Abstract
Animal imaging of brain systems offers exciting opportunities to better understand the neurobiology of pain and analgesia. Overall functional studies have lagged behind human studies as a result of technical issues including the use of anesthesia. Now that many of these issues have been overcome including the possibility of imaging awake animals, there are new opportunities to study whole brain systems neurobiology of acute and chronic pain as well as analgesic effects on brain systems de novo (using pharmacological MRI) or testing in animal models of pain. Understanding brain networks in these areas may provide new insights into translational science, and use neural networks as a “language of translation” between preclinical to clinical models. In this review we evaluate the role of functional and anatomical imaging in furthering our understanding in pain and analgesia.
1: Introduction
Small animal model imaging has an increasingly important role in biomedical research (Balaban and Hampshire, 2001). While studies using fMRI in animal imaging of pain and analgesia have lagged behind human imaging (Lowe et al., 2007), ever since some of the articles indicating the use of the approach (see (Tuor et al., 2000; Liu et al., 2007)) there has been a significant increases in the number of scientific reports in recent years. For example in the pain field, after the initial report of fMRI measures of pain in the spinal cord (Porszasz et al., 1997), there has been slow but steady increase in the number of reports on animal functional imaging in the literature (see Figure 1). The reason for this discrepancy relates to the technical difficulties of imaging pain and analgesia in animals that includes amongst other things, anesthesia. Developments for measures of pain, pain models, and brain states (resting states) have now become more widely used. As such these open tremendous opportunities for neurobiology of pain, translational medicine and the understanding of how drugs work on brain systems.
Figure 1. Research Reports on Preclinical Functional Imaging of Pain.

Numbers of papers published in the field of “functional magnetic resonance imaging and pain and rats” from before 2000 and through each year to 2009. Search Source PubMed (www.ncbi.nlm.nih.gov/pubmed/).
Great strides in understanding neural systems in animal models have been made possible with techniques such as electrophysiology, lesion studies, tract tracing, or measures of gene expression in neurons or glia. However, in these approaches whole brain systems studies are difficult to perform although some seem to be more useful such as functional multi-neuron calcium imaging (Ikegaya, 2008). While complementary to other techniques, fMRI allows the evaluation of disease state and drug effects in animal models in new and exciting ways. Such studies may be integrated into current techniques in preclinical studies. Perhaps more important is to use information gained from neural circuits as a “language of translation” for preclinical to clinical models. As such these imaging may provide more predictive data of therapeutic agents acting on the CNS because of a better understanding of brain function. In this review we evaluate the role of fMRI in furthering our understanding in pain and analgesia.
Benefits of Preclinical Imaging for Pain and Analgesia
The application of functional neuroimaging (fMRI) has opened doors to evaluating brain systems neuroscience in anesthetized or awake animals (Benveniste and Blackband, 2002; Benveniste and Blackband, 2006; Driehuys et al., 2008). While difficult and expensive there are a number of benefits that imaging neural systems can contribute to the field. These include: (i) Objective measures of CNS animal model disease state or drug effect; (ii) A translational method that can be used to define neural systems across species (see (Borsook et al., 2006)); (iii) insights into similarities and differences in animal models that may lead to the identification of a more predictive way to use conventional preclinical pain models; (iv) Differentiate CNS mechanisms across different pain disease states; (v) Evaluation of chronic/temporal changes in pain models; and (vi) Coupling imaging with behavioral pharmacology provides insights into dosing; (vii) Potential evaluation of CNS penetration and target engagement of novel drugs prior to development of a PET ligand.
2. The Methods
2.1. Imaging/Scanning Methods
Imaging methods as well as their advantages and disadvantages are described and summarized in Tables 1 and 2 and Figure 2. In this review, we will emphasize functional MRI techniques.
Table 1.
Imaging Technologies used in Preclinical Brain Studies
| Imaging Technology | Imaging Measures | Biological Measures | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Functional MRI | BOLD, CBV, CBF | Brain Activation following stimulation Brain networks in resting state | Non-invasive, repeatable, spatial, temporal resolution | Indirect brain activation measure. | (Borsook et al., 2006) |
| Pharmacological MRI (phMRI) | BOLD, CBV, CBF | De-novo effects in brain induced by drug | Non-invasive, repeatable, spatial, temporal resolution | Indirect brain activation measure. | (Wise and Tracey, 2006; Pohlmann et al., 2007; Martin and Sibson, 2008) |
| Structural MRI | Volumetric, fiber integrity, fiber connectivity | Atrophy, fiber degeneration | Non-invasive, repeatable, high spatial resolution | Time consuming, may require very high magnetic field scanners | (Strome and Doudet, 2007) |
| Near Infrared Spectroscopy (NIRS) | Hemoglobin changes | Brain activation following stimulation | Non-invasive, repeatable, spatial, temporal resolution | Indirect measure, could be invasive | (Crespi, 2007) |
| Magnetic Resonance Spectroscopy (MRS) | Metabolic concentrations | Alterations in brain chemistry | Can perform absolute measures, repeatable | Low spatial, temporal resolution, might be limited in certain brain structures | (Schaeffter and Dahnke, 2008) |
| Positron Emission Tomography (PET) | Radioligand concentration, displacement, blood volume changes | Brain activation following or not stimulation, | Absolute changes in blood volume, receptor displacement | Requires development of specific ligand for receptor studies | (Wang and Maurer, 2005; Cherry, 2006) |
| PET/MR | Combination of fMRI, phMRI, MRS, Structural MRI and PET | As described above | Simultaneous detection of PET and MRI measures in the same subject | Under development | (Wehrl et al., 2009) |
| Transcription MRI | Gene Activity in living brains using tMRI probes | Measures of endogenous gene transcription | Tracking, targeting and binding to intracellular mRNA | Use of contrast agents (e.g., SPION) as coupling agents may alter neuronal effects | (Liu et al., 2008) |
Table 2.
Analgesic Effects of Anesthetic Agents used in Preclinical Imaging Studies
| Stimulus | Mechanism of Action | Analgesic Effects | References |
|---|---|---|---|
| Isoflurane | Uncharacterized | None in sub-anesthetic levels in human models of pain | (Petersen-Felix et al., 1995) |
| Enhances Windup in Spinal Cord in Rats | (Ng and Antognini, 2006) | ||
| Etiomodate | Agonist at GABAA receptors | Depresses Lumbar dorsal horn neuronal activity to pain | (Mitsuyo et al., 2006) |
| Medetomidine | Alpha(2)-adrenergic agonist | Produces sedation and possible supraspinal mediation of analgesia | (Buerkle and Yaksh, 1998) |
| Alpha-chloralose | Unknown | Predominantly a sedative hypnotic with poor analgesic effects | (Strobel and Wollman, 1969) |
| Has been shown to suppresses dorsal horn neurons | (Collins et al., 1983) | ||
Figure 2. fMRI Measures of Acute Pain in the Rat Brain.
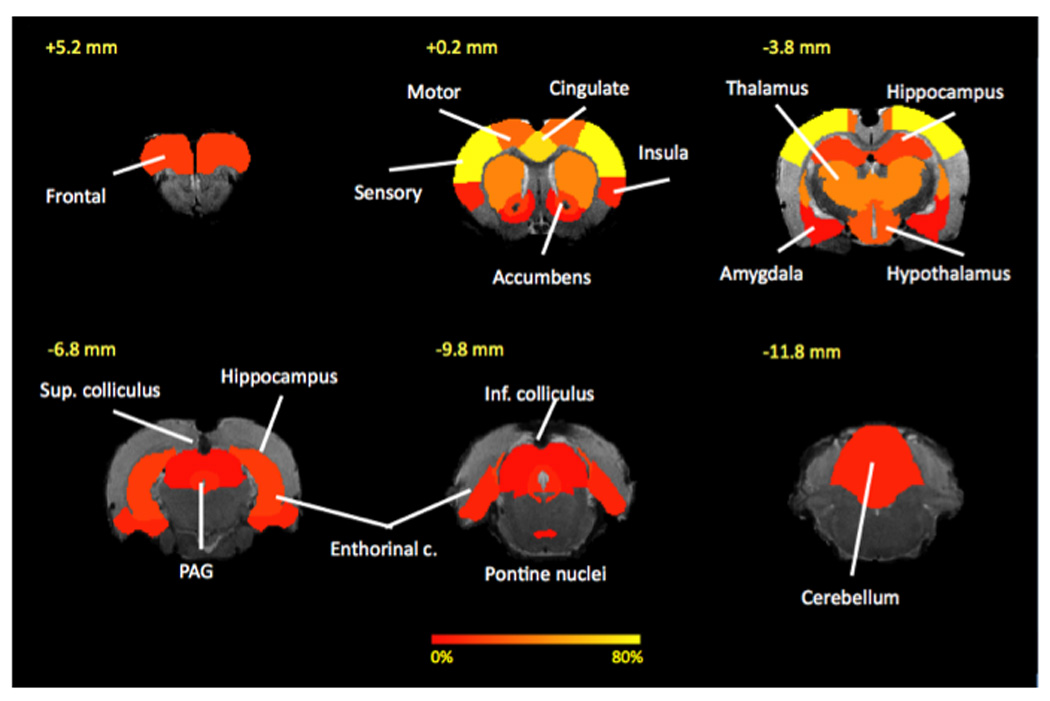
Common activated structures across evoked painful stimuli studies: Pseudo-colors represent percentage of 15 studies that a particular brain area has been to activate in response to an evoked painful stimulus. Cortical structures (sensory, motor, cingulate) tend to be most commonly activated that subcortical structures. See Table 4 for more details.
For fMRI, there are significant challenges in imaging functional activity in animals including, the use of anesthesia which may be a confound to functional studies because of alteration of brain metabolism and function (see below), the need to adopt special stimulation systems within the magnet, the intense noise levels produced by the scanner, and in general the lack of ability to directly observe the animal (since they are usually placed deep within the magnet core, unless appropriate equipment is in place).
For structural imaging, these constraints are less of an issue since anesthesia is not a confounding process on signal or neural processing.
Manganese (Mn(2+)) enhanced magnetic resonance imaging (MEMRI) has been used to define brain activity, trace neural connections and improve visualization of brain structure. It is administered systemically ((Silva et al., 2004; Watanabe et al., 2004)). The basic premise consists of acquiring an image after injection of manganese prior to the tests/experiments of interest that are carried out. The rodent is removed from the scanner and subject to the behavioral test and returned to the scanner to acquire a second image where Mn has been taken up by neurons and other cells. The difference of the pre- and post- behavioral images reflects brain areas that have been active. The use of enhancers such as manganese has been mitigated to some extent by toxicity and the difficulty of performing them (Silva and Bock, 2008). In addition to toxicity (that may include peripheral effects such as erythema), the use of enhancers such as magnesium chloride include the fact that is not a dynamic process (only the total uptake effects can be measured) , that there may be inhibition of neural systems via actions on receptor systems (e.g., NMDA), and that there may be metabolic changes such as weight loss or decreased motor performance ((Jackson et al., 2011)). Some of these side effects may be overcome by the use of slow release systems such as osmotic pumps ((Eschenko et al., 2010)).
Several researchers in other fields actively use paramagnetic nanoparticles to enhance fMRI signals (Boas et al., 2004; Liu et al., 2007;Mandeville et al., 2007) but these studies have been carried out under anesthesia.
Near infrared spectroscopy (NIRS) is another form of functional imaging based on blood flow (Boas et al., 2004; Ferrari et al., 2004), and currently it is limited to measures of cortical function. It is not well developed in terms of measures of deep structures and does not have a robust approach for localization of brain structures. NIRS offers some advantages over fMRI including temporal resolution and animals can be directly observed during the measures. It has been used to measure changes in brain function in rat models of cortical spreading depression (Wolf et al., 1997), sensory processing/mapping of SI in non-human primates (Shoham and Grinvald, 2001). Reports of correlation of NIRS with fMRI suggest a high correlation of the two methods in rats (Chen et al., 2003). The future of NIRS in pain and analgesia imaging may be in non-human primates and in rodent models once anatomical correlation methods and enhanced resolution have been defined.
Functional and ligand binding data may also be obtained using positron emission tomography (PET) in rodents (Jiang et al., 2009; Lancelot and Zimmer, 2010) and non-human primates (Fox et al., 2010). For PET ligand binding studies, anesthesia may interfere, but is not considered a major issue, although other concerns are well noted in the field (Ametamey and Honer, 2007). For PET functional studies (blood flow) the same considerations are similar to those noted for fMRI with respect to anesthesia. The use of microPET systems is now quite common and may be a useful technique going forward particularly with understanding neurotransmitter systems (Leriche et al., 2009).
Although the focus of this review is on functional MRI other approaches such as autoradiographic methods have been useful in defining brain function. Such methods have some advantages including the ability to evaluate behaviors in freely moving animals ((Holschneider and Maarek, 2004, 2008)). As noted in the latter reference, these approaches can now be used for to the functional brain mapping of behaviors providing three-dimensional reconstruction of the brain from autoradiographic sections and voxel-based analysis of the whole brain, and generation of maps of the flattened cortical regions. The disadvantage of autoradiographic methods, is that animals can only be studied once.
2.2. Technical Issues
MRI Imaging
The advantage of performing experiments at high and ultra-high magnetic fields include the ability to achieve resolutions of a few 10ths of microns (Silva and Koretsky, 2002; Schepkin et al., 2010; Yu et al., 2010), potentially allowing to investigate brain activation at cortical laminar level (Silva and Koretsky, 2002; Jin and Kim, 2008). However, issues related to susceptibility artifacts also increase with field strength rendering certain brain structures practically “unreadable”. Hence, the use of ultra-high magnetic fields for cortical structures is being used to advance significant the field. If whole brain or subcortical structures are of interest, lower fields might be more appropriate.
Animal Preparation
For pain imaging either anesthetized or the awake animal preparations have been used. In the case of anesthetized preparations, the most important issue relates to physiological stability (ETCO2, pH, HR and pO2 levels, and body temperature (see (Hildebrandt et al., 2008)) and the choice of anesthetic agent (since different agents may have different effects on BOLD signals (Masamoto et al., 2009; Tsurugizawa et al., 2010). Such changes may contribute to observed frequency of activations – for example, only 50% of SI activation was observed in a BOLD imaging experiment using forepaw stimulation in mice (Adamczak et al., 2010). In addition, stimulation parameters or anesthetic levels may either need to be adjusted for defining activation in anesthetized animals (Masamoto et al., 2007). For imaging awake animals, proper acclimation to the restraining procedures, stimulation type, and the MRI environment are all critical to achieve good results. The use of ultra-high fields will also bring the problem of noise levels exceeding the 120 dB creating a harmful and perturbing condition. There are developments to fabricate noise-cancelling enclosures to reduce noise in the scanner (Mechefske et al., 2002). Development of devices for conscious animal imaging have been reported (Khubchandani et al., 2003). Some of thee require parts of the device to be implanted in the skull, others require other considerations as noted below. The holder for the animal is an important component of the experiment, it has to hold the rodent snuggly without inducing pain or limit blood flow to the extremities. A mold fabricated out of a rodent of similar size and weight carved out to include stimulation devices seems to be a useful approach (Becerra et al., 2009). Equally, the electronics for RF and head restrain should be built in a manner that does not interfere with the experiment by adding additional pain or stress. Hence, the emphasis on acclimation cannot be underestimated.
