Abstract
Acetylcholine-induced endothelium-dependent vasodilation in conduit arteries primarily depends on nitric oxide (NO). However, the biochemical mediators in the microvasculature remain less well defined. We tested whether prostaglandins and NO are responsible for cutaneous acetylcholine mediated vasodilation, and if they interact to modulate vasodilation. We measured skin blood flow (SBF) using laser Doppler flow (LDF) with intradermal microdialysis in the calves of 23 healthy volunteers. We examined the response of SBF to different doses of acetylcholine (0.01mM to 100mM), the non-isoform specific NO synthase inhibitor nitro-L-arginine (NLA, 10mM), the nonspecific cyclooxygenase inhibitor ketorolac (Keto,10mM) and combined NLA+Keto. NLA had no effect on baseline SBF, while Keto increased baseline SBF approximately 150%. The increase was blunted with combined NLA+Keto. SBF increased approximately 700% with the highest acetylcholine concentration and reduced approximately 60% by NLA. Ketorolac alone also reduced the response to acetylcholine although the reduction varied between 10–20% at differing acetylcholine doses. NLA plus Ketorolac reduced the responses to different doses of acetylcholine by some 30%, which was intermediate to NOS or COX inhibition alone. These data suggest that cutaneous acetylcholine mediated endothelium-dependent vasodilation is highly nitric oxide dependent and is also strongly related to the interactions of NO with prostaglandins.
Keywords: prostaglandin, nitric oxide, acetylcholine, skin blood flow, microdialysis, laser Doppler flow
Introduction
Endothelial dysfunction is characterized by defective endothelium-dependent vasorelaxation in patients, which commonly precedes overt vascular disease (30, 45). Acetylcholine (ACh) is representative of naturally occurring receptor mediated endothelial dependent vasodilators(15, 44). The response to acetylcholine is therefore commonly used to assess endothelial function (24, 41). Kimura et. al recently measured cutaneous blood flow and sweat response to varying doses of acetylcholine and methacholine (28). Cutaneous microdialysis delivery of muscarinic agonists and antagonists has also been used to study the physiology of sweat gland physiology (35).
Implicit in this use is an understanding of the biochemical mechanisms by which acetylcholine produces endothelial-dependent vasodilation. In large arteries, particularly coronary arteries, the endothelial-dependent vasodilator response to acetylcholine is primarily due to receptor mediated nitric oxide (NO)(20, 30, 43). NO independent effects have also been documented (32).
Acetylcholine also produces microvascular vasodilation. While the direct local microvascular effects of acetylcholine are primarily mediated through the endothelium, this does not ensure that the effects are due solely to nitric oxide. For example, in the skin and coronary microcirculation NO, prostaglandins and endothelial dependent relaxation factor (EDHF) are each believed to exert important effects in varied proportion when studied in either human skin (26) or canine coronary vessels (37). In murine skeletal muscle NO and EDHF, but not prostaglandins, are thought to be most important (23). However, even in this well studied tissue results obtained in hamsters remain controversial (14) and additional factors such as the axon reflex also contribute to acetylcholine dependent microvascular vasodilation (4, 5).
Similar concerns exist for the cutaneous circulation concerning the contributions to acetylcholine mediated vasodilation arising from NO, from prostaglandins, and from other factors. Thus, Kellogg et al (26), using microdialysis catheters and laser Doppler flowmetry (LDF), found that both nitric oxide synthase (NOS) inhibition and prostaglandin inhibition attenuated acetylcholine vasodilation. Durand et al (16) demonstrated late attenuation to oral prostaglandin inhibition of vasodilation produced by acetylcholine delivered by iontophoresis.
Dalle-Ave et al (13) using iontophoretically delivered acetylcholine and oral prostaglandin inhibition showed a lack of effect of COX inhibition on acetylcholine induced vasodilation. In contrast, Holowatz et al (22) found that infusions of a nonselective cyclooxygenase (COX) inhibitor caused a significant attenuation of the vasodilation produced by acetylcholine when drugs were delivered by microdialysis. Significant attenuation of acetylcholine-induced vasodilation was also measured when a NOS-inhibitor was combined with COX inhibition.
While all investigations used LDF to measure changes in cutaneous blood flow, they relied on different doses of acetylcholine, different COX inhibitors, non-steady state kinetics, and different delivery routes to test vasodilatory responses.
We propose that these conflicting results reflect route and dose–dependent aspects of acetylcholine administration and prostaglandin inhibition and that data obtained using steady state conditions for medications delivered by direct intradermal microdialysis provide the most unequivocal information.
We tested the hypothesis that prostaglandins as well as NO are important to cutaneous acetylcholine mediated vasodilation and that NO and prostaglandins interact to exert modulatory effects on the response.
