Abstract
IL-10 is believed to underlie many of the immunologic defects in human visceral leishmaniasis (VL). We have identified CD4+CD25−Foxp3−T cells as the major source of IL-10 in the VL spleen. IL-27, a member of the IL-6/IL-12 cytokine family, has been shown to promote development of IL-10 producing T cells, in part by up-regulating their production of autocrine IL-21. We investigated whether IL-27 and IL-21 are associated with human VL. IL-27 was elevated in VL plasma, and at pre-treatment, spleen cells showed significantly elevated mRNA levels of both IL-27 sub-units, IL-27p28 and EBI-3, as well as IL-21, compared to post-treatment biopsies. CD14+ spleen cells were the main source of IL-27 mRNA, while CD3+T cells were the main source of IL-21. IL-27 mRNA could be strongly up-regulated in normal donor macrophages with IFN-γ and IL-1β, conditions consistent with those in the VL spleen. Lastly, a whole blood assay revealed that most VL patients could produce antigen-specific IFN-γ and IL-10, and that the IL-10 could be augmented with rhIL-21. Thus, pro-inflammatory cytokines acting on macrophages in the VL spleen have the potential to upregulate IL-27, which in turn can induce IL-21 to expand IL-10 producing T cells as a mechanism of feedback control.
Introduction
Visceral leishmaniasis (VL) or kala-azar, is a chronic infectious disease caused by the protozoan parasite, Leishmania donovani or Leishmania chagasi/infantum. VL is endemic in 62 countries, with a total of 200 million people at risk (1). Ninety percent of the global burden of kala-azar cases occurs in the Indian subcontinent and East Africa where the causative agent is L. donovani. Every year, more than 100,000 cases of VL occur in India alone, mainly amongst the poorest population groups living in rural areas (2).
Clinically, the disease is characterized by fever, hepatosplenomegaly, weight loss, anemia, leucopenia, and hypergammaglobulinemia, accompanied by high titers of anti-leishmanial antibodies. Following inoculation into the skin by vector sand flies, the parasite infiltrates vital organs, and replicates primarily in macrophages of the liver and spleen. Despite the progressive nature of the disease, elevated serum levels of IFN-γ and TNF-α, as well elevated mRNA levels of these cytokines in lesional tissue, are consistent findings in human VL (3–7), suggesting that immunosuppressive mechanisms that inhibit the production of adequate concentrations or functions of effector cytokines may be operating in VL.
Based on extensive experimental and clinical findings, IL-10 is believed to underlie many of the immunologic defects in kala-azar [reviewed in (8)]. IL-10 is a regulatory cytokine that has primarily suppressive effects on immune function, targeting multiple activation and antigen presentation pathways of macrophages and dendritic cells. Although induction of IL-10 by host cells during chronic infection is considered a homeostatic mechanism to limit the tissue damage caused by excessive inflammation, effective clearance of Leishmania can also be compromised. In mice, genetic ablation of IL-10 renders mice highly resistant to L. donovani infection, and treatment with anti-IL-10 receptor antibody promotes clinical cure (9, 10). In humans, patients with an advanced stage of disease have elevated levels of IL-10 in serum as well strongly enhanced IL-10 mRNA levels in spleen, lymph nodes, and bone marrow (3–7). A role for IL-10 in human VL pathology is supported by studies demonstrating the effect of IL-10 inhibition on enhancement of the IFN-γ response by antigen stimulated PBMC, and by the finding that IL-10 neutralization in VL serum inhibited L. donovani replication in macrophages (7, 11, 12).
IL-10 can be produced by many cell types, including innate cells, B-cells, and multiple T cell subsets. We have recently addressed the cellular source of IL-10 in human VL, and identified CD4+CD25−Foxp3− T cells as the major source of elevated IL-10 mRNA in the VL spleen (7). The findings suggest that IL-10 producing adaptive Treg or Tr1 cells, some of which may co-express IFN-γ, are important in suppression of anti-leishmanial immunity in human VL. Similar cells have been shown to be an important source of IL-10 in L. donovani infected mice, and in C57Bl/6 mice infected with a non-healing strain of L. major (13–15). There have been recent advances concerning the factors that regulate IL-10 production by Tr1 cells or Th1 cells in autoimmune or infection models in mice. While originally described as a co-factor with IL-12 for Th1 differentiation, a series of recent reports have implicated IL-27, a member of the IL-6/IL-12 cytokine family, as central to the regulation of T cell IL-10 production [reviewed in (16, 17)]. Further, autocrine IL-21 has been shown to amplify IL-27 induced T cell IL-10 expression in the mouse (18). IL-27 is a heterodimeric cytokine composed of an IL-12p35 related, p28 subunit, and an IL-12p40 related, Epstein Barr virus-induced 3 (EBI-3) subunit, and is produced primarily by innate cells such as macrophages and dendritic cells (DC) (19). IL-27 signals through a receptor containing the common IL-6 signal transducing subunit gp130 and the unique IL-27Rα subunit. Analysis of the expression patterns of IL-27p28 and EBI-3 has shown that these subunits can be differentially regulated; while some cell types and tissues express both, others express only one, suggesting that the subunits might dimerize extracellularly, or associate with different subunits of the IL-12 family (e.g. IL-35, composed of EBI-3 and p35 (20)). Several recent studies indicate that IL-27p28 is regulated at the transcriptional level by TLR ligation and TRIF mediated activation of IRF-3, and/or by types I and II IFN through recruitment of STAT-1/IRF1 and IRF8 to the human or mouse IL-27p28 promoter (21–23). In the mouse, EBI-3 is also strongly induced by TLR agonists via activation of transcription factors PU.1 and NF-kB (24).
In murine studies, whereas IL-27 exerts STAT1 dependent Th1 inducing signals in naïve cells, it appears to convert activated, inflammatory CD4+ T cells into IL-10 producing Th1 or Tr1 cells in a STAT1 and STAT3 dependent manner (25), presumably as a mechanism of feedback control. Thus, IL-27 receptor deficient mice can display both a Th1 response defect and exacerbated inflammation, depending on the nature and duration of the inflammatory stimulus. In IL-27Rα -deficient mice infected with L. donovani, the mice displayed enhanced resistance but developed severe liver immunopathology, consistent with a T cell IL-10 defect, although this was not specifically addressed (26). Severe cutaneous pathology was also associated with L. major infection in IL-27Rα -deficient mice, which in this case was related to reduced frequencies of IL-10 producing Th1 cells, and to a striking increase in antigen-specific IL-17 producing CD4+ T cells (27). The ability of IL-27 to suppress Th17 development in other inflammatory settings in the mouse has been reported (28, 29).
Apart from a series of in vitro studies demonstrating the role of IL-27 in promoting IL-10 production by human CD4+ T cells and in inhibiting Th17 development (30), only a few studies have investigated the pathophysiological roles of human IL-27, mainly in inflammatory conditions (31, 32). The study of IL-27 in human infection has been confined to assessment of circulating levels of the cytokine in patients with HIV or HBV (33, 34). The aim of the present study was to determine whether IL-27, IL-21, and Th17 related cytokines are upregulated in human VL, to understand the cellular source of these cytokines, and their possible role in regulation of T cell IL-10 production and in the pathogenesis of this disease.