Motion Artifact
A problem introduced by performing awake animals compared to anesthetized ones is that rodents are capable of flexing their muscles (providing a muscle paralyzer, as done in anesthetized animal experiments, is unethical) and, although they are restrained, it is not possible to completely avoid some motion of the head or the body. Motion of the head does not need to be significant to raise a potential issue with the images; voxel sizes are of the order of 500 or less microns, and movement of that order could be considered excessive for fMRI data. Acclimation of animals to the restraining device significantly reduces motion of the head ((Becerra et al., 2010)). Motion of the body could be driving motion of the head. Furthermore, motion of the body could be adding a confounding signal to the activation maps as body motion might be time locked with stimulus delivery. A potential solution to account for body motion-induced activation, is to track muscle flexion and utilize that information to account for motion in brain activation. Physiologically induced motion from respiration and cardiac processes can also introduce motion of the head. Standard equipment to measure heart and respiratory rate can be used to not only correlate them with brain activation but also to detect potentially induced changes induced by evoked stimuli.
Ancillary Equipment
In addition to the constrains of using non-ferromagnetic equipment for monitoring that can be electrically grounded or low-pass filtered to avoid spurious RF to be introduced and contaminate the images, the equipment should be small enough to be mounted on the holder to perform stimulation of the extremities or another body part. There are not many manufacturers of such equipment for thermal stimulation. For mechanical, automated von Frey filament devices that can be used in the scanner (Governo et al., 2007). Basic physiology (e.g. heart rate, respiratory rate) can be easily monitored in the scanner and there is a large variety of equipment and manufacturers that produce specific physiological equipment for MRI systems.
2.3. Data Analysis
Human imaging methods of the CNS have evolved over the years and have reached a level of standard that is becoming common among researchers. Application of novel systems including standardized analysis methods for functional, anatomical and MRS related approaches are well describe in human imaging (e.g., fMRIB software library (www.fmrib.ox.ac.uk/fsl/); SPM (http://www.fil.ion.ucl.ac.uk/spm/), Brainvoyager (http://www.brainvoyager.com/), Afni (http://afni.nimh.nih.gov/afni), or Freesurfer (http:surfer.nmr.mgh.harvard.edu/). In animal imaging, these techniques and software packages are being adapted.
3. Animal Preparation
A number of animal handling procedures need to be taken into consideration when designing and implementing an imaging experiment (for a review see (Hildebrandt et al., 2008)). These include animal strain and gender, fasting, anesthesia type and dose, and frequency of imaging, and duration of the study that need to taken into account increasing weights and size of the animals, particularly in rats.
3.1. Anesthetized vs. Awake Animal Imaging
While most imaging studies of rodents have used anesthesia, the effects of anesthesia on neural and hemodynamic responses are not well understood (Martin et al., 2006b). Compared with halothane, for example, alpha-chloralose anesthesia is associated with a greater BOLD response but there is variation of the response over time that may contribute to significant variability in studies (Austin et al., 2005). In a recent article by Heindl and colleagues (Heindl et al., 2008), the authors make the case for non-invasive imaging techniques to reduce the number of experimental animals used in research. They further note that this can be done “without pain”. Herein lies the conundrum: Do animals sense pain while anesthetized? Certainly, pain imaging studies (as noted below) show activation in sensory and emotional regions in anesthetized animals to painful stimuli (Adamczak et al., 2010), and thus beg the question as to whether the anesthesia is producing mainly motor inhibition. As noted in Table 2 currently used anesthetics either produce no analgesia (but sedation and decreased mobility) or mild analgesia. Also for some, the duration of the effect is not well defined and proper monitoring is required including direct observation. For proper monitoring anesthesia monitoring systems need to be free of ferromagnetic material or managed outside the field and special attention needs to be made in the MR situation (see (Hanusch et al., 2007)). The latter is of note, since in most cases animals are placed deep in the magnet core, and direct visualization without specialized equipment is not easy.
The use of fMRI in awake, trained rats has been performed by a number of investigators (Martin et al., 2006a; Chin et al., 2008). There are marked differences in the BOLD response in awake vs. anesthetized rats (Lahti et al., 1999; Sicard et al., 2003; Duong, 2007). Thus, anesthesia may also complicate the data sets either because of a diminished signal or as a result of drug-drug interactions on brain systems. Anesthesia itself may also be stressful, for a number or reasons; (i) anesthesia is known to produce apoptopic changes in the brain’s of animals (Dong et al., 2009; Lu et al., 2010); and (ii) it is stressful in that it may raise cortisol levels and that simple immobility may not be a measure of conscious sensation in the animal (Dobkin et al., 1966; Oyama, 1973). Anesthetic depth levels have been measured in rats using thalamocortical local field potentials (Silva et al., 2010) – burst suppression ratio (% suppression during burst suppression pattern) used as an index of anesthetic depth occurred in the somatosensory cortex and thalamic nuclei with the highest concentration of isoflurane (1.7%). It is noteworthy that in most animal studies with anesthesia, all brain systems are “activated” including cortical regions, presumably involved in conscious evaluation of the stimulus. In line with this, the cortex can show differential sensory responses during deep isoflurane anesthesia in rats (Rojas et al., 2008). While ideally more suited for phMRI studies, the trained awake animal can be used in pain experiments.
Different anesthetics have different effects on brain systems (Field et al., 1993) with regard to sedative, analgesic, of motor (immobility) function. Some anesthetics may enhance cortisol and other stress molecules such as norepinephrine (Hamstra et al., 1984; Diltoer and Camu, 1988; Kostopanagiotou et al., 2010) and others may inhibit such release (Wagner et al., 1984). Most fMRI experiments in rodents have used alpha-chloralose, which has no, or minimal, analgesic action but this is not possible in survival experiments and alternative drugs, medetomidine, an alpha-2-adrenegic antagonist, which does have analgesic effects has been adopted. The use of drugs that have analgesic effects may also confound the evaluation of the data. In addition, some anesthetics alter cerebral blood flow that may alter BOLD data, notwithstanding (Sharma et al., 1984). Table 3 summarizes pros and cons related to anesthetized vs. awake imaging.
Table 3.
Pros and Cons of Anesthetized vs. Awake Imaging
| Condition | Anesthetized | Awake |
|---|---|---|
| Training | No training required | Training required |
| RSN state | Δ by Anesthetic Agent | Not influenced by anesthetic agent |
| Hemodynamic Stability | Needs specialized processing | Not an issue |
| Mobility | Minimal | Movement may be an issue |
| Analgesic State | Unknown | Known |
| Anesthetic Depth | Difficult to Measure | Not an issue |
| Sensitized by Training | No | Unknown |
| Sensitized by Anesthetic | Yes (if survival experiments) | No |
| Repeated Exposure | Apoptosis | Unknown |
| Cortisol Release | Depends on anesthetic | Diminished by Training |
| Chemical modulation of Brain State by Anesthetic | Depends on anesthetic | Not affected |
| Stress State | Unknown since it depends on anesthetic depth | Measured |
4. Preclinical Functional Imaging of Pain
Approaches to imaging pain in animals have by and large followed the course that human imaging studies have reported. Since the first studies reported by Tuor and colleagues (Tuor et al., 2000) , a number of groups have reported functional imaging studies across a number of species from mouse to non-human primates in a number of acute and chronic preclinical pain models. Amongst the first preclinical imaging studies of pain Tuor and colleagues reported on the effects of electrical and chemical (formalin) stimulation of the forepaw in rats (Tuor et al., 2000). Subsequent studies evaluated pain in different models as noted below.
4.1. Acute Pain Models (Table 4, Figure 2)
Table 4.
fMRI CNS Activation in Acute Pain Studies
| REGION | STIMULUS | SPECIES | BOLD RESPONSE |
ANESTHETIC | FIELD STRENGTH |
REFERENCE |
|---|---|---|---|---|---|---|
| Cortical | ||||||
| Frontal Cortex | Capsaicin, dorsal forepaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Malisza and Docherty, 2001) |
| Electrical, forepaw | Rat | + | α-chloralose 25mg/kg/hr | 4.7T | (Lowe et al., 2007) | |
| Electrical, forepaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Tuor et al., 2000) | |
| Capsaicin, Intrajoint, ankle vs. hindpaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Malisza et al., 2003b) | |
| Cingulate Cortex | Capsaicin, dorsal forepaw | Rat | + | α-chloralose | 9.4T | (Malisza and Docherty, 2001) |
| Formalin 5%, hindpaw | Rat | + | α-chloralose 70mg/kg | 4.7T | (Shih et al., 2008b) | |
| Electrical, forepaw | Rat | + | α-chloralose 25mg/kg/hr | 4.7T | (Lowe et al., 2007) | |
| Zymosan s.c. Heat Hyperalgesia | Rat | + | Isoflurane | ? | (Hess et al., 2007) | |
| Formalin 5% | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Sciatic Nerve Stimulation Electrical | Rat | + | α-chloralose 50mg/kg | 4.7T | (Chang and Shyu, 2001) | |
| Formalin | Rat | + | Ketamine | 4.7T | (Shih et al., 2008b) | |
| Formalin 5% forepaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Tuor et al., 2000) | |
| Electrical, forepaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Tuor et al., 2000) | |
| Capsaicin, Intrajoint, ankle vs. hindpaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Malisza et al., 2003b) | |
| Somatosensory Cortex | Capsaicin, dorsal forepaw | Rat | + | Isoflurane 1.5–2% | 9.4T | (Malisza and Docherty, 2001) |
| Electrical, forepaw | Mouse | + | medetomidine | 9.4T | (Adamczak et al., 2010) | |
| Formalin, forepaw | Rat | + (early and late) | Isoflurane 1% | 7.05T | (Asanuma et al., 2008) | |
| Capsaicin, forepaw | Rat | + (early) | Isoflurane 1% | 7.05T | (Asanuma et al., 2008) | |
| Formalin 5%, hindpaw | Rat | + | α-chloralose 70mg/kg | 4.7T | (Shih et al., 2008b) | |
| Formalin | Rat | + | Ketamine | 4.7T | (Shih et al., 2008b) | |
| Formalin 5% | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Formalin 5% forepaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Tuor et al., 2000) | |
| Zymosan s.c. Heat Hyperalgesia | Rat | + | Isoflurane 1% | ? | (Hess et al., 2007) | |
| Electrical, forepaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Tuor et al., 2000) | |
| Capsaicin, Intrajoint, ankle vs. hindpaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Malisza et al., 2003b) | |
| Motor Cortex | Formalin 5%, hindpaw | Rat | + | α-chloralose 70mg/kg | 4.7T | (Shih et al., 2008b) |
| Formalin, 5% hindpaw | Rat | + | Ketamine | 4.7T | (Shih et al., 2008b) | |
| Zymosan s.c. Heat Hyperalgesia | Rat | + | medetomidine | 9.4T | (Hess et al., 2007) | |
| Formalin 5% forepaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Tuor et al., 2000) | |
| Electrical, forepaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Tuor et al., 2000) | |
| Capsaicin, Intrajoint, ankle vs. hindpaw | Rat | + | α-chloralose 80mg/kg | 9.4T | (Malisza et al., 2003b) | |
| Insula | Electrical, forepaw | Rat | + | α-chloralose 25mg/kg/h | 9.4T | (Luo et al., 2009) |
| Formalin 5%, hindpaw | Rat | + | α-chloralose 70mg/kg | 4.7T | (Shih et al., 2008b) | |
| Entorhinal Cortex | Punctate (noxious) Mechanical | Rat | − | Isoflurane 1.5% | 2.35T | (Governo et al., 2007) |
| Electrical, forepaw | Rat | + | α-chloralose 25mg/kg/hr | 4.7T | (Lowe et al., 2007) | |
| Subcortical | α-chloralose 25mg/kg/h | |||||
| Thalamus | Electrical, forepaw | Rat | + | α-chloralose 25mg/kg/h | 9.4 | (Luo et al., 2009) |
| Formalin | Rat | + | Ketamine | 4.7T | (Shih et al., 2008b) | |
| Electrical, forepaw | Rat | + | α-chloralose 25mg/kg/hr | 4.7T | (Lowe et al., 2007) | |
| Capsaicin 30µg Mechanical Allodynia | Rat | + | Isoflurane 1.5% | 2.35T | (Moylan Governo et al., 2006) | |
| Formalin 5% | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Zymosan s.c. Heat Hyperalgesia | Rat | + | Isoflurane | ? | (Hess et al., 2007) | |
| Hypothalamus | Capsaicin 30µg Mechanical Allodynia | Rat | + | Isoflurane 1.5% | 2.35T | (Moylan Governo et al., 2006) |
| Zymosan s.c. Heat Hyperalgesia | Rat | + | Isoflurane | ? | (Hess et al., 2007) | |
| Sciatic Nerve Stimulation Electrical | Rat | + | α-chloralose 50mg/kg | 4.7T | (Chang and Shyu, 2001) | |
| Formalin 5% | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Amygdala | Formalin 5% | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) |
| Hippocampus | Formalin 5%, hindpaw | Rat | + | α-chloralose 70mg/kg | 4.7T | (Shih et al., 2008a) |
| Formalin 5%, hindpaw | Rat | + | Ketamine | 4.7T | (Shih et al., 2008a) | |
| Punctate (noxious) Mechanical | Rat | + | Isoflurane 1.5% | 2.35T | (Governo et al., 2007) | |
| Caudate/Putamen | Formalin 5%, hindpaw | Rat | + | α-chloralose 70mg/kg | 4.7T | (Shih et al., 2008b) |
| Capsaicin 30µg Mechanical Allodynia | Rat | + | Isoflurane 1.5% | 2.35T | (Moylan Governo et al., 2006) | |
| Formalin 5% | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Formalin | Rat | + (early and late) | Isoflurane 1% | 7.05T | (Asanuma et al., 2008) | |
| Capsaicin | Rat | + Early | Isoflurane 1% | 7.05T | (Asanuma et al., 2008) | |
| Formalin | Rat | + | Ketamine | 4.7T | (Shih et al., 2008a) | |
| Nucleus Accumbens | Formalin | Rat | + | Ketamine | 4.7T | (Shih et al., 2008a) |
| Electrical, forepaw | Rat | + | α-chloralose 25mg/kg/hr | 4.7T | (Lowe et al., 2007) | |
| Formalin 5% | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Brainstem/Cerebellum | ||||||
| PAG | Capsaicin 30µg Mechanical Allodynia | Rat | + | Isoflurane 1.5% | 2.35T | (Moylan Governo et al., 2006) |
| Formalin 5% | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Zymosan s.c. Heat Hyperalgesia | Rat | + | Isoflurane | ? | (Hess et al., 2007) | |
| Cuneiform | Punctate (noxious) Mechanical | Rat | + | Isoflurane 1.5% | 2.35T | (Governo et al., 2007) |
| Parabrachial | Punctate (noxious) Mechanical | Rat | + | Isoflurane 1.5% | 2.35T | (Governo et al., 2007) |
| Pontine Nuclei | Punctate (noxious) Mechanical | Rat | + | Isoflurane 1.5% | 2.35T | (Governo et al., 2007) |
| Cerebellum | Punctate (noxious) Mechanical | Rat | + | Isoflurane 1.5% | 2.35T | (Governo et al., 2007) |
| Capsaicin 30µg Mechanical Allodynia | Rat | + | Isoflurane 1.5% | 2.35T | (Moylan Governo et al., 2006) | |
| Inferior Colliculus | Electrical, forepaw | Rat | + | α-chloralose 25mg/kg/hr | 4.7T | (Lowe et al., 2007) |
| Superior Colliculus | Capsaicin 30µg Mechanical Allodynia | Rat | + | Isoflurane 1.5% | 2.35T | (Moylan Governo et al., 2006) |
| Spinal Cord | ||||||
| Capsaicin 25µl, forepaw 75µl into ankle joint | Rat | + and − | Isoflurane 1.5–2% | 9.4T | (Malisza et al., 2003a) | |
| Formalin 50µl, hindpaw | Rat | + | Isoflurane 1% | 4.7T | (Porszasz et al., 1997) | |
| Electrical Stimulation, hindpaw + USPIO | Rat | + Lumbar cord (L3–L5) | α-chloralose 30mg/kg/hr | 4.7T | (Moylan Governo et al., 2006) (Zhao et al., 2008) | |
| Electrical Stimulation, hindpaw + USPIO | Rat | + Cervical cord (C4–C8) | α-chloralose 30mg/kg/hr | 4.7T | (Moylan Governo et al., 2006) (Zhao et al., 2009a) |
Electrical Stimulation
Electrical stimulation of the forepaw was initially used because of its relative ease of use in the scanner and has now been applied to mouse models (Adamczak et al., 2010) and rat (Chang and Shyu, 2001) models. Such studies have reported activation in contralateral somatosensory cortical regions to non-noxious stimuli and activation in somatosensory cortex, cingulate cortex, medial thalamus and hypothalamus to noxious stimuli that can be inhibited by the analgesic morphine; no activation was reported in other subcortical regions (Chang and Shyu, 2001).
