Abstract
Left ventricular remodeling that occurs after myocardial infarction (MI) and pressure overload is generally accepted as a determinant of the clinical course of heart failure. The molecular mechanism of this process, however, remains to be elucidated. Apoptosis signal-regulating kinase 1 (ASK1) is a mitogen-activated protein kinase kinase kinase that plays an important role in stress-induced apoptosis. We used ASK1 knockout mice (ASK-/-) to test the hypothesis that ASK1 is involved in development of left ventricular remodeling. ASK-/- hearts showed no morphological or histological defects. Echocardiography and cardiac catheterization revealed normal global structure and function. Left ventricular structural and functional remodeling were determined 4 weeks after coronary artery ligation or thoracic transverse aortic constriction (TAC). ASK-/- had significantly smaller increases in left ventricular end-diastolic and end-systolic ventricular dimensions and smaller decreases in fractional shortening in both experimental models compared with WT mice. The number of terminal deoxynucleotidyl transferase biotin-dUDP nick end-labeling-positive myocytes after MI or TAC was decreased in ASK-/- compared with that in WT mice. Overexpression of a constitutively active mutant of ASK1 induced apoptosis in isolated rat neonatal cardiomyocytes, whereas neonatal ASK-/- cardiomyocytes were resistant to H2O2-induced apoptosis. An in vitro kinase assay showed increased ASK1 activity in heart after MI or TAC in WT mice. Thus, ASK1 plays an important role in regulating left ventricular remodeling by promoting apoptosis.
Ventricular remodeling refers to changes in left ventricular (LV) geometry, mass, volume, and function, in response to myocardial injury or alteration in load. Although the remodeling process can be adaptive, the process becomes maladaptive when the stimuli are continuous and pathological, as in myocardial infarction (MI), hypertension, and valvular heart diseases. The extent of LV dilatation during remodeling is a strong predictor of both morbidity and mortality (1, 2). To improve prognosis in heart failure after LV remodeling after MI or hypertension, it is necessary to elucidate cellular and molecular mechanism underlying the progression of LV remodeling.
In MI, remodeling initially involves side-to-side slippage of myocytes in the wall, resulting in infarct expansion (3). Later, in response to volume overload and neurohumoral signals, the noninfarcted myocardium, remote from the infarct, undergoes hypertrophy, which initially helps to decrease wall stress. Ultimately, however, the left ventricle dilates, its walls thin, and contractile function deteriorates. Similarly, in response to pressure overload, the heart activates an adaptive physiological response in the form of cardiac hypertrophy to decrease wall stress. However, long-lasting or excessive exposure to mechanical stress results in chamber dilation and cardiac dysfunction. Independent of etiology, a similar remodeling process is seen in these myocardial disorders, suggesting common mechanisms for the development of LV remodeling (4).
The underlying mechanism responsible for LV remodeling after MI has been linked to myocyte apoptosis in human patients as well as in experimental models (5, 6). Apoptosis occurs in a scattered manner throughout the nonischemic region remote from the area of the ischemic injury (7) and the border zone (6). Similarly, it has been reported that apoptosis is activated in hearts after experimental pressure overload (8). In heart failure after pressure-overload-induced cardiac hypertrophy, cardiac myocyte apoptosis is proposed to be a critical point in the transition between compensatory hypertrophy and heart failure (9).
Apoptosis signal-regulating kinase 1 (ASK1) is a reactive oxygen species-sensitive mitogen-activated protein (MAP) kinase kinase kinase, which activates the c-Jun N-terminal kinase (JNK) and p38 MAP kinase (10). Overexpression of WT or constitutively active ASK1 induces apoptosis in various cells (11), whereas oxidative stress and tumor necrosis factor-induced apoptosis are suppressed in ASK1-/- cells (12).
In this study, we attempted to elucidate a role of ASK1 in LV remodeling by analyzing ASK1 knockout mice. Because LV remodeling after MI and pressure overload is the most clinically relevant etiology, we used two mouse experimental models leading to LV remodeling; MI by left coronary artery (LCA) ligation and pressure overload. We provide evidence for a critical role of the ASK1-dependent cell death pathway in LV remodeling.
Methods
Animals and in Vivo Assessment of Cardiac Functions. This study was carried out under the supervision of the Animal Research Committee in accordance with the Guideline on Animal Experiments of Osaka University and Japanese Government Animal Protection and Management Law (no. 105). Mice were maintained individually 4 weeks after birth and allowed access to water and mouse chow ad libitum. ASK1 knockout mice (ASK-/-) in the F6 generation on a C57Bl6/J background were described (12). Ten-week-old ASK-/- and age-matched C57Bl6/J mice (WT) were subject to surgery. Ligation of LCA (7) and thoracic transverse aortic constriction (TAC) (13) were performed as described. Hemodynamic evaluation was performed on mice as described (14). Echocardiography was performed on mice, anesthetized with 2.5% avertin (8 μl/g), by using ultra-sonography (SONOS-5500, equipped with a 15-MHz linear transducer, Philips Medical Systems). The heart was imaged in the two-dimensional parasternal short-axis view, and an M-mode echocardiogram of the midventricle was recorded at the level of the papillary muscles. Wall stress was calculated according to the equation: (wall stress) = (pressure × radius)/(2 × wall thickness).
Histological Analyses and Evaluation of Apoptosis. The heart samples were arrested in diastole and fixed immediately with buffered 3.7% formalin, embedded in paraffin, and sectioned into 3-μm thickness. Hematoxylin and eosin or Masson-trichrome staining was performed on serial sections. Paraffin-embedded heart sections were subjected to the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay using an in situ apoptosis detection kit (Takara). For some samples, triple staining with propidium iodide (Vector Laboratories), TUNEL, and anti-α-sarcomeric actin antibody (Sigma) was performed.