Methods
Overview
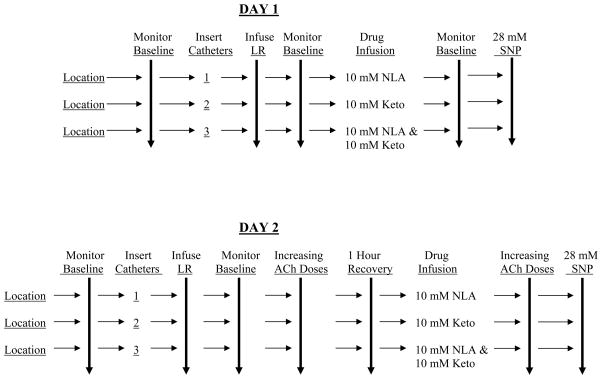
Experiments were performed on two separate days in each subject. All drugs were administered through microdialysis catheters and sites for catheter placement varied from day to day. A schematic of the maneuvers performed on each of the two experimental days is depicted in Figure 1. In brief, increasing doses (0.01, 0.1, 1.0, 10 and 100 mM) of ACh was perfused through microdialysis catheters and laser Doppler skin blood flow was measured. The effects of inhibition of NOS and COX on ACh-induced increases in skin blood flow was determined following perfusion of the non-isoform specific NOS inhibitor Nitro-L-arginine (NLA) and the non-selective cyclooxygenase inhibitor ketorolac (Keto). The concentrations of NLA and Keto used (10 mM) were based upon previous determinations in human skin utilizing microdialysis delivery of these, and similar categories of drugs (22, 26, 29, 40); we used the lowest dose that resulted in maximal inhibition. All drugs were dissolved in Lactated Ringers solution and perfused at a rate of 2 μl/min.
Figure 1.
shows a schematic representation of the described studies. First, locations on the leg were randomly designated 1–3, and baseline laser-Doppler flow was measured. Microdialysis catheters were then inserted and perfused with vehicle [sterile lactated Ringer’s solution (LR)], and laser-Doppler flow was monitored until levels returned to preinsertion values. All drugs were dissolved in LR and catheters were perfused at a rate of 2 μl/minute with the inhibitory drugs shown (Day 1), and acetylcholine (ACh) dose-response measured (Day 2) in the presence and absence of the inhibitory drugs as shown. Lastly, all catheters were perfused with 28 mM sodium nitroprusside (SNP). Skin laser-Doppler flow was recorded continuously during all of these determinations. NLA, nitro-L-arginine; Keto, ketorolac.
Subjects
We recruited 23 healthy volunteers aged 19–27 years (mean = 23.9±0.6 years). There were 9 men and 14 women. Only subjects found to be free from systemic diseases and cardiovascular disease were eligible. Subjects were not taking medication; specifically use of neurally active or vasoactive medications or exogenous hormones such as birth control pills were excluded from this study. Subjects also refrained from alcohol or caffeinated beverages for 24 hours prior to study. There were no smokers or trained competitive athletes. Informed consent was obtained and the Committee for the Protection of Human Subjects (IRB) of New York Medical College approved all protocols.
The average weight in males was 84.0±4.2 vs. 63.2±2.4 Kg in females. Average height in males was 178±2 vs. 166±2 cm in females. BMI was 26.6±1.4 in males vs. 23.2±1.0 Kg/m2 in females. Supine heart rate and blood pressure were not different in males compared to females (HR= 58±3 vs. 63±2 bpm, systolic BP=122±2 vs 117±3 mmHg, MAP= 87±2 vs 83±2 mmHg).
Protocol
Instrumentation
All testing was conducted in a temperature controlled room at 25°C at least two hours after a light breakfast. Experiments began after a 30 minute acclimatization period and all experiments were performed while the subject was supine and breathing spontaneously. Measurements were made in the lateral aspect of the left calf. Skin temperature was measured with a thermal probe to ensure neutral thermal conditions. On each day subjects were instrumented with 3 microdialysis probes placed at least 4 cm apart inserted in the dermal space of the lateral aspect of the left calf after hair was gently removed as needed from the insertion site. Probes comprised a 10 mm microdialysis membrane window placed in the intradermal space using a 25 gauge needle as an introducer and with the tip of the needle exiting the skin approximately 2 cm from the point of insertion. We threaded the microdialysis probe (LM-10 Linear Microdialysis Probes, Bioanalytical Systems, West Lafayette, IN) through the inserter needle. Typical intradermal insertion of microdialysis catheters results in placement that is between 0.3 and 1.0 mm below the surface of the skin (25). The probe has a membrane diameter of 320 μm and a nominal molecular weight cut-off of 30 kD. Catheters were randomly designated 1, 2 and 3. All catheters were initially perfused with Ringer’s solution at 2 μl/min. The leg was maintained at the level of the heart throughout all procedures. An integrating laser-Doppler flow-probe (Probe 413 Perimed, Stockholm) containing 7 individual elements separated by 0.25 mm was placed directly over each dialysis probe to measure SBF during recovery. Each of the elements has a transmitting fiber and a receiving fiber.
Recovery from probe insertion lasted for a minimum of 60 minutes and often up to 120 minutes until the SBF returned to baseline levels. Baseline resting flows and experimental flows were measured in arbitrary perfusion units (pfu). SBF data were converted to units of cutaneous vascular conductance (CVC) by dividing by the mean arterial pressure (MAP), measured as described below. The MAP of each subject did not change during the course of these determinations as they remained supine and inactive. CVC measurements were then converted to a percent maximum conductance (%CVCmax) by dividing CVC by the maximum CVC achieved by the administration of 28mM sodium nitroprusside at the end of experiments as shown in Figure 1. There were no differences in the maximal response elicited by 28 mM SNP alone compared to SNP with NLA, Ketorolac or NLA and Ketorolac combined. The average maximal laser-Doppler blood flow measured under all of these conditions was 185±12 pfu’s. This fraction was converted to a percentile by multiplication by 100. Conductance data are therefore displayed as %CVCmax.
Drugs were delivered through microdialysis catheters at a constant perfusion rate of 2 μl/min throughout all experiments. Continuous LDF data were collected at a sampling rate of 200 Hz during experiments multiplexed and interfaced to a personal computer through an A/D converter (DI-720, DATAQ industries, Milwaukee, WI) using custom data acquisition software, and generating binary files and computer displays of simultaneously collected data from all lasers and blood pressure data.