Algesic-Induced Pain
A similar approach using capsaicin-induced painful stimulation in anesthetized rats. Following intradermal capsaicin (25 microl), activation of the anterior cingulate (bilateral), frontal cortex (bilateral), and sensory motor cortex (contralateral) were observed; morphine reduced these activations (Malisza and Docherty, 2001). Others have observed additional structures in response to higher concentrations (30 microg) that included the periaqueductal gray, parabrachial nucleus and superior colliculus (Moylan Governo et al., 2006). Capsaicin has also been used to evaluate functional changes in the lumbar (Malisza et al., 2003a) and cervical spinal cord (Malisza and Stroman, 2002). Other algesic agents have been employed in fMRI imaging experiments. Formalin injected into the forepaw has been evaluated by a number of groups (Tuor et al., 2002; Asanuma et al., 2008). In the latter, pan early and late response in the SI cortex while only an early response is observed with capsaicin; both were abolished by morphine (Asanuma et al., 2008).
Inflammatory Pain
Reports of specific drivers of neuromodulators (e.g., dyorphin) have been employed in knockout vs. control mice in response to inflammatory stimuli (complete Freunds adjuvant) (Taketa et al., 2010); no activation was observed in regions such as the cingulate, somatosensory and insular cortices or thalamus in prodynorphin knockout mice. Other fMRI visceral pain studies have included those in acute pancreatitis (see Figure 3A) (Westlund et al., 2009), investigating the effects of a mu agonist (morphine) and mu antagonist (naloxone) and following a peripheral pain stimulus (intraplantar formalin).
Figure 3. Examples of Activations in clinical models of pain.
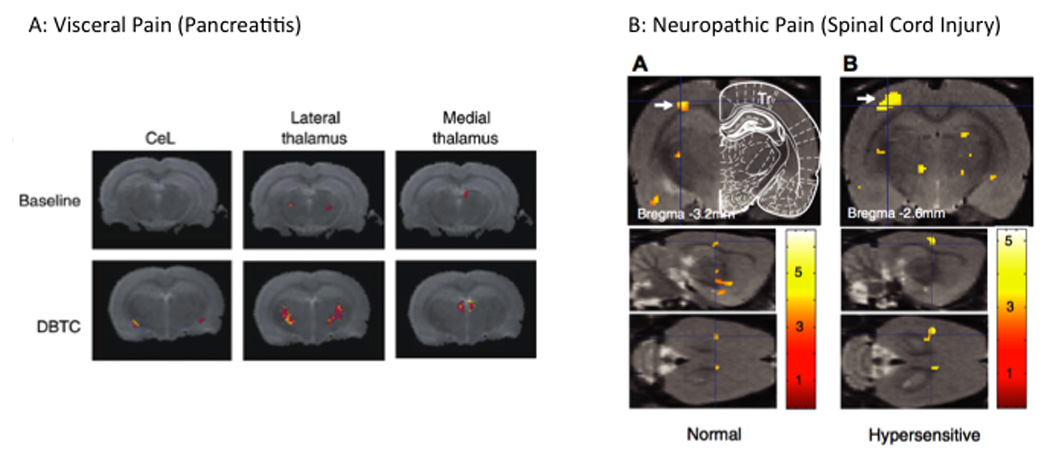
A: Visceral Pain: Activation in the thalamus in response to abdominal stimulation in a model of visceral pain ((Westlund et al., 2009); with permission, Neuroimage). Note the increase in the lateral and dorsal thalamus suggesting increased activation in lateral and medial pain pathways.
B: Neuropathic Pain: Activation in response to trunk stimulation in a rat model of spinal cord injury. Panel A shows activation in response to the stimuli in SI (arrow) in normal animals that is increased in the injured animal ((Endo et al., 2008a); with Permission, IASP Press).
Thermal Stimuli
Heat stimuli activate a number of regions including the cingulate, retrosplenial, sensory-motor and insular cortex, medial and lateral posterior thalamic nuclei, pretectal area, hypothalamus and periaqueductal gray (Hess et al., 2007). Following injection of zymosan a protein-carbohydrate complex that used to produce an experimental sterile inflammation), hyperalgesia was observed, with enhanced activation in these same areas consistent with the notion of sensitization. In a prior study from our group we evaluated the fMRI activation of the effects of heat on 5 drugs vs. placebo in humans and rats (Borsook et al., 2007). Using a whole brain and neural circuit (sensory, emotional, modulatory) activation, the effects of thermal heat applied to the hind paw in awake rats we observed that drugs that are clinically useful in neuropathic pain had a distinct profile (imipramine and gabapentin) compared with those that did not (topirimate, clonzaepam, rofecoxib). Such studies, if shown to be reproducible, show that functional imaging may be employed in ways we might not have previously considered.
Mechanical Stimuli
Systems to deliver punctate mechanical stimuli for animal fMRI studies have been developed (Governo et al., 2007). In rats, painful punctate stimulus produces activation in that included increased in cuneiform (involved in defensive responses including pain modulation) and parabrachial nuclei and decreased signal change in entorrhinal temporal and visual cortices (Governo et al., 2007). In addition to rodents, studies in primate fMRI have been performed. Mechanical nociceptive stimuli applied to the glabarous skin of the fingers have been used to map SI regions of the monkey brain using near infrared spectroscopy (NIRS). Specific SI regions including areas 3a, 3b, and 1 were activated and modulated by nociceptive stimulus intensity (Chen et al., 2009).
Taken together, these data represent a cohort of relatively early data sets that are consistent with what has been learned in human imaging in response to electrical, heat and algesic chemicals (see Figure 2), and in most studies, all activation patterns were reported to be reversed by morphine. Overall, acute imaging studies have shown consistent results for cortical regions but not for subcortical regions. To date, they have failed to depict a full image of CNS pain processing in a manner accomplished in human studies, perhaps the divergence of study designs, imaging, and analysis is at the root of that lack of a complete picture. Potentially, with the development of better imaging standards and analysis this gap will be filled.
4.2. Chronic Pain Models (Table 5)
Table 5.
fMRI CNS Activation in Chronic Pain/Disease Model Studies
| REGION | STIMULUS | SPECIES | BOLD RESPONSE |
ANESTHETIC | FIELD STRENGTH |
REFERENCE |
|---|---|---|---|---|---|---|
| Cortical | ||||||
| Cingulate Cortex | Pancreatic Inflammation Mechanical/Thermal Abdominal Skin | Rat | + | Isoflurane 1.2–1.5% | 4.7T SPIO | (Westlund et al., 2009) |
| Somatosensory Cortex | Spinal Cord Injury Mechanical and Cold Naloxone 1mg/kg Sensitized | Rat | + | α-chloralose 80mg/kg | 4.7T | (Endo et al., 2008a) |
| Spared Nerve Injury Model (SNI) | Rat | No change vs. sham | Isoflurane 2% | 2.35T | (Jones et al., 2009) | |
| Insular Cortex | Spared Nerve Injury Model (SNI) | Rat | No change vs. sham | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Subcortical | Spared Nerve Injury Model (SNI) | |||||
| Thalamus | Pancreatic Inflammation Mechanical/Thermal Abdominal Skin | Rat | + | Isoflurane 1.2–1.5% | 4.7T SPIO | (Westlund et al., 2009) |
| Spinal Cord Injury Mechanical and Cold Naloxone 1mg/kg sensitized | Rat | + | α-chloralose 80mg/kg Pancuronium | 4.7T | (Endo et al., 2008a) | |
| Amygdala | Pancreatic Inflammation Mechanical/Thermal Abdominal Skin | Rat | + | Isoflurane 1.2–1.5% | 4.7T SPIO | (Westlund et al., 2009) |
| Brainstem/Cerebellum | ||||||
| PAG | ||||||
| RVM | Pancreatic Inflammation Mechanical/Thermal Abdominal Skin | Rat | + | Isoflurane 1.2–1.5% | 4.7T SPIO | (Westlund et al., 2009) |
| Deep Mesencephalic Nucleus | Spared Nerve Injury Model (SNI) | Rat | No change vs. sham | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Spinal Cord | ||||||
| Dorsal Horn | Streptozotocin (STZ) induced Diabetes, Electrical | STZ Rats | STZ < controls | α-chloralose 40mg/kg | 7T | (Malisza et al., 2009) |
| Spinal Cord Injury (days-months), Electrical | Rat | + | α-chloralose 80mg/kg | 4.7T | (Endo et al., 2008a) |
Neuropathic Pain
As with the human experience, fMRI of neuropathic pain has followed the development of procedures for acute pain (see above). However a few studies have been reported in the literature. In a spinal cord injury of neuropathic pain (Xu et al., 1992), in which some rats exhibit spontaneous truncal mechanical and cold hypersensitivity and others are insensitive to these, but become hypersensitized when naloxone is administered, activation in the contralateral SI correlates with the level of the hypersensitive state (Endo et al., 2008b). See Figure 3B shows differences in activation in S1 in spinal neuropathic vs. sham control animals.
In addition to functional imaging, few anatomical morphometric measures have been used in neuropathic pain models in rats (Seminowicz et al., 2009). The approach is based on volumetric changes in gray matter reported in human studies of chronic pain (Apkarian et al., 2004). This study reports on anatomical changes in neuropathic rats over a five month period and demonstrated decreased gray matter volumes in the frontal retrosplenial and entorhinal cortices, S1 hind limb area, anterior cingulate cortex (ACC, areas 32 and 24), and the insula.
Visceral Pain
Visceral pain is a significant clinical problem and while a number of studies have been performed in humans (Aziz et al., 2000; Labus et al., 2009; Sharma et al., 2009), relatively few have been in rodents. While a number of labeled (e.g., [(14)C]-iodoantipyrine) studies have been performed (Wang et al., 2008; Wang et al., 2009), one study that uses fMRI (Johnson et al., 2010), in which a model of colonic distention is reported and another reported correlated brain changes of c-fos expression with fMRI using colorectal distention (Lazovic et al., 2005). fMRI activation was present in the amygdala, hypothalamus, thalamus, cerebellum and hippocampus and cfos present in amygdala and thalamus suggesting that these approaches may offer complementary information. The Willis group used fMRI to observe changes in the brain in response to dorsal column pathway manipulation (based on evidence that visceral information travels in these pathways from human data (reviewed in (Palecek, 2004)), reported blood volume changes in response to noxious stimulation of the pancreas and duodenum following before (sham) or after surgical lesions of the dorsal columns showed increased in the thalamus and brainstem (Willis et al., 1999).
As can be gleaned from the above discussion, preclinical imaging of pain is in its relative infancy, certainly when compared with human imaging. Other models would seem to be well suited for rat fMRI or MRI studies. For example arthritis models, cytokine models, genetic strain evaluation, and gender differences.
