Summary
DksA is a global transcriptional regulator that directly interacts with RNA polymerase (RNAP) and, in conjunction with an alarmone ppGpp, alters transcription initiation at target promoters. DksA proteins studied to date contain a canonical Cys4 Zn-finger motif thought to be essential for their proper folding and thus activity. In addition to the canonical DksA protein, the Pseudomonas aeruginosa genome encodes a closely-related paralog DksA2 that lacks the Zn-finger motif. Here, we report that DksA2 can functionally substitute for the canonical DksA in vivo in Escherichia coli and P. aeruginosa. We also demonstrate that DksA2 affects transcription by the E. coli RNAP in vitro similarly to DksA. The dksA2 gene is positioned downstream of a putative Zur-binding site. Accordingly, we show that dksA2 expression is repressed by the presence of exogenous Zn, deletion of Zur results in constitutive expression of dksA2, and Zur binds specifically to the promoter region of dksA2. We also found that deletion of dksA2 confers a growth defect in the absence of Zn. Our data suggest that DksA2 plays a role in Zn homeostasis and serves as a back-up copy of the canonical Zn-dependent DksA in Zn poor environments.
Keywords: DksA, Pseudomonas aeruginosa, Zur, zinc
Introduction
Tight control of zinc (Zn) homeostasis is critical for cell viability. Even though the activity of many essential proteins, such as DNA and RNA polymerases (RNAPs), ribosomal proteins and multiple metabolic enzymes is strictly dependent upon Zn, excess Zn is toxic. In environments with varying Zn concentrations, specific genes are turned off or on to maintain the optimal intracellular Zn quota. In all three kingdoms, these genes encode a variety of Zn transport systems, chaperones, and Zn-binding proteins (Blencowe & Morby, 2003).
Using both narrow and broad-specificity import systems, E. coli can actively accumulate Zn(II) to a level of 200,000 atoms/cell (Outten & O’Halloran, 2001), which corresponds to 0.2 mM, a 1,000-fold excess over the typical Zn concentration in the medium. However, biochemical measurements indicate that there is essentially no free Zn in an E. coli cell (Outten & O’Halloran, 2001), suggesting that, once imported, Zn becomes sequestered by cellular proteins. Zn-binding proteins account for 5% of the proteome (Andreini et al., 2006), and ribosomes most likely constitute the largest Zn reservoir. Indeed, a rapidly growing E. coli cell contains as many as 50,000 ribosomes (Bremer & Dennis, 2008), each with ~ three bound Zn ions, thereby tying up 75% of all Zn. Other abundant proteins must sequester the remaining Zn pool; RNAP (present at ~2000 copies/cell and bound to two Zn ions) is one of many examples.
Zn frequently plays a key role as a catalytic and/or structural cofactor in proteins essential for viability. Under conditions of Zn limitation, for example upon entry into vertebrate hosts that sequester Zn to guard against infection (Kehl-Fie & Skaar, 2010), cells must be able to acquire sufficient Zn. Adaptation to Zn depletion depends primarily on Zur, a transcriptional repressor from the Fur family of proteins; Zur orthologs are present in many bacterial species (Lee & Helmann, 2007). In the presence of Zn, Zur binds to operator sequences upstream of target genes, preventing binding of RNAP and thus transcription initiation. Conversely, upon Zn depletion, repression by Zur is lifted and expression of target genes is increased.
Simulating Zn-depleted environments in the laboratory has proven difficult because common metal chelators exhibit broad specificity that precludes targeted depletion of Zn from the culture medium. In most cases, the absence of the high-affinity Zn(II) transporter ZnuABC is required to observe growth defects linked to the deletion of genes involved in Zn homeostasis (Petrarca et al., 2010, Gabriel & Helmann, 2009). By growing E. coli under continuous culture conditions in a specially designed metal-free chemostat, sufficient Zn depletion was achieved to reveal growth defects in the wild-type znuABC background (Graham et al., 2009). However, this approach is labor-intensive and not amenable to a broad study of Zn homeostasis mechanisms across the bacterial kingdom. Fortunately, identification of putative Zur-binding sites has proven to be a productive way to identify novel proteins involved in the adaptation to Zn-limited environments (Panina et al., 2003, Haas et al., 2009).
The ZnuABC transporter imports Zn(II) in an ATP-dependent manner and is thought to be the main target for Zur regulation (Patzer & Hantke, 2000). However, the Zur regulon in several bacteria is not limited to Zn transporters, and other mechanisms that allow adaptation to Zn-depleted environments have been described. For example, comparative genomic approaches led to the initial identification of four ribosomal protein paralogs (Makarova et al., 2001, Panina et al., 2003). In contrast to the main copies that contain Cys4 Zn-ribbon motifs (and are thus called C+), the C− duplicates lack the key Cys residues, do not bind Zn, and are repressed by Zur. When Zn is scarce, these C− paralogs are expressed and substitute for the C+ proteins in ribosomes (Natori et al., 2007, Gabriel & Helmann, 2009). This mechanism is proposed to increase cell survival in Zn-limiting conditions by supplying functional copies of Zn-free proteins for the newly made ribosomes, while at the same time liberating the pool of Zn through dissociation from the existing ribosomes, and subsequent degradation of the C+ proteins.
Using a comparative genomic approach, we have recently identified six additional paralogs of Zn-dependent proteins in γ- and β-proteobacteria (Haas et al., 2009). It remains to be determined whether these proteins, whose genes are positioned downstream from putative Zur-binding sites, simply replace their Zn-dependent counterparts when the activity of the latter is adversely affected by the absence of Zn or play distinct roles in the cell. In this work, we focus on the division of labor between two DksA paralogs (DksA and DksA2) in P. aeruginosa and a possible role of DksA2 in Zn homeostasis.
