Abstract
Osteoblasts are continually recruited from stem cell pools to maintain bone. Although their immediate precursor is a plastic-adherent mesenchymal stem cell able to generate tissues other than bone, increasing evidence suggests the existence of a more primitive cell that can differentiate to both hematopoietic and mesenchymal cells. We show here that the “side population” (SP) of marrow stem cells, defined by their ability to rapidly expel a DNA-binding dye and to regenerate the hematopoietic compartment, can differentiate to osteoblasts through a mesenchymal intermediate. When transplanted into lethally irradiated mice, single gene-marked murine SP cells reconstituted depleted osteoprogenitor pools, such that a large proportion of the osteogenic cells in the epiphysis of long bone carried the donor SP cell marker. These findings suggest that the developmental capacity of SP cells is not restricted to the hematopoietic lineages but extends to osteogenic differentiation. This property not only elucidates a previously unrecognized step in osteoblast development, but also has intriguing implications for the use of SP cells in clinical orthopedics and stem cell-based disorders of bone.
Skeletal bone is unique among human tissues. It is continuously remodeled throughout life in a process that requires the recruitment and proliferation of stem cells with the capacity to differentiate to functional osteoblasts, which then deposit and mineralize extracellular bone matrix (1, 2). The identification of an osteogenic stem cell with competency for both self-renewal and robust differentiation to bone-forming osteoblasts has been elusive. Several studies have documented the ability of cells in whole bone marrow to form osteoblasts in vitro (3) and in vivo (4). Pittenger et al. (3) isolated mesenchymal stem cells (MSCs) that were able to differentiate to chondrocytes, adipocytes, and osteoblasts in culture, whereas the results of serial transplantation of single bone marrow-derived stem cells can be interpreted to suggest the existence of rare long-term repopulating cells that can regenerate not only the entire hematopoietic system, but also several different mesenchymal lineages (5, 6).
Our efforts to identify an osteogenic stem cell have focused on a side population (SP) of bone marrow cells that display strong hematopoietic reconstituting activity, as measured by competitive repopulation assays (7, 8). These so-called SP cells, which can be identified by their unique capacity to efflux fluorescent DNA-binding dye (7, 8), also have a limited capacity to differentiate in vivo to skeletal myocytes (9) as well as vascular endothelial cells (10), suggesting multilineage potential. To test the candidacy of these adult stem cells as progenitors of the osteoblast lineage, we tracked the fate of gene-marked SP both in vitro and in vivo. Donor-derived mesenchymal progenitors differentiated to osteoblasts in clonogenic medium, and immunostaining of long-bone sections after transplantation of SP cells into lethally irradiated mice demonstrated an abundance of gene-marked osteoblastic cells lining trabecular bone near the growth plate. These results suggest a model in which the osteoprogenitors required for bone remodeling are recruited from a pool of SP stem cells residing in bone marrow.
Methods
Isolation and Transplantation of SP Cells. SP cells were isolated from the bone marrow of 10 C57BL/6 CD45.2 Rosa26 mice according to the procedure of Goodell et al. (refs. 7 and 8; see also www.bcm.tmc.edu/genetherapy/goodell). Three thousand of these cells were injected retro-orbitally into 6- to 12-week-old C57BL/6 CD45.1 mice that had been given 11 Gy of radiation in a split dose. After ≈4 weeks, the mice were bled retro-orbitally, and the percentage of hematopoietic engraftment was determined by fluorescence-activated cell sorting analysis (11).
In single-cell transplantation studies, bone marrow was obtained from 2-month-old C57BL/6 CD45.2 Rosa26 mice. Single SP (CD45.2+ Sca-1+) cells were sorted directly into individual wells of a 96-well plate containing 150 μl of buffer by a single-cell deposition unit (Cytomation, Fort Collins, CO). Four hundred short-term repopulating (Lin- Sca1+ cKit+ CD34high) cells from a CD45.1 donor were then added to each well to support hematopoietic engraftment. The donor cells were transplanted into lethally irradiated 6- to 8-week-old C57BL/6 CD45.1 mice, and hematopoietic contribution was measured monthly by staining peripheral blood leukocytes with CD45.2FITC. Mice were killed 14 months after the first transplantation.