Isolation of Ventricular Myocytes and Survival Assay. Ventricular myocytes from 1- to 2-day-old Wistar rats or ASK-/- mice were prepared and cultured as described (15). Rat neonatal ventricular myocytes were infected with adenovirus vectors expressing a constitutively active form of ASK1 (AdASK-ΔN) or β-galactosidase (AdLacZ) (11) at a multiplicity of infection of 100 plaque forming units per cell for 1 h. Mouse neonatal cardiomyocytes isolated from ASK-/- were treated with H2O2 for 24 h. By using a 3-(4,5-dimethlthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay-based Cell Counting kit-8 (Dojindo, Kumamoto, Japan), survival cell numbers were determined in triplicate. Cells were stained by incubation with 160 μM Hoechst 33258. To measure cell area, adult mouse ventricular cells were isolated by retrograde perfusion with collagenase (14).
In Vitro Kinase Assay and Western Blots. The activity of ASK1 was measured by an immune complex kinase assay as described (15). Immunoprecipitation of endogenous ASK1 was performed on 1,000 μg of myocardial extracts with anti-ASK1 antibody (Santa Cruz Biotechnology). Then, immune complex kinase activity was measured by using MKK6 as a substrate. Total protein homogenates (50 μg per lane) were subjected to Western blot analysis using the antibodies against mouse p38 (N-20) and JNK1 (C-17) from Santa Cruz Biotechnology, phospho-p38 and phospho-JNK from Cell Signaling Technology, and anti-ASK1 antibody (12). Western blots were developed with the enhanced chemiluminescence (ECL) or ECL-Advance kit (Amersham Pharmacia).
Statistical Analysis. Results are shown as mean ± SEM. Paired data were evaluated by Student's t test. A one-way ANOVA with the Bonferroni's post hoc test or repeated-measures ANOVA was used for multiple comparisons. A value of P < 0.05 was considered statistically significant.
Results and Discussion
Characterization of ASK Knockout Mice. ASK knockout mice (ASK-/-) were born at the expected Mendelian frequency and were indistinguishable in appearance from age-matched control WT (12). There were no significant differences in the weight of body, LV, right ventricle, and atrium between ASK-/- and WT (Table 1). The ASK-/- hearts showed no evidence of any cardiac morphologic defects, nor did histological examination of the hearts demonstrate any myofibrillar disarray, necrosis, or ventricular fibrosis (data not shown). Our results thus indicated that there is no cardiomyocyte cell-autonomous requirement for the ASK1 signaling pathway during normal embryonic development. Furthermore, the ASK1 pathway also does not appear to be required for normal heart growth in the postnatal period.
Table 1. Physiological parameters and analysis of in vivo cardiac size and function by cardiac catheterization and echocardiography at basal level.
| ASK–/– | WT | |
|---|---|---|
| n = 5 | n = 5 | |
| Body weight, g | 23.0 ± 0.8 | 23.6 ± 0.2 |
| Heart weight, mg | 106.8 ± 3.8 | 106.0 ± 2.2 |
| LV weight, mg | 75.5 ± 2.0 | 74.1 ± 1.6 |
| RV weight, mg | 20.4 ± 2.0 | 18.4 ± 0.7 |
| Atrium weight, mg | 8.7 ± 1.3 | 10.1 ± 0.4 |
| Tibia length, mm | 16.8 ± 0.1 | 17.2 ± 0.2 |
| LV/BW, mg/g | 3.29 ± 0.07 | 3.14 ± 0.06 |
| LV/tibia length, mg/mm | 4.48 ± 0.11 | 4.30 ± 0.11 |
| LVSP, mmHg | 86.0 ± 2.0 | 83.2 ± 3.0 |
| LVEDP, mmHg | 0.9 ± 0.9 | 0.5 ± 0.3 |
| dp/dt max, mmHg/s | 6,600 ± 270 | 6,320 ± 490 |
| dp/dt min, mmHg/s | –5,300 ± 440 | –4,900 ± 360 |
| Heart rate, beats per min | 413 ± 28 | 393 ± 28 |
| n = 10 | n = 10 | |
| LVIDd, mm | 3.84 ± 0.08 | 3.92 ± 0.06 |
| LVIDs, mm | 2.33 ± 0.06 | 2.37 ± 0.06 |
| FS, % | 39.3 ± 0.8 | 39.5 ± 0.7 |
| IVSd, mm | 0.68 ± 0.03 | 0.70 ± 0.02 |
| LVPWd, mm | 0.62 ± 0.04 | 0.65 ± 0.03 |
| Heart rate, beats per min | 528 ± 14 | 531 ± 15 |
ASK–/–, ASK1 knockout mice; BW, body weight, LVSP, LV systolic pressure, LVEDP, LV end-diastolic pressure. The dp/dt max and dp/dt min are the maximum rates of pressure development during contraction and relaxation, respectively. IVSd, diastolic intraventricular septum thickness; LVPWd, diastolic left ventricular posterior wall thickness. Data are expressed as mean ± SEM. There are no significant differences in all listed parameters between ASK–/– and WT.