DksA was initially identified in E. coli (EC) as a suppressor of the dnaK phenotype (Kang & Craig, 1990). Since then, DksA was shown to act synergistically with (p)ppGpp to control the bacterial response to stress and starvation (Paul et al., 2004, Potrykus & Cashel, 2008). The X-ray structure of EC DksA (Perederina et al., 2004) revealed that it belongs to a class of bacterial transcription factors (which includes GreA, GreB and GfhI) that bind within the RNAP secondary channel near the active site located at the base of this channel. These regulators share a common two-domain organization and the mode of binding to the RNAP (Perederina et al., 2004, Laptenko et al., 2006, Stebbins et al., 1995, Symersky et al., 2006): their coiled-coil domains extend toward, and modulate various activities of, the RNAP active site whereas their globular domains likely bind to a β’ rim helices domain that lies at the entrance into the channel (Perederina et al., 2004, Laptenko et al., 2003, Vassylyeva et al., 2007). On the other hand, these proteins have very different effects on transcription: Gre factors act to rescue arrested (e.g., upon nucleotide misincorporation (Zenkin et al., 2006)) transcription elongation complexes, whereas DksA destabilizes initiation complexes (Paul et al., 2004, Paul et al., 2005).
The stringent response enables rapid and global change of gene expression following nutrient stress, which leads to a rapid increase in ppGpp levels (Magnusson et al., 2005, Srivatsan & Wang, 2008). DksA/ppGpp strongly inhibits transcription of rRNA genes while activating genes for amino acid biosynthesis and transport. Both effects utilize the main activity of DksA: destabilization of open promoter complexes. At the rrn promoters, open complexes are very unstable, and further destabilization essentially abolishes transcription of rRNA genes. Conversely, RNAP forms very stable complexes at amino acid promoters such as hisG. DksA and ppGpp destabilize these complexes and increase transcription in vitro (Paul et al., 2005). In vivo, a part of the control could be through liberating RNAP from rrnB promoters that account for 70% of the total RNA synthesis in rapidly growing cells (Zhou & Jin, 1998). The end result of this dual control is the restored balance between ribosome production and available amino acid pools. Interestingly, ppGpp and DksA may play independent, or even opposing, roles at some E. coli promoters (Magnusson et al., 2007, Aberg et al., 2008, Dalebroux et al., 2010) and during replication (Tehranchi et al., 2010) (Trautinger et al., 2005).
DksA proteins characterized to date contain a canonical Cys4 Zn-finger motif. Structural analysis of the EC DksA suggests that this motif plays a key role by maintaining the fold of the globular domain and its orientation relative to the “catalytic” coiled coil (Perederina et al., 2004). The Zn2+ ion is chelated by two cysteines from each domain and cannot be mobilized after extensive dialysis in the presence of chelators; mutation of any of the four Cys residues renders DksA nonfunctional (Paul et al., 2004, Perron et al., 2005). However, several DksA paralogs that lack the canonical Zn-finger exist (Fig. 1). For example, P. aeruginosa encodes both the Zn-finger DksA and its C− paralog DksA2. The dksA2 gene is located downstream of a putative Zur-binding site (Haas et al., 2009), suggesting that it may be induced upon Zn depletion. These observations suggest a model in which PA DksA and DksA2 have similar molecular mechanisms but act in different (Zn-rich and Zn-poor) conditions, respectively. In this work, we set out to investigate whether DksA2 is a functional paralog of DksA and is upregulated in Zn-depleted environments, as the model implies.
Figure 1.
Phylogenomic analysis of DksA and DksA2. Operon organization and location of putative Zur-binding site are given for putatively Zur-regulated dksA2 genes. Representative protein names for each branch are given in parentheses. To highlight that some organisms contain both DksA and DksA2, “I” (for DksA) or “II” (for DksA2) is given. For PA5536 (DksA2), in addition to the operon organization, the sequence of the predicted Zur-binding site is indicated.
Results
Phylogeny of the DksA family of proteins
Proteins belonging to the DksA/TraR superfamily are present throughout the bacterial kingdom (Marchler-Bauer et al., 2009) and the majority of these proteins are of unknown function. In addition to the characterized DksA protein (PA4723), which has been shown to be involved in the stringent response, the P. aeruginosa genome (Stover et al., 2000) encodes four other proteins from this superfamily (Fig. S1). Three of these DksA-like proteins (PA4577, PA4870, and PA0612) contain the characteristic CxxC-(x17)-CxxC Zn-finger motif but otherwise have low sequence homology to DksA (24%, 16%, and 14% identity, respectively). The fifth DksA-like protein (PA5536), which we refer to here as DksA2, has significant sequence homology to DksA (34% identity) but contains a CxxT-(x17)-CxxA motif instead of the typical Cys4 Zn-finger motif.
DksA2 is found in other bacteria and can be identified based on the presence of the Cxx[S/T]-(x17)-[C/S/T]xxA motif. We initially found dksA2 as part of the computationally reconstructed Zur regulon in several γ- and β-proteobacteria (Serratia, Klebsiella, Hahella, Pseudomonas spp., and Methylobacillus) (Haas et al., 2009). As such, dksA2 is often clustered physically on the chromosome with factors known to be involved in the response to Zn-depletion, such as znuABC (Fig. 1). In genomes that contain both dksA and dksA2, such as P. aeruginosa, dksA2 is usually found downstream of putative Zur-binding sites (Fig. 1). This situation is similar to the distribution pattern of folE and folE2 genes. The folE2 gene is found downstream of putative Zur-binding sites in genomes that contain both genes (Sankaran et al., 2009). Also like folE2, when only dksA2 is present in a genome, Zur-binding sites are not identified upstream. This appears to be the case for dksA2 in several α- and β-proteobacteria (Fig. 1).
