Abstract
Purpose
The symptom most frequently associated with sickle cell disease (SCD) is pain, but recent research is beginning to indicate that fatigue as an increasingly important symptom of this disease upon which to focus research efforts. This article explores biological and behavioral factors that can potentially contribute to fatigue in SCD.
Organizing Framework
A biobehavioral framework guides this discussion of factors that may contribute to SCD fatigue.
Findings
The pathophysiology of the disease process, such as the profound hemolytic anemia and unpredictable vasoocclusive crises, suggests that individuals with SCD are at risk for both acute and chronic fatigue. For example, hypoxemia can cause muscle weakness and produce oxidative stress, which, in turn, increases fatigue. Sickled erythrocytes disrupt the vascular endothelium and stimulate proinflammatory cytokines, which are linked to sleep disruptions. Pain, the most notorious symptom of SCD, has a complex and mechanistically poorly understood relationship with fatigue.
Conclusions
Little is known about the symptom of fatigue in SCD. Considering the biological and behavioral factors of SCD that could potentially contribute to fatigue, there is a great need for research on the nature and potential mechanisms of fatigue in SCD.
Clinical Relevance
Fatigue in SCD may negatively affect quality of life. Understanding factors that may contribute to fatigue aids the clinician in identifying causes and determining treatment.
Keywords: Fatigue, sickle cell disease, biomarkers
Fatigue in Sickle Cell Disease
Sickle cell disease (SCD) is a group of genetic hematologic disorders that is characterized by chronic hemolytic anemia and vasoocclusion. This extremely challenging disease is of global concern. The World Health Organization (2010) reported that “Each year over 300,000 babies with severe forms of these diseases are born worldwide.” In the United States, sickle cell anemia occurs in approximately 1 in 500 African American live births and 1 in 36,000 Hispanic American live births (National Heart, Lung, and Blood Institute, 2010).
The symptom most frequently associated with SCD is pain, which often occurs in the form of sudden, severe episodes called “crises.” Historically and justifiably, investigators have focused on improving sickle cell pain, either through palliation or disease remission. However, in the literature and on patient Web sites, another common symptom of SCD is fatigue (MedlinePlus, 2010; Redding-Lallinger & Knoll, 2006). Recent research is beginning to indicate that fatigue as an increasingly important symptom of this disease (Jacob, 2005; While & Mullen, 2004). In SCD, the pathophysiology of the disease process, such as the profound hemolytic anemia and unpredictable vasoocclusive crises, indicates that individuals with SCD may be at risk for both acute and chronic fatigue. Yet, the actual prevalence and nature of fatigue in SCD is unknown, and the research on improving fatigue, especially through palliative or lifestyle interventions, is virtually absent. Similarly, in cancer symptom research, initially the focus was on pain management, but once investigated, fatigue was recognized as a key symptom to address (Irvine, Vincent, Bubela, Thompson, & Graydon, 1991). In fact, compared with the 7,825 articles that appeared in PubMed in May 2010 when cancer and fatigue were entered as key words, only 21 appeared when SCD and fatigue were entered. In this article, we will discuss the impact of SCD fatigue and describe factors that may contribute to SCD fatigue, calling to action research in this area. Ultimately, such factors may be found to be responsive to biobehavioral interventions.
Impact of Fatigue
Fatigue is recognized as a major symptom of numerous chronic illnesses by the National Institute of Health (NIH) and defined by the NIH Patient-Reported Outcomes Measurement Information System (2010) initiative as “An overwhelming, debilitating, and sustained sense of exhaustion that decreases one's ability to carry out daily activities, including the ability to work effectively and to function at one's usual level in family or social roles.”
Fatigue in SCD is critical to address for a number of reasons. Over the past decade, fatigue has come to the forefront in symptom management across numerous diseases because of its recognized impact on quality of life and costs to society. The negative effects on quality of life, including interference with cognitive function, social withdrawal, modified daily activities, and decreased psychological well-being (Falk, Swedberg, Gaston-Johansson, & Ekman, 2007; Kralik, Telford, Price, & Koch, 2005; Ream & Richardson, 1997; Smith, Kupper, de Jonge, & Denollet, 2010), have been demonstrated in studies on such chronic diseases as cancer, chronic fatigue syndrome, fibromyalgia, and heart disease. Societal costs of fatigue include increased healthcare use, reduced work productivity, loss of work and school days, and high reliance on assistance of caregivers (McCrone, Darbishire, Ridsdale, & Seed, 2003; Scheeres, Wensing, Severens, Adang, & Bleijenberg, 2008).