To determine whether ASK1 ablation would affect cardiac function, cardiac performance was evaluated by means of echocardiography and cardiac catheterization in 10-week-old mice. Echocardiographic studies showed that there was no significant difference in end-diastolic (LVIDd) and end-systolic (LVIDs) internal dimensions of the LV, septal wall thickness, posterior wall thickness, and fractional shortening (FS) between ASK-/- and WT (Table 1 and Fig. 1). Furthermore, hemodynamic data did not indicate any differences in LV systolic and end-diastolic pressure, the maximum first derivative of LV pressure (LV dP/dtmax), the minimum first derivative of LV pressure (LV dP/dtmin), and heart rate between ASK-/- and WT (Table 1). These findings indicate that ASK-/- have normal global cardiac structure and function.
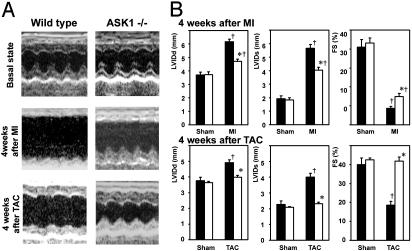
Fig. 1.
Morphological and functional consequences of MI and pressure overload in ASK-/- heart. (A) Transthoracic M-mode echocardiographic tracings from an ASK-/- (Right) and a WT (Left) before and 4 weeks after LCA ligation (Middle) and TAC (Bottom). (B) Changes in echocardiographic parameters such as end-diastolic (LVIDd) and end-systolic (LVIDs) LV diameters, and fractional shortening (FS) by LCA ligation (Upper) and TAC (Lower). Open and filled bars represent ASK-/- (n = 8 for LCA, n = 5 for TAC) and WT (n = 14 for LCA, n = 5 for TAC), respectively. *, P < 0.05 versus WT 4 weeks after surgery. †, P < 0.05 versus corresponding sham-operated controls.
Cardiac Function and Ventricular Anatomy After TAC and MI. MI was caused by ligation of LCA in ASK-/- and WT. No early operative mortality (within 24 h) was achieved in either ASK-/- or WT. During 7 days after the surgical procedure, mortality was not significantly different between ASK-/- and WT (18% for WT, 20% for ASK-/-). The mice died from LV rupture in all cases, as defined by excessive blood surrounding the heart or filling the chest cavity. No mice died between 1 and 4 weeks after surgery. The in vivo physiological consequences of the loss of ASK1 in LV remodeling after MI was assessed sequentially by echocardiography, applied before and 2 and 4 weeks after surgery (Table 2 and Fig. 1 A). The sham-operated WT and ASK-/- showed no significant changes in LVIDd and LVIDs. LVIDd and LVIDs gradually increased in WT and ASK-/- hearts, but the increases in WT were significantly higher than those in ASK-/-. The sham-operated animals showed no significant changes in FS. FS decreased 2 weeks after surgery and remains low thereafter in WT and ASK-/-. However, the decrease in FS was significantly smaller in ASK-/- than WT. Lung weight and the lung-to-body weight ratio in WT were increased significantly over those in ASK-/- 4 weeks after MI (lung weight, 204.5 ± 15.3 mg for WT, 153.3 ± 3.3 mg for ASK-/-; lung-to-body weight ratio, 7.65 ± 0.53 × 10-3 for WT, 5.63 ± 0.24 × 10-3 for ASK-/-).
Table 2. Physiological parameters and analysis of in vivo cardiac size and function by echocardiography 2 and 4 weeks after MI.
| ASK-/-, n = 14
|
WT, n = 8
|
|||||
|---|---|---|---|---|---|---|
| Treatment | Pre-MI | MI 2 wk | MI 4 wk | Pre-MI | MI 2 wk | MI 4 wk |
| Body weight, g | 24.6 ± 1.9 | 25.4 ± 1.0 | 27.4 ± 2.0* | 23.3 ± 0.8 | 24.5 ± 1.3 | 26.5 ± 2.3* |
| LVIDd, mm | 3.64 ± 0.09 | 4.33 ± 0.09* | 4.72 ± 0.16*† | 3.75 ± 0.06 | 5.72 ± 0.24* | 6.17 ± 0.3* |
| LVIDs, mm | 2.28 ± 0.06 | 3.78 ± 0.07* | 4.05 ± 0.19*† | 2.37 ± 0.15 | 5.23 ± 0.25* | 5.65 ± 0.3* |
| FS, % | 39.1 ± 1.7 | 12.8 ± 0.4* | 13.9 ± 1.6*† | 38.8 ± 1.0 | 8.8 ± 1.0* | 8.7 ± 1.0* |
| IVSd, mm | 0.68 ± 0.03 | 0.34 ± 0.04* | 0.37 ± 0.05* | 0.73 ± 0.02 | 0.33 ± 0.02* | 0.31 ± 0.01* |
| LVPWd, mm | 0.62 ± 0.03 | 0.73 ± 0.03 | 0.77 ± 0.03† | 0.65 ± 0.06 | 0.48 ± 0.06* | 0.55 ± 0.05* |
| SBP, mmHg | 107 ± 2.5 | 109 ± 3.1 | 108 ± 3.8 | 104 ± 4.8 | 104 ± 3.9 | 106 ± 4.0 |
| Heart rate, beats per min | 625 ± 20 | 615 ± 10 | 566 ± 14 | 604 ± 5 | 613 ± 4 | 576 ± 6 |
Echocaridography was sequentially performed on mice before and 2 and 4 weeks after LCA ligation. ASK-/-, ASK1 knockout mice; IVSd, diastolic intraventricular septum thickness; LVPWd, diastolic left ventricular posterior wall thickness; SBP, systolic blood pressure; wk, week. Data are expressed as mean ± SEM. *, P < 0.05 versus same genotype mice before MI. †, P < 0.05 versus WT 4 weeks after MI.