Exceptions to this pattern of distribution do occur in several Pseudomonas and Bordetella species. Pseudomonas fluorescens and Pseudomonas entomophila have one dksA and two dksA2 genes (dksA2-a and dksA2-b); dksA2-a but not dksA2-b is predicted to be regulated by Zur. The presence of two dksA2 genes could be explained by a late Pseudomonas clade-specific duplication of dksA2 as some Pseudomonas spp. have subsequently lost one of the two dksA2 paralogs. For instance, P. aeruginosa has retained dksA2-a, whereas Pseudomonas putida has kept dksA2-b. In several Bordetella species, the dksA2 gene may be a part of a Zur-regulated operon even though the canonical dksA gene is not present.
DksA2 in trans rescues the amino acid auxotrophy of E. coli ΔdksA
PA DksA (PA4723) has been the focus of several studies examining its roles in the quorum-sensing circuitry, rRNA transcription and survival during antibiotic stress (Branny et al., 2001, Jude et al., 2003, Perron et al., 2005, Viducic et al., 2006). DksA2 (PA5536), on the other hand, is annotated as a conserved hypothetical protein (Pseudomonas genome database (Winsor et al., 2009)). In order to determine whether the DksA2 protein of P. aeruginosa can functionally replace DksA, we took advantage of the amino acid auxotrophy of a ΔdksA E. coli strain, which is unable to grow in minimal media lacking leucine, valine, glycine, phenylalanine or threonine (Brown et al., 2002). The E. coli genome does not contain a dksA2 gene as discussed above.
As shown in Figure 2A, dksA2 expressed in trans from PBAD was able to rescue the growth defect of the E. coli ΔdksA strain grown in minimal medium lacking casamino acids. Compared to EC dksA or PA dksA, where uninduced expression from PBAD was sufficient for complementation, rescue by dksA2 required a higher concentration of the inducer arabinose (0.002 - 0.2%). As previously shown for EC dksA (Potrykus et al., 2006), overexpression of PA dksA was toxic in the presence of 0.2% arabinose. Toxicity was not observed when expressing dksA2 with the arabinose concentrations used in this study.
Figure 2.
Rescue of E. coli ΔdksA::TetR and P. aeruginosa ΔdksA phenotypes. A. Growth of E. coli ΔdksA::TetR derivatives on M9 medium with the indicated constructs in trans. B. Growth of P. aeruginosa derivatives on M9 medium with the indicated constructs in trans. C. Growth of P. aeruginosa mutants on M9 medium +/− 100 μM EDTA and +/− 25 μM metal. 10−3 dilutions of normalized cultures are shown for A-C. D. Pyocyanin production of WT PBAD–Ø, ΔdksA PBAD–Ø, and ΔdksA PBAD–dksA2 at various times during growth in LB at 37°C; corresponding growth curves are given in insert.
We next tested if changing the cysteines in the Cys4 Zn-finger motif of the PA DksA to the corresponding residues in DksA2 would abolish the ability to rescue growth of the E. coli ΔdksA strain. Substitutions of Cys132 and Cys135 to Ser in PA DksA were previously found to abolish function (Perron et al., 2005). We substituted the conserved Cys114 and Cys135 in PA DksA with Thr and Ala, respectively. We found that both substitutions eliminated the ability of PA DksA to restore growth of E. coli ΔdksA (Fig. S2). This result is consistent with a key role of the Zn-finger motif in the DksA structure and thus, function.
DksA2 can functionally replace P. aeruginosa DksA in vivo
We then turned to phenotypic studies directly in P. aeruginosa PAO1 and assessed whether dksA2 expression is regulated by Zn, as suggested by the presence of a putative Zur-binding site upstream. Like in E. coli, deletion of the dksA gene in P. aeruginosa leads to a growth defect in M9 minimal media with glucose (0.2% wt/vol) as a sole carbon source (Jude et al., 2003), an effect which we reproduced in M9 minimal medium with glycerol as a sole carbon source (Fig. 2B and Fig. S3A and B). We found that, similarly to the situation observed in a heterologous E. coli host, dksA2 expressed from PBAD complemented the growth phenotype of the P. aeruginosa ΔdksA strain (Fig. 2B and S3A), strongly suggesting that DksA2 can functionally replace PA DksA.
We found that the growth defect of ΔdksA could be suppressed by the addition of 100 μM ethylenediaminetetraacetic acid (EDTA) or by combining the dksA deletion with the deletion of the gene encoding the Zur homolog (PA5499, np20) (Fig. 2C and S3B and C). In both cases, suppression was dependent on the presence of dksA2 in cis (on the chromosome) (Fig. 2C and S3B) or in trans (expressed from PBAD) (Fig. S3E and F and S4). The addition of Zn, but not other transition metals tested, counteracted the suppression effect of EDTA (Fig. 2C and S3D). By contrast, Zn did not affect growth of the ΔdksA Δzur strain (Fig. 2C).
During these experiments, we noticed that pyocyanin was differentially produced in various strains and that these trends mimicked the growth defects observed above (Fig. S5). Pyocyanin is a secreted virulence factor that is thought to play a role in the tissue damage of infected hosts (Caldwell et al., 2009). The synthesis of this metabolite is regulated by quorum sensing, and PA DksA was initially characterized in a complementation screen of a quorum-sensing mutant (Branny et al., 2001). During growth in Luria broth at 37°C, the ΔdksA strain produced less than 10% of the pyocyanin produced by the parent strain (Fig. 2D). Pyocyanin production was restored by expressing dksA2 in trans (Fig. 2D).