The sparse literature on fatigue in SCD begins to expose the impact of this challenging symptom on this population. Ongoing or chronic fatigue was apparent in While and Mullen’s (2004) qualitative study of young people with SCD. The majority described being tired, not having energy, wanting to sleep, and often being unable to carry out activities of daily living. Fatigue-related concerns were expressed more often than pain among these young people.
In two studies on SCD, vitality was measured with the Medical Outcomes Short Form (SF-36). Developers of the SF-36 have defined vitality as “energy level and fatigue” (Ware & Sherbourne, 1992). In the Pain in Sickle Cell Disease Epidemiology Study (PiSCES) with adults in the United States, compared with national norms, men and women with SCD reported significantly lower levels of vitality (McClish et al., 2005). In addition, those with SCD were significantly lower on vitality compared with patients on hemodialysis, with cystic fibrosis, or with asthma. Similarly, in the United Kingdom vitality was significantly lower in individuals with SCD compared with the general population (Anie, Steptoe, & Bevan, 2002). In the SF-36, the assessment of fatigue is limited to the frequency of fatigue; other dimensions such as severity or emotional or functional impact are not measured. Despite measurement limitations, these findings suggest that fatigue in SCD is prevalent and may be both acute and chronic. To begin to formulate remittive (i.e., treatment that induces long periods of remission) and palliative treatment strategies for SCD fatigue, research is greatly needed to identify and investigate potential contributing factors.
Biobehavioral Framework
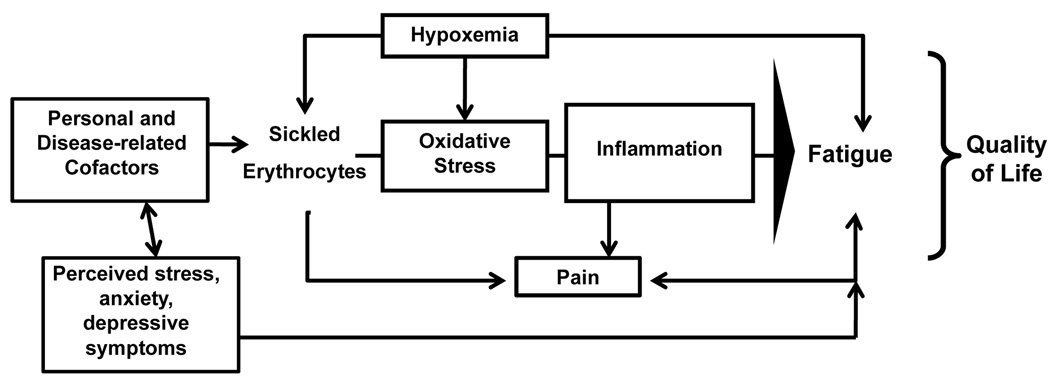
Based on the potential contributions of both biological and behavioral factors to fatigue, we will use a biobehavioral model of symptom management as an organizing framework for this discussion (Figure). The focus of biobehavioral research is on the “interactions between biological, behavioral, and social factors and their effects on outcomes” (Buerhaus, 2006, p. 210), as such is ideal for understanding fatigue in SCD. The model suggests that within the context of a critical health experience (e.g., SCD), personal and disease-related factors, and biological and behavioral moderators interact with biological mediators to explain symptoms or symptom constellations, and, in turn, health outcomes (e.g., quality of life). Specific biological and behavioral factors of SCD that can potentially lead to fatigue are hypoxemia, inflammation, pain, stress, depression, and anxiety. Potential personal and disease-related factors that may affect fatigue are age, sex, ethnicity, socioeconomic status, treatments, and disease severity. Following is a more in-depth discussion of how these factors may contribute to fatigue in SCD.
Figure 1.
Biobehavioral model of sickle cell disease fatigue.