The Effects of Acetylcholine on SBF
The purpose of these experiments was to examine the overall effects of endothelial dependent receptor mediated stimulation with acetylcholine on SBF in a dose-dependent fashion. These experiments were not conducted as classical “dose-response” and “inhibition” studies as derivation of pharmacological characteristics was not our intent. Rather, different doses of agonist (acetylcholine) and inhibitors (NLA and Keto) were used to elicit changes in skin blood flow responses thought to represent local signaling by chemical mediators such as NO and prostaglandins.
We anticipated a mix of effects due to receptor mediated NO release, prostaglandin stimulation, and other factors such as EDHF. After recovery, patients had baseline SBF data, measured by LDF collected for at least 10 minutes. Subjects then received perfusate containing 0.01, 0.10, 1.0, 10, and 100mM acetylcholine (designated .01▶ 100mM acetylcholine) dissolved in Ringer solution in ascending doses through each of catheters 1, 2, and 3 at a rate of 2 μl/min. The range of concentration of acetylcholine used (0.01–100 mM) is based upon previous determinations in human skin utilizing microdialysis delivery of this agonist (42). LDF monitoring continued and each dose was administered for 20 minutes during which steady state values of SBF were achieved. For purposes of analysis, only the last 5 minutes of data were averaged at each acetylcholine dose.
Monitoring
Heart rate was monitored by electrocardiography and right upper extremity blood pressures were measured by finger plethysmography intermittently recalibrated against oscillometry in the right arm. Mean arterial pressure (MAP) was calculated as the sum of the (systolic pressure + 2*diastolic pressure)/3.
Data Analysis and Statistical Analysis
Text and graphic results are reported as mean ± standard error of the mean. Changes in laser Doppler flow before and after drugs were compared by paired t-tests. Acetylcholine dose-response curves with and without different medications were analyzed by MANOVA with repeated measures, as were all determinations of multiple comparisons. Results were calculated using SPSS (Statistical Package for the Social Sciences) software version 11.0.
Results
The Effects of Acetylcholine on SBF
To establish the reproducibility over time of the SBF response to acetylcholine we performed preliminary studies in which an acetylcholine dose-response was measured, a one hour recovery period followed during which blood flow returned to baseline, and acetylcholine dose-response was repeated. These results are shown in Figure 2 as %CVCmax in which dose-response curves are depicted from a representative subject. This demonstrates that skin repeatedly vasodilates in response to agonist stimulation provided an adequate recovery time is allowed. Data are recorded for 20 minutes by which time the response reaches a plateau value. If in rare circumstances a plateau is not achieved, additional time is allowed. This determination was repeated in 3 additional subjects in experiments that were performed as preliminary studies. In subsequent determinations performed on all 23 subjects, SBF increased significantly from 18±3 to a maximum of 132±6 pfu (P<0.0001) with the infusion of 100 mM acetylcholine.
Figure 2.
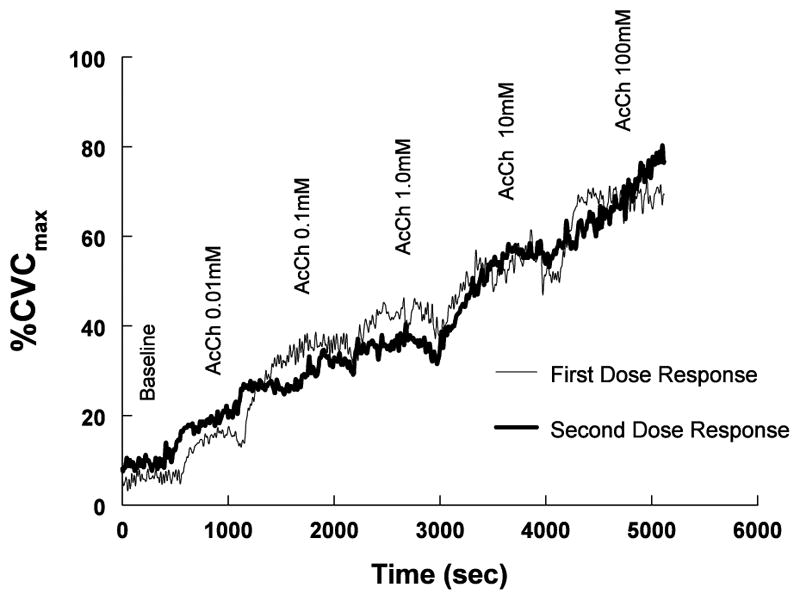
shows the response to increasing doses of acetylcholine in measured in one representative subject. Responses were initially recorded and repeated in one hour. The curves are similar indicating the repeatability of this response.
The Effects of NLA, Keto, NLA+Keto on SBF
In this and a previous study (31), Keto infusion resulted in a significant increase in baseline SBF causing an increase of Cutaneous Vascular Conduction (%CVC) that reached a plateau by 20 minutes and continues for at least 90 minutes. This response, expressed as %CVC max is shown in Figure 3 for a representative subject. In contrast, the infusion of 10 mM NLA (not shown) did not increase baseline above that determined in the absence of NOS inhibition.
Figure 3.