Mesenchymal Progenitor Cultures. Bone marrow was isolated from the leg bones of mice to generate plastic adherent cells in specialized medium [MesenCult basal medium for murine MSCs supplemented with MSC stimulatory supplement (murine), both from StemCell Technologies, Vancouver]. After 3 days, the culture supernatant, together with nonattached cells was withdrawn and the medium was replaced. When the cultures reached confluence, the medium in some cultures was changed to α-MEM (Invitrogen) with 10% FBS containing 100 nM dexamethasone and 50 μg/ml ascorbic acid, and the others remained untreated. With this procedure, an average of five bone-like nodules were identified per 107 whole bone marrow cells. Alkaline phosphatase was assayed with ELF 97 phosphatase substrate (Molecular Probes), which exhibits green fluorescence after cleavage, according to the manufacturer's instructions. Osteocalcin staining was carried out by using goat anti-mouse osteocalcin and detected by using fluorescein. An average of five nodules were obtained per 107 mouse whole bone marrow cells.
Immunohistochemical Analysis of Bone Sections. Six weeks after transplantation with either SP cells or whole bone marrow, mice were killed. Hind-limb paraffin-embedded (5 μm) sections were stained with a monoclonal antibody to Escherichia coli β-galactosidase (Roche Applied Sciences) by using a PowerVision Homo-Mouse IHC kit (ImmunoVision Technologies, Daly City, CA). The sections were counterstained with hematoxylin.
For double staining to detect both β-galactosidase and mouse osteocalcin, Vector Nova Red (Vector Laboratories) was substituted for the standard diaminobenzidine reagent. Sections were coated with Clearmount (Zymed, South San Francisco, CA) after staining for β-galactosidase, examined microscopically, photographed, incubated in water at 37°C to remove the mounting medium, and stained with goat anti-mouse osteocalcin antibody (Biomedical Technologies, Stoughton, MA), using an ABC Elite reagent (Vector Laboratories) and Vector SG (Vector Laboratories). Sections were viewed microscopically to identify the fields previously photographed after single staining.
Results
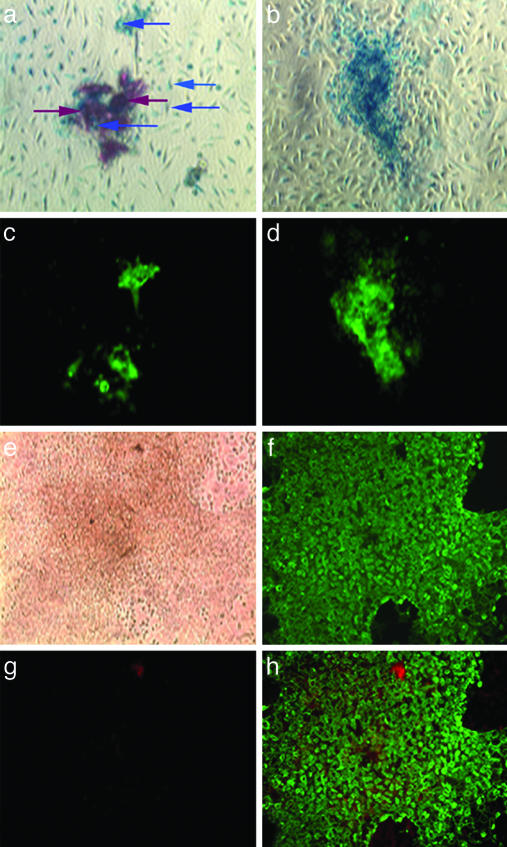
Characterization of SP and Mesenchymal Progenitor Populations. We first tested whether SP cells can give rise to mesenchymal progenitors capable of differentiating to osteoblasts. Irradiated C57BL/6 CD45.1 mice (n = 4) were transplanted with 3,000 SP cells isolated by Hoechst dye exclusion from C57BL/6 CD45.2 Rosa26 mice, which express the E. coli β-galactosidase marker in all of their tissues. After 4 weeks, hematopoietic engraftment in peripheral blood exceeded 90% in all animals. Two weeks later the mice were killed, and bone marrow was cultured to obtain plastic-adherent cells, which were placed in osteoinductive media. After 14 days, we observed bone-like nodules, approximately one-half of which stained positively for β-galactosidase (Fig. 1 a and b, blue), a marker of donor SP cells, as well as alkaline phosphatase (Fig. 1 c, d, and f, green) and mineral deposits (Fig. 1a, red-brown), indicative of an osteoblast phenotype and function, respectively. Further, the majority of cells in the single alkaline phosphatase-positive nodule shown in Fig. 1e lacked the definitive blood cell marker CD45 (Fig. 1 g and h, expected color, red), in contrast to the CD45+ phenotype of most cells in control cultures (not shown), which did not receive osteoinductive media and lacked bony nodules. These results suggest that SP cells may possess osteogenic potential realized through fibroblast intermediates.
Fig. 1.