ASK-/- and WT subjected to pressure overload by TAC were studied 1 and 4 weeks after TAC. No mice died during 4 weeks after TAC. TAC leads to hyperfunctional hypertrophy after 1 week without any signs of heart failure (16). Heart weight and heart-to-body weight ratio were increased to a similar degree in the WT and ASK-/- 1 week after TAC. The change in the myocyte cross-sectional area in ASK-/- 1 week after TAC was not different from that in WT (328 ± 12 to 445 ± 15 μm2 for WT and 338 ± 13 to 443 ± 18 μm2 for ASK-/-). Furthermore, the myocytes isolated from ASK-/- had no significant difference in the mean cell area compared with that from WT 1 week after either operation (2,380 ± 60 μm2 for sham-operated WT, 2,440 ± 60 μm2 for sham-operated ASK-/-, 3,240 ± 110 μm2 for TAC-operated WT, 3,070 ± 100 μm2 for TAC-operated ASK-/-). We have previously reported that ASK1 is involved in G protein-coupled receptor agonist-induced cardiomyocyte hypertrophy in an in vitro setting (15). Therefore, endogenous ASK1 does not play a role in the regulation of cardiomyocyte hypertrophy in this experimental model or its function in the hypertrophy process is compensated by that of other hypertrophic signaling molecules.
Four weeks after TAC, LVIDd increased significantly in WT (Table 3 and Fig. 1). Cardiac contractility was significantly depressed in WT 4 weeks after TAC, as assessed by FS. ASK-/- subjected to TAC exhibited no significant difference in LVIDd and FS compared with sham-operated ASK-/- and WT. Lung weight and lung-to-body weight ratio increased significantly in WT, but not in ASK-/- 4 weeks after TAC (Table 3).
Table 3. Physiological parameters and analysis of in vivo cardiac size and function by echocardiography 1 and 4 weeks after TAC.
| ASK–/–
|
WT
|
|||||
|---|---|---|---|---|---|---|
| Treatment | Sham 4 wk, n = 4 | TAC 1 wk, n = 5 | TAC 4 wk, n = 5 | Sham 4 wk, n = 3 | TAC 1 wk, n = 5 | TAC 4 wk, n = 5 |
| Body weight, g | 30.4 ± 0.4 | 27.1 ± 0.8 | 29.9 ± 0.6‡ | 27.7 ± 0.6 | 26.2 ± 0.2 | 28.3 ± 0.9 |
| Heart weight, mg | 146 ± 3 | 193 ± 13 | 218 ± 8* | 137 ± 10 | 182 ± 9 | 253 ± 17*‡ |
| Lung weight, mg | 152 ± 4 | ND | 162 ± 4† | 143 ± 7 | ND | 317 ± 41* |
| Liver weight, mg | 1,470 ± 50 | ND | 1,310 ± 50 | 1,360 ± 90 | ND | 1,350 ± 140 |
| Heart weight/body weight | 4.8 ± 0.1 | 7.1 ± 0.5 | 7.3 ± 0.2*† | 5.0 ± 0.3 | 6.9 ± 0.3 | 8.9 ± 0.3*‡ |
| Lung weight/body weight | 5.0 ± 0.1 | ND | 5.4 ± 0.1† | 5.2 ± 0.2 | ND | 11.1 ± 1.1* |
| Liver weight/body weight | 49 ± 1 | ND | 44 ± 1 | 49 ± 2 | ND | 47 ± 3 |
| LVIDd, mm | 3.63 ± 0.10 | 3.76 ± 0.09 | 3.98 ± 0.11† | 3.76 ± 0.21 | 3.64 ± 0.08 | 4.91 ± 0.19*‡ |
| LVIDs, mm | 2.09 ± 0.04 | 2.24 ± 0.07 | 2.32 ± 0.11† | 2.23 ± 0.24 | 2.22 ± 0.09 | 4.01 ± 0.23*‡ |
| FS, % | 42.4 ± 2.1 | 40.5 ± 1.1 | 41.9 ± 2.1† | 40.0 ± 3.5 | 39.1 ± 1.5 | 18.5 ± 2.1*‡ |
| IVSd, mm | 0.78 ± 0.03 | 1.00 ± 0.07 | 1.1 ± 0.02*† | 0.73 ± 0.01 | 0.92 ± 0.04 | 0.85 ± 0.08‡ |
| LVPWd, mm | 0.78 ± 0.04 | 0.91 ± 0.03 | 0.85 ± 0.03 | 0.67 ± 0.07 | 0.84 ± 0.05 | 0.79 ± 0.02 |
| Heart rate, beats per min | 554 ± 16 | 548 ± 24 | 563 ± 26 | 541 ± 26 | 541 ± 16 | 548 ± 30 |
ASK–/–, ASK1 knockout mice; IVSd, diastolic intraventricular septum thickness; LVPWd, diastolic left ventricular posterior wall thickness; ND, not determined; wk, week. Data are expressed as mean ± SEM. *, P < 0.05 versus sham-operated same genotype mice. †, P < 0.05 versus WT 4 weeks after TAC. ‡, P < 0.05 versus same genotype mice 1 week after TAC.
These findings indicate that ASK1 dose not play an essential role in TAC-induced cardiac hypertrophy, but in pressure overload- and MI-induced LV remodeling process that leads to cardiac dysfunction.