Contributing Factors to SCD Fatigue
Biological Factors
Hypoxemia
Key pathophysiological processes of SCD are hypoxemia and inflammation. Hypoxemia has been associated with fatigue in healthy individuals and those with pulmonary disease (Millett, Aubert, Favier, Busso, & Benoît, 2009; Vogiatzis et al., 2008). Precursors to hypoxemia of SDC include low hemoglobin levels from hemolytic anemia (premature destruction of sickled cells), hyperviscosity of whole blood, and decreased oxygen affinity of sickled hemoglobin. Long-term complications of SCD, particularly cardiopulmonary diseases, may contribute to hypoxemia as well.
Ubiquitous, sometimes profound hemolytic anemia and oxygen starvation to tissues occur in SCD. Hemolytic anemia of SCD is associated with oxyhemoglobin desaturation (Setty, Stuart, Dampier, Brodecki, & Allen, 2003) and is most commonly caused by acute splenic sequestration, transient red cell aplasia, and hyperhemolysis during infection (Neonatoet al., 2000). Anemia is generally defined as a hemoglobin < 12 g/dL in females 15 years and older and < 13 g/dL in males 15 years and older (Lanzkowsky, 2005), yet in a cooperative study of patients with SCD, the median hemoglobin concentration level was 9.1 (SD 1.8 g/dL) (Sebastiani et al., 2007). In cancer, a number of investigators have found that anemia is significantly associated with fatigue (Cella, Lai, Chang, Peterman, & Slavin, 2002; Yeh et al., 2008). In SCD, the relationship between fatigue and anemia has not been studied. Considering the acute and chronic state of anemia in this population, the risk for fatigue is potentially substantial, highlighting the need to investigate relationships between and mechanisms of anemia and fatigue in SCD.
Individuals with SCD are also at risk for hypoxemia if their hematocrit is too high, resulting in hyperviscosity—an elevation of whole blood viscosity. Blood flow through small vessels is compromised when viscosity increases. Hyperviscosity can result from simple and partial exchange transfusions that are commonly used for prevention and treatment of certain complications of SCD, such as stroke and priapism. For individuals with SCD, a hematocrit merely as high as 35% to 45% can result in reduced oxygen delivery (Rosse, Narla, Petz, & Steinberg, 2000). Thus, the dilemma in SCD is that both a high and a low hemoglobin level can cause hypoxemia.
Hypoxemia can be compounded because sickled hemoglobin (from oxyhemoglobin desaturation) has a reduced affinity for oxygen. Abdu, Gomez-Marquez, and Aldrich (2008) examined oxygen affinity among individuals with and without SCD and found that at normal arterial partial pressure, oxygen affinity was normal in both groups, but affinity was lower in those with SCD who were in hypoxic states.
Inflammation
Hypoxemia and inflammation are intricately linked. SCD has recently been considered an inflammatory disease because of mounting evidence of the inflammatory processes related to vascular endothelium disruption (Hebbel, Osarogiagbon, & Kaul, 2004). Sickle hemoglobin tetramers polymerize inside the red blood cell (RBC) in the deoxygenated state (Redding-Lallinger & Knoll, 2006). The polymer can alter both the red cell shape and membrane properties, leading to abnormal interactions of red cells with the vascular endothelium, resulting in vasoocclusion in small vessels (Evans & Mohandas, 1987). Stimulation and disruption of the vascular endothelium from the sickled erythrocytes and hypoxemia causes inflammation. In addition, sickled erythrocytes and hypoxemia can induce oxidative stress (Amer et al., 2006), which can further increase RBC adhesion to the endothelium (Fibach & Rachmilewitz, 2008), thereby amplifying the inflammatory response. Monocytes and leukocytes also interact with the vascular endothelium, further stimulating the inflammatory response (Zennadi, Chien, Xu, Batchvarova, & Telen, 2008).
Certain biomarkers involved in the inflammatory response have been linked to fatigue. See the Table for biomarkers of fatigue. The most prominent biomarkers are inflammatory cytokines, particularly interleukin (IL)-1, IL-6, and tumor necrosis factor-α (TNF-α), which respond to and are stimulated by activated endothelial cells. Evidence suggests that inflammation in SCD may be both acute and chronic because these cytokines are not only elevated during a crisis but are also elevated during steady state, that is, when there are no manifestations of a crisis (Croizat, 1994). Further, not only does the inflammatory response occur with hypoxia but also during reoxygenation (Kaul& Hebbel, 2000), suggesting a type of reperfusion injury, that is, tissue damage from free radical generation that occurs when the vascular supply is restored after a vasoocclusive event (Osarogiagbon et al., 2000).