DksA2 is repressed by Zn and Zur binds specifically to the dksA2 promoter region
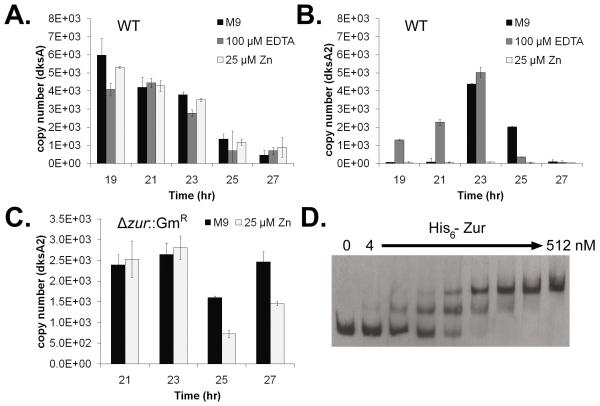
These results, together with the promoter region organization of the dksA2 gene (Fig. 1), suggest that dksA2 is regulated by Zur in P. aeruginosa, and that its expression may be activated under Zn limitation. We therefore analyzed the effect of EDTA and extracellular Zn on dksA and dksA2 transcript levels by qRT-PCR and on DksA and DksA2 protein levels by Western blotting. As shown in Fig. 3A, the abundance of dksA transcript was not significantly affected by either EDTA or Zn and was found to gradually decrease throughout the growth cycle as previously described (Perron et al., 2005) (growth curves available in Fig. S6). In contrast, as shown in Fig. 3B, dksA2 was not significantly expressed in the WT strain until late logarithmic/early stationary phase when grown in M9 medium, the same growth condition that gave rise to the ΔdksA growth defect described above. In the presence of EDTA, which was able to suppress the ΔdksA phenotype, the level of dksA2 transcript was increased during early/mid logarithmic phase (Fig. 3B). The dksA2 transcript was undetectable throughout the growth cycle when the cells were grown in the presence of 25 μM ZnSO4 (Fig. 3B). In the absence of Zur, Zn failed to repress dksA2 expression compared to the strain grown in M9 medium without supplementation (Fig. 3C). The abundance of DksA and DksA2 (as discerned from the Western blot) paralleled changes in the transcript levels (Fig. S7).
Figure 3.
Transcript abundance of PA DksA and DksA2 and binding of His6-Zur to the promoter region of dksA2. Average abundance of dksA (A) and dksA2 (B) transcript in total mRNA extracted from P. aeruginosa at 19, 21, 23, 25 and 27 hours of growth in M9 medium without supplementation (M9), plus 100 μM EDTA, or plus 25 μM ZnSO4. C. Average abundance of dksA2 transcript in total mRNA extracted from P. aeruginosa Δzur::GmR at 21, 23, 25 and 27 hours of growth in M9 medium without (M9) or with 25 μM ZnSO4. Copy number refers to the number of dksA or dksA2 transcripts per 0.1 ng total mRNA. Error bars represent ± one standard deviation of three replicates. D. Binding of purified His6-Zur to the promoter region of dksA2. Increasing concentrations of Zur (4, 8, 16, 32, 64, 128, 256, and 512 nM; corresponding to the monomeric unit) were incubated in the presence of 100 μM ZnSO4 with 1.5 ng of a biotin-labeled 223 bp fragment surrounding the putative Zur-binding site.
As expected from the expression results above, His6-Zur was found to bind specifically to the promoter region of dksA2 in the presence of Zn (Fig. 3D and S8). In the absence of Zn, binding is observed but only in the presence of higher concentrations of purified His6-Zur (Fig. S8A). In the presence of 100 μM EDTA, binding is abolished (Fig. S8A). Competition with the promoter DNA was achieved with specific competitor (annealed oligonucleotides containing the putative binding site) but not with non-specific competitor (control oligonucleotides which lack a consensus Zur-binding site) (Fig. S8B).
Deletion of dksA2 leads to an increased sensitivity to metal chelators
We hypothesized that DksA2 may be a condition-specific functional variant of PA DksA, and our analysis suggests that dksA2 is expressed during Zn depletion. Therefore, we wanted to test whether the deletion of dksA2 gives rise to a growth defect under metal depletion conditions.
We compared the growth of WT and ΔdksA2 P. aeruginosa strains in the presence of metal chelators. The absence of dksA2 resulted in an observable growth defect in the presence of EDTA or N,N,N’,N-tetrakis(2-pyridylmethyl) ethylenediamine (TPEN), the chelators used frequently to mimic Zn limitation (Fig. 4 and S9). To confirm that this phenotype was due specifically to Zn depletion (as opposed to that of another metal), we deleted znuA in the ΔdksA2 background. ZnuA encodes a homolog of the periplasmic chaperone component of the high-affinity Zn transporter ZnuABC, and its deletion impairs Zn uptake (Patzer & Hantke, 1998). Growth of WT, ΔdksA2, ΔznuA::GmR, and ΔdksA2 ΔznuA::GmR strains on minimal media were compared in the presence of various concentrations of EDTA and TPEN (Fig. S9). As shown in Figure 4, deletion of znuA exacerbated the growth defect due to the deletion of dksA2 in the presence of 1.5 mM EDTA or 5 μM TPEN. The growth defect was rescued by dksA2 expressed in trans from PBAD at 0.2% arabinose (Fig. 4). As further confirmation that the EDTA-sensitive phenotype of the dksA2 mutant is due to depletion of Zn, 25 μM of various transition metals were added to the medium containing either 1.25 mM or 1.5 mM EDTA. Only the addition of Zn was able to suppress the growth defect caused by the presence of EDTA (Fig. S9C and D).
Figure 4.
Growth defect of P. aeruginosa ΔdksA2 during metal depletion. Strains were grown on M9 medium with 0.2% arabinose without supplementation (M9), with 1.5 mM EDTA, or 5 μM TPEN. The 10−5 dilution of normalized cultures is shown.