5. Imaging Analgesics and Analgesic Effects (Table 6, Figures 4–6)
Table 6.
fMRI CNS Activation by Analgesics/Response to Analgesics
| REGION | DRUG/STIMULUS | SPECIES | BOLD RESPONSE |
MAINTENANCE ANESTHETIC |
FIELD STRENGTH |
REFERENCE |
|---|---|---|---|---|---|---|
| Cortical | ||||||
| Frontal Cortex | Desipramine 10mg/kg × 14d Mechanical allodynia in SNL model of NP pain | Rat | + | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Temporal Cortex | Desipramine 10mg/kg × 14d Mechanical allodynia in SNL model of NP pain | Rat | + | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Parietal Cortex | Morphine 5mg/kg Pancreatic Inflammation Mechanical/Thermal Abdominal Skin | Rat | Attenuated | Isoflurane 1.2–1.5% | 4.7T SPIO | (Westlund et al., 2009) |
| Cingulate Cortex | Morphine 5mg/kg Pancreatic Inflammation Mechanical/Thermal Abdominal Skin | Rat | Attenuated | Isoflurane 1.2–1.5% | 4.7T SPIO | (Westlund et al., 2009) |
| Morphine 5mg/kg | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Formalin 5% | Rat | + (attenuated by morphine) | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Morphine, Sciatic Nerve Stimulation Electrical | Rat | + Attenuated by morphine | α-chloralose 50mg/kg | 4.7T | (Chang and Shyu, 2001) | |
| Somatosensory Cortex | Lidocaine 1,4, 10mg/kg i.v. Electrical, forepaw | Rat | + | Isoflurane 1.5–2% | 9.4 | (Luo et al., 2009) |
| Desipramine 10mg/kg × 14d i.p. Mechanical allodynia in SNL model of NP pain | Rat | + | Isoflurane 2% | 2.35T | (Jones et al., 2009) | |
| Formalin 5% | Rat | + (attenuated by morphine) | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Morphine, Sciatic Nerve Stimulation Electrical | Rat | + Attenuated by morphine | α-chloralose 50mg/kg | 4.7T | (Chang and Shyu, 2001) | |
| Morphine 1mg/kg, Electrical, forepaw | Rat | − Not attenuated by morphine | α-chloralose 80mg/kg | 9.4T | (Tuor et al., 2000) | |
| Insula | Desipramine 10mg/kg × 14d i.p. Mechanical allodynia in SNL model of NP pain | Rat | + | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Gabapentin 30mg/kg i.v. | Rat | + | Isoflurane 1.5% | 2.35T | (Governo et al., 2008) | |
| Entorhinal Cortex | Gabapentin 30mg/kg i.v. | Rat | − | Isoflurane 1.5% | 2.35T | (Governo et al., 2008) |
| Morphine 5mg/kg | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Subcortical | ||||||
| Thalamus | Desipramine 10mg/kg × 14d Mechanical allodynia in SNL model of NP pain | Rat | − | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Morphine 5mg/kg Pancreatic Inflammation Mechanical/Thermal Abdominal Skin | Rat | Attenuated | Isoflurane 1.2–1.5% | 4.7T SPIO | (Westlund et al., 2009) | |
| Gabapentin 30mg/kg +100mg/kg i.v. | Rat | + | Isoflurane 1.5% | (Governo et al., 2008) | ||
| Gabapentin 30mg/kg | Rat | + | Isoflurane 1.5% | 2.35T | (Governo et al., 2008) | |
| Morphine 5mg/kg | Rat | + | Halothane 1–2% | (Shah et al., 2005) | ||
| Morphine, Sciatic Nerve Stimulation Electrical | Rat | + Attenuated by morphine | α-chloralose 50mg/kg | 4.7T | (Chang and Shyu, 2001) | |
| Hypothalamus | Desipramine 10mg/kg × 14d i.p. Mechanical allodynia in SNL model of NP pain | Rat | + | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Morphine 5mg/kg | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Morphine, Sciatic Nerve Stimulation Electrical | Rat | + Attenuated by morphine | α-chloralose 50mg/kg | 4.7T | (Chang and Shyu, 2001) | |
| Formalin 5% | Rat | + Attenuated by morphine | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Amygdala | Morphine 5mg/kg Pancreatic Inflammation Mechanical/Thermal Abdominal Skin | Rat | Attenuated | Isoflurane 1.2–1.5% | 4.7T SPIO | (Westlund et al., 2009) |
| Gabapentin 30mg/kg i.v. | Rat | − | Isoflurane 1.5% | 2.35T | (Governo et al., 2008) | |
| Morphine 5mg/kg | Rat | + | Halothane 1–2% | (Shah et al., 2005) | ||
| Formalin 5% | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Caudate/Putamen | Desipramine 10mg/kg × 14d Mechanical allodynia in SNL model of NP pain | Rat | + | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Formalin 5% | Rat | + Attenuated by morphine | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Hippocampus | Desipramine 10mg/kg × 14d Mechanical allodynia in SNL model of NP pain | Rat | + | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Morphine 5mg/kg/Pancreatic Infalammation | Isoflurane 1.2–1.5% | 4.7T SPIO | (Westlund et al., 2009) | |||
| Nucleus Accumbens | Morphine 5mg/kg | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) |
| Formalin 5% | Rat | + Attenuated by morphine | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Brainstem/Cerebellum | ||||||
| PAG | Desipramine 10mg/kg × 14d Mechanical allodynia in SNL model of NP pain | Rat | + | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Gabapentin 30mg/kg i.v. | Rat | + | Isoflurane 1.5% | 2.35T | (Governo et al., 2008) | |
| Morphine 5mg/kg | Rat | + | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| Morphine, Formalin 5% | Rat | + Attenuated by morphine | Halothane 1–2% | 2.35T | (Shah et al., 2005) | |
| VTA | Morphine 5mg/kg | Rat | − | Halothane 1–2% | 2.35T | (Shah et al., 2005) |
| RVM | Morphine 5mg/kg. Pancreatic Inflammation Mechanical/Thermal Abdominal Skin | Rat | + Attenuated | Isoflurane 1.2–1.5% | 4.7T SPIO | (Westlund et al., 2009) |
| DR | Morphine, Pancreatic Inflammation Mechanical/Thermal Abdominal Skin | Rat | + Attenuated by Morphine | Isoflurane 1.2–1.5% | 4.7T SPIO | (Westlund et al., 2009) |
| Parabrachial Nucleus | Desipramine 10mg/kg × 14d Mechanical allodynia in SNL model of NP pain | Rat | − | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Inferior Colliculus | Desipramine 10mg/kg × 14d Mechanical allodynia in SNL model of NP pain | Rat | − | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Cerebellum | Desipramine 10mg/kg × 14d Mechanical allodynia in SNL model of NP pain | Rat | − | Isoflurane 2% | 2.35T | (Jones et al., 2009) |
| Spinal Cord | ||||||
| Lidocaine 3mg/kg i.v., Electrical Stimulation, hindpaws (USPIO) | Rat | + Blocked by Lidocaine | Isoflurane 2% | 4.7T | (Zhao et al., 2008) |
Figure 4. phMRI showing Activation Maps.

A: Remifentanil. The figure shows positive (red-yellow) and negative (blue-green) changes in CBV following remifentanil infusion (10µg/kg). Activation patterns are noted in piriform cortex (white rostral slices), ventral tegmental areas (yellow), hippocampus (green rostral areas), raphe (red), reticular formation (green caudal areas). From ((Liu et al., 2007); with permission, Neuroimage).
B: Gabapentin. The figure shows BOLD signal activation following 100mg/kg infusion of gabapentin vs. saline resulting in activation in a number of regions including the thalamus, PAG, hippocampus and tegmental area (From (Governo et al., 2008); permission, British Journal of Pharmacology, with minor modifications). Numbers indicate distance from Bregma.
Figure 6. Differentiating Drugs.
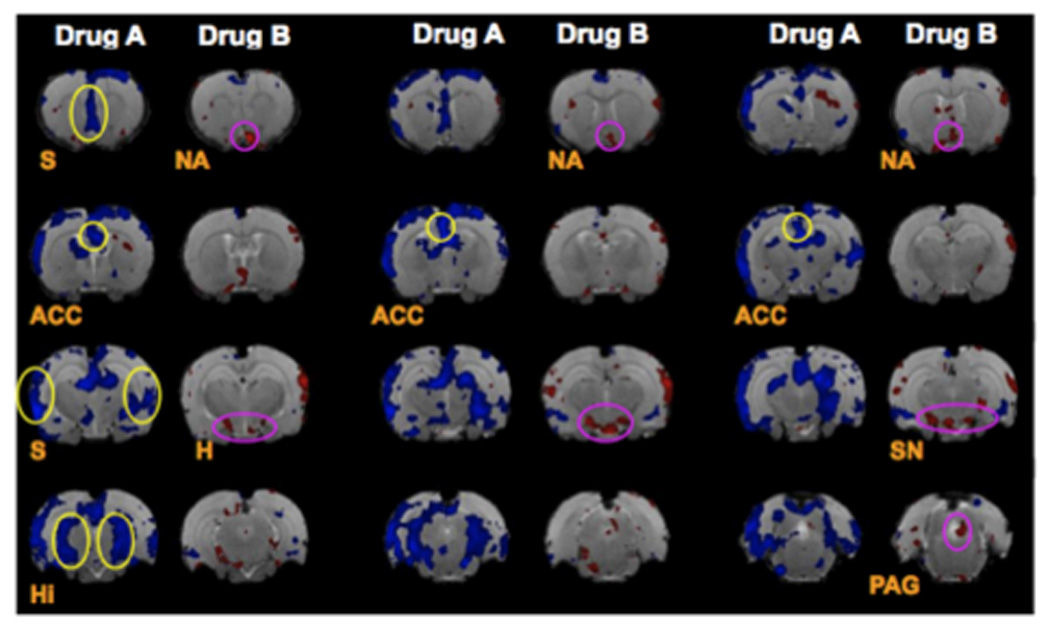
fMRI images through the rat brain following infusion of a tricyclic antidepressant imipramine 20mg/kg (Drug A) opioid morphine 5mg/kg (Drug B) and showing different activation patterns. The experiments were conducted in trained awake rats. (Borsook et al., unpublished data). Key: ACC = anterior cingulated cortex; H = hypothalamus; Hi = Hippocampus; S = septal region; PAG = periaqueductal gray; SN = substantia nigra; DB = Diagonal band of Broca;
A number of analgesic drugs have been evaluated using fMRI. As noted above, many of the models used morphine as a “blocker” of pain stimuli. Anesthetics alter brain neurotransmitter systems and brain metabolism (Choi et al., 2002; Shulman et al., 2003) and cellular metabolism (Luo et al., 2007). What would seem as an important use of animal models is the ability to measure drug effects on brain neural circuits, particularly in the awake model where the pharmacological and physiological effects of anesthetic drugs are not an issue (see above). Most of the current fMRI reports on direct drug action on brain systems in rodent models do however employ anesthesia. These have focused on opioids (remifentanil, morphine, and heroin), gabapentinoids (pregabalin), antidepressants (desipramine), NMDA antagonists (ketamine), and membrane stabilizers (lidocaine) (For further details, including references, See Table 6). Most of these drugs, or similar drugs have been evaluated in human imaging studies as noted in the discussion that follows, perhaps not surprisingly since these fill the main categories of analgesics used clinically.
One of the earliest preclinical fMRI studies on opioids was with the drug heroin (Xu et al., 2000). Following intravenous heroin, where under artificial respiration a region-specific increase in BOLD signal was reported. Of significance was that the fMRI activation observed corresponded with the distribution of opiate mu-receptors in rat brain as previously reported by autoradiography. More recent studies on of the de novo effects of opioid analgesics include a study on remifentanil and morphine (Liu et al., 2007).
In the study evaluating the cerebral blood volume (CBV) effects of remifentanil (10umg/kg) and morphine (200µg/kg) on brain activation in anesthetized rats, the initial effects of remifentanil resulted in a slow component with decreased CBV in a number of brain regions, the largest of which occurred in the caudate, accumbens, ventral hippocampus, cingulate, and piriform cortex (Liu et al., 2007). Figure 4A shows results from this study for remifentanil. A faster temporal increase in CBV response occurred in the hippocampus (thought to be due to inhibition of GABAergic neurons). The study demonstrated common activation patterns for two opioids, and provided a temporal model for evaluating these effects.
Another pharmacological MRI (phMRI) study on the gabapentinoid gabapentin (Governo et al., 2007), perhaps the most commonly used agent for the clinical treatment of neuropathic pain, resulted in increases in brain activity in sensory (thalamus) and modulatory (PAG) regions, and decreased BOLD signal in emotional areas (amygdala and entorhinal cortex) (Figure 4B). These results indicate supraspinal effects of a drug and may explain not only some of the analgesic effects but also some of the effects on regions of the brain that may be involved in ‘pain memory’ and anxiety. Such insights are not possible from behavioral studies alone. A similar approach by the same group, evaluated chronic effects of the antidepressant drug desipramine (10 mg/kg × 14 days) in a model of chronic neuropathic pain (SNL - spinal nerve ligation). Desipramine treatment diminished mechanical allodynia in SNL rats that correlated with greater activation of the midbrain region, primary somatosensory cortex, insular cortex, medial globus pallidus, inferior colliculus, perirhinal cortex and cerebellum when compared with sham treated animals (in the latter regions activated included those associated with “learning and memory”.
The sodium channel blocker, lidocaine, has been used to evaluate its effects on non-noxious and noxious stimulation of the forepaw in anesthetized rats (Luo et al., 2009). The results in healthy rats, as reported, indicate that lidocaine produced enhanced responses similar to those reported for cocaine. The same drug (3mg/kg) was used to determine the effects on painful electrical stimulation in a model of spinal cord neuropathic pain (see above). Local application blocked all CNS activity, while systemic application affected activation (Zhao et al., 2009b).
In a related field of anesthesia, the direct effects of anesthetics (etiomodate, isoflurane) on brain activity in rodent models have been investigated. Anesthetic agents used in animal studies have differing analgesic potency or effects (Table 4). Comparisons between equithesin and isoflurane on air puffs applied to the face demonstrated that the former reduced brain activation in response the stimulus, while isoflurane include the anesthetics equithesin and with the use of isoflurane (1.5%) robust activations were detected (Dashti et al., 2005). Furthermore, repeated exposure of the animals to isoflurane, results in altered cerebro-vascular responses even if the exposures are separated by five days (Wegener and Wong, 2008). This type of data is very important since it suggests that different anesthetics can influence sensory responses. No such comparisons have been reported for the effects of different anesthetics on painful stimuli.
6. Preclinical imaging of pain and analgesics in Future Research
The ability to measure changes in multiple brain areas in the brainstem and higher centers (cortical and subcortical) with new imaging modalities, such as functional magnetic resonance imaging (fMRI), allows for a ‘global brain response’ that captures activation patterns in numerous functional circuits (e.g., sensory, emotional) that may provide an objective evaluation of an organisms response to pain or analgesics. As the use of NMR technologies in the preclinical phase increases, significant advances in understanding the neurobiology of pain, understanding of how and where in the brain these drugs affect pain processing, improving an understanding of direct effects of analgesics on brain systems and implicitly in analgesic drug development. The impact of using this technology for measures of whole brain systems in the context of healthy vs. preclinical animal models is just beginning to be realized. The use of neural circuit as “a language of translation” (see (Borsook et al., 2006)) has implications for refining old and defining new models in translational medicine. Such translations may be bidirectional (see Figure 5).
Figure 5. Human – Rat Correlations using fMRI (Box).