Bone-like nodules are derived from donor SP cells in cultures treated with osteogenic medium. Nodules were stained for both β-galactosidase (a and b, blue) and mineral deposits (a, red-brown) and costained for alkaline phosphatase, a marker of differentiated osteoblasts (c and d, green fluorescence). A bright-field image of a typical nodule is shown in e. This nodule was later stained for alkaline phosphatase (f) and the CD45 surface antigen (g, red). h is a composite of f and g. (Magnification, ×10.)
Osteogenic Differentiation by a Single Bone Marrow-Derived SP Cell. We next performed experiments in which a single SP cell was transplanted into recipient mice. Whole bone marrow cells from C57BL/6 CD45.2 Rosa26 mice were stained with Hoechst 33342 dye, and single CD45+ Sca-1+ SP cells were sorted into the individual wells of a 96-well plate. The single SP cells were transplanted into lethally irradiated C57BL/6 CD45.1 mice together with short-term repopulating cells derived from the same mouse strain, to provide committed progenitors that would assist in bone marrow regeneration during the early phase of recovery from radiation treatment (12). Approximately 14 months after transplantation, whole bone marrow was isolated, and plastic-adherent cells were cultured in osteoinductive media.
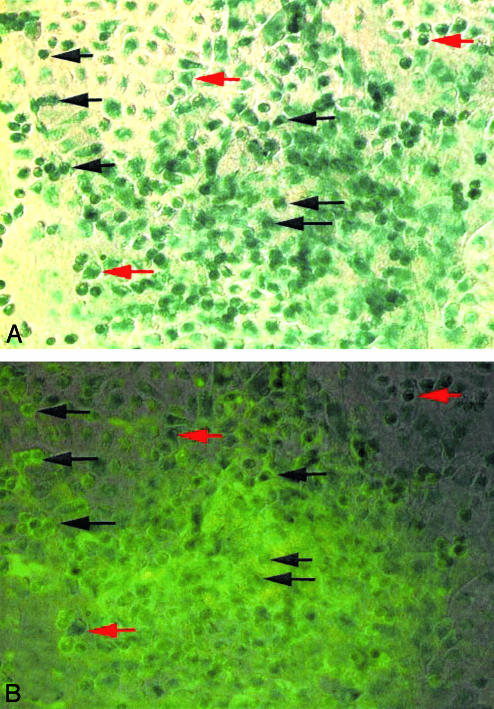
Bone-like nodules that stained positively for β-galactosidase and osteocalcin were observed in cell cultures treated with osteoinductive media (Fig. 2). The major fraction of cells in the apex of a given nodule stained positively for both markers (black arrows), as did some cells in the periphery. Other peripheral cells showed only β-galactosidase activity (red arrows). Noncellular osteocalcin staining in the apex of the nodule is probably associated with bone matrix. This staining pattern is identical to that described by Pockwinse et al. (13), who noted that osteocalcin synthesis occurs postproliferatively and is therefore mostly restricted to cells within the apex of the nodule. Some nodules had only a few cells at the apex that were positive for both β-galactosidase and osteocalcin, suggesting that these nodules were in an earlier state of osteoblastic differentiation (data not shown). All bony nodules in parallel cultures of marrow from nontransplanted C57BL/6 CD45.1 mice were positive for osteocalcin but not β-galactosidase (data not shown). These findings clearly demonstrate that a single SP cell can give rise to repopulating mesenchymal progenitors with the capacity to differentiate to functional osteoblasts.
Fig. 2.
A single transplanted SP can differentiate to osteogenic MSCs. Cultures of bone marrow from mice undergoing single SP-cell transplantation were established and subsequently treated with osteoinductive medium (α-MEM containing 100 nM dexamethasone and 50 μg/ml ascorbic acid). After 2 weeks, cultures were stained for mouse osteocalcin, a recognized marker of osteoblasts. Individual bone-like nodules (≈10–20) in the cultures were photographed and stained for β-galactosidase (A, blue-green). B includes an overlay of A showing the results of osteocalcin staining (green). Black arrows denote individual cells staining for both β-galactosidase and osteocalcin, and red arrows indicate peripheral cells staining for β-galactosidase only. The major fraction of cells in the apex of any nodule staining positively for β-galactosidase reacted with both reagents.
Both SP Cells and Whole Bone Marrow Generate Osteoblasts for Endochondral Bone Formation. To track the fate of SP-derived osteoblasts in vivo and to compare their prevalence in bone with that of osteoblasts from whole marrow, we irradiated C57BL/6 CD45.1 mice and transplanted them with either 3 × 103 SP cells or 1 × 106 unmanipulated marrow cells from C57BL/6 CD45.2 Rosa26 mice (n = 3 per treatment group). Nontransplanted C57BL/6 CD45.1 mice served as controls. Four weeks after transplantation, the percentage of hematopoietic engraftment was >90% in both groups.