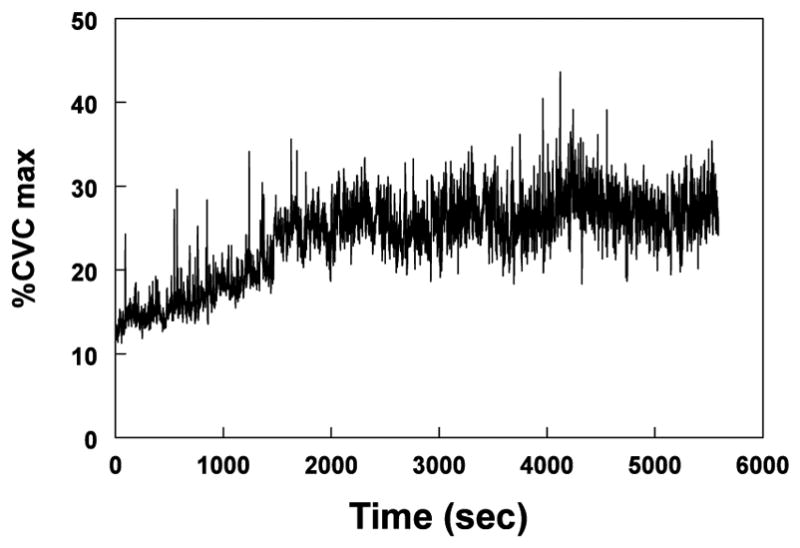
shows skin blood flow (SBF), expressed as a percentage of the maximum Cutaneous Vascular Conduction (%CVCmax) during the infusion of 10 mM Ketorolac (Keto) in one representative subject. Keto caused an increase of %CVCmax that reached a plateau by 20 minutes and continues for at least 90 minutes.
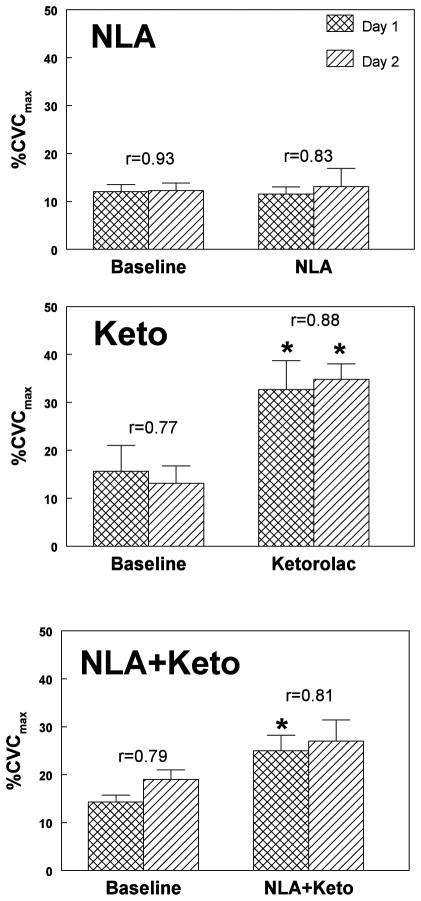
Baseline data and the effects of NLA, Keto and NLA+Keto on SBF, expressed as %CVCmax are shown in Figure 4. Neither baseline nor post-drug data were different on the two days of experimentation for any drug. Data from subjects correlated well from day to day as indicated by the correlation coefficients (ranging from 0.77 to 0.93) comparing determinations of LDF measurements made on different days thus assuring that comparison of measurements made on different days was appropriate.
Figure 4.
shows baseline data and the effects of nitro-L-arginine (NLA, top panel), ketorolac (Keto, middle panel) and NLA+Keto (bottom panel) on skin blood flow (SBF) expressed as a percentage of the maximum Cutaneous Vascular Conduction (%CVCmax). Data are shown for each drug on both experiment days. Correlation coefficients comparing pre-drug and post-drug data for subjects on different days appear on the top of the error bars. There was no difference between data obtained on different days. Data from subjects correlated well from day to day. NLA had no effect on baseline data. Keto increased SBF. NLA+Keto also increased SBF but the increase was blunted compared to ketorolac alone. *=P<0.05, n=23 compared to baseline.
While NLA had no significant effect on baseline SBF, Keto significantly increased SBF (p<0.0001). When NLA + Keto were jointly administered, a significant increase in skin blood flow also occurred (P<0.01) but this increase was significantly blunted (P<0.05) compared to the increase when Keto was given alone.
The Effects of NLA, Keto and NLA+Keto on LDF during Acetylcholine Dose-Response
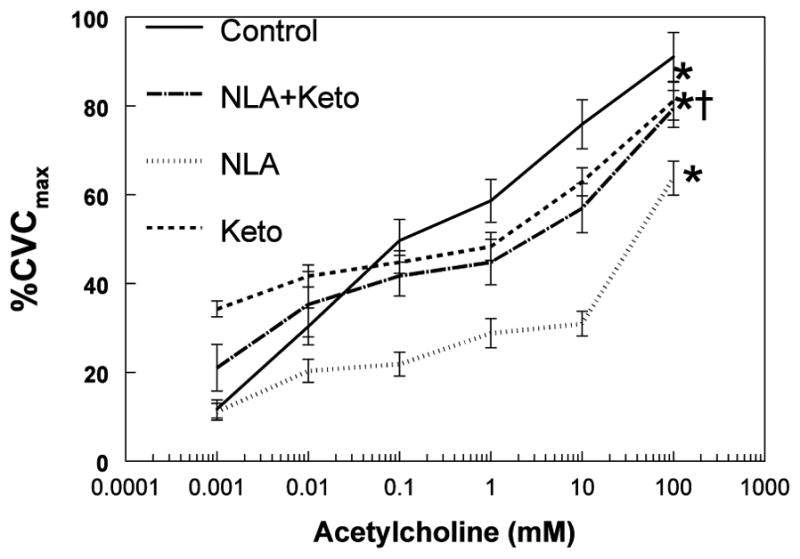
Figure 5 shows averaged data from all 23 subjects depicting the effects of NLA, Keto, and combined NLA+Keto on the acetylcholine dose response. This was computed by using control acetylcholine dose-responses averaged over all probes prior to NLA, Keto, or NLA+Keto. Differences between acetylcholine dose-response curves for NLA, Keto, or NLA+Keto were evaluated by subtracting paired percent changes for each subject and then averaging the results for all subjects. MANOVA for multiple comparisons demonstrated significant differences for NLA compared with control (P<0.00001), for Keto compared with control (P<0.025), and for NLA+Keto compared to control (P<0.0002). Also, we found that the NLA+Keto acetylcholine dose-response differed from the NLA acetylcholine dose-response (P<0.0002). The shape of the acetylcholine dose-response curves deserves comment: the control curve was nearly log-linear. The NLA curve had a small slope at lower doses of acetylcholine, which increased markedly at high dose. Keto and NLA + Keto dose-responses showed qualitatively similar patterns.