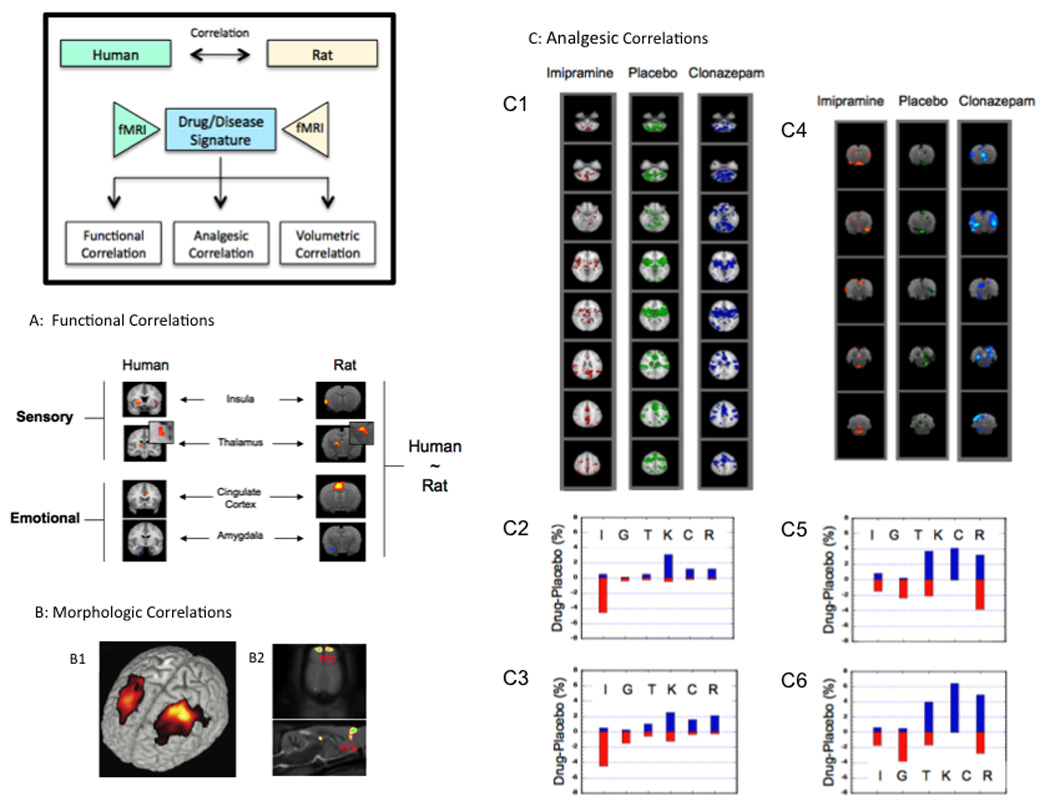
A: Functional Correlations (from (Borsook et al., 2007); with permission, Drug Discovery Research). The figure shows the thermal response of rats and humans to a 46°C stimulus applied to the dorsum of the foot. Note that the activation patterns in the regions of interest (primary somatosensory cortex (SI), thalamus (Th), insula (I), anterior cingulate cortex (aCG) and amygdala (A)) is similar in each species. Furthermore, the signal sign (i.e., increase or decrease in BOLD signal) is similar in both species).
B: Morphological Correlations: B1: The Figure shows cortical gray matter volume loss (gray matter density) in the dorsolateral prefrontal cortex (DLPF) in patients with chronic back pain (From (Apkarian et al., 2004); with permission, Journal of Neuroscience). B2: Data from a rat neuropathic pain model (SNI) showing cortical volume loss in the prefrontal cortex (From (Seminowicz et al., 2009); with permission, Neuroimage).
C: Analgesic Correlations (from (Borsook et al., 2006); with permission, Drug Discovery Research). Effects of Drugs on Thermal Stressor in Humans. C1: Sample axial slices depicting activation maps for two drugs (imipramine and clonazepam) and placebo. Visual inspections indicate that there is an overall decrease in activation for imipramine vs. placebo and an overall increase in activation for clonazepam vs. placebo. C2: Voxel count for 5 drugs vs. placebo for whole brain (WHB) activation. Note that for imipramine (I) and gabapentin (G) more voxels are activated in drug vs. placebo while for clonazepam (C), rofecoxib (R) and ketorolac (K) more voxels are activated in the drug vs. placebo. Topiramate (T) has an intermediate or mixed effect. C3: Voxel count for pathway activation (PB). Note a similar pattern is present when compared with whole brain activation.
Effects of Drugs on Thermal Stressor in and Rats. C4: Sample axial slices depicting activation maps for two drugs (imipramine and clonazepam) and placebo. Visual inspection indicates that there is an overall decrease in activation for imipramine vs. placebo and an overall increase in activation for clonazepam (C) vs. placebo. C5: Voxel count for 4 drugs vs. placebo for rat whole brain (RWHB) activation. Note that for imipramine (I) more voxels are activated in placebo vs. drug while for clonazepam (C), rofecoxib (R) and ketorolac (K) more voxels are activated in the drug vs. placebo. C6: Voxel count for pathway (RPB) activation in rats, showing a similar pattern to that for RWHB. Key: Red bar = voxels activated in drug > placebo; blue bar = drugs < placebo.
Implications for Pain Neurobiology
From a systems neuroscience point of view, functional imaging offers a unique window into brain function. Aside from traditional approaches that include evoked and resting state measures the addition of anatomical and chemical measures allows for evaluation of plasticity of the nervous system. In addition, the use of stimulation and ablation techniques in combination with imaging opens new vistas into circuit based measures (Figure 3). New approaches including measures of resting state networks may enable new research and insights into chronic pain processing.
Differentiating Drug Classes
Potentially, one of the most useful applications of phMRI of the brain is to segregate classes of drugs. Figure 6 shows one such example of different brain activation patterns for an opioid vs. a tricyclic antidepressant. Such approaches may help evaluate novel drugs, and combination treatments. With novel drug evaluation, differentiation of potential molecules being developed can be evaluated this way as an additional method of profiling candidate molecules.
Electrophysiology - Brain Recording and Stimulation in the Magnet
fMRI is a surrogate for measures of neural activity (Logothetis, 2002). Coupling of the BOLD response and electrophysiology has been reported in rats (Huttunen et al., 2008) and primates (Shmuel et al., 2006). Combining imaging with electrophysiology or stimulation of a targeted region of interest offer additional research. Stimulation of specific regions, defined by imaging, may provide preclinical evidence for efficacy of such approaches that are now common across a number of diseases affecting the central nervous system.
Using glass-coasted carbon fiber electrodes methods for direct stimulation of brain structures have been described in which the authors report little distortion of images acquired (Shyu et al., 2004b; Shyu et al., 2004a). Stimulation of the medial thalamus resulted in an increase in signal in the anterior cingulate cortex (ACC). Such approaches may be used not only to show such connectivity, but also may be important in evaluating targets for neurostimulation therapies for chronic pain.
Longitudinal studies for Pain
Pain conditions such as neuropathic pain, evolve following an injury and an understanding of how the brain changes over time through repeated fMRI measures. As noted above, longitudinal studies are possible with functional and anatomical imaging and may be limited with PET because of issues related to radioactivity exposure. Such possibilities are important in measures of changes that occur with the evolution of chronic pain in animal models. Repeated imaging needs to take into account issues of the potential effects of multi-dose effects of anesthetic drugs, or in the case of awake animals, repeated training for the imaging (Weber et al., 2006).
“Dynamic” and “Static” Lesion Studies
Animal models offer approaches that allow for experiments to answer questions that are difficult or impossible to perform in humans. Lesions of pain pathways have contributed enormously to our understanding including modulatory pain pathways (Porreca et al., 2002). We use the term “static” lesion studies as those where a lesion is produced in the routine manner (electrolytic or chemical) and “dynamic” refers to manipulations such as genetic switches introduced into a brain region (Bernstein et al., 2008) that can be turned on or off. By introducing genes into specified neurons, the latter may be silenced or activated in a controlled temporal fashion and thus allows for studies of neural regions as they relate to systems biology in surrogate models of pain.
Species and Gender Considerations
Imaging across species including rats, mice and non-human primates is now possible with advantages amongst each group. Rats have formed the backbone of much of pain research. Since genetic strains and manipulations of gene in mouse strains, these clearly offer additional advantages on evaluation of changes resulting from gene manipulation (Nadler et al., 2006; Badea et al., 2009). For some specific issues, non-human primates may be the species of choice, for example, PET studies of ligands or novel pharmacological agents. Given that human imaging is now pervasive and most experiments can now be done in human volunteers and patients, the need for experiments in non-human primates is relatively low. Differences and commonalities across species clearly need to be taken into account (Keller et al., 2009; Pinsk et al., 2009). In addition, well known gender differences need to be considered as have been shown in human studies (Lv et al., 2010).
Neuroplasticity
Pain is now considered a disease that affects CNS function ad structure. These neuroplastic changes observed across a number of human studies (phantom limb pain, neuropathic pain, fibromyalgia, migraine) have recently been applied to the rodent model of neuropathic pain. In general, structural (Antonini et al., 1999; De Groof et al., 2006; Ghosh et al., 2009) and functional imaging (Kuo et al., 2005; Van der Linden et al., 2009) in rodents has provided excellent readouts of brain plasticity. In a rodent model of spared nerve injury (SNI) significant changes in measures of brain volume were reported (Seminowicz et al., 2009). This is highly significant since the information provides another human-rodent correlation, it allows for strategies to be developed in order to fully understand these changes (Metz et al., 2009). Similar approaches to prior human models evaluating cortical plasticity are also observed in humans has been reported in animal models (Sydekum et al., 2009). In the latter study, spinal cord injury resulted in changes in fMRI responses in SI (enlargement of the activated area) and persistence of the fMRI signal in the wake of behavioral recovery. Such functional reorganization has been shown in humans to continue brain even in the light of abnormal brain function (Lebel et al., 2008). However, such recovery markers may prove to be useful in long-term imaging/behavioral trials.
Understanding Signal Change
The nature of decreased or negative BOLD fMRI signals is not been well understood (Harel et al., 2002; Boorman et al., 2010). In a novel approach to this issue using a pain model, the effects of intravenous dopaimine-2-receptor agonists (D2RA) that normally produce decreased BOLD in the basal ganglia (caudate-putamen) was evaluated in rats (Shih et al., 2009). By combining pharmacological applications of the D2RA (which would produce decreased activations), markers of neuronal activation (c-fos), and electrophysiological recordings, decreases in BOLD were associated with increases in c-fos expression and electrical activity in neurons. The implication of this study is that endogenous neurotransmission may be responsible for the diminished BOLD signal. In addition, differences in BOLD signal may be the result of the anesthetic state (Figure 7) and attempts to understand not only BOLD signal biology, but also differences in pain and analgesic processing will require further understanding of the benefits of awake, trained animal imaging.
Figure 7. Resting State Networks in the Rat.
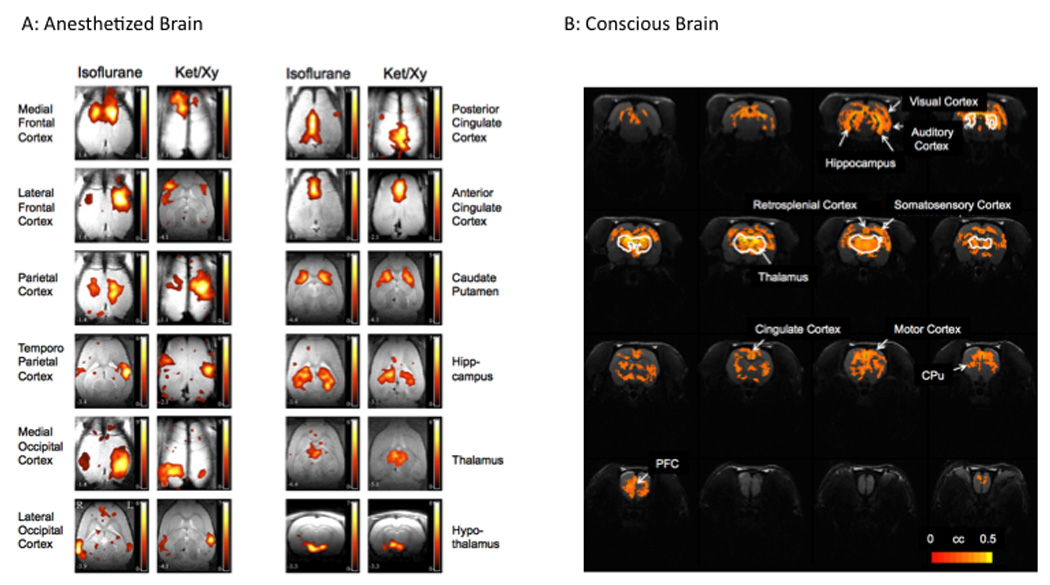
A: Anesthetized Rat. Horizontal images (except for lower right showing hypothalamus) through the rat brain showing differences in activation patterns in different brain regions to 1% Isoflurane (left columns) and ketamine 50mg/kg/h i.p./xylazine 6mg/kg/h i.p. (right columns) (From (Hutchison et al., 2010) ;with permission, Journal of Neurophysiology, with modification).
B: Conscious Rat. Funcitonal connectivity map using the thalamus as a seed region. A number of regions connected to the thalamus including the hippocampus, auditory, motor somatosensory, retrosplenial cingulate, prefrontal regions and the caudate putaminal regions (From (Zhang et al., 2010); with permission, Journal Neurosci Methods, with modification).
Genetic Strains
A great deal of new information on the genetics of pain and analgesia has been reported in animal rodent models (Lariviere and Mogil, 2010). An understanding of how strain and genetic differences affect pain processing at neural circuit levels is clearly an opportunity that is exciting. Most work on genetic manipulation has been carried out in mice strains. Species and strain differences produce different manifestations related to drug effects and disease models (e.g., neuropathic pain) (Rigaud et al., 2008). In a recent example of these approaches, evaluation of dynophin over-expressing mice brain activations to heat were compared with wild type mice with an overall decrement in signal in pain structures (Heindl-Erdmann et al., 2010). Of interest, was that administration of a kappa receptor antagonist reversed this observation to parallel changes observed in wild type mice. Another example of use of imaging in evaluating genetic modulation relates to protein kinase C (PKC) that has been reported to significantly alter pain processing (Zou et al., 2002). In mice lacking PKC gamma gene, activation of PKC produces decreased paw withdrawal latency to heat but not in knockout mice lacking the gene (Niikura et al., 2008). fMRI analyses reported early increases in brain activity in sensory and thalamic regions and delayed activation increases in emotional brain regions (cingulate, accumbens and ventral tegmental area) in wild type mice in response to the intrathecal injection of the PKC activator. None of these effects were observed in the knockout mice. Such approaches offer tremendous advantages in understanding genetic modification on brain systems in acute and chronic pain models.
Implications for Translational Uses in Drug Development – Mapping Animal-Human Equivalence
In the past 50 years there have been few novel therapeutic agents for chronic pain and patients are in need of more effective therapies. The lack of facile surrogate endpoints for potential CNS therapeutics means that making the right choice of molecule and enhancing early attrition of inappropriate targets so that the best molecules survive in development (Pritchard et al., 2003) is critical. For the CNS, the problem is also confounded by the paucity of processes and technologies that can provide a true translational bridge between preclinical and clinical testing and evaluation (Bleicher et al., 2003). See Figure 6. A number of models now show cross-species equivalence in spinal cord and higher centers. With respect to the former, much of our understanding of chronic pain has come from studies on the dorsal horn for both somatic and visceral pain (Willis and Westlund, 2001). Both human (Bouwman et al., 2008) and animal imaging studies (Zhao et al., 2009a) allow for measures of fMRI activation in the spinal cord offering further insights into intact animal evaluation of pain processing at this level. For examples of spinal cord imaging see Tables 4–6. Additional examples are in painful heat responses in the brain (Becerra et al., 2010). Studies on cross species equivalence in drug and disease state are now warranted.