DksA2 inhibits transcription from the rrnB P1 promoter in vitro
The rescue experiments in E. coli and P. aeruginosa (see above) suggest that DksA2 can functionally replace DksA by binding to the RNAP and altering its properties. This idea is consistent with the common origin of the two proteins; other paralogous transcription regulators such as σ initiation factors (Burgess & Anthony, 2001) or NusG/RfaH elongation factors (Belogurov et al., 2009) have been shown to bind to a shared target on RNAP, even though they have lower identity scores. It is also possible that DksA2 exerts its effect indirectly, through some alternative pathway. To distinguish between these possibilities, we tested the effect of DksA2 in a purified in vitro system.
The main targets of DksA are the ribosomal rrn promoters, which account for ~70% of the transcription in the cell. The EC DksA effect at the rrnB P1 promoter has been extensively studied both in vivo and in vitro. Since our genetic analysis indicates that DksA2 functions in E. coli, we used a well established in vitro transcription assay to test the effect of DksA2 on initiation from the E. coli rrnB P1 promoter. We found that, similarly to DksA, DksA2 inhibited transcription from rrnB P1 (Fig. 5). The effects of both proteins were potentiated in the presence of 50 μM ppGpp. The concentration of DksA2 required for half-maximal inhibition was ~ three-fold higher as compared to that of the EC DksA, suggesting that it binds more weakly to the EC RNAP (Fig. 5A). This is not surprising given that the DksA and RNAP regions that are proposed to mediate their interactions (Perederina et al., 2004) display significant sequence divergence.
Figure 5.
Inhibition of transcription from the E .coli rrnB P1 promoter by DksA and DksA2. A. E. coli DksA or P. aeruginosa DksA2 were incubated with RNAP for 15 minutes prior to the addition of the DNA template. B. Different concentrations of DksA or DksA2 were added to RNAP in the presence or absence of 50 μM ppGpp. The concentration of DksA at which 50% of transcription is inhibited (IC50) was calculated from three repeats using the Scientist software (MicroMath).
DksA2 destabilizes open promoter complexes
Transcription initiation is a multi-step process that proceeds through several intermediates, which differ in interactions between the RNAP and the promoter DNA. In the initial encounter complex, the double-stranded DNA is loosely bound on the RNAP surface via sequence-specific contacts to the σ factor. This closed promoter complex (called RPc) is highly unstable and the RNAP is in equilibrium with the free enzyme in solution. Through a series of isomerization steps (which may differ at different promoters), the RNAP melts the promoter DNA segment between the −12 and +2 positions (relative to the transcription start site) to form a catalytically-competent open promoter complex (RPo).
E. coli DksA was shown to reduce the RPo stability at different promoters but the regulatory outcome of this destabilization differs depending on the specific features of the promoters. At promoters which form very stable open complexes (such as λ PR with the half-life on the order of hours in vitro), EC DksA may not have any effect or act as an activator (Paul et al, 2005, (Łyzen et al., 2009). In contrast, EC DksA (particularly in the presence of ppGpp) reduces transcription from promoters that form highly unstable open complexes (such as rrnB P1, t1/2 on the order of seconds). To test the effect of DksA2 on the open complex stability we used a model lacUV5 promoter, which forms an RPo with an intermediate, readily measurable half-life (Fig. 6A). To measure the rate of RPo dissociation, we preformed the complexes in the presence or in the absence of EC DksA or DksA2 and then challenged them with heparin, a nonspecific competitor that would trap the free RNAP that had dissociated from the DNA. The open promoter complex was resistant to heparin challenge whereas RPc was highly sensitive. Following heparin addition, nucleotide substrates were added to measure the fraction of the remaining active RPo (Fig. 6). We found that DksA2 destabilized lacUV5 open complexes (t1/2 = 44 sec in the absence of added factors) somewhat more efficiently than EC DksA; the half-life was decreased to 11 and 16 sec, respectively (Fig. 6B). As in the case of DksA (Paul et al., 2005, Rutherford et al., 2009), DksA2 destabilized complexes at another model promoter (Fig. S10).
Figure 6.
Destabilization of the open promoter complex by DksA and DksA2 at the lacUV5 promoter. A. Schematics of the assay. B. Decay of the open complexes in presence or absence of 2 μM DksA or DksA2 was monitored by following the formation of short abortive transcripts radiolabeled with [α-32P]UMP at the selected times following the addition of heparin. C. Quantification of the open complexes stability, with and without DksA proteins. The errors (± one standard deviation) were determined from three independent experiments.
DksA2 traps the closed promoter complex at rrnB P1
Heparin sensitivity of the T7A1 transcription complexes suggests that DksA2 affects a step in the open complex formation pathway. However, this pathway includes several intermediate complexes which can be variably sensitive to heparin challenge. We decided to evaluate the effect of DksA2 on the promoter complex formation pathway in more detail using rrnB P1 promoter, the main physiological target of DksA, as a model.
Studies in the Gourse lab revealed that RPo formed at the rrn P1 promoters are highly unstable in the absence of the nucleotide substrates (Barker & Gourse, 2001); this property ties the rRNA synthesis to the cellular pool of ATP and GTP (Schneider et al., 2002), and thus the translational capacity of the cell. Additional level of regulation by ppGpp and DksA serves to coordinate the rRNA synthesis with the nutrient availability and other environmental factors. Gourse and coauthors argued that EC DksA inhibits transcription of the rrn genes by stabilizing a transcriptionally-inactive promoter complex at rrnB P1 (Rutherford et al., 2009). Their DNaseI footprinting analysis revealed that, in the absence of the initiating substrates which lock the complex in the open conformation, EC DksA shifted the downstream boundary of DNaseI protection from ~ +12 to ~+1 even at 37°C. This footprinting pattern is characteristic for the closed RPc complex and readily explains the inhibitory effect of EC DksA at the rrnB P1 promoter.