Table.
Biomarkers of Fatigue in Sickle Cell Disease
| Variable | Biomarker |
|---|---|
| Hypoxemia | |
| Deoxygenation | Partial pressure of oxygen (PaO2) -Arterial blood gas |
| Arterial oxygen saturation (SaO2) -Pulse oximetry |
|
| Anemia and hyperviscosity | Hemoglobin |
| Hematocrit | |
| Inflammation | IL-1 |
| IL-6 | |
| TNF-α | |
| Stress | Cortisol |
| Heart rate variability |
These inflammatory cytokines, IL-1, IL-6, and TNF-α, may contribute to fatigue in several ways. Elevated levels of both IL-1 and TNF-α have been linked to decreased muscle strength (Visser et al., 2002) and decreased exercise capacity (Carmichael et al., 2006). With regard to sleep, IL-6 appears to interfere with slow-wave stages of rapid eye movement (REM) sleep (Spath-Schwalbe et al., 1998), TNF-α alters slow-wave activity, a trait of non-rapid eye movement (NREM) sleep (Yoshida et al., 2004), and IL-1 affects hippocampal activity, which is integral to sleep regulation (Luk et al., 1999). In addition, inflammation of SCD appears to increase resting energy expenditure (REE; Akohoue et al., 2007), but whether this affects an individual’s perceived energy level has not been investigated. In summary, inflammation may contribute to fatigue because of the effects on REE, muscle strength, and sleep architecture.
Associated Symptoms (Pain, Stress, Depression and Anxiety)
Pain
Pain is a common and unpredictable symptom of SCD. Ubiquitous vasoocclusive ischemia due to red cell obstruction of small vessels may produce ischemic pain in almost any organ, muscle, or bone in persons with SCD. Pain may also come in the form of chronic ischemic leg ulcers; avascular necrosis of the hip, knee, and shoulder; and bony necrosis of the spine, easily recognizable as “fishmouthing” of the vertebrae on x-rays. This pain is often severe in nature and can be challenging to control (Smith et al., 2008).
Few studies exist on the cooccurrence of pain and fatigue in SCD, and only limited dimensions of fatigue have been assessed. In two studies with adults, investigators found significant inverse correlations between pain and vitality (measured with the SF-36) in adults, such that as pain intensity increased, vitality decreased (Ballas et al., 2006; McClish et al., 2005). Children and adolescents with SCD have reported disruptions in sleep or poor sleep during painful crises such that greater pain intensity during the day was related to poor sleep quality the same night, and poor sleep quality correlated with greater SCD pain the next day (Valrie, Gil, Redding-Lallinger, & Daeschner, 2007), suggesting that sleep disruption plays a mediating role between pain and fatigue. Ostensibly, both pain and fatigue in SCD are explainable through shared pathophysiological mechanisms that suggest an approach to management of both. Research is needed to unravel the mechanisms between these symptoms.
Stress
Stress, known to be associated with chronic illness, is prevalent in individuals with SCD (Gil et al., 2004; Porter, Gil, Carson, Anthony, & Ready, 2000). Stress has been linked to greater fatigue in both clinical and nonclinical populations, but the underlying pathways remain unclear (Aaronson, Pallikkathayil, & Crighton, 2003; Kerr & Mattey, 2008). Consistent with the biobehavioral model, one proposed pathway is that perceived stress stimulates the neuroendocrine system to release cortisol, which in turn stimulates the immune system to activate cytokines, resulting in negative effects on health outcomes such as greater fatigue. Beginning evidence to support this pathway was demonstrated in a study of patients with rheumatoid arthritis, in which interpersonal relationship stress was significantly correlated with increased stimulated IL-6 production (i.e., in vitro stimulation of mononuclear cell production of IL-6) but not with plasma IL-6 levels (Davis et al., 2008). Further, stimulated production of IL-6 was linked to higher levels of perceived fatigue. Similar significant correlations have been found between stress and elevated levels of proinflammatory cytokines (Hirano, Nagashima, Ogawa, & Yoshino, 2001), which have ultimately been linked to poorer health outcomes (Zautra et al., 2004). Thus, stress has the potential to compound the effects of those same cytokines that are activated during inflammation on fatigue.