Figure 5.
shows the response to increasing doses of acetylcholine averaged over all subjects. Skin blood flow (SBF) was decreased by NLA at all doses above baseline. The decrease in dose-response was significantly blunted by NLA+Keto. SBF was significantly different from control for Keto, and for NLA+Keto. *=P<0.05, n=23compared to control. †=P<0.05, n=23 compared to NLA.
These data are presented in a somewhat different format in Figure 6 using paired data and are shown as percent change from control acetylcholine infusion at each dose and with each inhibitor. Significant percent changes are present at each dose of NLA. Significant percent changes are present at all but the lowest dose of NLA+Keto when compared to acetylcholine alone. Results differ for Keto in which, if acetylcholine doses are viewed individually, only the second to highest dose is significantly different by t-test and that at only P<0.05. However, if ANOVA are applied to all of the Keto data, overall significant changes from control are present.
Figure 6.
presents data showing percent differences from control at every dose of acetylcholine for NLA (upper panel), Keto (middle panel) and NLA+Keto (lower panel). ANOVA with repeated measures revealed significant difference from control for all medications. The percent change during the dose-response is different for each drug combination.
Discussion
We measured the ability of acetylcholine, a naturally occurring receptor-mediated endothelium-dependent vasodilator, to increase skin blood flow when delivered locally through microdialysis catheters. These studies show that the vasodilatory response to ACh in skin is repeatable over time and is likely mediated by several mechanisms. We showed that skin blood flow of the leg increased in a dose-dependent manner over the range of concentrations tested. These studies are of interest as they are the first to combine measurement of the response of skin blood flow and the dose response to acetylcholine with NOS and COX inhibition with drugs delivered through microdialysis catheters. Some previous “inhibition” studies have employed one dose of agonist, while others have relied on delivering escalating doses of acetylcholine iontophoretically (36). This experimental design, using a wide range of acetylcholine concentrations helps to illustrate the large contribution of NO to ACh-induced vasodilatation.
In the present study, we used NLA instead of Lnitroarginine methyl ester (L-NAME) because the latter can act as a muscarinic receptor antagonist (8, 10). A major finding of our study is that NLA, a nitric oxide synthase inhibitor, ketorolac, a nonselective COX-1 and COX-2 inhibitor, alone or in combination reduce the response to all doses of acetylcholine tested when delivered directly into the intradermal space and studied using repeated measures analysis. In addition, Keto, when given alone, exerts small effects on the acetylcholine dose-response, but when added to NLA greatly attenuates the reduction of the acetylcholine dose-response compared to NLA alone. These effects are similar to that shown previously by Holowatz who measured the effects of NOS and COX inhibition using only one dose of acetylcholine (22). Laser Doppler measurements of acetylcholine receptor mediated endothelium-dependent vasodilation are highly nitric oxide dependent and strongly relate to the interactions of NO with prostaglandins.
To substantiate the adequacy of COX inhibition in our current experimental design, we performed additional experiments (not shown) in which we compared the effects of 10 mM Ketorolac and 10 mM aspirin on skin blood flow. The results showed that non-specific COX inhibition with aspirin caused a reduction in the skin blood flow response to acetylcholine, similar to that show for Ketorolac, but did result in an increase of baseline skin blood flow.
Skin Blood Flow Increases with Cyclooxygenase Inhibition
We also found that baseline SBF was significantly increased by ketorolac. This is qualitatively similar to the data of Kellogg and associates (26), although their flow increments did not reach significance at similar doses of Keto. COX inhibition with ketorolac non-specifically blocks both vasodilator prostaglandins (e.g. prostacyclin) and vasoconstrictor prostaglandins (e.g. thromboxanes) (34). The data support the conclusion that at room temperature, vasoconstrictor rather than vasodilator prostaglandins predominate and that cyclooxygenase inhibition enables ischemic dilation through the reduction of vasoconstrictive prostaglandins. This vasoconstrictive effect does not take into account potential effects of epoxyeicosatrienoic acids derived from arachidonic acid - which is believed to comprise potent EDHF’s (17) - because ketorolac exerts no influence on their synthesis. The combination of NLA with Keto blunted increases in LDF which could relate to interactions between NO and prostaglandin synthesis (33) or relate to the effects of NO on EDHF (3, 37).