Similar effects of the EC DksA and DksA2 on transcription from rrnB P1 (Fig. 5) suggest that the two proteins may share the molecular mechanism of inhibition. To test this idea, we performed DNaseI footprinting analysis on a linear rrnB P1 promoter fragment (Fig. 7). As reported by Rutherford et al., the contacts between the RNAP and the downstream duplex DNA became destabilized upon the addition of DksA, as evidenced by the loss of protection downstream from the +1 position. Addition of DksA2 led to a similar loss of the downstream protection. As previously observed for EC DksA (Rutherford et al., 2009), DksA2 did not change the pattern of protection in the presence of ATP and CTP, the nucleotides incorporated at the +1 and +2 positions of the nascent RNA (data not shown). These results support the hypothesis that DksA2 shares the molecular mechanism with the “primary” DksA and can functionally substitute for it when Zn becomes scarce.
Figure 7.
Stabilization of the RPc complex at the rrnB P1 promoter by DksA and DksA2. A linear DNA fragment encompassing positions −60 to +10 of the rrnB P1 promoter was generated by PCR; the non-template DNA strand was end-labeled with [32P]-ATP (see Experimental Procedures). The footprint boundaries in the promoter region shown are indicated by black (RPc), gray (RPo), and white (RPinit, stabilized with ApC) bars on the left. DksA and DksA2 apparently destabilize the RPo and shift the complex toward an RPc-like state. The dideoxy-sequencing ladder is shown for reference.
Purified DksA2 does not contain Zn(II)
Previously, EC DksA was found by ICP-MS (Inductively Coupled Plasma – Mass Spectrometry) to have one Zn per monomer and was crystallized with the Zn bound to the four cysteines of the Zn-finger motif (Paul et al., 2004, Perederina et al., 2004). As two of those four cysteines are absent from DksA2, Zn binding may have been lost. We tested EC DksA and PA DksA2 preparations for metal content using ICP-MS. As shown in Table 1, Zn was found in association with EC DksA (at a ratio of DksA to Zn of 1:0.67) but not with DksA2. Furthermore, X-ray crystallographic analysis of DksA2 (O. Tsodikov, personal communication) also failed to reveal a bound metal ion.
Table 1.
ICP-MS analysis of EC DksA and DksA2
| Isotope | DksA | DksA2 |
|---|---|---|
| K39 | 8.57a | 30.0 |
| Ca42 | 0 | 23.7 |
| Mn55 | 0 | 0 |
| Fe56 | 0.173 | 0.146 |
| Fe57 | 0.0951 | 0.0877 |
| Co59 | 0 | 0 |
| Ni60 | 0 | 0 |
| Cu63 | 0.118 | 0 |
| Zn66 | 12.5 | 0 |
Units are in μg/L
Discussion
Zn depletion can have detrimental effects on bacterial cell viability and can impede its ability to infect a vertebrate host. P. aeruginosa is a significant human opportunistic pathogen and the primary cause of mortality among cystic fibrosis patients. As part of the acute immune response, the host actively sequesters Zn, limiting its availability to the invading pathogen. One common solution is to induce the expression of a high-affinity Zn(II)-transporter and transport available Zn from the environment. Indeed, high-affinity Zn(II)-transporters have been shown to be required for virulence of several pathogens (Dahiya & Stevenson, 2010, Davis et al., 2009, Ammendola et al., 2007, Yang et al., 2006, Kim et al., 2004, Campoy et al., 2002). Other strategies may include expression of Zn-independent functional copies of key proteins, sometimes coupled to mobilization of protein-bound Zn (Panina et al., 2003, Gabriel & Helmann, 2009, Akanuma et al., 2006, Sankaran et al., 2009). Our analysis argues that P. aeruginosa utilizes the DksA2 protein as a part of the adaptation to Zn-limited environments.
DksA2 was initially discovered as an unknown protein putatively regulated by the Zn(II)-responsive transcription factor Zur (Haas et al., 2009). In support of this prediction, transcript analysis reveals that dksA2 is expressed during metal depletion and repressed in the presence of Zn (Fig. 3B). This response is mediated by Zur, as expression of dksA2 is not repressed by Zn in the absence of Zur and Zur was found to bind specifically to the promoter region of dksA2 (Fig. 3C and D). These results suggest that DksA2 exerts its activity when cells encounter an environment poor in available Zn. In agreement with this hypothesis, DksA2 is induced during growth in cystic fibrosis sputum (Palmer et al., 2005). Sputum from cystic fibrosis patients was shown to have high levels of calprotectin (Gray et al., 2008), a neutrophil protein that chelates Zn making it unavailable to pathogens (Clohessy & Golden, 1995).
DksA2 lacks a conserved Zn-finger motif and, consequently, the bound Zn. Thus, DksA2 function should be independent of Zn, in contrast to DksA where it appears to be critical. Our growth assays and in vitro results reveal that DksA2 can (at least under some conditions) functionally replace DksA. These observations suggest a simple model wherein DksA2 substitutes for Zn-dependent DksA when Zn is scarce. This scenario is reminiscent of the failsafe mechanism of action of a C– S14 ribosomal protein paralog. In this model, the S14 paralog, which is missing the Zn-binding motif and does not require Zn, is expressed in the absence of Zn and enables active ribosome biosynthesis (Natori et al., 2007, Gabriel & Helmann, 2009).
