Summary
In view of previous reports of changes in seizure susceptibility in adult rats exposed to phenobarbital or diazepam as pups, we examined the effects of early-life exposure to lamotrigine and phenytoin, two commonly used antiepileptic drugs (AEDs), for their effect on seizure threshold in adult rats. We found that pups exposed to lamotrigine for six days during the second postnatal week had a significantly lower threshold for pentylenetetrazol-evoked seizures when tested as adults. In contrast, phenytoin exposure during the second postnatal week was without a significant effect on seizure threshold in adults. Seizure scores at threshold were comparable across all groups tested. The dose of lamotrigine used in our study (20 mg/kg) was below that required to cause developmental neuronal apoptosis, while the dose of phenytoin used (50 mg/kg) was above that required for developmental neurotoxicity. Thus, our findings suggest that neurodevelopmental alterations in seizure susceptibility may occur via mechanisms that are independent of those responsible for neural injury or teratogenesis. Our findings support the possibility that therapy with certain AEDs during pregnancy or infancy may alter seizure susceptibility later in life, a possibility that should be taken into account when examining early-life factors that contribute to seizure susceptibility in adulthood.
Keywords: pentylenetetrazol, development, antiepileptic drug
Introduction
Antiepileptic drugs (AEDs), when administered to pregnant women or infants, can influence brain development during sensitive periods. The brain is especially sensitive to developmental alterations during the “brain growth spurt” period, when massive synaptogenesis and pruning take place. This period corresponds to the third trimester of gestation through early infancy in humans, and to the first two postnatal weeks in rats (Dobbing & Sands, 1979). Previous reports show that exposure of rats to phenobarbital, barbital, diazepam, or ethanol during this period results in a decreased threshold in response to seizures evoked by pentylenetetrazole (PTZ) later in life (Tagashira et al., 1982, Bonthius et al., 2001). This raises serious concerns regarding the choice of AED therapy during pregnancy and for neonatal seizures. Lamotrigine and phenytoin are AEDs that have been used to treat pregnant women (Ornoy, 2006), and phenytoin is used as an adjunct or alternative to phenobarbital for neonatal seizures (Bartha et al., 2007). Therefore, in the present study, we examined the effects of exposure of rats to lamotrigine or phenytoin during the second postnatal week on PTZ seizure threshold measured in adulthood.
Methods
Animals
Timed-pregnant female (GD16-18) Sprague-Dawley rats (Harlan) were housed in a temperature-controlled (21°C) room with a 12-h light cycle (0600-1800 Lights on). All manipulations occurred during the light phase. Date of parturition was designated as P0. Beginning on P7, male pups were weighed, labeled, and given one of three treatments via intraperitoneal (i.p.) injection: lamotrigine isethionate (20mg/kg; 2mg/ml), phenytoin (5,5-diphenylhydantoin, 50mg/kg; 5mg/ml in alkalinized saline), or vehicle (saline, 10ml/kg). Drugs (and saline for control pups) were injected at a volume of 0.01ml/g body weight. Drug doses are the same as those we and others have used previously for assessment of acute cellular toxicity (Bittigau et al., 2002, Katz et al., 2007), and fall within the anticonvulsant range in rat pups (Stankova et al., 1992). Pups were treated once per day on P7,8,9,11,12,13. Treatments were balanced within and across litters. Pups were weaned on P21 into cages of 2–3 littermates. Seizure threshold testing started on P90.
Seizure threshold testing
To establish the CD50 for a threshold seizure response to i.p. pentylenetetrazol (PTZ), a staircase procedure of escalating doses (20mg/kg to 50mg/kg, in 2.5mg/kg increments) was employed until a threshold seizure was observed. A threshold seizure was defined as multiple myoclonic jerks with or without facial and forelimb clonus (a partial seizure). Seizures were rated according to a modified Racine scoring procedure for limbic motor seizures (Racine, 1972): 1 = single myoclonic jerk [subthreshold response]; 2 = multiple myoclonic jerks [threshold seizure]; 3 = facial and forelimb clonus (FFC); 3.5 = FFC with a body twist; 3.75 = FFC with a full body roll; 4 = FFC with rearing; 4.5 = FFC with rearing and a body twist; 5 = FFC with rearing and loss of balance; 6 = running bouncing seizure with tonic forelimb extension. Scores for all seizures were recorded in order to permit a comparison of seizure scores at threshold. Repeated testing with the same dose within subjects produced no evidence of either tolerance or sensitization. All experiments were performed in compliance with the American Association for Accreditation of Laboratory Animal Care standards, and were approved by the Georgetown University Animal Care and Use Committee.