Our findings are different from those of Kellogg who showed the greatest inhibition of ACh vasodilation in the presence of L-NAME and Keto (26). The reasons for these differences may be due to the significant increase in baseline skin blood flow caused by Ketorolac in our determinations. We also used NLA instead of L-NAME because of our previous experience with this agent, as well as our concern about L-NAME acting as a muscarinic receptor antagonist (8, 10). Because of these differences in experimental design, a direct comparison of these studies is not possible at the present time, but may be the subject of future investigations.
Cutaneous Acetylcholine Vasodilation is Differentially Affected by NO and Prostaglandins
The bulk of evidence indicates that a large reduction of acetylcholine augmented cutaneous blood flow occurs with the administration of nonselective NOS inhibitors independent of the route of delivery (5, 7, 22, 26). Similar data exists for methacholine (29). Evidence also supports an increase in NO production with intradermal acetylcholine administration (5).
The role of prostaglandins appears to be more controversial (9, 13, 16, 21, 22, 27) with some investigators proposing an intermediate permissive role (9). Our data indicate that NLA reduced acetylcholine-stimulated flow by about 50–60% at moderate doses, but that COX inhibition with Keto only reduced acetylcholine mediated flow by 10–20%. However, when repeated measure analysis is applied there is significant reduction of the acetylcholine dose-response by ketorolac. Combined with NLA, Keto significantly blunted the reduction in vasodilation produced by NOS inhibition alone. This suggests either interactions between NO and dilator prostaglandins (33), superoxide mediated interactions (6) or the vasodilating actions of EDHF (14, 23). Thus prostaglandins seem to exert a restraining effect on acetylcholine mediated vasodilation.
In the present study, we showed a differential effect on acetylcholine-induced skin blood flow when of NOS inhibition was combined with prostaglandin inhibition. This may be due to the interactions between NO and PG biosynthetic pathways involving an active back modulation operated by reaction end products, including NO, PGs, and cyclic nucleotides (33), or even from combined effects of COX metabolites and NO on EDHF (18, 19, 33).
While other investigators have studied the effects COX and NOS inhibition on acetylcholine-induced vasodilation, the present study examines these effects using a wide range of acetylcholine concentrations. As such, the shape of the acetylcholine dose-response curves merits further discussion. As shown in Figures 4 and 5, the inhibiting effects of NLA, Keto and NLA+Keto on acetylcholine dose response are attenuated at high dose acetylcholine. This suggests that at high doses acetylcholine mediated vasodilation may relate to factors other than nitric oxide and COX metabolites. Potential factors include acetylcholine activation of nociceptive C fibers that can cause an axon-reflex-mediated vasodilation of the skin and direct effects of acetylcholine on smooth muscles responses (4, 5).
Limitations
The local control of cutaneous blood flow is undoubtedly more complex than interactions between non-isoform selective nitric oxide synthase, nonselective COX-1, COX-2 prostaglandin inhibitors. Further analysis using isoform specific NOS inhibitors, selective vasodilator and vasoconstrictor prostaglandin inhibitors, and EDHF will be required.
Microdialysis is invasive, alters the interstitial milieu and a uniform depth of catheter insertion can not be assured. The work of Anderson et al (2) suggests that flow responses return to baseline levels within approximately 1 hour. This environment likely remains changed for some time despite a return of skin blood flow to pre-catheter insertion values. However, in pilot experiments we measured baseline flows, removed the LDF probes, instrumented the same site with microdialysis catheters, replaced the probes, waited at least an hour and repeated the LDF measurements with (on average) similar results. Another limitation of the current experimental design is that we are not measuring the identical region as first measured to determine baseline, however, the region measured is very similar as repeated removal and replacement of the probe over one area yields near-identical values for baseline blood-flow determinations. After initial laser measurement, the sites were instrumented with microdialysis probes, we waited until the instrumentation effect abated and thereafter used the very same positioning to measure all laser flows. It is however impossible to assure that the laser measures the same exact area from which skin blood flow was recorded before and after microdialysis catheter placement.
Relative to these concerns, there is evidence that NO and arachidonic acid metabolites may be generated at different locations within the skin, and there are interactions between their enzymatic pathways (7, 33, 37). The inability to assure uniform delivery of agonist and antagonist to each receptor via microdialysis catheters may be responsible for differential stimulation and blockade of skin blood flow, thus influencing tissue responsiveness.
We used calves instead of forearms for purposes of microdialysis and laser Doppler flowmetry. While previous studies have shown there are many similarities, particularly in cutaneous vascular conductance, cutaneous vascular resistance or electrodermal activity comparing arm to leg.(38, 39) this does not eliminate the possibility that there are differences in the response of these different vascular beds to varying stimuli.
Our current experimental design of drug delivery through microdialysis catheters depends upon varying dynamics that at a minimum include, the hydrodynamic force of the perfusate, the physiochemical properties of the solute and solvent, the size and charge of the molecule, and the nature of the membrane through which they move (11). Therefore, our presentation of the response of skin blood flow to agonist stimulation and its inhibition should be considered within the context of these factors. In the same regard, perfusion of the intravascular microdialysis catheters may have a direct influence on local blood flow and the availability of local mediators that influence the responses measured.
Noninvasive macrovascular markers of NO such as measurements of brachial artery flow mediated dilation (9) are well known. Why add microvascular measures of NO? Arguably, endothelial dysfunction is primarily a microvascular disease and may be preclinically evident in the microvasculature (12). Also, microvascular measures of endothelial function and specifically NO-dependent function may not necessarily correlate very well with macrovascular measures (1).