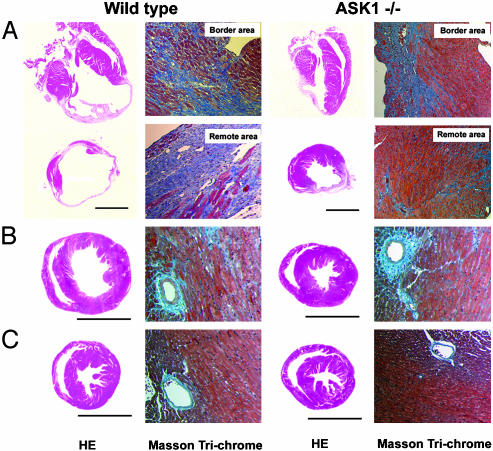
Histological Examination. Four weeks after LCA ligation, WT hearts had become obviously enlarged compared with those of ASK-/- (Fig. 2A). The cross sections show a WT heart with massive infarct that included most of free wall, apex, and some septum, whereas the ASK-/- hearts showed infarction of only the free wall. We observed no difference in the area at risk between ASK-/- (51.3 ± 5.7%) and WT (49.1 ± 4.9%), which was measured immediately after LCA ligation by Evans blue staining. This indicates that initial infarct area upon LCA occlusion was not different in both group, but subsequent LV remodeling caused the extension of the infarct and that the beneficial phenotype of ASK ablation is unlikely to be related to the development of collateral circulation. In WT, a part of the area remote from ischemic injury was replaced by fibrous tissue, whereas the remote area appeared to be intact in ASK-/- (Fig. 2 A). Only slight intermuscular and perivascular fibrosis was observed in the remote area in ASK-/- hearts.
Fig. 2.
Histological examination of hearts after MI or TAC. Macroscopic hematoxylin-eosin-stained (HE) and Masson-trichrome-stained histological section of hearts from WT (Left) and ASK1 knockout (ASK-/-) (Right) mice 4 weeks after LCA ligation (A), TAC (B), or sham-operation (C). (Bar represents 5 mm.)
Histological examination of the heart 4 weeks after TAC demonstrated LV enlargement in WT, whereas chamber dilatation was prevented in ASK-/- (Fig. 2 B and C). Intermuscular fibrosis, as well as perivascular fibrosis, was observed in a scattered manner in WT and ASK-/- hearts. The extent of fibrosis was similar in WT and ASK-/- hearts (8.9 ± 0.6% for ASK-/-, 8.3 ± 0.6% for WT).
In both experimental models, morphological and histological studies confirmed the results obtained by means of echocardiography. ASK1 ablation reduced replacement fibrosis in MI hearts but not in TAC hearts, indicating the effect of ASK1 on the development of fibrosis appears to be indirect.
Mechanical Stress. The main stimulus for myocardial remodeling is mechanical overload. We examined whether applied mechanical stress was similar in WT and ASK-/- hearts upon LCA ligation or TAC. In the MI model, mechanical overload imposed on the surviving myocardium is estimated as the product of LV systolic pressure and heart rate. Neither LV systolic pressure nor heart rate was significantly different between ASK-/- and WT 1 week after LCA ligation (systolic pressure, 110.2 ± 6.0 mmHg for ASK-/-, 108.2 ± 2.8 mmHg for WT; heart rate, 582 ± 10 per min for ASK-/-, 559 ± 24 per min for WT; 1 mmHg = 133 Pa). Wall stress, calculated according to Laplace's law, in WT was larger than that in ASK-/- at 1 week after LCA ligation, because WT hearts then demonstrated LV enlargement. Taking into consideration that we observed no differences in basal LV size, configuration, and area at risk between ASK-/- and WT, we can assume that wall stress in ASK-/- will be similar with that in WT before LV enlargement.
The mechanical stress produced during TAC was estimated by measuring in vivo transstenotic pressure gradients 7 days after TAC. Although the TAC procedure increased pressure gradients significantly between the two carotid arteries, there were no significant differences in pressure gradients between ASK-/- and WT (55.5 ± 5.7 mmHg for ASK-/-, 57.3 ± 4.6 mmHg for WT). Furthermore, wall stress was not significantly different between ASK-/- and WT (8.75 ± 0.25 mmHg for ASK-/-, 9.00 ± 0.65 mmHg for WT).
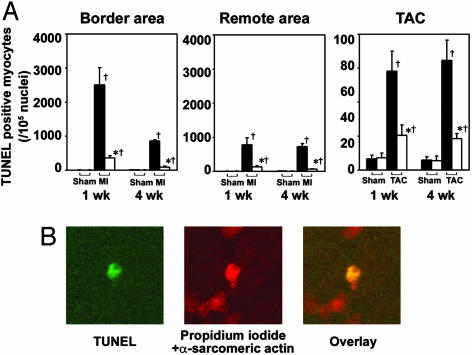
Apoptosis in LV Remodeling. It has been reported that LV remodeling is associated with increased apoptosis in the myocardium. We examined the effect of ASK1 ablation on apoptosis induced by LCA ligation or TAC by using the TUNEL assay (Fig. 3). In the MI model, there was a significant increase in the number of apoptotic cells in the border area, as well as in the myocardium remote from the area of ischemic injury. The number of TUNEL-positive cells was significantly higher in WT hearts than in ASK-/- hearts 1 and 4 weeks after LCA ligation (Fig. 3A). TUNEL-positive cells showed condensed chromatin, characteristic of apoptosis. They were identified as cardiac myocytes by anti-α-sarcomeric actin staining (Fig. 3B). In the TAC model, TUNEL-positive cells were observed to be ubiquitous in the LV wall. The number of TUNEL-positive cells was significantly higher in WT hearts than that in ASK-/- hearts 1 and 4 weeks after TAC (Fig. 3A). TUNEL-positive cells were identified as cardiomyocytes by anti-α-sarcomeric actin staining in the TAC model (data not shown). These results strongly indicate that stress-induced apoptosis through the ASK1 signaling pathway is essential for LV remodeling. The number of apoptotic cells observed in the MI model was much higher than that in the TAC model. This might explain the fact that we detected no difference in fibrosis between TAC-operated WT and ASK-/-, but a marked reduction in ASK-/- in the MI model.
