome formulations. Prog Lipid Res. 2001;40:95–123. doi: 10.1016/s0163-7827(00)00014-x. [DOI] [PubMed] [Google Scholar]
- 71.Gabizon A, Chisin R, Amselem S, et al. Pharmacokinetic and imaging studies in patients receiving a formulation of liposome-associated adriamycin. Br J Cancer. 1991;64:1125–32. doi: 10.1038/bjc.1991.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laverman P, Brouwers AH, Dams ET, et al. Preclinical and clinical evidence for disappearance of long-circulating characteristics of polyethylene glycol liposomes at low lipid dose. J Pharmacol Exp Ther. 2000;293:996–1001. [PubMed] [Google Scholar]
- 73.Phillips WT, Rudolph AS, Goins B, et al. A simple method for producing a technetium-99m labeled liposome which is stable in vivo. Nucl Med Biol. 1992;19:539–47. doi: 10.1016/0883-2897(92)90149-s. [DOI] [PubMed] [Google Scholar]
- 74.Morrie E, Kricun MD. Red yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14:10–19. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]
- 75.Oyajobi BO, Muñoz S, Kakonen R, et al. Detection of myeloma in skeleton of mice by whole-body optical fluorescence imaging. Mol Cancer Ther. 2007;6:1701–8. doi: 10.1158/1535-7163.MCT-07-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupta S, Pal A, Vyas SP. Drug delivery strategies for therapy of visceral leishmaniasis. Expert Opin Drug Deliv. 2010;7:371–402. doi: 10.1517/17425240903548232. [DOI] [PubMed] [Google Scholar]
- 77.Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–8. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 79.Legendre JY, Szoka FC. Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: comparison with cationic liposomes. Pharm Res. 1992;9:1235–42. doi: 10.1023/a:1015836829670. [DOI] [PubMed] [Google Scholar]
- 80.Obata Y, Tajima S, Takeoka S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. J Control Release. 2010;142:267–76. doi: 10.1016/j.jconrel.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 81.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60:1197–201. [PubMed] [Google Scholar]
- 82.Andresen TL, Jensen SS, Jorgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–70. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-assosiated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 85.Dove A. Cell-based therapies go live. Nature Biotechnol. 2002;20:339–43. doi: 10.1038/nbt0402-339. [DOI] [PubMed] [Google Scholar]
- 86.Burke B, Sumner S, Maitland N, Lewis CE. Macrophages in gene therapy: cellular delivery vehicles and in vivo targets. J Leukoc Biol. 2002;72:417–28. [PubMed] [Google Scholar]
- 87.Hinds KA, Hill JM, Shapiro EM, et al. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood. 2003;102:867–72. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 88.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–64. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]