Material and Methods
Study subjects
All patient presented with symptoms of VL at the Kala-azar Research Center, Muzaffarpur, Bihar, India. Their diagnosis was in each case confirmed by detection of amastigotes in splenic aspirates smears and/or by detection of antibodies against the recombinant antigen, K39. In total, 108 patients were included in one or more of the various aspects of the study. Splenic needle aspirates were collected for diagnostic purposes before treatment and 3–4 weeks after initiation of anti-leishmanial treatment to evaluate parasitologic cure. All of the patients included in the study demonstrated parasitologic cure as defined by the absence parasites in the post-treatment splenic aspirate smears, and all remained free of symptoms at 6 mo. followup. Control spleen cells (n = 10), were obtained from healthy organ donors (HODs) as described elsewhere (7). Heparinized blood was obtained from VL patients (n = 54) before treatment and from endemic control (EC) volunteers (n = 29) who were in each case healthy household family members of an active VL case. Aggregate clinical data of patients are presented in Table 1. All patients were seronegative for HIV. This work was conducted with ethical approval obtained from institutional review committees in India and the United States and informed consent was obtained from all patients or their guardians.
Table 1.
Aggregate clinical data for VL patients and Endemic controls
| VL (pre-treatinent)A | VL (post-treatment) | EC | control spleen | ||
|---|---|---|---|---|---|
| n | 100 | 39 | 29 | 10 | |
| Age | 25.91 ± 15.2(25)B | 27 ± 13.4(25) | 33.5 ± 9.2(35) | ND | |
| Sex (M/F) | 64/36 | 25/14 | 18/11 | ND | |
| Duration of illness (days) | 24.3 ± 30.3(15) | 27.2 ± 23.2(20) | N/A | NA | |
| Infection scoreC Day 0 | 2 ± 1.1(2) | 1.92 ± 1(2) | N/A | N/A | |
| Day dis | 0 | 0 | NA | NA | |
| N/A | N/A | ||||
| Spleen size (cm) Day 0 | 4.8 ± 3.9(3) | 4.8 ± 3.3(4) | |||
| Day dis | 0.57 ± 1.3 | 0.8 ± 1.6 | N/A | N/A | |
| WBC(X103/mm3) Day 0 | 3.2 ± 1.3(3) | 3.1 ± 0.9(3) | ND | ND | |
| Day dis | 7 ± 2.3(7) | 6.3 ± 2.1(6.2) | ND | N/A | |
N/D, not done: N/A, not applicable.
Includes 39 donors from whom post-treatment samples were also available
Mean values ± SD of aggregated data are shown, median values are in parenthesis.
Scoring of parasite load is on a logarithmic scale from 1 to 6, were 0 is no parasites per 1,000 microscopic fields (1,000X), 1 is 1–10 parasites per 1,000 fields, and 6 is >100 parasites per field.
Detection of cytokine in Plasma
Plasma levels of IFN-γ, IL-10 and IL-17 were measured by multiplex analysis (Aushon Biosystems, Billerica, MA). IL-27 and IL-21 plasma levels were measured by dual antibody sandwich-ELISA kits according to manufacturer’s instruction (R&D Systems, Minneapolis, MN and e-Biosciences, San Diego, CA, respectively). The IL-27 ELISA detects the heterodimeric cytokine. Each sample was tested in duplicate and cytokine concentrations were calculated using a standard curve generated from recombinant cytokines. Cytokine values were expressed as picograms/milliliter (pg/ml).
Real-time PCR with whole and sequentially enriched splenic cell subsets
Whole splenic needle aspirates (100–150 µl) were directly placed in 1 ml RNAlater (Qiagen, Valencia, CA) and stored at –70°C until use. Total RNA was isolated using the RNeasy minikit and Qiashredder homogenizers (Qiagen) according to the manufacturer’s protocol. The quality of RNA was assessed by denaturing agarose gel electrophoresis. cDNA synthesis was performed in 20µl reactions on 0.5–1.0 µg RNA using High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) that uses random primers and Mutliscribe TM MuLV reverse transcriptase enzyme. Incubation conditions for reverse transcription were 10 min at 25°C, followed by 2 hours at 37°C and were performed on a MasterCycler Gradient (Eppendrof North America, Westbury, NY).
RNA was prepared from enriched fractions of splenic aspirate cells, isolated using a modification of the sequential selection protocol described previously (7). Briefly, cell subsets were enriched by sequential positive selection of CD19+ (B-cells) followed by CD3+ (T cells) cell selection from the CD19- depleted fraction. CD14+ (Monocytes/Macrophages) cells were selected from the CD19- and CD3- depleted fraction followed by CD1c+ (DCs) cell selection from the CD19-, CD3- and CD14- depleted fraction. Finally, CD56+ (NK cells) cells were selection from the CD19-, CD3-, CD14-, and CD1c- depleted fraction. All selections were carried out using MACS beads (Miltenyi Biotec) and Magnetic LS columns (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. Cells remaining after the removal of CD19+, CD3+, CD14+, CD1c+, and CD56+ cells were referred to as ‘depleted’. The positively selected subsets and depleted cells were re-suspended in 200–300µl RNA later and stored at −70°C until use. RNA isolation and cDNA preparation was carried out as described above, and the extent of subset enrichment was validated by PCR amplification of cell specific markers. Real-time PCR was performed on an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster city, CA) using cDNA specific FAM-MGB-labeled primer/probe for IL-10, IFN-γ, IL-27p28, EBI-3, IL-21, IL-12p19, IL-1β, IRF-1, IL-17, RORγt, CD14, CD19, CD1c and VIC-MGB-labeled 18S mRNA. All reagents were purchased from Applied Biosystems. The relative quantification of products was determined by the number of cycles over 18S mRNA endogenous control required to detect the mRNA of interest.
In vitro generation of monocyte-derived macrophages
CD14+ monocytes were obtained by elutriation from normal volunteer blood donors at the National Institutes of Health Clinical Center Department of Transfusion Medicine. Cells were frozen at 1×108 cells/ml until use. Briefly, monocytes were thawed, and 50 × 106 cells were cultured in six-well tissue culture plates in a volume of 2 ml. All cells were cultured in complete RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 2% heat inactivated Human AB serum (Gemini Bio-Products, Woodland, CA), 20 mM L-glutamine, 100U/ml penicillin and 100µg/ml streptomycin (Invitrogen) at 37°C under 5% CO2. For generation of macrophages (MF), human rhM-CSF (PeproTech, Rocky Hill, NJ) was added to cultures on days 0, 3, and 5 at 62.5 ng/ml. Cells were harvested on day 8. Cell numbers and viability were determined by trypan blue exclusion.
In vitro activation of macrophages and infection
Harvested MFs were resuspended at 1–2 × 106/ml in complete RPMI, plated in a 48-well tissue culture plate and stimulated with IFN-γ (10ng/ml), TNF-α (10ng/ml), (PeproTech) their combination, or medium alone for 8 h prior to infection with hamster derived, tissue-purified amastigotes (4:1 ratio) of L. donovani strain 9515 (MHOM/IN/95/9515), isolated from a patient with VL in Bihar, India. Macrophages were cultured for another 16 h and either lysed for mRNA expression analysis, or harvested for Wright-Giemsa staining of cytospin preparations to monitor infections by light microscopy.