Depression and Anxiety
Depression and anxiety are highly comorbid conditions that are not uncommon in chronic illness (Stevinson et al., 2009) and are strongly linked to fatigue. In SCD, depression tends to occur at higher rates (18%–27%; Levenson et al., 2008; Wilson et al., 1999) compared with African Americans in general (7%–8%; Riolo, Nguyen, Greden, & King, 2005). In one study, 80% of those individuals with SCD who had depression also had an anxiety disorder (Levenson et al.). But the nature of the relationship between depression and fatigue is unclear, and differentiating fatigue related to depression from physical illness symptoms can be challenging. For example, depression screening tools such as the Center for Epidemiologic Studies Depression Scale (Radloff, 1977) assess for some symptoms that are common to both depression and physical illness (e.g., fatigue and poor appetite). One screening tool that may be more appropriate is the Hospital Anxiety and Depression Scale (Zigmond & Snaith, 1983). This tool is designed to screen for depression in individuals with physical illnesses in that it excludes physical problems such as energy impairments and sleep disturbances.
Fatigue and depression may share common etiologies. For example, in SCD pain may contribute to both. In addition, evidence suggests that proinflammatory cytokines may play a role in depression and fatigue, in particular, IL-1, IL-6, and TNF-α (Wichers & Maes, 2002). Considering the inflammatory nature of SCD, the role of these cytokines in depression and fatigue seem critical to explore.
Personal and Disease-Related Factors
A number of personal and disease-related factors may contribute to fatigue, including but not limited exclusively to age, sex, ethnicity, socioeconomic status, treatments, disease severity, time with disease, medications, long-term complications, and cumulative organ damage. In other chronic illnesses, such as heart disease, cancer, and HIV/AIDS, findings have been mixed regarding fatigue and the variables age, sex, ethnicity, and education (Smith et al., 2010; Stone, Richards, A’Hern & Hardy, 2000; Voss, 2005). Little is known about the exact relationship between fatigue and these factors in SCD, thus indicating a great need for research.
Summary and Implications for Research
Sickled erythrocytes initiate a cascade of events that can lead to deleterious health outcomes, one of which may be fatigue. In qualitative studies, individuals with SCD have reported that fatigue is frequently present and interferes with daily life, but the extent of fatigue in SCD is unknown, and causal mechanisms are not well understood. Considering the societal impact of fatigue and its negative effects on quality of life in other chronic illnesses, the need for research on fatigue in SCD is great. Researchers of cancer-related fatigue have demonstrated that a systematic approach to the investigation of this symptom is necessary for understanding this phenomenon in SCD. A first step would be to validate fatigue measures in individuals with SCD. A number of reliable fatigue measures exist, but none have been validated in the SCD population.
Initial steps to increase understanding of fatigue include conducting descriptive studies to examine prevalence, severity, frequency, and interference, and conducting qualitative studies to explore the experience of fatigue, including perceived causes and consequences, and strategies used to manage fatigue. Studies are needed to identify key biological and behavioral factors associated with SCD fatigue. The extent to which fatigue and other symptoms occur in concert is unknown. Thus, once fatigue is described, there will be a need for research to explore symptoms cooccurring with fatigue in SCD. Increasing understanding of SCD fatigue may lead to early identification and management of this difficult symptom.
Clinical Resources.
World Health Organization http://www.sicklecelldisease.org/about_scd/index.phtml
National Institute of Health http://www.nhlbi.nih.gov/health/dci/Diseases/Sca/SCA_WhatIs.html
Acknowledgments
The authors thank Dr. Nancy McCain and Dr. Debra Lyon for their thoughtful review and comments on this article. This work was funded through Grant #P30 NR011403, National Institute of Nursing Research, NIH.