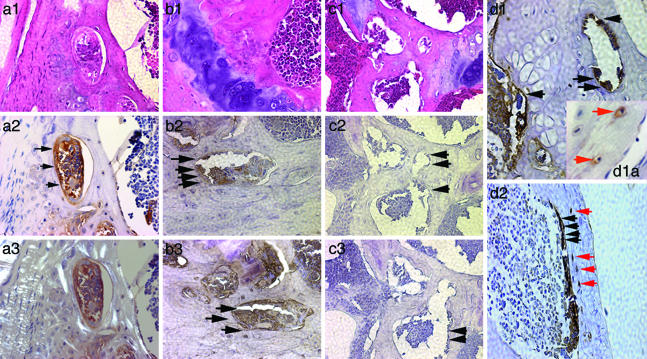
Figs. 3a1 and a2 (SP cells) and b1 and b2 (whole bone marrow) show representative positive results of staining osteoblasts and other osteogenic cells (e.g., bone lining cells) lining trabeculae (arrows) near the growth plate. The rim of newly formed bone apparent in Fig. 3a2 is confirmed in a3 by the decreased birefringence evident under polarized light. By contrast, in sections of C57BL/6 CD45.1 mouse femur (no transplantation), the osteogenic cells lining trabeculae did not react with the β-galactosidase antibody (Fig. 3 c2 and c3, arrows). All sections were counterstained with hematoxylin. The use of horseradish peroxidase to detect β-galactosidase activity did not produce background staining (Fig. 3 c2 and c3). Finally, analysis of additional donor-derived osteogenic cells revealed positively staining osteocytes within lacunae (red arrows) in mice transplanted with either SP cells (Fig. 3d1a) or whole bone marrow (Fig. 3d2), suggesting the ability of donor-derived osteoblasts to undergo terminal differentiation.
Fig. 3.
Detection of donor SP cell-derived osteoblasts lining the trabeculae of hind-limb bone. Serial hind-limb sections from transplanted mice were stained with an antibody against β-galactosidase, detected with horseradish peroxidase (brown) and hematoxylin (blue). Positively staining osteogenic cells were readily apparent in trabecular bone (arrows) whether mice were transplanted with marked SP cells (a1–a3) or whole bone marrow (b1–b3). a3 duplicates a2 using polarized light, and b3 shows the results with an anti-osteocalcin antibody. c2 (five sections away from the section depicted in c1) and c3 show representative bone sections from nontransplanted C57BL/6 CD45.1 mice stained with anti-β-galactosidase or an anti-human mitochondrial antigen, respectively. Although osteogenic cells were evident in trabecular bone, they lacked staining altogether. c1 is an adjacent section stained with hematoxylin and eosin. d1 and d2 show representative bone sections of mice transplanted with either SP cells or whole bone marrow, respectively, and stained with anti-β-galactosidase antibody. Red arrows in d1a and d2 indicate the position of osteocytes staining positively for β-galactosidase. (Magnification, ×40, except d1a, ×100.)
To confirm that the positively staining cells were indeed osteoblasts, we costained immediately adjacent serial sections for the osteoblast-specific gene product osteocalcin (14, 15), which is synthesized only by late-stage osteoblasts and, in its secreted form, serves as an integral component of bone matrix (16, 17). The results are presented in Fig. 3b3 (whole bone marrow).