Whole blood culture assay and cytokine measurement
Heparinized whole blood was collected from active VL patients and endemic controls. Since plasma from VL cases contain pre-existing levels of cytokines, the plasma was removed and the blood cells were washed once with saline. The autologous plasma was replaced with an equivalent volume of plasma pooled from healthy individuals living in a non-endemic area for VL. Whole blood cells were cultured in the absence of antigen or stimulated with L. donovani soluble antigen (SLA, 10µg/ml) prepared from L. donovani stationary-phage promastigotes (prepared as described previously, (7)). Where indicated, recombinant human IL-27 (100ng/ml) (R&D Systems) or human IL-21 (25ng/ml) (PeproTech) alone or in combination, were added to the whole blood cultures. Cultures were incubated at 37°C with 5% CO2. After 24h, culture supernatants were collected and stored at −70°C until ELISA measurement. The frozen culture supernatant from whole blood assay was thawed and levels of IFN-γ and IL-10 were measured using Biolegend kits (Biolegend, San Diego, CA) according to manufacturer’s instruction. Cytokine concentrations in the samples were calculated with a standard curve generated from recombinant cytokines.
Statistics
For statistical analysis, data were analyzed using GraphPad Prism 5 software (GraphPad Software). Differences between groups or paired pre- and post- treatment groups were compared by Mann-Whitney and Wilcoxon singed-rank tests for unpaired and paired analyses.
Results
Active VL is associated with elevated levels of IL-27 in plasma and IL-27 mRNA in spleen
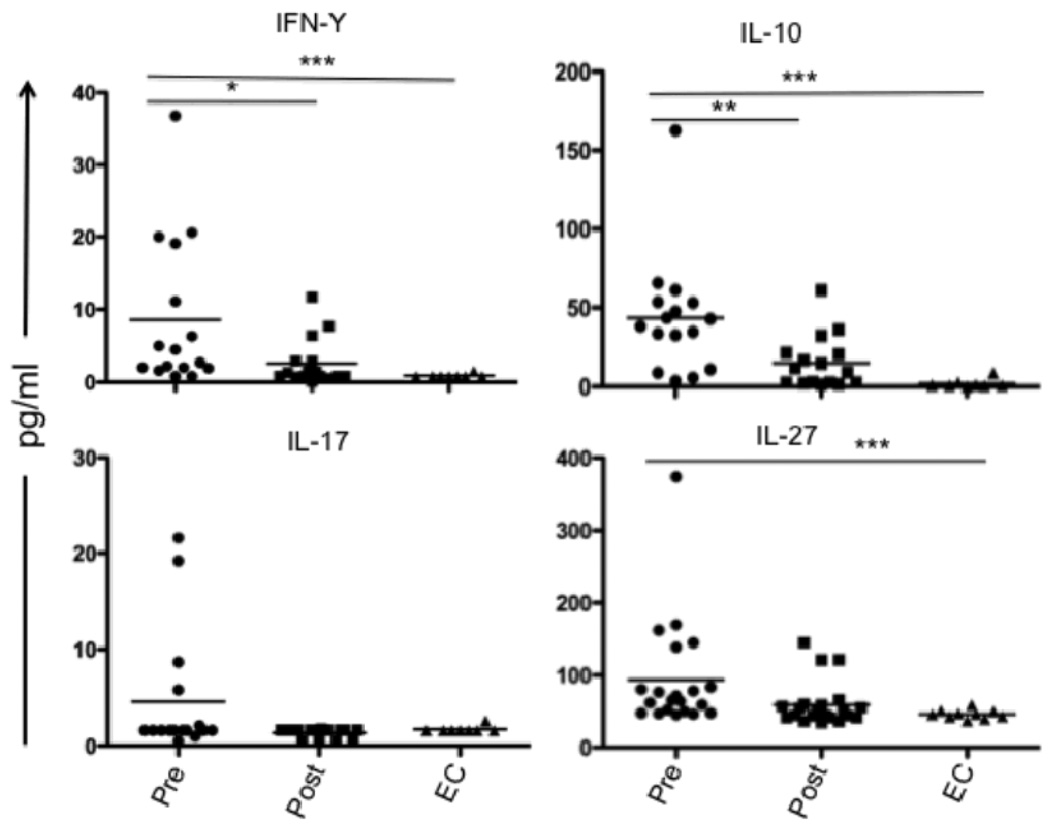
We and others have previously demonstrated elevated plasma levels of IL-10, as well as elevated mRNA levels for IL-10 and IFN-γ in splenic biopsies from VL patients (7, 35). We further demonstrated that T cells are the main source of IL-10 and IFN-γ in the VL spleen. In the mouse, IL-27 was recently found to induce Th1 cell differentiation and T cell IL-10 production, and to counter-regulate Th17 lineage development. We were interested to know if a similar pathway might regulate T cell IL-10 production in VL patients. Screening of plasma for IL-27, IL-10, IFN-γ, IL-17, and IL-21 by ELISA and multiplex assays, revealed significantly elevated circulating levels of IL-27, IL-10 and IFN-γ in pre-treatment VL samples as compared to post-treatment VL or EC (Fig. 1). Circulating IL-27 has not been previously investigated in human VL, and its elevation was reflected in 18 of 21 paired, pre- and post-treatment samples. In the case of IL-17, low levels were detected in only 4 of the VL plasma, and in none of the plasma from post-treatment cases or ECs. Circulating IL-21 was not detectable in any group (data not shown).
FIGURE 1.
Cytokines in plasma from VL patients. IFN-γ, IL-10 and IL-17 were measured by multiplex analysis of plasma obtained from paired VL patients at pre- and post -treatment (n = 16) and from ECs (n = 10). Dual antibody sandwich ELISA was used to measure plasma IL-27 levels in paired VL patients (n = 21) and ECs (n = 10). Significant differences are indicated with *, p < 0.05; **, p < 0.01, ***, p < 0.001.