By analogy, since Zn is likely required for the proper folding and function of DksA, the newly synthesized DksA would be inactive in a Zn-depleted environment, leading to defects in gene expression control. Under these conditions, it could be advantageous to express a back-up, Zn-independent DksA variant. Our data are consistent with a model in which DksA2 plays such a role in P. aeruginosa. In vivo, DksA2 compensates for the absence of DksA in both E. coli and P. aeruginosa during growth in minimal media and suppresses the pyocyanin deficiency phenotype of the P. aeruginosa ΔdksA strain (Fig. 2). These data suggest that DksA2 functions similarly to DksA in regulation of starvation and quorum sensing responses, respectively. In vitro, purified DksA2 stabilizes the closed promoter complex at, and thus inhibits transcription from, the rrnB P1 promoter (Figs. 5 and 7), the main target of DksA, as well as destabilizes open promoter complexes at the lacUV5 and T7A1 promoters (Figs. 6 and S10), suggesting that DksA2 acts directly on RNAP.
These observations are consistent with a model where DksA (that requires Zn) can be functionally replaced with its paralog DksA2 (that does not require Zn) when cellular Zn levels are low. However, it is quite possible that DksA2 plays additional roles in cellular physiology. For example, DksA2 may regulate promoters required for adaptation to various stresses, in essence acting as a specialized version of DksA. The growth defects observed in the presence of EDTA and TPEN could result from altered regulation at yet unknown promoters.
The discovery of the DksA paralog, DksA2, adds an extra level onto the already complex global regulation of gene expression by RNAP-binding factors. Uniquely, DksA2 brings to light the potential for novel gene regulation during Zn limitation, a condition that triggers an increase in DksA2 levels (Fig. 3). It is also possible that DksA2 (or other DksA paralogs) may be upregulated in response to other environmental stresses, such as nutrient starvation or reactive oxygen species. The DksA family of regulators may be the key players of an elaborate gene expression program designed to integrate diverse environmental cues and balance the cell’s growth under a large variety of conditions.
Experimental Procedures
Bioinformatics and phylogenetics
The complete genomes of proteobacteria were downloaded from Genbank (Benson et al., 2009). Identification of putative Zur-binding sites and regulon reconstruction was performed using the RegPredict web server (http://regpredict.lbl.gov/RegPredict/) (Novichkov et al., 2010) as previously described (Schröder et al., 2010). The deduced regulatory interactions were stored in the RegPrecise database (http://regprecise.lbl.gov/RegPrecise) (Novichkov et al., 2010). Genomic neighborhood of the dksA genes in bacterial genomes was analyzed using the SEED database and its tools (Overbeek et al., 2005). The phylogenetic tree of the DksA family was constructed by the maximum likelihood method implemented in the PROML program of the PHYLIP package (Felsenstein, 1997) using multiple sequence alignments of protein sequences produced by the MUSCLE program (Edgar, 2004). Sequence identity between the DksA homologs of P. aeruginosa was determined with the Needleman-Wunsch Global Sequence Alignment Tool available at NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Bacterial strains, plasmids, and growth conditions
E. coli K-12 MG1655 and P. aeruginosa PAO1 were used as WT strains and routinely grown at 37°C on LB-Lennox medium (LB) or minimal medium containing 1X M9 salts (Sambrook & Russell, 2001), 0.1 mM CaCl2, 2 mM MgSO4, 3 mg/L FeSO4×7H20 and 0.2% (wt/vol) glycerol as the carbon source. Media were solidified with 15 g of agar/liter. Construction of E. coli ΔdksA strain was performed by P1 transduction of the dksA::TetR allele from strain RLG8124 (Rutherford et al., 2009) into MG1655 as described by Miller (Miller, 1972). For plasmid maintenance in E. coli, the medium was supplemented with 100 μg ml−1 ampicillin (Amp), 10 μg ml−1 tetracycline (Tet), or 15 μg ml−1 gentamicin (Gm). For P. aeruginosa, 30 μg ml−1 Gm was used for marker selection and 200 μg ml−1 Amp was used for plasmid maintenance. Strains used in this study are listed in Table 2.
Table 2.
Strains and Plasmids
| Strain | Genotype | Source |
|---|---|---|
|
E. coli K12 MG1655 |
F, λ, rph1 |
E. coli Genetic Stock Center |
|
P. aeruginosa PAO1 |
Wild-type strain |
P. aeruginosa type strain (ATCC 33351) |
| VDC4098 | MG1655 ΔdksA::TetR | This study |
| VDC4133 | VDC4098 pBAD24 | This study |
| VDC4241 | VDC4098 pCH078 | This study |
| VDC4263 | VDC4098 pCH080 | This study |
| VDC4614 | VDC4098 pCH040 | This study |
| VDC4239 | VDC4098 pCH071 | This study |
| VDC4240 | VDC4098 pCH075 | This study |
| VDC4489 | PAO1 ΔPA4723 | This study |
| VDC4525 | VDC4489 ΔPA5536::GmR | This study |
| VDC4499 | VDC4489 ΔPA5499 | This study |
| VDC4510 | VDC4499 ΔPA5536::GmR | This study |
| VDC4607 | PAO1 pHERD20T | This study |
| VDC4666 | VDC4489 pHERD20T | This study |
| VDC4667 | VDC4489 pCH115 | This study |
| VDC4610 | VDC4525 pHERD20T | This study |
| VDC4611 | VDC4525 pCH115 | This study |
| VDC4612 | VDC4510 pHERD20T | This study |
| VDC4613 | VDC4510 pCH115 | This study |
| VDC4640 | PAO1 ΔPA5499::GmR | This study |
| VDC4615 | PAO1 ΔPA5536 | This study |
| VDC4635 | VDC4615 ΔPA5498:: GmR | This study |
| VDC4650 | ΔPA5498:: GmR pHERD20T | This study |
| VDC4652 | VDC4635 pHERD20T | This study |
| VDC4653 | VDC4635 pCH115 | This study |
| Plasmid | Description | Reference |
|---|---|---|
| pBAD24 | E. coli expression vector, AmpR | (Guzman et al., 1995) |