Fig. 3.
Apoptosis in ASK1-null mouse heart after LCA ligation or TAC. (A) Relative number of TUNEL-positive cells in ASK-/- hearts compared with that in WT in border area and remote area 1 or 4 weeks after LCA ligation (Left and Center) or in myocardium 1 or 4 weeks after TAC (Right). Open and filled bars represent ASK-/- (n = 6) and WT (n = 6), respectively. *, P < 0.05 versus corresponding WT. †, P < 0.05 versus corresponding sham-operated controls. (B) Confocal analysis of ASK-/- ventricular myocardium 1 week after LCA ligation. Triple staining (propidium iodide, TUNEL, and anti-α-sarcomeric actin antibody) was performed. Staining for propidium iodide and anti-α-sarcomeric actin antibody is shown in red, and that for TUNEL is shown in green. In the overlay image, a nucleus stained by both TUNEL and propidium iodide is shown in yellow.
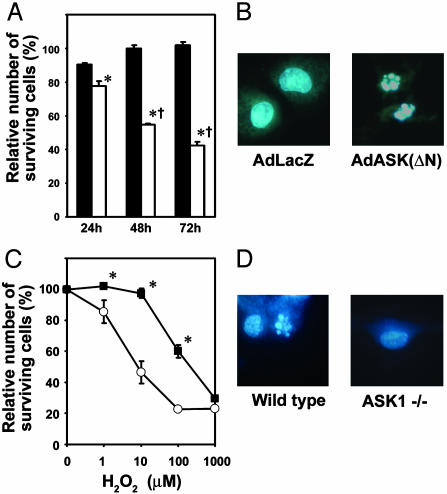
Induction of Apoptosis by ASK1 Activation. Previous studies demonstrated that overexpression of WT or constitutively active ASK1 induced apoptosis in noncardiomyocytes (11). We previously reported that infection of rat neonatal cardiomyocytes with adenovirus expressing a constitutively active mutant of ASK1 (AdASK-ΔN) at a multiplicity of infection of 10 plaque forming units per cell resulted in cardiomyocyte hypertrophy without any signs of apoptosis (15). We examined whether higher expression of activated ASK1 is able to induce apoptosis in cardiomyocytes. We infected isolated rat neonatal cardiomyocytes with AdASK-ΔN or LacZ at a multiplicity of infection of 100 plaque forming units per cell. Infection of AdASK-ΔN led to a decrease in viable cell number (Fig. 4A). Hoechst dye 33258-stained nuclei demonstrated a varying degree of condensed nuclear chromatin and fragmented nuclei (Fig. 4B). Next, we assessed the requirement of ASK1 for H2O2-induced apoptosis by using neonatal cardiomyocytes derived from ASK-/- (Fig. 4 C and D). Apoptotic cell death was clearly induced by H2O2, as determined by nuclear morphology (Fig. 4D). ASK-/- cardiomyocytes were more resistant to H2O2 as determined by an MTT assay as compared with WT cells (Fig. 4C). These results clearly indicate a role of ASK1 in cardiomyocyte apoptosis. Thus, the phenotype resulting from ASK1 activation may depend on the quantitative nature of the stimulus. We can hypothesize that excessive activation of ASK1 caused by pathological stress such as ischemia and pressure overload play a pivotal role in cardiomyocyte apoptosis and resultant cardiac remodeling.
Fig. 4.
Involvement of ASK1 in apoptosis in cardiomyocytes. (A and C) Cell viability was assessed by using a Cell Counting kit. Data show means ± SEM of three independent experiments from different cell preparations. (A) Viability of cells is expressed as the percentage of viability of cells without adenoviral infection. Rat neonatal cardiomyocytes were infected with AdASK-ΔN (open bars) or AdLacZ (filled bars) at a multiplicity of infection of 100 plaque forming units per cell. *, P < 0.05 versus corresponding AdLacZ-infected cells. †, P < 0.05 versus AdASK(ΔN)-infected cells at 24 h. (C) Viability of cells is expressed as the percentage of viability of cells in the absence of H2O2, when mouse neonatal cardiomyocytes isolated from ASK-/- (filled squares) or WT (open circles) were incubated with various concentrations of H2O2 for 24 h. *, P < 0.05 versus corresponding WT. (B and D) Hoechst staining. (B) Forty-eight hours after infection with the adenovirus, rat neonatal cardiomyocytes were stained with Hoechst dye 33258. (D) ASK-/- or WT neonatal cardiomyocytes treated with 10 μM H2O2 for 24 h were stained with the dye.