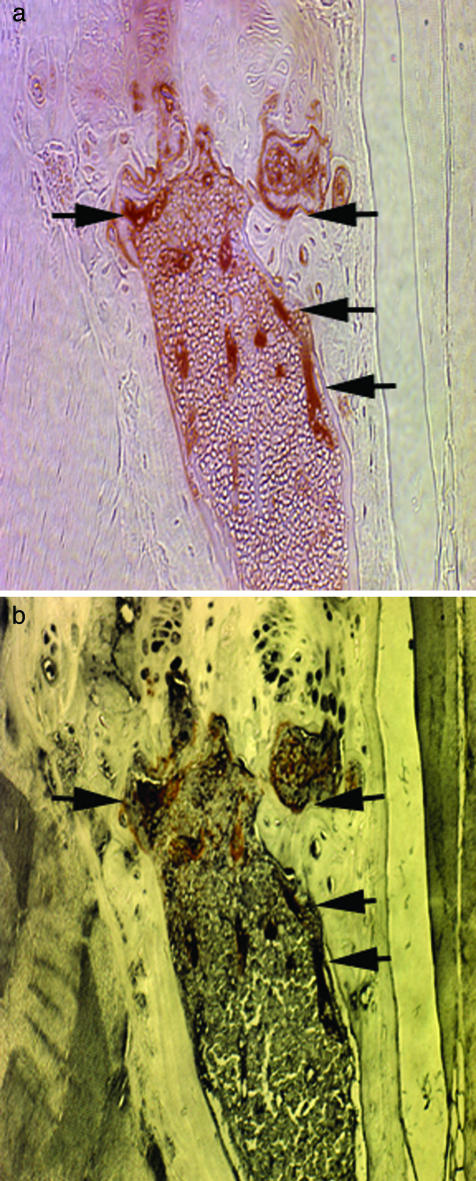
To demonstrate more rigorously the osteoblast identity of donor-derived cells lining trabeculae, we stained the same section of bone with both anti-β-galactosidase and anti-mouse osteocalcin antibodies. Long-bone sections from C57BL/6 CD45.1 mice (n = 4) that were lethally irradiated and repopulated with 3,000 SP cells from C57BL/6 CD45.2 Rosa26 mice were first stained with anti-β-galactosidase antibody (red-brown) and then with anti-mouse osteocalcin antibody (black). Fig. 4 depicts osteocalcin-positive cells (osteoblasts) lining trabeculae near the growth plate (b), many of which also stained positively for the β-galactosidase marker gene (a).
Fig. 4.
Osteocalcin analysis of bone from C57BL/6 CD45.1 mice transplanted with marked SP cells for the presence of marked osteoblasts. Mice were irradiated and transplanted with SP cells derived from C57BL/6 CD45.2 Rosa26 mice. Paraffin-embedded sections of normal long bone were then costained with an anti-β-galactosidase antibody and an antibody against mouse osteocalcin, a definitive marker of osteoblasts. A shows a representative field from a section stained with the anti-β-galactosidase antibody and Vector Nova Red (red-brown). B shows the same field after removal of the mounting medium and staining with the antibody against mouse osteocalcin and Vector SG (black). Bone sections from nontransplanted mice were also double stained and shown to be negative for β-galactosidase, and other murine tissues failed to react with the anti-osteocalcin antibody (not shown). (Magnification, ×40.)
A major question in this study was whether isolated SP cells possess the same capacity as whole bone marrow to generate new osteoblast populations. Thus, proportions of β-galactosidase-positive osteoblasts were determined in mice transplanted with either SP cells or whole bone marrow. As shown in Table 1, a substantial proportion of the osteoblasts were donor-derived whether the animals received gene-marked SP cells (13 ± 3%) or whole bone marrow (8 ± 4%). One explanation for these data is that the entire osteoblast regenerating power of whole bone marrow is represented by SP cells. However, this interpretation is subject to the bias inherent in counting immunostained cells. Formal comparison of the capacity of whole bone marrow and of the SP cells it contains to produce osteoblasts in vivo would require additional large-scale experiments with MSCs themselves, whole bone marrow depleted of MSCs, and whole bone marrow depleted of MSCs and SP cells. The results also show (Table 1) that the vast majority of donor-derived osteoblasts (>60%) were found in the epiphysis and metaphysis; only 1.3 ± 1% of the osteoblasts in the diaphysis were of donor origin. The overall percentage of donor-derived osteoblasts was 13 ± 3%. Several previous studies have reported that transplantation with either whole bone marrow or MSCs results not only in the incorporation of donor-derived cells into bone (18), but also that the donor label is transferred to bone lining cells, osteoblasts, and osteocytes within bone (4, 19, 20). In contrast to the current study, however, these investigations used mice with osteogenesis imperfecta (4, 18, 20), did not perform marrow ablation (21), or injected marked cells directly into the marrow space (19).
Table 1. Engraftment of donor-derived osteoblasts in the long bones of mice transplanted with either SP cells or whole bone marrow.
| Mean (±SD) percentage of osteoblasts derived from donor cells*
|
||||
|---|---|---|---|---|
| Mice transplanted with | Epiphysis | Metaphysis | Diaphysis | Total |
| 3 × 103 SP cells from C57BL/6 CD45.2 Rosa26 mice | 46 ± 20 | 18 ± 15 | 1.3 ± 1 | 13 ± 3 |
| 1 × 106 whole bone marrow cells from C57BL/6 CD45.2 Rosa26 mice | 33 ± 2 | 11 ± 0 | 0.12 ± 0.09 | 8 ± 4 |
A total of 6 C57BL/6 CD45.1 Rosa26 mice (6 weeks old, three per group) were lethally irradiated (11 Gy of γ-irradiation in a split dose) and transplanted with the indicated numbers of SP cells or unsorted bone marrow cells. Six weeks later, the percent engraftment was determined by fluorescence-activated cell sorting analysis of peripheral blood for the β-galactosidase marker with the fluorescent substrate FDG (Molecular Probes) and found to be >90% in all cases. The animals were killed, and the bones of the hind limbs were processed for immunohistochemistry.