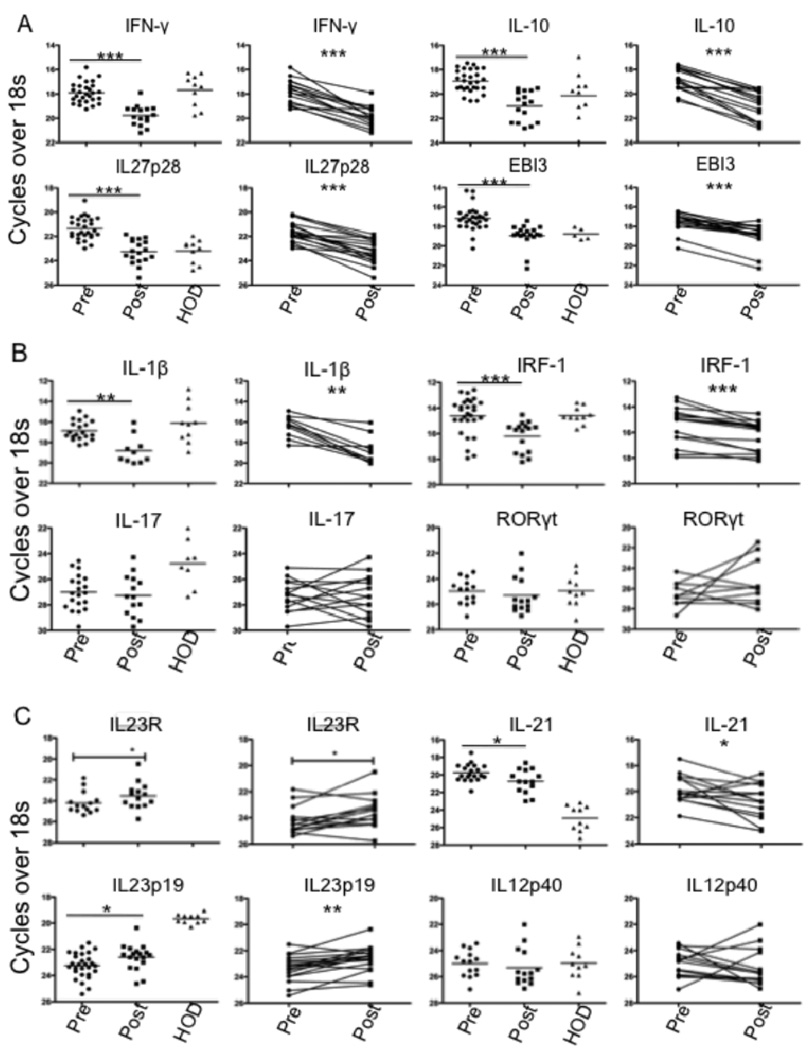
Analysis of mRNA by real-time PCR confirmed elevated mRNA for both IFN-γ and IL-10 in pre-treatment splenic biopsies compared to post-treatment samples, and to levels in splenic cells obtained from healthy organ donors (HOD) (Fig. 2A). The mRNA elevation was reflected in 15 of 16 paired samples for IFN-γ and 14 of 16 for IL-10. Analysis of IL-27 subunits revealed significant elevation of splenic mRNA levels for both IL-27p28 and EBI-3 in pre-treatment samples compared to post-treatment or to HOD (Fig. 2A). Further, the difference was reflected in 16 of 18 paired samples for IL-27p28, and 17 of 18 for EBI-3. We also analyzed IRF-1 and IL-1β mRNA, involved in transcriptional up-regulation of IL-27. Both transcripts were significantly elevated in pre-treatment compared to post-treatment samples, reflected in 12 of 16 paired samples for IRF-1 and 9 of 10 paired samples for IL-1β (Fig. 2B).
FIGURE 2.
Cytokine mRNA expression in VL spleen. (A–C) Ex-vivo analysis of relative mRNA levels for indicated genes in splenic aspirates from VL patients before (n = 28) or 21–30 d after treatment (n = 18) and in HOD spleen samples (n = 10). Paired pre- and post- treatment samples(n = 18) are also shown separately. Significant differences are indicated with *, p < 0.05; **, p < 0.01; ***, p < 0.001.
IL-27 has inhibitory effects on mouse Th17 lineage development, and a role for Th17 cells in protection against human VL has been suggested (36). Analysis of mRNA levels of RoRγt and IL-17 revealed no difference between the pre- and post-treatment groups, and in fact both transcripts were below or at the limits of detection in the majority of samples (Fig. 2B). By contrast, mRNA levels of IL-21, which has been proposed as a growth factor for Th17 cells (37, 38), was significantly elevated in pretreatment samples, reflected in 10 of 14 paired samples analyzed separately (Fig. 2C). Since IL-21 has also been shown to promote the IL-27-induced expansion of Tr1 cells, its elevated expression may have relevance to the regulation of T cell IL-10 production in VL patients. Finally, we analyzed the mRNA levels of IL-23, a heterodimeric, IL-12 family cytokine member that is also thought to promote Th17 cell differentiation. Distinct from the other cytokine transcripts analyzed, mRNA levels for the IL-23p19 subunit were significantly reduced in pre-treatment as compared to post- treatment samples, reflected in 14 of 18 paired samples (Fig. 2C). By contrast, the IL-12p40 subunit, which is shared with IL-12, was not differentially expressed. The potential functional relevance of the elevated IL-23 expression is reinforced by the coordinated elevated expression of IL-23R mRNA on the post-treatment cells, reflected in 8 of the 14 paired samples analyzed (Fig. 2C).
Cellular source of IL-27 and IL-21 mRNA in VL splenic aspirate cells
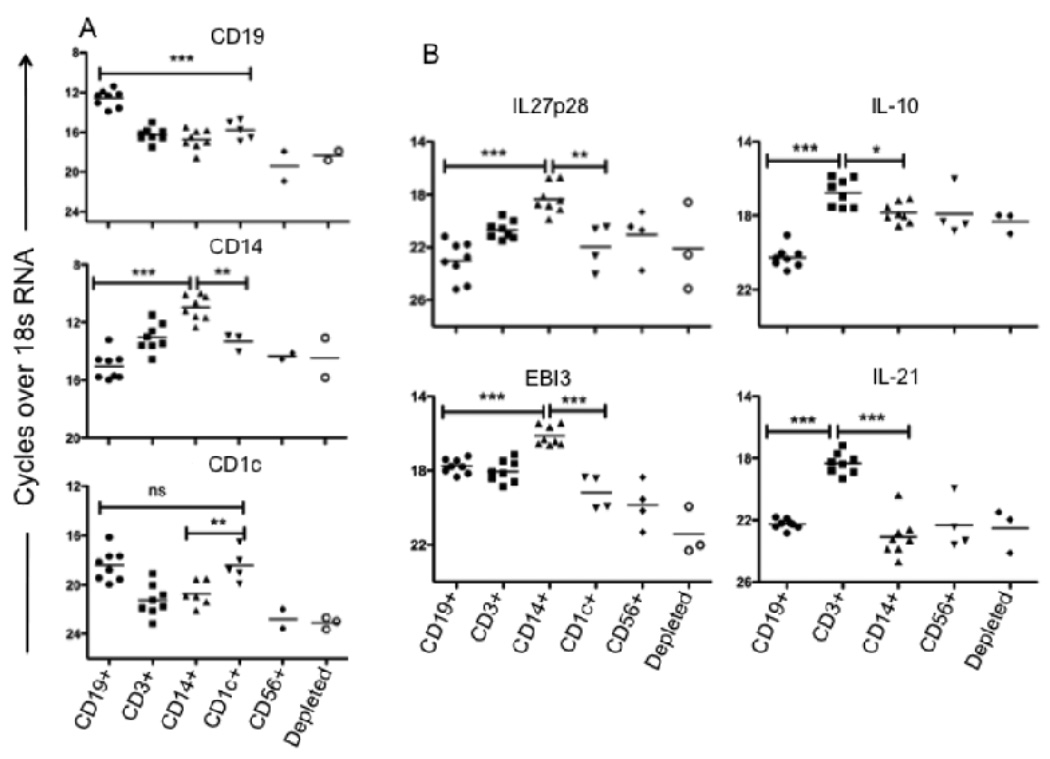
Since elevated levels of mRNA for IL-27 subunits and IL-21 were observed in splenic cells from active VL patients, and both cytokines have been implicated in regulating T cell IL-10 secretion, we were interested to know the cellular source of these transcripts. Residual mRNA preperations from the sequentially selected, splenic cell subsets obtained from the patient series examined previously (7), comprised of whole spleen, CD19+, CD19−CD25−CD3+, and B- and T- cells depleted, were taken advantage of to analyze these additional cytokine transcripts by RT-PCR. Ex-vivo mRNA analysis clearly showed enhanced IL-27p28 expression in the B- and T- cells depleted, macrophage and DC enriched fraction (Fig 3A). The EBI-3 transcripts were also elevated in these cells relative to the T cell enriched fraction, though not in comparison to the B cells, for which upregulation of EBI-3 on activated human B cells has been described (31). By contrast, CD3+ T cells (depleted of CD25+ cells), were the main cellular source of IL-21 mRNA in the VL spleen.