Results
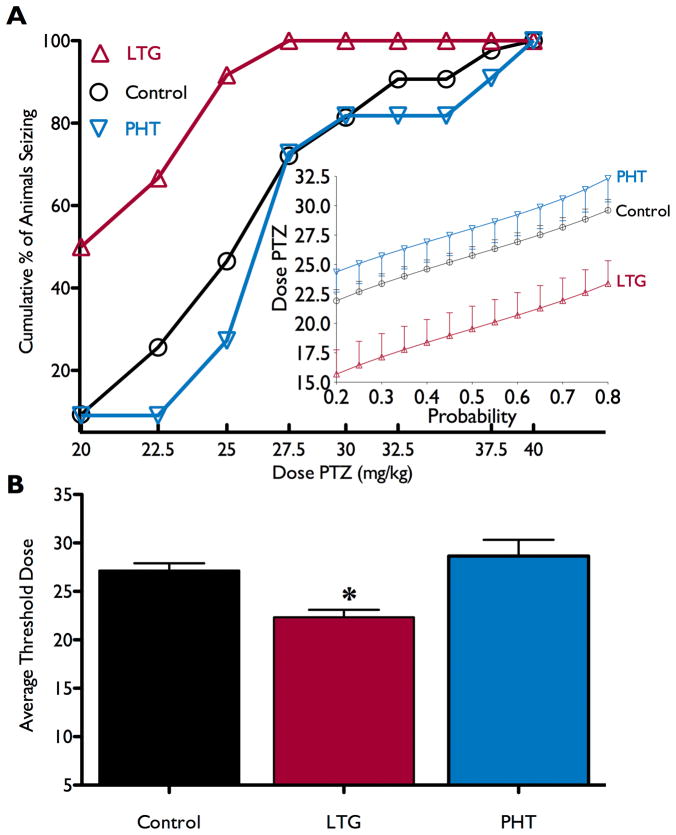
Figure 1a shows cumulative percent of animals exhibiting a threshold seizure as a function of PTZ dose for each treatment group. For animals that had been exposed to lamotrigine, the PTZ dose-response function is left-shifted as compared to control and phenytoin-exposed animals. The average seizure-threshold dose for each treatment group is shown in Figure 1b. There was a significant reduction in seizure threshold for animals exposed as pups to lamotrigine as compared to all other groups. One-way analysis of variance revealed a main effect of drug exposure (F2,63=6.188, p<0.005). Dunnet’s multiple comparison test revealed a significant difference between the lamotrigine and vehicle (p<0.01), and between the lamotrigine and phenytoin groups (p<0.01), but no difference between vehicle and phenytoin groups. The mean seizure score at threshold did not significantly differ across treatment groups (mean±standard deviation; control 3.7±1.4; phenytoin 3.6±0.89; lamotrigine 2.6±1.1; Kruskal-Wallis test; H=5.163, p>0.08). The proportion of animals exhibiting a seizure response to 25mg/kg PTZ (CD50 for control animals) is show in Table 1. Consistent with the data in Figure 1, the lamotrigine-exposed group showed a significantly higher incidence of seizures than controls (p<0.05, Fisher’s Exact Test).
Figure 1.
Effects of neonatal AED exposure on adult seizure threshold. A) cumulative percent of the group seizing as a function of dose of PTZ. Insert: a probit model of the seizure threshold data, with the curves for vehicle (○) and phenytoin (PHT; ▽)-exposed groups overlapping, and the lamotrigine (LTG; △) curve showing a reduction in seizure threshold. B) average threshold dose needed to induce a seizure for animals exposed to vehicle (control: 27.2±0.8, median=27.5), lamotrigine (LTG: 22.3±0.8, median=21.3), or phenytoin (PHT: 28.6±1.7, median=27.5).
Table 1.