References
- Aaronson LS, Pallikkathayil L, Crighton F. A qualitative investigation of fatigue among healthy working adults. Western Journal of Nursing Research. 2003;25:419–433. doi: 10.1177/0193945903025004007. [DOI] [PubMed] [Google Scholar]
- Abdu A, Gomez-Marquez J, Aldrich TK. The oxygen affinity of sickle hemoglobin. Respiratory Physiology & Neurobiology. 2008;161(1):92–94. doi: 10.1016/j.resp.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Akohoue SA, Shankar S, Milne GL, Morrow J, Chen KY, Ajayi WU, et al. Energy expenditure, inflammation, and oxidative stress in steady-state adolescents with sickle cell anemia. Pediatric Research. 2007;61:233–238. doi: 10.1203/pdr.0b013e31802d7754. [DOI] [PubMed] [Google Scholar]
- Amer J, Ghoti H, Rachmilewitz E, Koren A, Levin C, Fibach E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. British Journal of Haematology. 2006;132:108–113. doi: 10.1111/j.1365-2141.2005.05834.x. [DOI] [PubMed] [Google Scholar]
- Anie K, Steptoe A, Bevan D. Sickle cell disease: Pain, coping and quality of life in a study of adults in the UK. British Journal of Health Psychology. 2002;7:331–334. doi: 10.1348/135910702760213715. [DOI] [PubMed] [Google Scholar]
- Ballas S, Barton F, Waclawiw M, Swerdlow P, Eckman J, Pegelow C, et al. Hydroxyurea and sickle cell anemia: Effect on quality of life. Health and Quality of Life Outcomes. 2006;4(1):59. doi: 10.1186/1477-7525-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerhaus PI. A discussion with Patricia A. Grady on the 20th anniversary of the National Institute of Nursing Research. Journal of Nursing Scholarship. 2006;38:208–212. doi: 10.1111/j.1547-5069.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- Carmichael MD, Davis JM, Murphy EA, Brown AS, Carson JA, Mayer EP, et al. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2006;291(5):R1344–R1248. doi: 10.1152/ajpregu.00141.2006. [DOI] [PubMed] [Google Scholar]
- Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. 2002:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- Croizat H. Circulating cytokines in sickle cell patients during steady state. British Journal of Haematology. 1994;87:592–597. doi: 10.1111/j.1365-2141.1994.tb08318.x. [DOI] [PubMed] [Google Scholar]
- Davis MC, Zautra AJ, Younger J, Motivala SJ, Attrep J, Irwin MR. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: Implications for fatigue. Brain, Behavior, and Immunity. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Mohandas N. Membrane-associated sickle hemoglobin: A major determinant of sickle erythrocyte rigidity. Blood. 1987;70:1443–1449. [PubMed] [Google Scholar]
- Falk K, Swedberg K, Gaston-Johansson F, Ekman I. Fatigue is a prevalent and severe symptom associated with uncertainty and sense of coherence in patients with chronic heart failure. European Journal of Cardiovascular Nursing. 2007;6:99–104. doi: 10.1016/j.ejcnurse.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Fibach E, Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Current Molecular Medicine. 2008;8:609–619. doi: 10.2174/156652408786241384. [DOI] [PubMed] [Google Scholar]
- Gil KM, Carson JW, Porter LS, Scipio C, Bediako SM, Orringer E. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychology. 2004;23:267–274. doi: 10.1037/0278-6133.23.3.267. [DOI] [PubMed] [Google Scholar]
- Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: Inflammation and a chronic vasculopathy. Microcirculation. 2004;11:129–151. [PubMed] [Google Scholar]
- Hirano D, Nagashima M, Ogawa R, Yoshino S. Serum levels of interleukin 6 and stress related substances indicate mental stress condition in patients with rheumatoid arthritis. Journal of Rheumatology. 2001;28:490–495. [PubMed] [Google Scholar]
- Irvine DM, Vincent L, Bubela N, Thompson L, Graydon J. A critical appraisal of the research literature investigating fatigue in the individual with cancer. Cancer Nursing. 1991;14:188–199. [PubMed] [Google Scholar]
- Jacob E, Miaskowski C, Savedra M, Beyer JE, Treadwell M, Styles L. Quantification of analgesic use in children with sickle cell disease. Clinical Journal of Pain. 2007;23:8–14. doi: 10.1097/01.ajp.0000210938.58439.dd. [O5] [DOI] [PubMed] [Google Scholar]
- Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. Journal of Clinical Investigation. 2000;106:411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JR, Mattey DL. Preexisting psychological stress predicts acute and chronic fatigue and arthritis following symptomatic parvovirus B19 infection. Clinical Infectious Diseases. 2008;46(9):e83–e87. doi: 10.1086/533471. [DOI] [PubMed] [Google Scholar]
- Kralik D, Telford K, Price K, Koch T. Women's experiences of fatigue in chronic illness. Journal of Advanced Nursing. 2005;52:372–380. doi: 10.1111/j.1365-2648.2005.03602.x. [DOI] [PubMed] [Google Scholar]
- Lanzkowsky P. Manual of pediatric hematology and oncology. Burlington, MA: Elsevier Academic Press; 2005. [Google Scholar]
- Levenson JL, McClish DK, Dahman BA, Bovbjerg VE, de ACV, Penberthy LT, et al. Depression and anxiety in adults with sickle cell disease: The PiSCES project. Psychosomatic Medicine. 2008;70:192–196. doi: 10.1097/PSY.0b013e31815ff5c5. [DOI] [PubMed] [Google Scholar]
- Luk WP, Zhang Y, White TD, Lue FA, Wu C, Jiang CG, et al. Adenosine: A mediator of interleukin-1beta-induced hippocampal synaptic inhibition. Journal of Neuroscience. 1999;19:4238–4244. doi: 10.1523/JNEUROSCI.19-11-04238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClish D, Penberthy L, Bovbjerg V, Roberts J, Aisiku I, Levenson J, et al. Health related quality of life in sickle cell patients: The PiSCES project. Health and Quality of Life Outcomes. 2005;3:50. doi: 10.1186/1477-7525-3-50. [O7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrone P, Darbishire L, Ridsdale L, Seed P. The economic cost of chronic fatigue and chronic fatigue syndrome in UK primary care. Psychological Medicine. 2003;33:253–261. doi: 10.1017/s0033291702006980. [DOI] [PubMed] [Google Scholar]
- Medline Plus. Sickle cell anemia. 2010 Retrieved from http://www.nlm.nih.gov/medlineplus/ency/article/000527.htm.
- Millet G, Aubert D, Favier F, Busso T, Benoît H. Effect of acute hypoxia on central fatigue during repeated isometric leg contractions. Scandinavian Journal of Medicine & Science in Sports. 2009;19:695–702. doi: 10.1111/j.1600-0838.2008.00823.x. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute. Who is at risk for sickle cell anemia? 2010 Retrieved from http://www.nhlbi.nih.gov/health/dci/Diseases/Sca/SCA_WhoIsAtRisk.html.
- National Institute of Health Patient Reported Outcomes Measurement Information System. PROMIS domain definitions. 2010 Retrieved from http://www.nihpromis.org/Web%20Pages/Domain%20Definitions.aspx.
- Neonato MG, Guilloud-Bataille M, Beauvais P, Begue P, Belloy M, Benkerrou M, et al. Acute clinical events in 299 homozygous sickle cell patients living in France. French Study Group on Sickle Cell Disease. European Journal of Haematology. 2000;65:155–164. doi: 10.1034/j.1600-0609.2000.90210.x. [DOI] [PubMed] [Google Scholar]
- Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96(1):314–320. [PubMed] [Google Scholar]
- Porter LS, Gil KM, Carson JW, Anthony KK, Ready J. The role of stress and mood in sickle cell disease pain: An analysis of daily diary data. Journal of Health Psychology. 2000;5:53–63. doi: 10.1177/135910530000500109. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ream E, Richardson A. Fatigue in patients with cancer and chronic obstructive airways disease: A phenomenological enquiry. International Journal of Nursing Studies. 1997;34:44–53. doi: 10.1016/s0020-7489(96)00032-6. [DOI] [PubMed] [Google Scholar]
- Redding-Lallinger R, Knoll C. Sickle cell disease-Pathophysiology and treatment. Current Problems in Pediatric and Adolescent Health Care. 2006;36:346–376. doi: 10.1016/j.cppeds.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Riolo SA, Nguyen TA, Greden JF, King CA. Prevalence of depression by race/ethnicity: Findings from the national health and nutrition examination survey III. American Journal of Public Health. 2005;95:998–1000. doi: 10.2105/AJPH.2004.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse WF, Narla M, Petz LD, Steinberg MH. New views of sickle cell disease pathophysiology and treatment. Hematology. 2000;2000(1):2–17. doi: 10.1182/asheducation-2000.1.2. [DOI] [PubMed] [Google Scholar]