Appropriate preclinical models that translate into successful clinical programs are fraught with difficulties related to predictability. Imaging offers a view of the invisible that is perhaps best described by an example. Rats do not have a robust vomiting behavior, and as a result, other species (e.g., dogs or ferrets) may be used to evaluate this side effect. A report showing that a marker for vomiting in awake rats showed activation in the well-known brainstem “vomiting centers” the area postrema and the nucleus tractus solitarius in response to the emetic drug apomorphine (Chin et al., 2006). This study captures some notable issues important in the use of imaging in drug development; including, (i) evaluation of novel drugs based on known neural circuit activations; (ii) the ability to predict potential side effects.
However, new correlation metrics will need to be determined including: (i) A correlative segmentation or functional activity domains integrating human and rat atlases; (ii) Standardization of fMRI signals: humans and rats will be required since they are imaged in very different MRI systems. In order to accomplish this, methods such as the following may be introduced – determination of activation through standard fMRI analysis for noxious and mechanical stimulation for both humans and rats; normalization of activity on a voxel-by-voxel by scaling sensory activity with BOLD amplitude generated by BOLD-control scan: breath holding in humans and CO2 challenge in rats; and summary of each brain structure by defined metrics such as peak amplitude, volume of activation (as a fraction of the volume of the structure).
Direct Effects of Analgesics on Brain Activation (phMRI)
While few studies have been reported in this area, it would seem an area of great opportunity since resting state or phMRI experiments can be performed in anesthetized (Hutchison et al., 2010) or awake animals where no stimuli are required (Ferris and Stolberg, 2010). Examples are discussed above and shown in Figures 4–7. A major obstacle in the development of new drugs for chronic pain as well as better understanding of chronic pain disease conditions relates to the poor relationship that exists between clinical and preclinical studies. Behavioral measures, although very in formative, reduce significantly the dimensions of pain. It is very difficult to assess sensory, emotional, attention, modulatory characteristics in preclinical models in an integrative manner that could begin to predict a clinical condition or the effects of a drug. An integrative approach that could in principle provide such level of information relies in neuroimaging of the Central Nervous System.
Drug Sorting
Imaging may be able to distinguish different drug effects, by class (e.g., membrane stabilizers vs. antidepressants) or within a class (e.g., antidepressants). This approach could enhance drug selection for further development by selecting best in class for CNS effects for pain and analgesia.
Drug Effects Across Species
Determination of signals within and between species is a critical process that needs to be evaluated and imaging studies perhaps provide one of the best platforms in which to do this (see (Borsook et al., 2007)). See Figure 6C.
Costs, Speed and Integrative Approaches
Clearly imaging is not a stand-alone entity that can answer all questions related to drug development. Animal imaging can now be performed relatively quickly and analysis tools are becoming standardized and efficient. Thus, the technology may be employed so that it can be a contributor to evaluating CNS acting drugs, including analgesics, relatively fast and in an objective manner. In addition, early observation of CNS effects in drugs “designed to not have any side effects” can be done early on prior to human experiments.
Detection of side effects
CNS side effects that can be predicted include (i) emesis (see (Chin et al., 2006); (ii) sedation; and (iii) potential rewarding (addictive) or aversive responses. The sensitivity and specificity need further investigation and definition. Pharmacological modulation of side effects
7. Closing the Gaps
There is a need for standardized approaches so that data can be compared across institutions/centers. Standards, reproducibility, benefits, application and opportunities for imaging awake vs. anesthetized rats in order to study brain effects of pain and analgesia. Ideally, initial data supporting similarities and differences across species and within species would be a significant contribution. Advantages of having animal imaging processes that are robust and reproducible (particularly with inferences to the human condition) allow for manipulations that are not possible in humans. Can imaging reduce animal experimentation using these technologies (Gruber and Hartung, 2004; Heindl et al., 2008)? While this is not clear, the idea of obtaining more objective data on brain effects may help focus research to accomplish this.
The Need for Animal Imaging Studies
Ideally, we would not need animal studies to further define brain systems involved in pain and analgesia (see (Langley et al., 2008)). The necessity of animal models in pain research has recently been reviewed (Mogil et al., 2010) and supports the complementary role, inherent advantages and relevance of translation that animal models may afford. On the one hand, despite significant use of animals in basic research, the benefits in terms of effective treatments with few side effects for chronic pain remain elusive. Part of the issue has been the use of animal models that have not provided useful translation into the human condition – both from a drug evaluation point of view and from a disease state point of view. Most drugs defined in humans for pain have great efficacy in animals (Kobierski et al., 2003; Borsook and Edwards, 2004). However, as suggested previously, given that chronic pain is now considered a disease of the brain, functional imaging may be used as a language of translation to help overcome some of these deficits. Furthermore, other considerations include costs of human studies, inability to test novel drugs in humans until regulatory approval (IND) is made, the ability to perform genetic manipulations in humans and inability to produce invasive procedures in initial testing of either procedures or of lesioning brain regions to better understand pain processing. Taken together, it would seem that preclinical imaging has much to offer in understanding basic neurobiological aspects of chronic pain and providing new opportunities in evaluating potential opportunities. However, as with any animal model use, optimizing animal welfare and scientific validity for research clearly deserves further attention (Auer et al., 2007).
8. Conclusions
Functional neuroimaging provides an objective readout of CNS function (neuroinformatics) that can inform neurobehavioral studies of CNS disorders such as chronic pain and provide a novel framework to evaluate therapeutic hypotheses rapidly. A better understanding of the neurobiology of pain particularly CNS changes in chronic pain has clearly made significant advances in recent times. However, the big question is whether preclinical fMRI can help provide a focus and a level of specific functionality that can guide therapeutic approaches to chronic pain treatment in the clinic? Continued efforts are poised to make functional neuroimaging of pain and analgesia a significant contributor to this end.
Research Highlights
Preclinical Imaging of Pain and Analgesia should advance systems neural science
Review of Methodological Approaches using fMRI including technical issues
Effects on brain systems in Anesthetized Animal vs. Acclimated Awake Animal Imaging
Current Review of Acute and Chronic Pain models as well as Drug effects
Evaluation of Translational models that overlap the human data
Acknowledgements
Supported by (NS064050) to DB and the L Herlands Fund to the P.AI.N. Group (DB, LB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamczak JM, Farr TD, Seehafer JU, Kalthoff D, Hoehn M. High field BOLD response to forepaw stimulation in the mouse. Neuroimage. 2010;51:704–712. doi: 10.1016/j.neuroimage.2010.02.083. [DOI] [PubMed] [Google Scholar]
- Ametamey SM, Honer M. Pharmacological prerequisites for PET ligands and practical issues in preclinical PET research. Ernst Schering Res Found Workshop. 2007:317–327. doi: 10.1007/978-3-540-49527-7_12. [DOI] [PubMed] [Google Scholar]
- Antonini A, Fagiolini M, Stryker MP. Anatomical correlates of functional plasticity in mouse visual cortex. J Neurosci. 1999;19:4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma T, Yasui H, Sato M, Inanami O, Kuwabara M. A BOLD-fMRI study of cerebral activation induced by injection of algesic chemical substances into the anesthetized rat forepaw. Jpn J Vet Res. 2008;56:99–107. [PubMed] [Google Scholar]
- Auer JA, Goodship A, Arnoczky S, Pearce S, Price J, Claes L, von Rechenberg B, Hofmann-Amtenbrinck M, Schneider E, Muller-Terpitz R, Thiele F, Rippe KP, Grainger DW. Refining animal models in fracture research: seeking consensus in optimising both animal welfare and scientific validity for appropriate biomedical use. BMC Musculoskelet Disord. 2007;8:72. doi: 10.1186/1471-2474-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin VC, Blamire AM, Allers KA, Sharp T, Styles P, Matthews PM, Sibson NR. Confounding effects of anesthesia on functional activation in rodent brain: a study of halothane and alpha-chloralose anesthesia. Neuroimage. 2005;24:92–100. doi: 10.1016/j.neuroimage.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Schnitzler A, Enck P. Functional neuroimaging of visceral sensation. J Clin Neurophysiol. 2000;17:604–612. doi: 10.1097/00004691-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Badea A, Johnson GA, Williams RW. Genetic dissection of the mouse brain using high-field magnetic resonance microscopy. Neuroimage. 2009;45:1067–1079. doi: 10.1016/j.neuroimage.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Hampshire VA. Challenges in small animal noninvasive imaging. ILAR J. 2001;42:248–262. doi: 10.1093/ilar.42.3.248. [DOI] [PubMed] [Google Scholar]
- Becerra L, Chang PC, Bishop J, Borsook D. CNS activation maps in awake rats exposed to thermal stimuli to the dorsum of the hindpaw. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.08.056. [DOI] [PubMed] [Google Scholar]
- Becerra L, Chang P-C, Bishop J, Crown E, Tsai Y, Urban M, Coimbra A, Klimas M, Hargreaves R, Iyengar S, Simmons R, Peters S, Schwarz A, Bleakman D, Borsook D. Validation of awake rat model for measures of pain and analgesia with fMRI. Honolulu, HI: ISMRM; 2009. [Google Scholar]
- Benveniste H, Blackband S. MR microscopy and high resolution small animal MRI: applications in neuroscience research. Prog Neurobiol. 2002;67:393–420. doi: 10.1016/s0301-0082(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Blackband SJ. Translational neuroscience and magnetic-resonance microscopy. Lancet Neurol. 2006;5:536–544. doi: 10.1016/S1474-4422(06)70472-0. [DOI] [PubMed] [Google Scholar]
- Bernstein JG, Han X, Henninger MA, Ko EY, Qian X, Franzesi GT, McConnell JP, Stern P, Desimone R, Boyden ES. Prosthetic systems for therapeutic optical activation and silencing of genetically-targeted neurons. Proc Soc Photo Opt Instrum Eng. 2008;6854:68540H. doi: 10.1117/12.768798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicher KH, Bohm HJ, Muller K, Alanine AI. Hit and lead generation: beyond high-throughput screening. Nat Rev Drug Discov. 2003;2:369–378. doi: 10.1038/nrd1086. [DOI] [PubMed] [Google Scholar]
- Boas DA, Dale AM, Franceschini MA. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage. 2004;23 Suppl 1:S275–S288. doi: 10.1016/j.neuroimage.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Boorman L, Kennerley AJ, Johnston D, Jones M, Zheng Y, Redgrave P, Berwick J. Negative blood oxygen level dependence in the rat: a model for investigating the role of suppression in neurovascular coupling. J Neurosci. 2010;30:4285–4294. doi: 10.1523/JNEUROSCI.6063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Edwards AD. Antineuropathic effects of the antibiotic derivative spicamycin KRN5500. Pain Med. 2004;5:104–108. doi: 10.1111/j.1526-4637.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov. 2006;5:411–424. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- Borsook D, Pendse G, Aiello-Lammens M, Glicksman M, Gostic J, Sherman S, Korn J, Shaw M, Stewart K, Gostic R, Shelly Bazes S, Hargreaves R, Becerra L. CNS response to a thermal stressor in human volunteers and awake rats may predict clinical utility of analgesics. Drug Development Research. 2007;68:23–41. [Google Scholar]
- Bouwman CJ, Wilmink JT, Mess WH, Backes WH. Spinal cord functional MRI at 3 T: gradient echo echo-planar imaging versus turbo spin echo. Neuroimage. 2008;43:288–296. doi: 10.1016/j.neuroimage.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Buerkle H, Yaksh TL. Pharmacological evidence for different alpha 2-adrenergic receptor sites mediating analgesia and sedation in the rat. Br J Anaesth. 1998;81:208–215. doi: 10.1093/bja/81.2.208. [DOI] [PubMed] [Google Scholar]
- Chang C, Shyu BC. A fMRI study of brain activations during non-noxious and noxious electrical stimulation of the sciatic nerve of rats. Brain Res. 2001;897:71–81. doi: 10.1016/s0006-8993(01)02094-7. [DOI] [PubMed] [Google Scholar]
- Chen LM, Friedman RM, Roe AW. Area-specific representation of mechanical nociceptive stimuli within SI cortex of squirrel monkeys. Pain. 2009;141:258–268. doi: 10.1016/j.pain.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tailor DR, Intes X, Chance B. Correlation between near-infrared spectroscopy and magnetic resonance imaging of rat brain oxygenation modulation. Phys Med Biol. 2003;48:417–427. doi: 10.1088/0031-9155/48/4/301. [DOI] [PubMed] [Google Scholar]
- Cherry SR. The 2006 Henry N. Wagner Lecture: Of mice and men (and positrons)--advances in PET imaging technology. J Nucl Med. 2006;47:1735–1745. [PubMed] [Google Scholar]
- Chin CL, Fox GB, Hradil VP, Osinski MA, McGaraughty SP, Skoubis PD, Cox BF, Luo Y. Pharmacological MRI in awake rats reveals neural activity in area postrema and nucleus tractus solitarius: relevance as a potential biomarker for detecting drug-induced emesis. Neuroimage. 2006;33:1152–1160. doi: 10.1016/j.neuroimage.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Chin CL, Tovcimak AE, Hradil VP, Seifert TR, Hollingsworth PR, Chandran P, Zhu CZ, Gauvin D, Pai M, Wetter J, Hsieh GC, Honore P, Frost JM, Dart MJ, Meyer MD, Yao BB, Cox BF, Fox GB. Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI. Br J Pharmacol. 2008;153:367–379. doi: 10.1038/sj.bjp.0707506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Lei H, Gruetter R. Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: on the correlation of total glucose consumption with glutamatergic action. J Cereb Blood Flow Metab. 2002;22:1343–1351. doi: 10.1097/01.WCB.0000040945.89393.46. [DOI] [PubMed] [Google Scholar]