One additional concern is that of the phase of the menstrual cycle of the female subjects and the effects of altered hormonal status among subjects. While this was not controlled for, since determinations were done on 1 or 2 sequential days, individual measurements the response to RH were not likely to vary in each subject.
Conclusions
We have shown, using agonist alone and in combination with various inhibitors, that skin blood flow is responsive to increasing doses of acetylcholine delivered locally by microdialysis catheters. We have also demonstrated that these responses are reliably reproducible over subsequent days and that the microvascular response to acetylcholine is importantly inhibited following inhibition of NO using NLA and prostaglandins using Keto. This supports our contention that that acetylcholine receptor mediated endothelium-dependent vasodilation is highly nitric oxide dependent and is related to the interactions of NO with prostaglandins.
Acknowledgments
Grants
Supported by 1RO1HL074873 from the National Heart Lung and Blood Institute and by 1R21DK071647 from the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health.
Footnotes
Disclosures
The authors have nothing to disclose to the APS Publications Office concerning any potential conflict of interest (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, lack of access to data, or lack of control of the decision to publish).
Literature Cited
- 1.Agewall S, Henareh L, Kublickiene K. Endothelial function in conduit and resistance arteries in men with coronary disease. Atherosclerosis. 2006;184:130–6. doi: 10.1016/j.atherosclerosis.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Invest Dermatol. 1994;102:807–11. doi: 10.1111/1523-1747.ep12378630. [DOI] [PubMed] [Google Scholar]
- 3.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–7. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- 4.Berghoff M, Kathpal M, Kilo S, Hilz MJ, Freeman R. Vascular and neural mechanisms of ACh-mediated vasodilation in the forearm cutaneous microcirculation. J Appl Physiol. 2002;92:780–8. doi: 10.1152/japplphysiol.01167.2000. [DOI] [PubMed] [Google Scholar]
- 5.Boutsiouki P, Georgiou S, Clough GF. Recovery of nitric oxide from acetylcholine-mediated vasodilatation in human skin in vivo. Microcirculation. 2004;11:249–59. doi: 10.1080/10739680490425958. [DOI] [PubMed] [Google Scholar]
- 6.Bratz IN, Kanagy NL. Nitric oxide synthase-inhibition hypertension is associated with altered endothelial cyclooxygenase function. Am J Physiol Heart Circ Physiol. 2004;287:H2394–H2401. doi: 10.1152/ajpheart.00628.2004. [DOI] [PubMed] [Google Scholar]
- 7.Buus NH, Simonsen U, Pilegaard HK, Mulvany MJ. Nitric oxide, prostanoid and non-NO, non-prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br J Pharmacol. 2000;129:184–92. doi: 10.1038/sj.bjp.0703041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buxton IL, Cheek DJ, Eckman D, Westfall DP, Sanders KM, Keef KD. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993;72:387–95. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- 9.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 10.Chang HY, Chen CW, Hsiue TR. Comparative effects of L-NOARG and L-NAME on basal blood flow and ACh-induced vasodilatation in rat diaphragmatic microcirculation. Br J Pharmacol. 1997;120:326–32. doi: 10.1038/sj.bjp.0700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clough GF, Boutsiouki P, Church MK, Michel CC. Effects of blood flow on the in vivo recovery of a small diffusible molecule by microdialysis in human skin. J Pharmacol Exp Ther. 2002;302:681–6. doi: 10.1124/jpet.102.035634. [DOI] [PubMed] [Google Scholar]
- 12.Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–34. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalle-Ave A, Kubli S, Golay S, Delachaux A, Liaudet L, et al. Acetylcholine-induced vasodilation and reactive hyperemia are not affected by acute cyclo-oxygenase inhibition in human skin. Microcirculation. 2004;11:327–36. doi: 10.1080/10739680490449268. [DOI] [PubMed] [Google Scholar]
- 14.de WC, Esser N, Lehr HA, Bolz SS, Pohl U. Pentobarbital-sensitive EDHF comediates ACh-induced arteriolar dilation in the hamster microcirculation. Am J Physiol. 1999;276:H1527–H1534. doi: 10.1152/ajpheart.1999.276.5.H1527. [DOI] [PubMed] [Google Scholar]
- 15.Drexler H, Hornig B. Endothelial dysfunction in human disease. J Mol Cell Cardiol. 1999;31:51–60. doi: 10.1006/jmcc.1998.0843. [DOI] [PubMed] [Google Scholar]
- 16.Durand S, Tartas M, Bouye P, Koitka A, Saumet JL, Abraham P. Prostaglandins participate in the late phase of the vascular response to acetylcholine iontophoresis in humans. J Physiol. 2004;561:811–9. doi: 10.1113/jphysiol.2004.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming I, Busse R. Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension. 2006;47:629–33. doi: 10.1161/01.HYP.0000208597.87957.89. [DOI] [PubMed] [Google Scholar]
- 18.Fuloria M, Eckman DM, Leach DA, Aschner JL. 20-hydroxyeicosatetraenoic acid is a vasoconstrictor in the newborn piglet pulmonary microcirculation. Am J Physiol Lung Cell Mol Physiol. 2004;287:L360–L365. doi: 10.1152/ajplung.00358.2003. [DOI] [PubMed] [Google Scholar]
- 19.Fuloria M, Smith TK, Aschner JL. Role of 5,6-epoxyeicosatrienoic acid in the regulation of newborn piglet pulmonary vascular tone. Am J Physiol Lung Cell Mol Physiol. 2002;283:L383–L389. doi: 10.1152/ajplung.00444.2001. [DOI] [PubMed] [Google Scholar]