Cells that stained positively for β-galactosidase (SP cell marker) were counted in various sections of one femur of each mouse, and the average (±SD) was determined. Any cells that stained positively for β-galactosidase but not for osteocalcin (an osteoblast marker) were excluded
Discussion
These studies demonstrate that SP cells, a self-renewing population of bone marrow hematopoietic stem cells with a Lin- Sca1+ cKit+ CD45+ surface phenotype (22, 23), can regenerate functional osteoblasts. The percentage of bone-forming osteoblasts that expressed the C57BL/6 CD45.2 Rosa26 marker of the donor SP cells was quite high in the epiphysis and metaphysis and represented all of the osteogenic activity in whole bone marrow (Table 1). Most of the donor-derived osteoblasts were found along the margins of trabeculae of the epiphyseal and metaphyseal compartments, the most metabolically active region during longitudinal bone growth as well as during the remodeling of mature bone (24, 25). Jilka et al. (26) showed that osteoblasts in mouse bone have a lifespan of only 300 h, a turnover rate that could account, at least in part, for the high engraftment levels we observed. Very few osteocytes in cortical bone contained the donor marker, presumably because of the low turnover of osteocytes compared to osteoblasts (26). Nevertheless, our detection of even rare donor-derived osteocytes (Fig. 3 d1a and d2) suggests that SP cell-derived osteoblasts are competent to undergo further maturation, a requisite step in the formation of new bone.
The percent engraftment of SP cell-derived osteoblast in the epiphysis and metaphysis was remarkably higher than SP cell contributions to other tissues, including 0.02% of cardiomyocytes (10), 3.3% of endothelial cells adjacent to an infarct (10), and <1% in regenerating muscle fibers (27), suggesting that the developmental capacity of SP cells is primarily restricted to the hematopoietic and osteoblast lineages, and does not extend to mesenchymal lineages in general. An osteogenic potential for this ostensibly blood-restricted side population of stem cells is supported by the loss of the CD45 surface marker during SP cell differentiation to osteoblasts, by the close anatomic alignment of the bone and marrow compartments, and by the monocytic derivation of osteoclasts (28). Wagers et al. (29), in a recent study of tissue reconstitution after transplantation of a single marked donor stem cell, concluded that there is little developmental plasticity of adult hematopoietic stem cells. Although they did examine many different organs and tissues for the presence of donor-derived cells, these authors did not search for donor osteoblasts in bone. Thus, we would argue that our results do not reflect rare stochastic events after SP cell transplantation into radiation-conditioned hosts (30), but rather a normal functional activity of SP cells, i.e., providing an osteoblast reservoir from which the constant demands for bone remodeling can be met. We did not determine chromosome numbers within these cells and therefore cannot rule out stem cell–osteoblast fusion (31, 32). This explanation seems unlikely not only because the number of positively staining osteoblasts was quite high, in contrast to the rarity of known cell fusion events (9, 33), but also because osteoblasts have not been implicated in such fusion.
Friedenstein et al. (34), in 1968, showed that on transplantation of bone marrow under the renal capsule, new bone is formed from donor cells, demonstrating that whole bone marrow possesses a stem cell that can give rise to osteoblasts. Subsequent studies by Caplan (35) and others (36) delineated the mesogenic process of cellular differentiation from precursor cells to multiple mesenchymal lineages, leading to the concept of an adult MSC able to generate osteoblasts (3, 37). Unlike SP cells, MSCs constitute a heterogeneous population, lack the CD45 marker, and do not possess unique surface antigens that can be used to distinguish between lineage-committed and uncommitted progenitors. Our data suggest that SP cells differentiate to a fibroblast intermediate before becoming osteoblasts. Whether this pathway is identical to or independent of the well described generation of osteoblasts directly from a multipotent MSC (3, 37) is unclear and warrants further investigation.
The use of isolated mesenchymal progenitors for therapeutic purposes has been hampered by their relatively low engraftment rate (18), the heterogeneity of the MSC population (38), and the lack of reliable markers for early-stage osteoprogenitors (39). We suggest that SP cells may have the potential to override these obstacles. For example, the level of osteogenic stem cells decreases during aging, which may contribute to osteoporosis (40). The ability to increase the number of functional osteoblasts in bone by transplantation of SP cells might offset this deficiency and help attenuate the disease phenotype, in contrast to most current therapies, which only prevent the loss of existing bone (41). High-level engraftment of normal osteoblasts might also improve therapy for diseases of bone in which the major defect is the abnormal production of bone matrix (42).