- Collins JG, Kawahara M, Homma E, Kitahata LM. Alpha-chloralose suppression of neuronal activity. Life Sci. 1983;32:2995–2999. doi: 10.1016/0024-3205(83)90651-3. [DOI] [PubMed] [Google Scholar]
- Crespi F. Near-infrared spectroscopy (NIRS): a non-invasive in vivo methodology for analysis of brain vascular and metabolic activities in real time in rodents. Curr Vasc Pharmacol. 2007;5:305–321. doi: 10.2174/157016107782023398. [DOI] [PubMed] [Google Scholar]
- Dashti M, Geso M, Williams J. The effects of anaesthesia on cortical stimulation in rats: a functional MRI study. Australas Phys Eng Sci Med. 2005;28:21–25. doi: 10.1007/BF03178860. [DOI] [PubMed] [Google Scholar]
- De Groof G, Verhoye M, Van Meir V, Tindemans I, Leemans A, Van der Linden A. In vivo diffusion tensor imaging (DTI) of brain subdivisions and vocal pathways in songbirds. Neuroimage. 2006;29:754–763. doi: 10.1016/j.neuroimage.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Diltoer M, Camu F. Glucose homeostasis and insulin secretion during isoflurane anesthesia in humans. Anesthesiology. 1988;68:880–886. doi: 10.1097/00000542-198806000-00008. [DOI] [PubMed] [Google Scholar]
- Dobkin AB, Byles PH, Neville JF., Jr Neuroendocrine and metabolic effects of general anaesthesia during spontaneous breathing, controlled breathing, milk hypoxia, and mild hypercarbia. Can Anaesth Soc J. 1966;13:130–171. doi: 10.1007/BF03003441. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, Culley DJ, Crosby G, Tanzi RE, Xie Z. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66:620–631. doi: 10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driehuys B, Nouls J, Badea A, Bucholz E, Ghaghada K, Petiet A, Hedlund LW. Small animal imaging with magnetic resonance microscopy. ILAR J. 2008;49:35–53. doi: 10.1093/ilar.49.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong TQ. Cerebral blood flow and BOLD fMRI responses to hypoxia in awake and anesthetized rats. Brain Res. 2007;1135:186–194. doi: 10.1016/j.brainres.2006.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Spenger C, Westman E, Tominaga T, Olson L. Reorganization of sensory processing below the level of spinal cord injury as revealed by fMRI. Exp Neurol. 2008a;209:155–160. doi: 10.1016/j.expneurol.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Endo T, Spenger C, Hao J, Tominaga T, Wiesenfeld-Hallin Z, Olson L, Xu XJ. Functional MRI of the brain detects neuropathic pain in experimental spinal cord injury. Pain. 2008b;138:292–300. doi: 10.1016/j.pain.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Canals S, Simanova I, Beyerlein M, Murayama Y, Logothetis NK. Mapping of functional brain activity in freely behaving rats during voluntary running using manganese-enhanced MRI: implication for longitudinal studies. Neuroimage. 2010;49:2544–2555. doi: 10.1016/j.neuroimage.2009.10.079. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T. Imaging the immediate non-genomic effects of stress hormone on brain activity. Psychoneuroendocrinology. 2010;35:5–14. doi: 10.1016/j.psyneuen.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Field KJ, White WJ, Lang CM. Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab Anim. 1993;27:258–269. doi: 10.1258/002367793780745471. [DOI] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30:7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zorner B, Schneider R, Baltes C, Rudin M, Weber B, Schwab ME. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J Neurosci. 2009;29:12210–12219. doi: 10.1523/JNEUROSCI.1828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Governo RJ, Morris PG, Marsden CA, Chapman V. Gabapentin evoked changes in functional activity in nociceptive regions in the brain of the anaesthetized rat: an fMRI study. Br J Pharmacol. 2008;153:1558–1567. doi: 10.1038/bjp.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Governo RJ, Prior MJ, Morris PG, Marsden CA, Chapman V. Validation of an automated punctate mechanical stimuli delivery system designed for fMRI studies in rodents. J Neurosci Methods. 2007;163:31–37. doi: 10.1016/j.jneumeth.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Gruber FP, Hartung T. Alternatives to animal experimentation in basic research. ALTEX. 2004;21 Suppl 1:3–31. [PubMed] [Google Scholar]
- Hamstra GH, Peper A, Grimbergen CA. Low-power, low-noise instrumentation amplifier for physiological signals. Med Biol Eng Comput. 1984;22:272–274. doi: 10.1007/BF02442756. [DOI] [PubMed] [Google Scholar]
- Hanusch C, Hoeger S, Beck GC. Anaesthesia of small rodents during magnetic resonance imaging. Methods. 2007;43:68–78. doi: 10.1016/j.ymeth.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Heindl C, Hess A, Brune K. Refinement and reduction in animal experimentation: options for new imaging techniques. ALTEX. 2008;25:121–125. doi: 10.14573/altex.2008.2.121. [DOI] [PubMed] [Google Scholar]
- Heindl-Erdmann C, Axmann R, Kreitz S, Zwerina J, Penninger J, Schett G, Brune K, Hess A. Combining functional magnetic resonance imaging with mouse genomics: new options in pain research. Neuroreport. 2010;21:29–33. doi: 10.1097/WNR.0b013e3283324faf. [DOI] [PubMed] [Google Scholar]
- Hess A, Sergejeva M, Budinsky L, Zeilhofer HU, Brune K. Imaging of hyperalgesia in rats by functional MRI. Eur J Pain. 2007;11:109–119. doi: 10.1016/j.ejpain.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Hildebrandt IJ, Su H, Weber WA. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J. 2008;49:17–26. doi: 10.1093/ilar.49.1.17. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Maarek JM. Mapping brain function in freely moving subjects. Neurosci Biobehav Rev. 2004;28:449–461. doi: 10.1016/j.neubiorev.2004.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DP, Maarek JM. Brain maps on the go: functional imaging during motor challenge in animals. Methods. 2008;45:255–261. doi: 10.1016/j.ymeth.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Mirsattari SM, Jones CK, Gati JS, Leung LS. Functional networks in the anesthetized rat brain revealed by independent component analysis of resting-state FMRI. J Neurophysiol. 2010;103:3398–3406. doi: 10.1152/jn.00141.2010. [DOI] [PubMed] [Google Scholar]
- Huttunen JK, Grohn O, Penttonen M. Coupling between simultaneously recorded BOLD response and neuronal activity in the rat somatosensory cortex. Neuroimage. 2008;39:775–785. doi: 10.1016/j.neuroimage.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y. Large-scale recordings for drug screening in neural circuit systems. Yakugaku Zasshi. 2008;128:1251–1257. doi: 10.1248/yakushi.128.1251. [DOI] [PubMed] [Google Scholar]
- Jackson SJ, Hussey R, Jansen MA, Merrifield GD, Marshall I, Maclullich A, Yau JL, Bast T. Manganese-enhanced magnetic resonance imaging (MEMRI) of rat brain after systemic administration of MnCl(2): Hippocampal signal enhancement without disruption of hippocampus-dependent behavior. Behav Brain Res. 2011;216:293–300. doi: 10.1016/j.bbr.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Jiang J, et al. Potent, brain-penetrant, hydroisoindoline-based human neurokinin-1 receptor antagonists. J Med Chem. 2009;52:3039–3046. doi: 10.1021/jm8016514. [DOI] [PubMed] [Google Scholar]
- Jin T, Kim SG. Cortical layer-dependent dynamic blood oxygenation, cerebral blood flow and cerebral blood volume responses during visual stimulation. Neuroimage. 2008;43:1–9. doi: 10.1016/j.neuroimage.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Myers B, Lazovic J, Towner R, Greenwood-Van Meerveld B. Brain activation in response to visceral stimulation in rats with amygdala implants of corticosterone: an FMRI study. PLoS One. 2010;5:e8573. doi: 10.1371/journal.pone.0008573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Finn DP, Governo RJ, Prior MJ, Morris PG, Kendall DA, Marsden CA, Chapman V. Identification of discrete sites of action of chronic treatment with desipramine in a model of neuropathic pain. Neuropharmacology. 2009;56:405–413. doi: 10.1016/j.neuropharm.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Keller SS, Roberts N, Hopkins W. A comparative magnetic resonance imaging study of the anatomy, variability, and asymmetry of Broca's area in the human and chimpanzee brain. J Neurosci. 2009;29:14607–14616. doi: 10.1523/JNEUROSCI.2892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khubchandani M, Mallick HN, Jagannathan NR, Mohan Kumar V. Stereotaxic assembly and procedures for simultaneous electrophysiological and MRI study of conscious rat. Magn Reson Med. 2003;49:962–967. doi: 10.1002/mrm.10441. [DOI] [PubMed] [Google Scholar]
- Kobierski LA, Abdi S, DiLorenzo L, Feroz N, Borsook D. A single intravenous injection of KRN5500 (antibiotic spicamycin) produces long-term decreases in multiple sensory hypersensitivities in neuropathic pain. Anesth Analg. 2003;97:174–182. doi: 10.1213/01.ane.0000066359.83348.f1. table of contents. [DOI] [PubMed] [Google Scholar]
- Kostopanagiotou G, Kalimeris K, Christodoulaki K, Nastos C, Papoutsidakis N, Dima C, Chrelias C, Pandazi A, Mourouzis I, Pantos C. The differential impact of volatile and intravenous anaesthetics on stress response in the swine. Hormones (Athens) 2010;9:67–75. doi: 10.14310/horm.2002.1255. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Chen JH, Tsai CY, Liang KC, Yen CT. BOLD signals correlate with ensemble unit activities in rat's somatosensory cortex. Chin J Physiol. 2005;48:200–209. [PubMed] [Google Scholar]
- Labus JS, Naliboff BD, Berman SM, Suyenobu B, Vianna EP, Tillisch K, Mayer EA. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. Neuroimage. 2009;47:952–960. doi: 10.1016/j.neuroimage.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Comparison of evoked cortical activity in conscious and propofol-anesthetized rats using functional MRI. Magn Reson Med. 1999;41:412–416. doi: 10.1002/(sici)1522-2594(199902)41:2<412::aid-mrm28>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lancelot S, Zimmer L. Small-animal positron emission tomography as a tool for neuropharmacology. Trends Pharmacol Sci. 2010 doi: 10.1016/j.tips.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Langley CK, Aziz Q, Bountra C, Gordon N, Hawkins P, Jones A, Langley G, Nurmikko T, Tracey I. Volunteer studies in pain research--opportunities and challenges to replace animal experiments: the report and recommendations of a Focus on Alternatives workshop. Neuroimage. 2008;42:467–473. doi: 10.1016/j.neuroimage.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Lariviere WR, Mogil JS. The genetics of pain and analgesia in laboratory animals. Methods Mol Biol. 2010;617:261–278. doi: 10.1007/978-1-60327-323-7_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazovic J, Wrzos HF, Yang QX, Collins CM, Smith MB, Norgren R, Matyas K, Ouyang A. Regional activation in the rat brain during visceral stimulation detected by c-fos expression and fMRI. Neurogastroenterol Motil. 2005;17:548–556. doi: 10.1111/j.1365-2982.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, Jasciewicz J, Stein M, Aiello-Lammens M, Grant E, Berde C, Borsook D. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008;131:1854–1879. doi: 10.1093/brain/awn123. [DOI] [PubMed] [Google Scholar]
- Leriche L, Bjorklund T, Breysse N, Besret L, Gregoire MC, Carlsson T, Dolle F, Mandel RJ, Deglon N, Hantraye P, Kirik D. Positron emission tomography imaging demonstrates correlation between behavioral recovery and correction of dopamine neurotransmission after gene therapy. J Neurosci. 2009;29:1544–1553. doi: 10.1523/JNEUROSCI.4491-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Greve DN, Dai G, Marota JJ, Mandeville JB. Remifentanil administration reveals biphasic phMRI temporal responses in rat consistent with dynamic receptor regulation. Neuroimage. 2007;34:1042–1053. doi: 10.1016/j.neuroimage.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PK, Mandeville JB, Guangping D, Jenkins BG, Kim YR, Liu CH. Transcription MRI: a new view of the living brain. Neuroscientist. 2008;14:503–520. doi: 10.1177/1073858407309746. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AS, Beech JS, Williams SC. Small animal, whole brain fMRI: innocuous and nociceptive forepaw stimulation. Neuroimage. 2007;35:719–728. doi: 10.1016/j.neuroimage.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wu X, Dong Y, Xu Z, Zhang Y, Xie Z. Anesthetic sevoflurane causes neurotoxicity differently in neonatal naive and Alzheimer disease transgenic mice. Anesthesiology. 2010;112:1404–1416. doi: 10.1097/ALN.0b013e3181d94de1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Li Z, Treistman SN, Kim YR, King JA, Fox GB, Ferris CF. Confounding effects of volatile anesthesia on CBV assessment in rodent forebrain following ethanol challenge. J Magn Reson Imaging. 2007;26:557–563. doi: 10.1002/jmri.21083. [DOI] [PubMed] [Google Scholar]
- Luo Z, Yu M, Smith SD, Kritzer M, Du C, Ma Y, Volkow ND, Glass PS, Benveniste H. The effect of intravenous lidocaine on brain activation during non-noxious and acute noxious stimulation of the forepaw: a functional magnetic resonance imaging study in the rat. Anesth Analg. 2009;108:334–344. doi: 10.1213/ane.0b013e31818e0d34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv B, Li J, He H, Li M, Zhao M, Ai L, Yan F, Xian J, Wang Z. Gender consistency and difference in healthy adults revealed by cortical thickness. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Docherty JC. Capsaicin as a source for painful stimulation in functional MRI. J Magn Reson Imaging. 2001;14:341–347. doi: 10.1002/jmri.1192. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Stroman PW. Functional imaging of the rat cervical spinal cord. J Magn Reson Imaging. 2002;16:553–558. doi: 10.1002/jmri.10185. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Stroman PW, Turner A, Gregorash L, Foniok T, Wright A. Functional MRI of the rat lumbar spinal cord involving painful stimulation and the effect of peripheral joint mobilization. J Magn Reson Imaging. 2003a;18:152–159. doi: 10.1002/jmri.10339. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Jones C, Gruwel ML, Foreman D, Fernyhough P, Calcutt NA. Functional magnetic resonance imaging of the spinal cord during sensory stimulation in diabetic rats. J Magn Reson Imaging. 2009;30:271–276. doi: 10.1002/jmri.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisza KL, Gregorash L, Turner A, Foniok T, Stroman PW, Allman AA, Summers R, Wright A. Functional MRI involving painful stimulation of the ankle and the effect of physiotherapy joint mobilization. Magn Reson Imaging. 2003b;21:489–496. doi: 10.1016/s0730-725x(03)00074-2. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Leite FP, Marota JJ. Spin-echo MRI underestimates functional changes in microvascular cerebral blood plasma volume using exogenous contrast agent. Magn Reson Med. 2007;58:769–776. doi: 10.1002/mrm.21380. [DOI] [PubMed] [Google Scholar]
- Martin C, Sibson NR. Pharmacological MRI in animal models: a useful tool for 5-HT research? Neuropharmacology. 2008;55:1038–1047. doi: 10.1016/j.neuropharm.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Martin C, Jones M, Martindale J, Mayhew J. Haemodynamic and neural responses to hypercapnia in the awake rat. Eur J Neurosci. 2006a;24:2601–2610. doi: 10.1111/j.1460-9568.2006.05135.x. [DOI] [PubMed] [Google Scholar]