- Scheeres K, Wensing M, Severens H, Adang E, Bleijenberg G. Determinants of health care use in chronic fatigue syndrome patients: A cross-sectional study. Journal of Psychosomatic Research. 2008;65:39–46. doi: 10.1016/j.jpsychores.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Sebastiani P, Nolan VG, Baldwin CT, Abad-Grau MM, Wang L, Adewoye AH, et al. A network model to predict the risk of death in sickle cell disease. Blood. 2007;110:2727–2735. doi: 10.1182/blood-2007-04-084921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty BN, Stuart MJ, Dampier C, Brodecki D, Allen JL. Hypoxaemia in sickle cell disease: Biomarker modulation and relevance to pathophysiology. Lancet. 2003;362:1450–1455. doi: 10.1016/S0140-6736(03)14689-2. [DOI] [PubMed] [Google Scholar]
- Smith ORF, Kupper N, de Jonge P, Denollet J. Distinct trajectories of fatigue in chronic heart failure and their association with prognosis. European Journal of Heart Failure. 2010;12:841–848. doi: 10.1093/eurjhf/hfq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, et al. Daily assessment of pain in adults with sickle cell disease. Annals of Internal Medicine. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, et al. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. Journal of Clinical Endocrinology and Metabolism. 1998;83:1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- Stevinson CP, Steed HMD, Faught WMD, Tonkin KMD, Vallance JKP, Ladha ABM, et al. Physical activity in ovarian cancer survivors: Associations with fatigue, sleep, and psychosocial functioning. International Journal of Gynecological Cancer. 2009;19:73–78. doi: 10.1111/IGC.0b013e31819902ec. [DOI] [PubMed] [Google Scholar]
- Stone P, Richards M, A'Hern R, Hardy J. A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Annals of Oncology. 2000;11:561–567. doi: 10.1023/a:1008331230608. [DOI] [PubMed] [Google Scholar]
- Valrie CR, Gil KM, Redding-Lallinger R, Daeschner C. Brief report: Sleep in children with sickle cell disease: An analysis of daily diaries utilizing multilevel models. Journal of Pediatric Psychology. 2007;32:857–861. doi: 10.1093/jpepsy/jsm016. [DOI] [PubMed] [Google Scholar]
- Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: The health ABC study. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Athanasopoulos D, Boushel R, Guenette JA, Koskolou M, Vasilopoulou M, et al. Contribution of respiratory muscle blood flow to exercise-induced diaphragmatic fatigue in trained cyclists. Journal of Physiology. 2008;586:5575–5587. doi: 10.1113/jphysiol.2008.162768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JG. Predictors and correlates of fatigue in HIV/AIDS. Journal of Pain and Symptom Management. 2005;29:173–184. doi: 10.1016/j.jpainsymman.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- While AE, Mullen J. Living with sickle cell disease: The perspective of young people. British Journal of Nursing. 2004;13(6):320–325. doi: 10.12968/bjon.2004.13.6.12528. [DOI] [PubMed] [Google Scholar]
- Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. International Journal of Neuropsychopharmacology. 2002;5(04):375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- Wilson JJ, Gil KM, Burchinal M, Kramer KD, Nash KB, Orringer E, et al. Depression, disease severity, and sickle cell disease. Journal of Behavioral Medicine. 1999;22:115–126. doi: 10.1023/a:1018755831101. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Sickle-cell disease and other haemoglobin disorders. 2010 Retrieved from http://www.who.int/mediacentre/factsheets/fs308/en/
- Yeh CH, Chiang YC, Lin L, Yang CP, Chien LC, Weaver MA, et al. Clinical factors associated with fatigue over time in paediatric oncology patients receiving chemotherapy. British Journal of Cancer. 2008;99(1):23–29. doi: 10.1038/sj.bjc.6604434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Peterfi Z, Garcia-Garcia F, Kirkpatrick R, Yasuda T, Krueger JM. State-specific asymmetries in EEG slow wave activity induced by local application of TNFalpha. Brain Research. 2004;1009(1–2):129–136. doi: 10.1016/j.brainres.2004.02.055. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Yocum DC, Villanueva I, Smith B, Davis MC, Attrep J, et al. Immune activation and depression in women with rheumatoid arthritis. Journal of Rheumatology. 2004;31(3):457–463. [PubMed] [Google Scholar]
- Zennadi R, Chien A, Xu K, Batchvarova M, Telen MJ. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood. 2008;112(8):3474–3483. doi: 10.1182/blood-2008-01-134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]