FIGURE 3.
Ex-vivo analysis of cytokine mRNA levels in splenic lymphocytes subsets. (A) Relative expression of IL-10, IL-21, IL-27p28 and EBI-3 mRNA levels in sequential, positively selected CD19+, CD3+(CD25-) and in the remaining depleted (Innate cells: NK, Macrophages, DCs) splenic cell population before treatment (n = 8). (B) Relative expression of cell specific markers (CD19/B cell, CD14/ Monocytes/ Macrophages and CD1c/DCs on sequential, positively selected CD19+, CD3+, CD14+, CD1c+, CD56+ and in the remaining ‘depleted’ splenic cell population. (C) Relative expression of mRNA levels for IL-10, IL-21, IL-27p28, and EBI-3 in positively selected splenic cell fractions from VL patients prior to treatment (n = 8). Significant differences are indicated with *, p < 0.05; **, p < 0.01; ***, p < 0.001, ns, not significant.
To further support and extend the findings regarding the cellular source of elevated IL-27 and IL-21 mRNA in the VL spleen, splenic aspirate cells from a new series of patients was submitted to a sequential enrichment protocol that included positive selection of CD14+ monocytes/macrophages, CD1c+DC, and CD56+ NK cells. To confirm the enrichment of the intended subsets, we amplified the cell specific markers targeted by the MACs beads used for the sequential selection. In each case, the mRNA was expressed at highest levels in the appropriate enriched fraction, with CD1c also expressed at high levels in the CD19+ enriched cells, although the CD1c+ selected population will have been depleted of B cells during the sequential enrichment (Fig. 3B). The relative transcript abundance for IL-27 subunits clearly demonstrates CD14+ cells (monocytes/mcarophages) to be the main source of both IL-27p28 and EBI-3 mRNA (Figure 3C). By contrast, CD3+ T cells were the main cellular source of IL-10, and especially IL-21 mRNA, in the spleen of active VL patients (Fig. 3B).
IFN-γ and IL-1β induces IL-27 mRNA in human monocyte derived macrophages
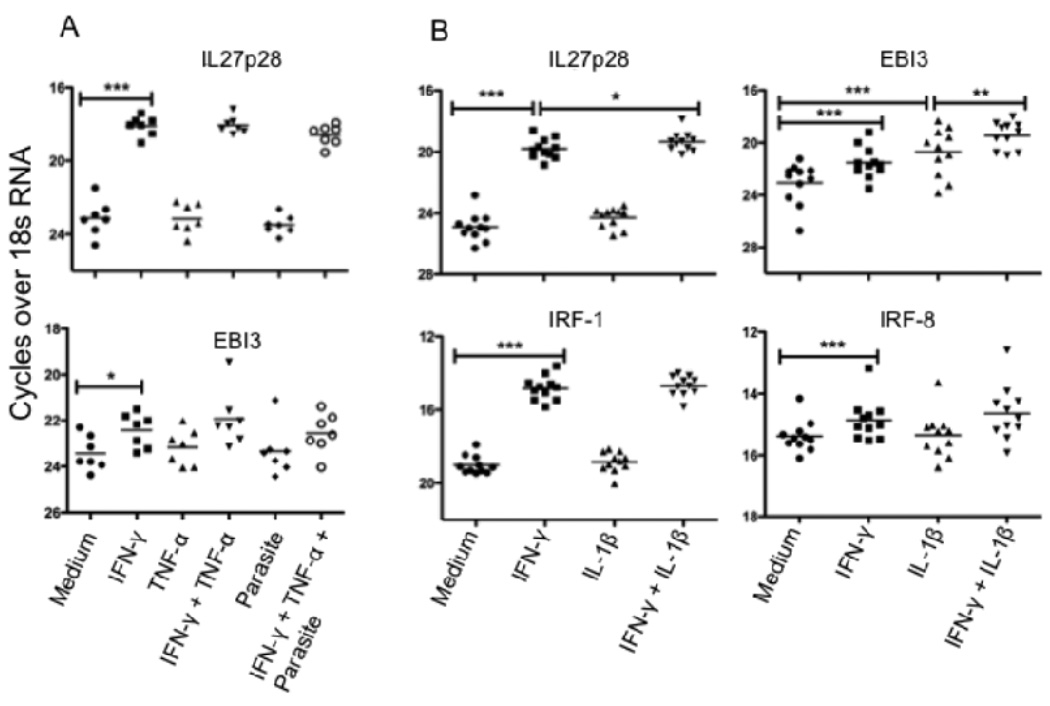
Having determined that CD14+ cells are an important source of IL-27 mRNA in the VL spleen, we were interested to know if L. donovani amastigotes, and/or pro-inflammatory cytokines upregulated in VL (IFN-γ, TNF-α or IL-1β), have a role in IL-27 induction in human macrophages that may be relevant to its elevated expression in the VL spleen. Monocyte derived macrophages from normal donors were treated with IFN-γ, TNF-α, IL-1β, their combination or medium alone for 8 h prior to infection with L. donovani amastigotes. Macrophages were cultured for an additional 16 h before being lysed for mRNA expression analysis. The results demonstrate a strong induction of IL-27p28 mRNA in the presence of IFN-γ, and a somewhat weaker induction of EBI-3 (Fig. 4A). TNF-α had no impact on either subunit, either alone or in combination with IFN-γ. Notably, L. donovani amastigotes did not induce IL-27 or enhance IL-27 expression. In an additional set of normal donor macrophages, IL-1β significantly upregulated EBI-3 mRNA expression, and produced a synergistic effect on induction of IL-27p28, and especially EBI-3, when used in conjunction with IFN-γ (Fig. 4B). Analysis of IRF-1 and IRF-8 mRNA also revealed enhanced expression of these transcription factors which have each been shown to bind to the p28 promoter to induce IL-27 expression in mouse macrophages (23). Thus, the conditions appropriate for upregulation of both IL-27 subunits in infected or uninfected human macrophages are present in the VL spleen.
FIGURE 4.
IFN-γ and IL-1β induces IL-27 mRNA in human monocyte derived macrophages. (A) Monocyte derived macrophages from normal donors (n = 7) were stimulated with rhIFN-γ or rhTNF-α or their combination or medium alone for 8 h prior to infection with L. donovani amastigotes. Macrophages were cultured for another 16 h before cells were lysed for mRNA expression analysis. (B) Monocyte derived macrophages from normal donors (n = 11) were stimulated with rhIFN-γ or rhIL-1β or their combination or medium alone for 24 h before cells were lysed for mRNA expression analysis. Significant differences are indicated with *, p < 0.05; **, p < 0.01; ***, p < 0.001.