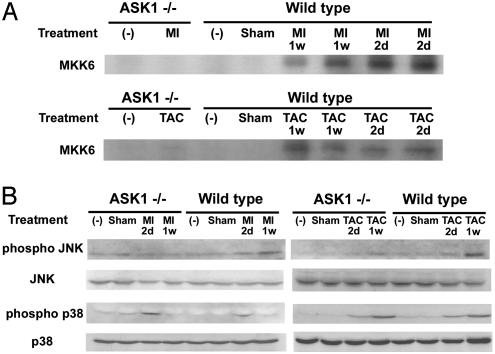
ASK1 Activation After TAC and MI. We examined whether LV remodeling was accompanied by the activation of ASK1. We measured ASK1 activity in hearts after LCA ligation or after TAC. Two days after MI, a significant 17.1 ± 4.4-fold increase in ASK1 activity was detected in WT hearts. Activation of ASK1 remained increased above the sham level (10.4 ± 4.5-fold) 7 days after MI. In response to pressure overload, WT hearts had a significant increase in total ASK1 activity compared with sham-operated hearts at 2 days (18.0 ± 2.7-fold increase) and 1 week (15.2 ± 3.8-fold increase) after TAC (Fig. 5A). No change in the protein level of ASK1 was observed after MI or TAC (data not shown). On the other hand, no activation of ASK1 was observed in ASK-/- after TAC or LCA ligation. A previous study has shown that TNF- and H2O2-induced activation of JNK and p38 are lost in ASK-/- embryonic fibroblasts (12). There were no differences in the basal protein levels of JNK and p38 in ASK-/- and WT hearts, nor in the basal level of phosphorylated JNK and p38 (Fig. 5B). To examine the activation status of JNK and p38 in ASK-/- hearts, the protein level of phosphorylated JNK and p38 was measured 2 days and 1 week after LCA ligation or TAC (Fig. 5B). The JNK phosphorylation level was significantly increased in WT hearts 2 days and 1 week after LCA ligation or TAC. The increases in phosphorylation level of JNK were significantly attenuated in ASK-/- hearts after LCA ligation or TAC. On the other hand, p38 phosphorylation was increased to a similar degree in the WT and ASK-/- after LCA ligation or TAC. This indicates that ASK1-JNK signaling pathway plays a pivotal role in the stress-induced apoptosis, leading to cardiac remodeling.
Fig. 5.
ASK1, JNK, and p38 activation after LCA ligation and TAC. (A) ASK1 activity was measured by immune complex assay, using His-MKK6 as a substrate. Upper and Lower represent ASK1 activation 2 days and 1 week after LCA ligation and TAC in ASK-/- or WT, respectively. (B) The activity of JNK and p38 was assessed by immunoblotting with anti-phospho-specific antibody after LCA ligation (Left) or TAC (Right). The filters were reprobed with anti-non-phopho-specific antibody.
Accumulating evidence suggests that apoptosis may be an important mode of cell death during heart failure in both human and animal models (17). However, the extent to which apoptosis plays a critical role in the pathogenesis of remodeling has remained an open question. Our study demonstrated that ASK1-mediated apoptosis leads to the development and progression of cardiac dysfunction and dilatation after MI or in response to pressure overload.
Molecules responsible for triggering apoptosis after MI or TAC remain to be elucidated. Several factors that may be important for the remodeling have been shown to stimulate myocyte apoptosis in vitro, including increased mechanical strain, neurohormones (angiotensin II, norepinephrine), reactive oxygen species, and tumor necrosis factor (18). Reactive oxygen species, tumor necrosis factor (12), and angiotensin II (15) are capable of activating ASK1. It has been reported that LV remodeling is associated with a progressive increase in the frequency of apoptosis in the myocardium remote from the infarct (7), as well as in the region adjacent to the infarct myocardium (6, 19, 20). Although composition of stimuli to trigger apoptosis in the region adjacent to the infarct myocardium and remote from the infarct should be different, our result indicates that ASK1 is involved in apoptosis in both regions.
Our results indicate that the downstream target of ASK1 appears to be JNK signaling pathway. ASK1-JNK signaling pathway has been reported to be a crucial element of neuronal apoptosis (21). Furthermore, the role of JNK pathway in proapoptotic signaling has been demonstrated by knockout studies (22). Hatai et al. (23) have reported that overexpression of ASK-ΔN induced cytochrome c release from mitochondria and activation of caspase-9 and caspase-3 but not of caspase-8 in nonmuscle cells. This finding indicates that mitochondria-initiated activation of caspases might be a main mechanism operating in the execution of ASK1-inuced apoptosis in cardiomyocytes. The molecular target for activated JNK to induce apoptosis remains to be elucidated. Recently, Bcl-2 is phosphorylated in response to microtubule-interfering agents via ASK1-JNK signaling pathway (24). Thus, ASK1 might lead to apoptosis mediated through Bcl-2 family members. Further investigations, however, will be necessary to elucidate the issue of cell-type specificity in ASK1 signaling.
In conclusion, this study provides clear evidence that ASK1 is an essential component of a stress-medicated myocyte death pathway. Because patients with major remodeling demonstrate progressive worsening of cardiac function, preventing, slowing or reversing remodeling will be a goal of heart failure therapy. The inhibition of ASK1 will be a valid target for the development of novel therapeutic agents for cardiac remodeling to suppress the onset of heart failure.
Acknowledgments
We are grateful to Ms. Ritsuko Okamoto and Atsuko Nakai for their expert technical assistance. This work was supported by Grant-in-Aid 13470145 for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to K.O.).
Abbreviations: ASK1, apoptosis signal-regulating kinase 1; MI, myocardial infarction; LCA, left coronary artery; TAC, thoracic transverse aortic constriction; LV, left ventricle; JNK, c-Jun N-terminal kinase; TUNEL, terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling; LVIDd, left ventricular end-diastolic internal dimension; LVIDs, left ventricular end-systolic internal dimension; FS, fractional shortening.