Proportion of Animals Seizing at 25mg/kg PTZ (CD50 for controls)
| Postnatal Treatment | Proportion Seizing | % Seizing |
|---|---|---|
| Vehicle | 27/50 | 54 |
| Lamotrigine | 13/15* | 87 |
| Phenytoin | 3/11 | 27 |
Significantly (p<0.05) different than control, Fisher’s Exact Test
Discussion
We found that exposure of neonatal rat pups to lamotrigine, but not to phenytoin, resulted in a decrease in seizure threshold in response to PTZ when the animals were tested as adults. The distinct effects of lamotrigine and phenytoin suggest that their shared mechanism of action [i.e., sodium channel blockade, (Leach et al., 1986)] is not likely to account for the change in seizure threshold after neonatal lamotrigine exposure. In addition, the effect of lamotrigine, but not phenytoin, on seizure threshold is in sharp contrast to the relative lack of toxicity of lamotrigine in developmental models and the notable developmental toxicity of phenytoin. In particular, phenytoin exhibits profound neuronal toxicity with acute neonatal administration in rats (Bittigau et al., 2002, Katz et al., 2007) and is associated with significant somatic and behavioral teratogenic effects in humans (Ornoy, 2006). In contrast, lamotrigine does not cause neurotoxicity in neonatal rats (Katz et al., 2007) and appears devoid of significant teratogenic effects in humans (Meador et al., 2009, Ornoy, 2006). Taken together, these findings indicate that alterations in seizure susceptibility may occur via mechanisms that are independent of those responsible for neural injury or teratogenesis. It should be noted that the effect of early life exposure to lamotrigine may differ depending upon the method of seizure initiation in adults, as the threshold for clonic seizures evoked by focal electrical stimulation of sensorimotor cortex was not significantly changed in adult rats exposed to lamotrigine as pups (Tsenov et al., 2009).
In the clinical setting, increased seizure susceptibility in adulthood has been associated with a history of early life seizures. This has been interpreted as evidence for early life seizures either as a cause of later life seizure susceptibility, or as a marker for an underlying predisposition to seizures (Ben-Ari and Holmes, 2006). However, since early life seizures are typically treated with AEDs, our results suggest that AED exposure should be considered as a confounding variable for evaluating the impact of early life seizures on later seizure susceptibility. Our results with lamotrigine exposure taken together with previous reports on phenobarbital and diazepam exposure during infancy, raise the possibility that exposure to AEDs during early life may alter subsequent seizure susceptibility (Tagashira et al., 1982). These results suggest it is possible that medication used to treat seizures in infancy may be an important determinant of seizure susceptibility later in life.
Acknowledgments
This research was supported by NIH F31NS066822 and Epilepsy Foundation Fellowship EFA123098 to PAF, Research grant from GlaxoSmithKline to AK, and NIH training grants T32DA007291 and T32NS041231.
Footnotes
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose. “We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.”
References
- Bartha AI, Shen J, Katz KH, Mischel RE, Yap KR, Ivacko JA, Andrews EM, Ferriero D, Ment LR, Silverstein F. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90. doi: 10.1016/j.pediatrneurol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–1063. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, Woodhouse J, Bonthius NE, Taggard DA, Lothman EW. Reduced seizure threshold and hippocampal cell loss in rats exposed to alcohol during the brain growth spurt. Alcohol Clin Exp Res. 2001;25:70–82. [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Katz I, Kim J, Gale K, Kondratyev A. Effects of lamotrigine alone and in combination with MK-801, phenobarbital, or phenytoin on cell death in the neonatal rat brain. J Pharmacol Exp Ther. 2007;322:494–500. doi: 10.1124/jpet.107.123133. [DOI] [PubMed] [Google Scholar]
- Leach MJ, Marden CM, Miller AA. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action. Epilepsia. 1986;27:490–497. doi: 10.1111/j.1528-1157.1986.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A. Neuroteratogens in man: an overview with special emphasis on the teratogenicity of antiepileptic drugs in pregnancy. Reprod Toxicol. 2006;22:214–226. doi: 10.1016/j.reprotox.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroen Clin Neuro. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Stankova L, Kubova H, Mares P. Anticonvulsant action of lamotrigine during ontogenesis in rats. Epilepsy Res. 1992;13:17–22. doi: 10.1016/0920-1211(92)90003-c. [DOI] [PubMed] [Google Scholar]
- Tagashira E, Nakao K, Urano T, Hiramori T, Yanaura S. Alteration of convulsive threshold and sensitivity to CNS acting drugs in sedative-hypnotics-experienced rat offspring. Jpn J Pharmacol. 1982;32:263–271. doi: 10.1254/jjp.32.263. [DOI] [PubMed] [Google Scholar]
- Tsenov G, Redkozubova O, Kubova H, Mares P. Effects of lamotrigine on cortically-elicited phenomena in adult rats: Differences between acute application and late consequences of early postnatal administration. Brain Res. 2009;1258:65–70. doi: 10.1016/j.brainres.2008.12.054. [DOI] [PubMed] [Google Scholar]