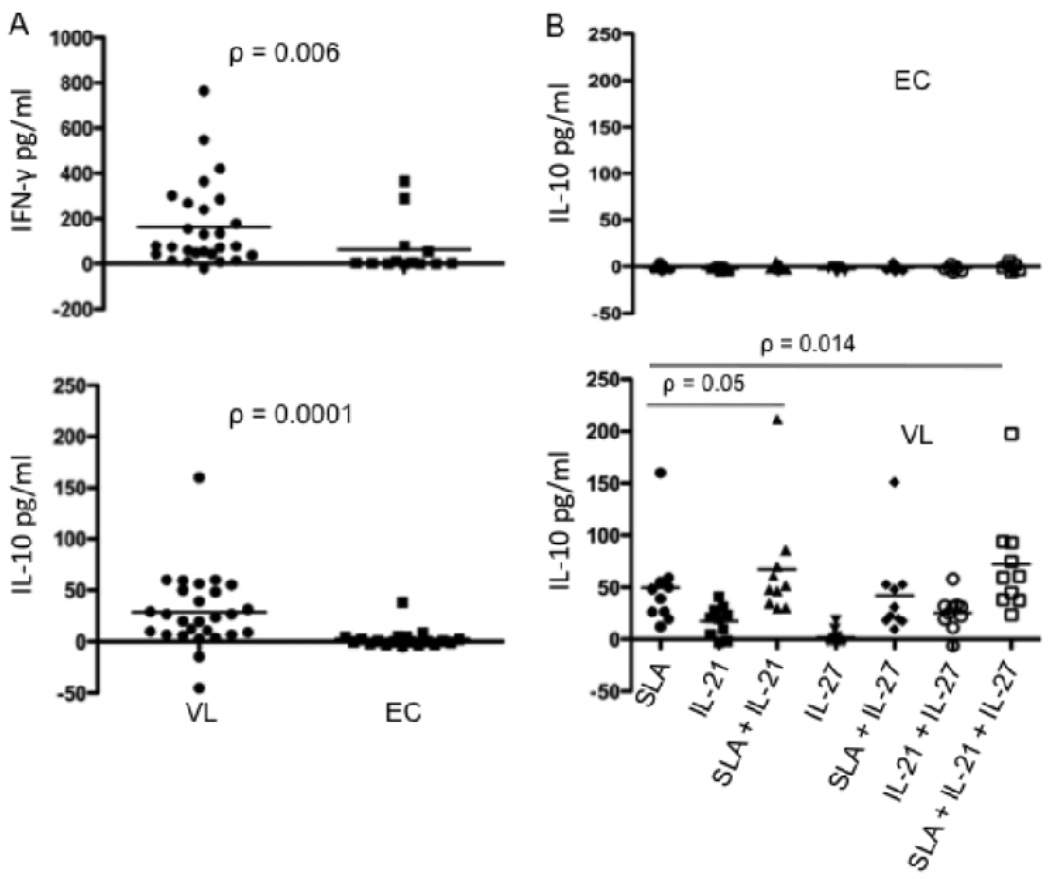
IL-21 enhances antigen specific IL-10 production in active VL cases
Autocrine IL-21 has recently been shown to amplify IL-27 induced T cell IL-10 expression in the mouse. Since we observed elevated mRNA for IL-27, IL-21 and IL-10 in active VL cases, we were interested to determine whether rhIL-27 or rhIL-21, used alone or in combination with soluble antigen prepared from L. donovani promastigotes (SLA), had the potential to stimulate IL-10 production in active VL cases or endemic controls. We used a whole blood assay that has recently been shown to detect antigen-specific IFN-γ secretion by peripheral blood cells from the majority of active VL cases (K. Gidwani et al., manuscript submitted) to determine if antigen-specific IL-10 might also be detected in these patients. ELISA results confirmed the antigen driven secretion of IFN-γ by peripheral blood cells from VL patients, with 22 of 27 patients producing cytokine above background levels (Fig. 5A), while cells from only 4 of 13 healthy endemic controls produced detectable levels of IFN-γ, reflecting their possible subclinical exposure to infection. Following stimulation with SLA, peripheral blood cells from the majority of active VL cases also produced IL-10, with 23 of 27 patients secreting low, but detectable levels of cytokine compared with only 2 of 19 endemic controls (Figure 5A). Interestingly, the peripheral blood cells from an independent series of VL patients also produced IL-10 when cultured in the presence of exogenous rhIL-21, while no response was detected in the cells from endemic controls (Fig. 5B). While a few of the VL patients also upregulated IL-10 secretion in response to rhIL-27, the majority did not, likewise none of the ECs. Treatment with IL-21 alone, and especially in combination with IL-27, significantly enhanced the antigen driven IL-10 response. Cells from ECs remained IL-10 unresponsive to all combinations of stimuli, indicating that patient cells had been specifically primed under conditions that made them more responsive to IL-10 inducing signals. The effect of IL-27 in combination with IL-21 to enhance IL-10 production by antigen stimulated cells was associated with significantly higher levels of IL-21 compared to the antigen stimulated cells treated with IL-21 alone (Fig. 5C). The supernatants from 11 of 13 VL patients contained elevated levels of IL-21 under these conditions, emphasizing the likely role of IL-27 in upregulation of autocrine IL-21 by T cells to amplify their IL-10 expression. We were not able to detect IL-21 secreted by the antigen stimulated cells treated with IL-27 alone (data not shown), perhaps due to rapid consumption of this cytokine in the cultures lacking exogenous IL-21.
FIGURE 5.
IL-21 upregulates antigen specific IL-10 production in whole blood cell cultures from active VL cases. (A) IFN-γ and IL-10 production by peripheral blood cells from pre-treatment VL patients (n = 27) and EC (n = 13 or 19) in response to SLA. (B) IL-10 production by peripheral blood cells from pre-treatment VL patients (n = 18) and ECs (n = 7) in response to SLA, rhIL-27 or rhIL-21 alone or in combination. (C) IL-21 production by peripheral blood cells from pre-treatment VL patients (n = 13). Whole blood cells were cultured in the absence or presence of 10µg/ml L. donovani soluble antigen and/or exogenous rhL-27(100ng/ml) and/or rhIL-21 (25ng/ml). Twenty four hours later supernatant was collected for cytokine analysis. Cytokine concentrations shown are the values in the stimulated cultures minus the medium control. Significant differences are indicated with p values.
Discussion
We have previously addressed the cellular source of elevated IL-10 in human VL, and identified CD4+CD25−Foxp3− T cells as the major producers of IL-10 in the VL spleen (7). The findings suggest that Tr1 cells, or Th subsets that co-express IL-10, are important in suppression of anti-leishmanial immunity in human VL. In the mouse, various conditions that can promote T cell IL-10 secretion have recently been described that have in common the involvement of IL-12 family members that may self-regulate inflammatory responses via their ability to instruct activated T cells to produce IL-10. Thus, IL-12 induced STAT4 signaling together with high antigen dose can activate Th1 cells to co-express IL-10 (39). An alternative IL-12 family member, IL-27, which activates Th1 transcription factors T-bet and STAT1, was shown to up-regulate IL-10 expression by Th1 cells (25, 40) and to induce IL-10-producing anti-inflammatory Tr1-like cells (41, 42). While IL-27 has been shown to promote IL-10 secretion by human CD4+ T cells in vitro (30), its expression in human infection has been confined to the detection of elevated serum levels in individuals infected with HIV or HBV (33, 34), and its association with IL-10 regulation in any human infectious disease setting has not been investigated. The current studies were designed to address the possible role of IL-27 in T cell IL-10 regulation in human VL.