References
- 1.Solomon, S. D. & Pfeffer, M. A. (1997) Basic Res. Cardiol. 92, 61-65. [DOI] [PubMed] [Google Scholar]
- 2.Patten, R. D., Udelson, J. E. & Konstam, M. A. (1998) Curr. Opin. Cardiol. 13, 162-167. [PubMed] [Google Scholar]
- 3.Mani, K. & Kitsis, R. N. (2003) J. Am. Coll. Cardiol. 41, 761-764. [DOI] [PubMed] [Google Scholar]
- 4.Florea, V. G., Mareyev, V. Y., Samko, A. N., Orlova, I. A., Coats, A. J. & Belenkov, Y. N. (1999) Int. J. Cardiol. 68, 281-287. [DOI] [PubMed] [Google Scholar]
- 5.Olivetti, G., Quaini, F., Sala, R., Lagrasta, C., Corradi, D., Bonacina, E., Gambert, S. R., Cigola, E. & Anversa, P. (1996) J. Mol. Cell. Cardiol. 28, 2005-2016. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, W., Kajstura, J., Nitahara, J. A., Li, B., Reiss, K., Liu, Y., Clark, W. A., Krajewski, S., Reed, J. C., Olivetti, G., et al. (1996) Exp. Cell Res. 226, 316-327. [DOI] [PubMed] [Google Scholar]
- 7.Sam, F., Sawyer, D. B., Chang, D. L.-F., Eberli, F. R., Ngoy, S., Jain, M., Amin, J., Apstein, C. S. & Colucci, W. S. (2000) Am. J. Physiol. 279, H422-H428. [DOI] [PubMed] [Google Scholar]
- 8.Teiger, E., Dam, T.-V., Richard, L., Wisnewsky, C., Tea, B.-S., Gaboury, L., Tremblay, J., Schwartz, K. & Hamet, P. (1996) J. Clin. Invest. 97, 2891-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirota, H., Chen, J., Betz, U. A., Rajewsky, K., Gu, Y., Ross, J., Jr., Muller, W. & Chien, K. R. (1999) Cell 97, 189-198. [DOI] [PubMed] [Google Scholar]
- 10.Ichijo, H., Nishida, E., Irie, K., Dijike, P. T., Saitoh, M., Moriguchi, T., Takagi, M., Matsumoto, K., Miyazono, K. & Gotoh, Y. (1997) Science 275, 90-94. [DOI] [PubMed] [Google Scholar]
- 11.Saitoh, M., Nishitoh, H., Fujii, M., Takeda, K., Tobiume, K., Sawada, Y., Kawabata, M., Miyazono, K. & Ichijo, H. (1998) EMBO J. 17, 2596-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobiume, K., Matsuzawa, A., Takahashi, T., Nishitoh, H., Morita, K.-I., Takeda, K., Minowa, O., Miyazono, K., Noda, T. & Ichijo, H. (2001) EMBO Rep. 2, 222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Date, M., Morita, T., Yamashita, N., Nishida, K., Yamaguchi, O., Higuchi, Y., Hirotani, S., Matsumura, Y., Hori, M., Tada, M., et al. (2002) J. Am. Coll. Cardiol. 39, 907-912. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama, H., Otsu, K., Yamaguchi, O., Nishida, K., Date, M., Hongo, K., Kusakari, Y., Toyofuku, T., Hikoso, S., Kashiwase, K., et al. (2002) FASEB J., 02–0474fje. [DOI] [PubMed]
- 15.Hirotani, S., Otsu, K., Nishida, K., Higuchi, Y., Morita, T., Nakayama, H., Yamaguchi, O., Mano, T., Matsumura, Y., Ueno, H., et al. (2002) Circulation 105, 509-515. [DOI] [PubMed] [Google Scholar]
- 16.Rockman, H., Ross, R., Harris, A., Knowlton, K., Steinhelper, M., Field, L., Ross, J., Jr., & Chien, K. (1991) Proc. Natl. Acad. Sci. USA 88, 8277-8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haunstetter, A. & Izumo, S. (1998) Circ. Res. 82, 1111-1129. [DOI] [PubMed] [Google Scholar]
- 18.Willenheimer, R. (2000) Int. J. Cardiol. 72, 143-150. [DOI] [PubMed] [Google Scholar]
- 19.Bialik, S., Geenen, D. L., Sasson, I. E., Cheng, R., Horner, J. W., Evans, S. M., Lord, E. M., Koch, C. J. & Kitsis, R. N. (1997) J. Clin. Invest. 100, 1363-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, Q., Li, B., Wang, X., Leri, A., Jana, K. P., Liu, Y., Kajstura, J., Baserga, R. & Anversa, P. (1997) J. Clin. Invest. 100, 1991-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanamoto, T., Mota, M., Takeda, K., Rubin, L. L., Miyazono, K., Ichijo, H. & Bazenet, C. E. (2000) Mol. Cell. Biol. 20, 196-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuan, C., Yang, D., Roy, D., Davis, R., Rakic, P. & Flavell, R. (1999) Neuron 22, 667-676. [DOI] [PubMed] [Google Scholar]
- 23.Hatai, T., Matsuzawa, A., Inoshita, S., Mochida, Y., Kuroda, T., Sakamaki, K., Kuida, K., Yonehara, S., Ichijo, H. & Takeda, K. (2000) J. Biol. Chem. 275, 26576-26581. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto, K., Ichijo, H. & Korsmeyer, S. J. (1999) Mol. Cell. Biol. 19, 8469-8478. [DOI] [PMC free article] [PubMed] [Google Scholar]