Acknowledgments
We thank Brian Newsom and Mike Cubbage for operation of the Cytomation MoFlow Cell Sorter, Tatiana Goltsova for assistance with flow cytometry, Robert McAlhany for technical assistance, Dorthy Burton for assistance with mice in the barrier facility, and John Gilbert for his critical review of the manuscript. This work was supported by National Institutes of Health Grants R03 AR47463-01 (to E.A.O.-D.) and R21 AR484-01 (to A.R.D.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MSC, mesenchymal stem cell; SP, side population.
References
- 1.Wagner, E. F. & Karsenty, G. (2001) Curr. Opin. Genet. Dev. 11, 527-532. [DOI] [PubMed] [Google Scholar]
- 2.Karsenty, G. & Wagner, E. F. (2002) Dev. Cell 2, 389-406. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., Moorman, M. A., Simonetti, D. W., Craig, S. & Marshak, D. R. (1999) Science 284, 143-147. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz, E. M., Prockop, D. J., Fitzpatrick, L. A., Koo, W. W., Gordon, P. L., Neel, M., Sussman, M., Orchard, P., Marx, J. C., Pyeritz, R. E. & Brenner, M. K. (1999) Nat. Med. 5, 309-313. [DOI] [PubMed] [Google Scholar]
- 5.Jiang, Y., Jahagirdar, B. N., Reinhardt, R. L., Schwartz, R. E., Keene, C. D., Ortiz-Gonzalez, X. R., Reyes, M., Lenvik, T., Lund, T., Blackstad, M., et al. (2002) Nature 418, 41-49. [DOI] [PubMed] [Google Scholar]
- 6.Krause, D. S., Theise, N. D., Collector, M. I., Henegariu, O., Hwang, S., Gardner, R., Neutzel, S. & Sharkis, S. J. (2001) Cell 105, 369-377. [DOI] [PubMed] [Google Scholar]
- 7.Goodell, M. A., Brose, K., Paradis, G., Conner, A. S. & Mulligan, R. C. (1996) J. Exp. Med. 183, 1797-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodell, M. A., Rosenzweig, M., Kim, H., Marks, D. F., DeMaria, M., Paradis, G., Grupp, S. A., Sieff, C. A., Mulligan, R. C. & Johnson, R. P. (1997) Nat. Med. 3, 1337-1345. [DOI] [PubMed] [Google Scholar]
- 9.Gussoni, E., Soneoka, Y., Strickland, C. D., Buzney, E. A., Khan, M. K., Flint, A. F., Kunkel, L. M. & Mulligan, R. C. (1999) Nature 401, 390-394. [DOI] [PubMed] [Google Scholar]
- 10.Jackson, K. A., Majka, S. M., Wang, H., Pocius, J., Hartley, C. J., Majesky, M. W., Entman, M. L., Michael, L. H., Hirschi, K. K. & Goodell, M. A. (2001) J. Clin. Invest. 107, 1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiering, S. N., Roederer, M., Nolan, G. P., Micklem, D. R., Parks, D. R. & Herzenberg, L. A. (1991) Cytometry 12, 291-301. [DOI] [PubMed] [Google Scholar]
- 12.Osawa, M., Hanada, K., Hamada, H. & Nakauchi, H. (1996) Science 273, 242-245. [DOI] [PubMed] [Google Scholar]
- 13.Pockwinse, S. M., Wilming, L. G., Conlon, D. M., Stein, G. S. & Lian, J. B. (1992) J. Cell. Biochem. 49, 310-323. [DOI] [PubMed] [Google Scholar]
- 14.Frendo, J. L., Xiao, G., Fuchs, S., Franceschi, R. T., Karsenty, G. & Ducy, P. (1998) J. Biol. Chem. 273, 30509-30516. [DOI] [PubMed] [Google Scholar]
- 15.Ducy, P., Desbois, C., Boyce, B., Pinero, G., Story, B., Dunstan, C., Smith, E., Bonadio, J., Goldstein, S., Gundberg, C., et al. (1996) Nature 382, 448-452. [DOI] [PubMed] [Google Scholar]
- 16.Desbois, C., Hogue, D. A. & Karsenty, G. (1994) J. Biol. Chem. 269, 1183-1190. [PubMed] [Google Scholar]
- 17.Hauschka, P. V., Lian, J. B., Cole, D. E. & Gundberg, C. M. (1989) Physiol. Rev. 69, 990-1047. [DOI] [PubMed] [Google Scholar]