We were able to detect elevated circulating levels of IL-27 in VL patients (Fig. 1), as well as significantly elevated mRNA levels for both IL-27p28 and EBI-3 subunits in the VL spleen during active disease. Expression of IL-21 mRNA was also significantly enhanced in pre- as compared to post-treatment splenic aspirate cells, and especially in comparison to cells from healthy spleens. The IL-27 transcripts were enriched in CD14+ cells (monocytes /macrophages), while the IL-21 and IL-10 transcripts were enriched in CD3+ cells (T cells). The role for IL-21 in amplifying IL-27 induced, T cells IL-10 production has been recently recognized in mice (43). These data are consistent with the possibility that IL-27 from innate cells induces T cells to produce IL-21 that acts as an autocrine growth factor to expand IL-10 producing T cells in the VL spleen (18). As IL-21 is also known to critically regulate Ig production (44), it could be a contributing factor to the high titers of anti-leishmanial antibodies in VL patients.
The influence of these regulatory cytokines on antigen-specific IL-10 secretion in vitro was investigated. We utilized a whole blood assay that has been shown to detect antigen-specific IFN-γ secretion by peripheral blood cells from cured VL and cutaneous leishmaniasis (CL) cases (45, 46). More recently, the assays has been found to detect antigen-specific IFN-γ production by cells from active VL cases (K. Gidwani et al., manuscript submitted), in striking contrast to the extensive experience regarding the lack of this response in VL patients when purified peripheral blood mononuclear cells have been employed. We confirm the IFN-γ responsiveness of VL patients in the whole blood assay, and further report secretion of detectable levels of IL-10 in the majority of patients. So far as we are aware, this is the first demonstration of antigen driven IL-10 production by cells from VL patients in vitro. While the findings are consistent with antigen-specific T cell IL-10 expression, it is possible that T cells responding to antigen stimulate IL-10 production from other cells. This might be especially relevant to the regulation of IL-10 production in target organs such as the spleen where macrophages constitute an expanded population of lymphoid cells. We were unable to demonstrate that neutralizing antibodies against either IL-27 or IL-21 could inhibit the IL-10 response (not shown), reflecting either that these cytokines were not involved, or that the assay detects peripheral cells already activated to produce IL-10, and are not influenced by the low concentrations of IL-27 or IL-21 produced by APCs or T cells present in the whole blood assay. By contrast, the peripheral blood cells were IL-10 responsive to exogenous rIL-21, which was able to induce IL-10 from VL patients even in the absence of antigen. These culture conditions may mimic the high levels of IL-21 plus antigen present in the VL spleen, which might therefore be expected to drive T cell IL-10 secretion during active disease.
The antigen-specific IFN-γ response detected in VL patients is consistent with the elevated levels of IFN-γ in VL plasma, as wells as elevated IFN-γ mRNA expression in lesional tissue. The coordinate production of antigen-specific IFN-γ and IL-10 might suggest that, while undoubtedly essential for acquired resistance to L. donovani, IFN-γ might also be involved in the regulation of T cell IL-10 expression as a homeostatic mechanism of restrain inflammation. Several recent studies in mice and humans have indicated that IL-27p28 is regulated at the transcriptional level. Given the high expression of p28 mRNA in CD14+ enriched splenic cells from VL patients, we were specifically interested in IFN-γ regulation of IL-27p28 gene expression in human macrophages. In the mouse, IFN-γ signaling in macrophages can contribute to activation of the p28 gene through recruitment of IRF1 and IRF8 to an IFN-stimulated regulatory element (ISRE) in the p28 promoter (23, 47). In humans, an effect of IFN-γ on IL-27p28 upregulation in a retinal cell line has been reported (21), however, effects on human macrophages have not been described. Using human monocyte derived macrophages from a series of healthy donors, we could demonstrate a consistent and powerful induction of p28 mRNA by IFN-γ, accompanied by a more modest upregulation of EBI3. Other pro-inflammatory mediators, either alone or in combination with IFN-γ, had only a slight effect on p28 expression. By contrast, EBI3 mRNA was substantially upregulated in the presence of both IFN-γ and IL-1β, consistent with the reported effect of IL-1β on EBI-3 expression in a human pre-dendritic cell line (48). Thus, the conditions suitable for activation of both IL-27 sub-units in human macrophages appear to be present in the VL spleen. Further, the elevated IRF1 transcripts that we detected in the VL spleen are consistent with the strongly elevated levels of IRF1 mRNA observed in the donor macrophages following treatment with IFN-γ, reinforcing a possible role for IFN-γ induced signaling to promote IL-27 production in human VL. Importantly, exposure of the macrophages to L. donovani amastigotes, either alone or in combination with IFN-γ, produced no discernable effects on IL-27p28 or EBI3 gene expression, consistent with the generally low stimulatory potential of these parasites and emphasizing the role of host derived agonists in upregulating IL-27 as a mechanism of feedback control.
The increased production of IL-27 in human VL might have regulatory consequences beyond the generation of IL-10 producing T cells. Studies in mice have demonstrated that IL-27 can inhibit the differentiation of Th17 cells involved in autoimmunity and pathogenic responses to infection (28, 29). In human CD4+ T cells, IL-27 suppressed IL-17 and IL-22 secretion under Th17 polarizing conditions, i.e. exposure of anti-CD3 stimulated cells to IL-1β and IL-23 (30). In the VL patients, IL-17 transcripts were expressed in low abundance in both the pre- and post- treatment splenic samples. By contrast, IL-23p19 and IL-23R mRNA were expressed at significantly increased levels in the post-treatment biopsies, unique amongst the selected transcripts we analyzed in its down regulated expression during active disease. The low or down regulated expression of Th17 associated cytokines in patients with active disease may be particularly significant in light of recent clinical studies in Sudan showing that L. donovani antigen stimulated production of IL-17 and IL-22 by PBMCs was strongly and independently associated with protection against VL (36). While we have no direct evidence that IL-27 inhibited the Th17 response in VL patients, L. major infections in mice have clearly revealed that in the absence of IL-27 signaling, the infection drives a robust Th17 response that in this instance exacerbated cutaneous pathology (27).
In conclusion, IL-27 produced by macrophages, along with IL-21 from T cell sources, are suggested to be disease promoting cytokines in VL by virtue of their roles in promoting the differentiation and expansion of antigen specific, IL-10 producing T cells. The studies support the notion that IL-27 is a key instructional cytokine involved in regulating the balance between immunity and pathology in human VL.
Acknowledgements
We thank the hospital staff at the Kala-azar Medical Research Center, Muzaffarpur, for their assistance in the collection of patient samples, Ms S. Mehrotra for help in preparation of the samples, and Dr. L. Eidsmo for providing control spleen samples. The care of the patients was supported by the Sitaram Memorial Trust, Muzaffarpur, India.