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage. 2006b;32:33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Fukuda M, Vazquez A, Kim SG. Dose-dependent effect of isoflurane on neurovascular coupling in rat cerebral cortex. Eur J Neurosci. 2009;30:242–250. doi: 10.1111/j.1460-9568.2009.06812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- Mechefske CK, Geris R, Gati JS, Rutt BK. Acoustic noise reduction in a 4 T MRI scanner. MAGMA. 2002;13:172–176. doi: 10.1007/BF02678593. [DOI] [PubMed] [Google Scholar]
- Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyo T, Antognini JF, Carstens E. Etomidate depresses lumbar dorsal horn neuronal responses to noxious thermal stimulation in rats. Anesth Analg. 2006;102:1169–1173. doi: 10.1213/01.ane.0000204764.13637.d4. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Moylan Governo RJ, Morris PG, Prior MJ, Marsden CA, Chapman V. Capsaicin-evoked brain activation and central sensitization in anaesthetised rats: a functional magnetic resonance imaging study. Pain. 2006;126:35–45. doi: 10.1016/j.pain.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Zou F, Huang H, Moy SS, Lauder J, Crawley JN, Threadgill DW, Wright FA, Magnuson TR. Large-scale gene expression differences across brain regions and inbred strains correlate with a behavioral phenotype. Genetics. 2006;174:1229–1236. doi: 10.1534/genetics.106.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KP, Antognini JF. Isoflurane and propofol have similar effects on spinal neuronal windup at concentrations that block movement. Anesth Analg. 2006;103:1453–1458. doi: 10.1213/01.ane.0000247732.33602.f5. [DOI] [PubMed] [Google Scholar]
- Niikura K, Kobayashi Y, Okutsu D, Furuya M, Kawano K, Maitani Y, Suzuki T, Narita M. Implication of spinal protein kinase Cgamma isoform in activation of the mouse brain by intrathecal injection of the protein kinase C activator phorbol 12,13-dibutyrate using functional magnetic resonance imaging analysis. Neurosci Lett. 2008;433:6–10. doi: 10.1016/j.neulet.2007.12.049. [DOI] [PubMed] [Google Scholar]
- Oyama T. Endocrine responses to anaesthetic agents. Br J Anaesth. 1973;45:276–281. doi: 10.1093/bja/45.3.276. [DOI] [PubMed] [Google Scholar]
- Palecek J. The role of dorsal columns pathway in visceral pain. Physiol Res. 2004;53 Suppl 1:S125–S130. [PubMed] [Google Scholar]
- Petersen-Felix S, Arendt-Nielsen L, Bak P, Roth D, Fischer M, Bjerring P, Zbinden AM. Analgesic effect in humans of subanaesthetic isoflurane concentrations evaluated by experimentally induced pain. Br J Anaesth. 1995;75:55–60. doi: 10.1093/bja/75.1.55. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Arcaro M, Weiner KS, Kalkus JF, Inati SJ, Gross CG, Kastner S. Neural representations of faces and body parts in macaque and human cortex: a comparative FMRI study. J Neurophysiol. 2009;101:2581–2600. doi: 10.1152/jn.91198.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann A, Barjat H, Tilling LC, James MF. Pharmacological fMRI--challenges in analysing drug-induced single-event BOLD responses; Conf Proc IEEE Eng Med Biol Soc; 2007. pp. 3411–3416. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Porszasz R, Beckmann N, Bruttel K, Urban L, Rudin M. Signal changes in the spinal cord of the rat after injection of formalin into the hindpaw: characterization using functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1997;94:5034–5039. doi: 10.1073/pnas.94.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JF, Jurima-Romet M, Reimer ML, Mortimer E, Rolfe B, Cayen MN. Making better drugs: Decision gates in non-clinical drug development. Nat Rev Drug Discov. 2003;2:542–553. doi: 10.1038/nrd1131. [DOI] [PubMed] [Google Scholar]
- Rigaud M, Gemes G, Barabas ME, Chernoff DI, Abram SE, Stucky CL, Hogan QH. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain. 2008;136:188–201. doi: 10.1016/j.pain.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas MJ, Navas JA, Greene SA, Rector DM. Discrimination of auditory stimuli during isoflurane anesthesia. Comp Med. 2008;58:454–457. [PMC free article] [PubMed] [Google Scholar]
- Schaeffter T, Dahnke H. Magnetic resonance imaging and spectroscopy. Handb Exp Pharmacol. 2008:75–90. doi: 10.1007/978-3-540-72718-7_4. [DOI] [PubMed] [Google Scholar]
- Schepkin VD, Brey WW, Gor'kov PL, Grant SC. Initial in vivo rodent sodium and proton MR imaging at 21.1 T. Magn Reson Imaging. 2010;28:400–407. doi: 10.1016/j.mri.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009;47:1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah YB, Haynes L, Prior MJ, Marsden CA, Morris PG, Chapman V. Functional magnetic resonance imaging studies of opioid receptor-mediated modulation of noxious-evoked BOLD contrast in rats. Psychopharmacology (Berl) 2005;180:761–773. doi: 10.1007/s00213-005-2214-6. [DOI] [PubMed] [Google Scholar]
- Sharma A, Lelic D, Brock C, Paine P, Aziz Q. New technologies to investigate the brain-gut axis. World J Gastroenterol. 2009;15:182–191. doi: 10.3748/wjg.15.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Singh J, Singh AP, Peshin PK. Haemodynamics, blood gas and metabolic changes after anaesthesia with chloral hydrate and magnesium sulphate in camels (Camelus dromedarius) Res Vet Sci. 1984;36:12–15. [PubMed] [Google Scholar]
- Shih YY, Chang C, Chen JC, Jaw FS. BOLD fMRI mapping of brain responses to nociceptive stimuli in rats under ketamine anesthesia. Med Eng Phys. 2008a;30:953–958. doi: 10.1016/j.medengphy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Shih YY, Chen YY, Chen CC, Chen JC, Chang C, Jaw FS. Whole-brain functional magnetic resonance imaging mapping of acute nociceptive responses induced by formalin in rats using atlas registration-based event-related analysis. J Neurosci Res. 2008b;86:1801–1811. doi: 10.1002/jnr.21638. [DOI] [PubMed] [Google Scholar]
- Shih YY, Chen CC, Shyu BC, Lin ZJ, Chiang YC, Jaw FS, Chen YY, Chang C. A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. J Neurosci. 2009;29:3036–3044. doi: 10.1523/JNEUROSCI.3447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Shoham D, Grinvald A. The cortical representation of the hand in macaque and human area S-I: high resolution optical imaging. J Neurosci. 2001;21:6820–6835. doi: 10.1523/JNEUROSCI.21-17-06820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Cerebral metabolism and consciousness. C R Biol. 2003;326:253–273. doi: 10.1016/s1631-0691(03)00071-4. [DOI] [PubMed] [Google Scholar]
- Shyu BC, Lin CY, Sun JJ, Sylantyev S, Chang C. A method for direct thalamic stimulation in fMRI studies using a glass-coated carbon fiber electrode. J Neurosci Methods. 2004a;137:123–131. doi: 10.1016/j.jneumeth.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Shyu BC, Lin CY, Sun JJ, Chen SL, Chang C. BOLD response to direct thalamic stimulation reveals a functional connection between the medial thalamus and the anterior cingulate cortex in the rat. Magn Reson Med. 2004b;52:47–55. doi: 10.1002/mrm.20111. [DOI] [PubMed] [Google Scholar]
- Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab. 2003;23:472–481. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A, Cardoso-Cruz H, Silva F, Galhardo V, Antunes L. Comparison of anesthetic depth indexes based on thalamocortical local field potentials in rats. Anesthesiology. 2010;112:355–363. doi: 10.1097/ALN.0b013e3181ca3196. [DOI] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc Natl Acad Sci U S A. 2002;99:15182–15187. doi: 10.1073/pnas.222561899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Bock NA. Manganese-enhanced MRI: an exceptional tool in translational neuroimaging. Schizophr Bull. 2008;34:595–604. doi: 10.1093/schbul/sbn056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Lee JH, Aoki I, Koretsky AP. Manganese-enhanced magnetic resonance imaging (MEMRI): methodological and practical considerations. NMR Biomed. 2004;17:532–543. doi: 10.1002/nbm.945. [DOI] [PubMed] [Google Scholar]
- Strobel GE, Wollman H. Pharmacology of anesthetic agents. Fed Proc. 1969;28:1386–1403. [PubMed] [Google Scholar]
- Strome EM, Doudet DJ. Animal models of neurodegenerative disease: insights from in vivo imaging studies. Mol Imaging Biol. 2007;9:186–195. doi: 10.1007/s11307-007-0093-4. [DOI] [PubMed] [Google Scholar]
- Sydekum E, Baltes C, Ghosh A, Mueggler T, Schwab ME, Rudin M. Functional reorganization in rat somatosensory cortex assessed by fMRI: elastic image registration based on structural landmarks in fMRI images and application to spinal cord injured rats. Neuroimage. 2009;44:1345–1354. doi: 10.1016/j.neuroimage.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Taketa Y, Niikura K, Kobayashi Y, Furuya M, Shimizu T, Narita M, Imai S, Kuzumaki N, Maitani Y, Yamazaki M, Inada E, Iseki M, Suzuki T. Direct evidence for the ongoing brain activation by enhanced dynorphinergic system in the spinal cord under inflammatory noxious stimuli. Anesthesiology. 2010;112:418–431. doi: 10.1097/ALN.0b013e3181ca31d9. [DOI] [PubMed] [Google Scholar]
- Tsurugizawa T, Uematsu A, Uneyama H, Torii K. Effects of isoflurane and alpha-chloralose anesthesia on BOLD fMRI responses to ingested L-glutamate in rats. Neuroscience. 2010;165:244–251. doi: 10.1016/j.neuroscience.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Tuor UI, McKenzie E, Tomanek B. Functional magnetic resonance imaging of tonic pain and vasopressor effects in rats. Magn Reson Imaging. 2002;20:707–712. doi: 10.1016/s0730-725x(02)00599-4. [DOI] [PubMed] [Google Scholar]
- Tuor UI, Malisza K, Foniok T, Papadimitropoulos R, Jarmasz M, Somorjai R, Kozlowski P. Functional magnetic resonance imaging in rats subjected to intense electrical and noxious chemical stimulation of the forepaw. Pain. 2000;87:315–324. doi: 10.1016/S0304-3959(00)00293-1. [DOI] [PubMed] [Google Scholar]
- Van der Linden A, Van Meir V, Boumans T, Poirier C, Balthazart J. MRI in small brains displaying extensive plasticity. Trends Neurosci. 2009;32:257–266. doi: 10.1016/j.tins.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415–1421. doi: 10.1056/NEJM198405313102202. [DOI] [PubMed] [Google Scholar]
- Wang J, Maurer L. Positron Emission Tomography: applications in drug discovery and drug development. Curr Top Med Chem. 2005;5:1053–1075. doi: 10.2174/156802605774297056. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bradesi S, Maarek JM, Lee K, Winchester WJ, Mayer EA, Holschneider DP. Regional brain activation in conscious, nonrestrained rats in response to noxious visceral stimulation. Pain. 2008;138:233–243. doi: 10.1016/j.pain.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Guo Y, Bradesi S, Labus JS, Maarek JM, Lee K, Winchester WJ, Mayer EA, Holschneider DP. Sex differences in functional brain activation during noxious visceral stimulation in rats. Pain. 2009;145:120–128. doi: 10.1016/j.pain.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Frahm J, Michaelis T. Functional mapping of neural pathways in rodent brain in vivo using manganese-enhanced three-dimensional magnetic resonance imaging. NMR Biomed. 2004;17:554–568. doi: 10.1002/nbm.937. [DOI] [PubMed] [Google Scholar]
- Weber R, Ramos-Cabrer P, Wiedermann D, van Camp N, Hoehn M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage. 2006;29:1303–1310. doi: 10.1016/j.neuroimage.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Wegener S, Wong EC. Longitudinal MRI studies in the isoflurane-anesthetized rat: long-term effects of a short hypoxic episode on regulation of cerebral blood flow as assessed by pulsed arterial spin labelling. NMR Biomed. 2008;21:696–703. doi: 10.1002/nbm.1243. [DOI] [PubMed] [Google Scholar]
- Wehrl HF, Judenhofer MS, Wiehr S, Pichler BJ. Pre-clinical PET/MR: technological advances and new perspectives in biomedical research. Eur J Nucl Med Mol Imaging. 2009;36 Suppl 1:S56–S68. doi: 10.1007/s00259-009-1078-0. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Vera-Portocarrero LP, Zhang L, Wei J, Quast MJ, Cleeland CS. fMRI of supraspinal areas after morphine and one week pancreatic inflammation in rats. Neuroimage. 2009;44:23–34. doi: 10.1016/j.neuroimage.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Al-Chaer ED, Quast MJ, Westlund KN. A visceral pain pathway in the dorsal column of the spinal cord. Proc Natl Acad Sci U S A. 1999;96:7675–7679. doi: 10.1073/pnas.96.14.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Jr, Westlund KN. The role of the dorsal column pathway in visceral nociception. Curr Pain Headache Rep. 2001;5:20–26. doi: 10.1007/s11916-001-0006-1. [DOI] [PubMed] [Google Scholar]
- Wise RG, Tracey I. The role of fMRI in drug discovery. J Magn Reson Imaging. 2006;23:862–876. doi: 10.1002/jmri.20584. [DOI] [PubMed] [Google Scholar]
- Wolf T, Lindauer U, Reuter U, Back T, Villringer A, Einhaupl K, Dirnagl U. Noninvasive near infrared spectroscopy monitoring of regional cerebral blood oxygenation changes during peri-infarct depolarizations in focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1997;17:950–954. doi: 10.1097/00004647-199709000-00004. [DOI] [PubMed] [Google Scholar]
- Xu H, Li SJ, Bodurka J, Zhao X, Xi ZX, Stein EA. Heroin-induced neuronal activation in rat brain assessed by functional MRI. Neuroreport. 2000;11:1085–1092. doi: 10.1097/00001756-200004070-00036. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Aldskogius H, Seiger A, Wiesenfeld-Hallin Z. Chronic pain-related syndrome in rats after ischemic spinal cord lesion: a possible animal model for pain in patients with spinal cord injury. Pain. 1992;48:279–290. doi: 10.1016/0304-3959(92)90070-R. [DOI] [PubMed] [Google Scholar]
- Yu X, Wang S, Chen DY, Dodd S, Goloshevsky A, Koretsky AP. 3D mapping of somatotopic reorganization with small animal functional MRI. Neuroimage. 2010;49:1667–1676. doi: 10.1016/j.neuroimage.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Rane P, Huang W, Liang Z, Kennedy D, Frazier JA, King J. Mapping resting-state brain networks in conscious animals. J Neurosci Methods. 2010;189:186–196. doi: 10.1016/j.jneumeth.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Williams M, Meng X, Welsh DC, Grachev ID, Hargreaves R, Williams DS. Pain fMRI in rat cervical spinal cord: an echo planar imaging evaluation of sensitivity of BOLD and blood volume-weighted fMRI. Neuroimage. 2009a;44:349–362. doi: 10.1016/j.neuroimage.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Zhao F, Williams M, Meng X, Welsh DC, Coimbra A, Crown ED, Cook JJ, Urban MO, Hargreaves R, Williams DS. BOLD and blood volume-weighted fMRI of rat lumbar spinal cord during non-noxious and noxious electrical hindpaw stimulation. Neuroimage. 2008;40:133–147. doi: 10.1016/j.neuroimage.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Zhao F, Williams M, Welsh DC, Meng X, Ritter A, Abbadie C, Cook JJ, Reicin AS, Hargreaves R, Williams DS. fMRI investigation of the effect of local and systemic lidocaine on noxious electrical stimulation-induced activation in spinal cord. Pain. 2009b;145:110–119. doi: 10.1016/j.pain.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience. 2002;115:775–786. doi: 10.1016/s0306-4522(02)00490-6. [DOI] [PubMed] [Google Scholar]