- 18.Pereira, R. F., O'Hara, M. D., Laptev, A. V., Halford, K. W., Pollard, M. D., Class, R., Simon, D., Livezey, K. & Prockop, D. J. (1998) Proc. Natl. Acad. Sci. USA 95, 1142-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onyia, J. E., Clapp, D. W., Long, H. & Hock, J. M. (1998) J. Bone Miner. Res. 13, 20-30. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz, E. M., Prockop, D. J., Gordon, P. L., Koo, W. W., Fitzpatrick, L. A., Neel, M. D., McCarville, M. E., Orchard, P. J., Pyeritz, R. E. & Brenner, M. K. (2001) Blood 97, 1227-1231. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson, S. K., Dooner, M. S., Weier, H. U., Frenkel, B., Lian, J. B., Stein, G. S. & Quesenberry, P. J. (1999) J. Exp. Med. 189, 729-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirschi, K. & Goodell, M. (2001) Differentiation 68, 186-192. [DOI] [PubMed] [Google Scholar]
- 23.Ramos, C. A., Venezia, T. A., Camargo, F. A. & Goodell, M. A. (2003) BioTechniques 34, 572-591. [DOI] [PubMed] [Google Scholar]
- 24.Fleisch, H. (1995) Bisphosphonates in Bone Disease: From the Laboratory to the Patient (Academic, New York).
- 25.Steiner, E., Jergas, M. & Genant, H. K. (1996) Radiology of Osteoporosis (Academic, New York).
- 26.Jilka, R. L., Weinstein, R. S., Bellido, T., Parfitt, A. M. & Manolagas, S. C. (1998) J. Bone Miner. Res. 13, 793-802. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari, G., Cusella-De Angelis, G., Coletta, M., Paolucci, E., Stornaiuolo, A., Cossu, G. & Mavilio, F. (1998) Science 279, 1528-1530. [DOI] [PubMed] [Google Scholar]
- 28.Duong, L. T. & Rodan, G. A. (2001) Rev. Endocr. Metab. Disorders 2, 95-104. [DOI] [PubMed] [Google Scholar]
- 29.Wagers, A. J., Sherwood, R. I., Christensen, J. L. & Weissman, I. L. (2002) Science 297, 2256-2259. [DOI] [PubMed] [Google Scholar]
- 30.Orkin, S. H. & Zon, L. I. (2002) Nat. Immunol. 3, 323-328. [DOI] [PubMed] [Google Scholar]
- 31.Terada, N., Hamazaki, T., Oka, M., Hoki, M., Mastalerz, D. M., Nakano, Y., Meyer, E. M., Morel, L., Petersen, B. E. & Scott, E. W. (2002) Nature 416, 542-545. [DOI] [PubMed] [Google Scholar]
- 32.Ying, Q. L., Nichols, J., Evans, E. P. & Smith, A. G. (2002) Nature 416, 545-548. [DOI] [PubMed] [Google Scholar]
- 33.LaBarge, M. A. & Blau, H. M. (2002) Cell 111, 589-601. [DOI] [PubMed] [Google Scholar]
- 34.Friedenstein, A. J., Petrakova, K. V., Kurolesova, A. I. & Frolova, G. P. (1968) Transplantation 6, 230-247. [PubMed] [Google Scholar]
- 35.Caplan, A. I. (1994) Clin. Plast. Surg. 21, 429-435. [PubMed] [Google Scholar]
- 36.Prockop, D. J. (1997) Science 276, 71-74. [DOI] [PubMed] [Google Scholar]
- 37.Dennis, J. E., Merriam, A., Awadallah, A., Yoo, J. U., Johnstone, B. & Caplan, A. I. (1999) J. Bone Miner. Res. 14, 700-709. [DOI] [PubMed] [Google Scholar]
- 38.Phinney, D. G. (2002) J. Cell. Biochem. Suppl. 38, 7-12. [DOI] [PubMed] [Google Scholar]
- 39.Devine, S. M. (2002) J. Cell. Biochem. Suppl. 38, 73-79. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez, J. P., Garat, S., Gajardo, H., Pino, A. M. & Seitz, G. (1999) J. Cell. Biochem. 75, 414-423. [DOI] [PubMed] [Google Scholar]
- 41.Rezka, A. A. & Rodan, G. A. (2003) Curr. Rheumatol. Rep. 5, 65-74. [DOI] [PubMed] [Google Scholar]
- 42.Mundlos, S. & Olsen, B. R. (1997) FASEB J. 11, 227-233. [PubMed] [Google Scholar]