This research was supported in part by the Intramural Research program of the NIH, NIAID; by NIAID, NIH TMRC Grant No. 1P50AI074321, Extramural Research Program of the NIAID, NIH, USA; by ICMR, (New Delhi, India); and by the Swedish Society for Medicine.
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, Wasunna MK, Bryceson AD. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002;2:494–501. doi: 10.1016/s1473-3099(02)00347-x. [DOI] [PubMed] [Google Scholar]
- 2.Sundar S, Rai M. Advances in the treatment of leishmaniasis. Curr Opin Infect Dis. 2002;15:593–598. doi: 10.1097/00001432-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Ansari NA, Saluja S, Salotra P. Elevated levels of interferon-gamma, interleukin-10, and interleukin-6 during active disease in Indian kala azar. Clin Immunol. 2006;119:339–345. doi: 10.1016/j.clim.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Caldas A, Favali C, Aquino D, Vinhas V, van Weyenbergh J, Brodskyn C, Costa J, Barral-Netto M, Barral A. Balance of IL-10 and Interferon-gamma plasma levels in human visceral leishmaniasis: implications in the pathogenesis. BMC Infect Dis. 2005;5:113. doi: 10.1186/1471-2334-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghalib HW, Piuvezam MR, Skeiky YA, Siddig M, Hashim FA, el-Hassan AM, Russo DM, Reed SG. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993;92:324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karp CL, el-Safi SH, Wynn TA, Satti MM, Kordofani AM, Hashim FA, Hag-Ali M, Neva FA, Nutman TB, Sacks DL. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993;91:1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nylen S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007 doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28:378–384. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol. 2001;31:2848–2856. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Murray HW, Flanders KC, Donaldson DD, Sypek JP, Gotwals PJ, Liu J, Ma X. Antagonizing deactivating cytokines to enhance host defense and chemotherapy in experimental visceral leishmaniasis. Infect Immun. 2005;73:3903–3911. doi: 10.1128/IAI.73.7.3903-3911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghalib HW, Whittle JA, Kubin M, Hashim FA, el-Hassan AM, Grabstein KH, Trinchieri G, Reed SG. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623–4629. [PubMed] [Google Scholar]
- 12.Carvalho EM, Bacellar O, Brownell C, Regis T, Coffman RL, Reed SG. Restoration of IFN-gamma production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–5956. [PubMed] [Google Scholar]
- 13.Stager S, Maroof A, Zubairi S, Sanos SL, Kopf M, Kaye PM. Distinct roles for IL-6 and IL-12p40 in mediating protection against Leishmania donovani and the expansion of IL-10+ CD4+ T cells. Eur J Immunol. 2006;36:1764–1771. doi: 10.1002/eji.200635937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranatunga D, Hedrich CM, Wang F, McVicar DW, Nowak N, Joshi T, Feigenbaum L, Grant LR, Stager S, Bream JH. A human IL10 BAC transgene reveals tissue-specific control of IL-10 expression and alters disease outcome. Proc Natl Acad Sci U S A. 2009;106:17123–17128. doi: 10.1073/pnas.0904955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida H, Miyazaki Y. Regulation of immune responses by interleukin-27. Immunol Rev. 2008;226:234–247. doi: 10.1111/j.1600-065X.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 18.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 20.Collison LW, Vignali DA. Interleukin-35: odd one out or part of the family? Immunol Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 22.Molle C, Goldman M, Goriely S. Critical role of the IFN-stimulated gene factor 3 complex in TLR-mediated IL-27p28 gene expression revealing a two-step activation process. J Immunol. 184:1784–1792. doi: 10.4049/jimmunol.0902005. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Qian X, Ning H, Yang J, Xiong H, Liu J. Activation of IL-27 p28 gene transcription by interferon regulatory factor 8 in cooperation with interferon regulatory factor 1. J Biol Chem. 2010;285:21269–21281. doi: 10.1074/jbc.M110.100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wirtz S, Becker C, Fantini MC, Nieuwenhuis EE, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-kappa B activation. J Immunol. 2005;174:2814–2824. doi: 10.4049/jimmunol.174.5.2814. [DOI] [PubMed] [Google Scholar]
- 25.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 26.Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, de Sauvage FJ, Satoskar AR. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. Am J Pathol. 2006;168:158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson CF, Stumhofer JS, Hunter CA, Sacks D. IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. J Immunol. 2009;183:4619–4627. doi: 10.4049/jimmunol.0804024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 29.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 30.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larousserie F, Bardel E, Coulomb L'Hermine A, Canioni D, Brousse N, Kastelein RA, Devergne O. Variable expression of Epstein-Barr virus-induced gene 3 during normal B-cell differentiation and among B-cell lymphomas. J Pathol. 2006;209:360–368. doi: 10.1002/path.1995. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, Meuer SC, Stallmach A. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflamm Bowel Dis. 2005;11:16–23. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Guzzo C, Hopman WM, Mat NF, Wobeser W, Gee K. Impact of HIV infection, highly active antiretroviral therapy, and hepatitis C coinfection on serum interleukin-27. AIDS. 2010;24:1371–1374. doi: 10.1097/QAD.0b013e3283391d2b. [DOI] [PubMed] [Google Scholar]
- 34.Zhu C, Zhang R, Liu L, Rasool ST, Mu Y, Sun W, Hao Q, Liu F, Zhu Y, Wu J. Hepatitis B virus enhances interleukin-27 expression both in vivo and in vitro. Clin Immunol. 2009;131:92–97. doi: 10.1016/j.clim.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Kenney RT, Sacks DL, Gam AA, Murray HW, Sundar S. Splenic cytokine responses in Indian kala-azar before and after treatment. J Infect Dis. 1998;177:815–818. doi: 10.1086/517817. [DOI] [PubMed] [Google Scholar]
- 36.Pitta MG, Romano A, Cabantous S, Henri S, Hammad A, Kouriba B, Argiro L, el Kheir M, Bucheton B, Mary C, El-Safi SH, Dessein A. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J Clin Invest. 2009;119:2379–2387. doi: 10.1172/JCI38813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 39.Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O'Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 42.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 43.Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 45.Kumar R, Goto Y, Gidwani K, Cowgill KD, Sundar S, Reed SG. Evaluation of ex vivo human immune response against candidate antigens for a visceral leishmaniasis vaccine. Am J Trop Med Hyg. 2010;82:808–813. doi: 10.4269/ajtmh.2010.09-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahmoodi M, Rajabalian S, Fekri A, Esfandiarpour I. Evaluation of In Vitro Production of IFN-gamma, IL-10, IL-12 and IL-13 by Blood Cells in Patients with Cutaneous Leishmaniasis Lesions. Iran J Allergy Asthma Immunol. 2005;4:15–21. [PubMed] [Google Scholar]
- 47.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poleganov MA, Bachmann M, Pfeilschifter J, Muhl H. Genome-wide analysis displays marked induction of EBI3/IL-27B in IL-18-activated AML-derived KG1 cells: critical role of two kappaB binding sites in the human EBI3 promotor. Mol Immunol. 2008;45:2869–2880. doi: 10.1016/j.molimm.2008.01.021. [DOI] [PubMed] [Google Scholar]