- 20.Furchgott RF, Cherry PD, Zawadzki JV, Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984;6(Suppl 2):S336–S343. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- 21.Hendry RG, Marshall JM. Vasoconstrictor products of cyclo-oxygenase activity limit acetylcholine-induced cutaneous vasodilatation in young men. Clin Sci (Lond) 2004;107:323–30. doi: 10.1042/CS20040077. [DOI] [PubMed] [Google Scholar]
- 22.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol. 2005;563:965–73. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang A, Sun D, Smith CJ, Connetta JA, Shesely EG, et al. In eNOS knockout mice skeletal muscle arteriolar dilation to acetylcholine is mediated by EDHF. Am J Physiol Heart Circ Physiol. 2000;278:H762–H768. doi: 10.1152/ajpheart.2000.278.3.H762. [DOI] [PubMed] [Google Scholar]
- 24.Katz SD, Schwarz M, Yuen J, LeJemtel TH. Impaired acetylcholine-mediated vasodilation in patients with congestive heart failure. Role of endothelium-derived vasodilating and vasoconstricting factors. Circulation. 1993;88:55–61. doi: 10.1161/01.cir.88.1.55. [DOI] [PubMed] [Google Scholar]
- 25.Kellogg DL, Jr, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–90. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 26.Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol. 2005;98:629–32. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- 27.Khan F, Green FC, Forsyth JS, Greene SA, Morris AD, Belch JJ. Impaired microvascular function in normal children: effects of adiposity and poor glucose handling. J Physiol. 2003;551:705–11. doi: 10.1113/jphysiol.2003.045351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura K, Low DA, Keller DM, Davis SL, Crandall CG. Cutaneous blood flow and sweat rate responses to exogenous administration of acetylcholine and methacholine. J Appl Physiol. 2007;102:1856–61. doi: 10.1152/japplphysiol.01069.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee K, Mack GW. Role of nitric oxide in methacholine-induced sweating and vasodilation in human skin. J Appl Physiol. 2006;100:1355–60. doi: 10.1152/japplphysiol.00122.2005. [DOI] [PubMed] [Google Scholar]
- 30.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–51. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 31.Medow MS, Taneja I, Stewart JM. Cyclooxygenase and Nitric Oxide Synthase Dependence of Cutaneous Reactive Hyperemia in Humans. Am J Physiol Heart Circ Physiol. 2007;293(1):H425–H432. doi: 10.1152/ajpheart.01217.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ming Z, Parent R, Lavallee M. Nitric oxide-independent dilation of conductance coronary arteries to acetylcholine in conscious dogs. Circ Res. 1997;81:977–87. doi: 10.1161/01.res.81.6.977. [DOI] [PubMed] [Google Scholar]
- 33.Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–52. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- 34.Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978;30:293–331. [PubMed] [Google Scholar]
- 35.Morgan CJ, Friedmann PS, Church MK, Clough GF. Cutaneous microdialysis as a novel means of continuously stimulating eccrine sweat glands in vivo. J Invest Dermatol. 2006;126:1220–5. doi: 10.1038/sj.jid.5700197. [DOI] [PubMed] [Google Scholar]
- 36.Morris SJ, Shore AC. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol. 1996;496 ( Pt 2):531–42. doi: 10.1113/jphysiol.1996.sp021704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol Heart Circ Physiol. 2000;279:H459–H465. doi: 10.1152/ajpheart.2000.279.2.H459. [DOI] [PubMed] [Google Scholar]
- 38.Okazaki K, Fu Q, Martini ER, Shook R, Conner C, et al. Vasoconstriction during venous congestion: effects of venoarteriolar response, myogenic reflexes, and hemodynamics of changing perfusion pressure. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1354–R1359. doi: 10.1152/ajpregu.00804.2004. [DOI] [PubMed] [Google Scholar]
- 39.Ray CA, Wilson TE. Comparison of skin sympathetic nerve responses to isometric arm and leg exercise. J Appl Physiol. 2004;97:160–4. doi: 10.1152/japplphysiol.00699.2003. [DOI] [PubMed] [Google Scholar]
- 40.Shibasaki M, Crandall CG. Effect of local acetylcholinesterase inhibition on sweat rate in humans. J Appl Physiol. 2001;90:757–62. doi: 10.1152/jappl.2001.90.3.757. [DOI] [PubMed] [Google Scholar]
- 41.Shoemaker JK, Pozeg ZI, Hughson RL. Forearm blood flow by Doppler ultrasound during test and exercise: tests of day-to-day repeatability. Med Sci Sports Exerc. 1996;28:1144–9. doi: 10.1097/00005768-199609000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous Neuronal Nitric Oxide is Specifically Decreased in Postural Tachycardia Syndrome. Am J Physiol Heart Circ Physiol. 2007;113(11):449–57. doi: 10.1152/ajpheart.00600.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoen R, Lossius K, Karlsson JO. Acetylcholine-induced vasodilation may depend entirely upon NO in the femoral artery of young piglets. Br J Pharmacol. 2003;138:39–46. doi: 10.1038/sj.bjp.0705001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanhoutte PM. Could the absence or malfunction of vascular endothelium precipitate the occurrence of vasospasm? J Mol Cell Cardiol. 1986;18:679–89. doi: 10.1016/s0022-2828(86)80940-3. [DOI] [PubMed] [Google Scholar]
- 45.Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–92. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]