| pHERD20T | P. aeruginosa shuttle vector, AmpR | (Qiu et al., 2008) |
| pCH078 | PA4723 cloned into NcoI/XbaI sites of pBAD24 | This study |
| pCH080 | EC dksA cloned ineto NcoI/XbaI sites of pBAD24 | This study |
| pCH040 | PA5536 cloned into NcoI/XbaI sites of pBAD24 | This study |
| pCH071 | Product of PA4723 C114T mutagenesis cloned into NcoI/XbaI sites of pBAD24 |
This study |
| pCH075 | Product of PA4723 C135A mutagenesis cloned into NcoI/XbaI sites of pBAD24 |
This study |
| pCH115 | PA5536 cloned into NcoI/XbaI sites of pHERD20T | This study |
| pCH103 | pEX18Tc derivative carrying PA4723 deletion construct | This study |
| pCH107 | pEX18Tc derivative carrying PA5536 deletion construct | This study |
| pCH108 | pEX18Tc derivative carrying PA5499 deletion construct | This study |
| pCH131 | pEX18Tc derivative carrying PA5498 deletion construct | This study |
Plasmid Construction
Genes of interest were amplified by polymerase chain reaction (PCR) using Phusion® High-Fidelity DNA polymerase (Finnzymes, Oy, Espoo, Finland) according to the manufacturer’s guidelines from MG1655 or PAO1 genomic DNA using the primers listed in Table S1 in the supplemental material. Oligonucleotides were obtained from Integrated DNA Technologies (Coralville, IA, USA). PCR products were purified using the QIAquick PCR purification kit (Qiagen, Chatsworth, CA, USA) and cloned into pBAD24 (Guzman et al., 1995) for expression in E. coli or pHERD20T (Qiu et al., 2008) for expression in P. aeruginosa. For site-specific mutagenesis of PA dksA, the megaprimer PCR method (Sambrook & Russell, 2001) was employed with primers listed in Table S1. Plasmids used in this study are listed in Table 2.
Construction of P. aeruginosa mutants
Primers used in gene deletion constructs are listed in Table S1 in supplemental material. For gene replacement, a sacB-based strategy (Schweizer & Hoang, 1995) was employed. The gentamicin resistance gene was PCR amplified from pEX18Gm with primers containing FRT sites at the 5′ end (Hoang et al., 1998). Upstream and downstream regions flanking the gene of interest were PCR amplified from PAO1 genomic DNA and gel extracted using the QIAquick gel extraction kit (Qiagen). Generation of the gene deletion construct was performed by PCR overlap extension as previously described (Choi & Schweizer, 2005) and cloned into pEX18Tc (Hoang et al., 1998). The appropriate parent strain was transformed by electroporation as previously described (Choi et al., 2006) with the following changes. Instead of using an overnight culture, 50 ml of LB were inoculated with 1 ml of overnight culture and grown for 2 hours at 37°C then washed. Colonies were selected on gentamicin and screened for tetracycline sensitivity. If only single homologous recombination events occurred, GmR/TetR colonies were inoculated into 5 ml LB, grown overnight, and plated onto LB (minus NaCl) and 10% (wt/vol) sucrose to select for excision of the plasmid from the chromosome. Deletions were confirmed by PCR using both primers external to the upstream and downstream flank regions and primers internal to the gene. When required, the gentamicin cassette was excised with FLP recombinase (Hoang et al., 1998).
E. coli and P. aeruginosa growth assays
Overnight cultures grown in LB were washed with M9 medium and normalized to an optical density at 600nm of 1.0 for E. coli and optical density at 660nm (OD660nm) of 1.0 for P. aeruginosa. Washed and normalized cultures were then serially diluted and 10 μL were plated onto appropriate media. Serial dilutions were also plated on minimal medium plus 0.2% (wt/vol) casamino acids (for the E. coli and P. aeruginosa ΔdksA rescue experiments) or on minimal medium without chelator (for the P. aeruginosa ΔdksA2 Zn-depletion experiments) to ensure normalization of cell number between the different strains was achieved. Growth was imaged at 24 hours after plating for E. coli and 36 hours for P. aeruginosa. For the Zn-depletion experiments, plates were imaged after 67 hours. All experiments were repeated independently at least three times.
Pyocyanin Assay
Overnight cultures of WT pHERD20T, ΔdksA pHERD20T and ΔdksA pCH115 grown in LB were washed, normalized to an OD660nm of 1.0 and then diluted 1/100th into fresh LB. Pyocyanin was isolated from 5 ml samples as described previously (Farrell et al., 2010) and detected at an absorbance of 520nm in an acidic solution. The concentration of pyocyanin was calculated by multiplying the absorbance at 520nm by 17.072 (Kurachi, 1958).
qRT-PCR and Western blot analysis
One liter cultures of PAO1 or ΔPA5499::GmR in M9 media with or without 100 μM EDTA or 25 μM ZnSO4 were inoculated with 10 ml of an overnight culture grown in LB that was washed with M9 media and diluted to an OD660nm of 1.0. Two samples were harvested at each time point from each culture. One sample was used for RNA isolation and subsequent qRT-PCR, while the other sample was analyzed by Western blot as described below. For RNA isolation, 5 ml of culture were spun down and resuspended in TRIzol®-LS reagent (Invitrogen, Carlsbad, CA, USA) then froze at −80°C. Samples were thawed and RNA was extracted with chloroform and purified using the RNeasy mini kit (Qiagen). Trace DNA was removed with Turbo™ DNase (Ambion, Austin, TX, USA). qRT-PCR was performed using iScript cDNA synthesis kit and SYBR Green, according to the manufacture’s guidelines (Bio-Rad, Richmond, CA, USA). One nanogram of total mRNA was reverse transcribed then 1 μl of cDNA was added to a 20 μl SYBR Green reaction mix containing 2.5% DMSO. qPCR on the generated cDNA was conducted in the iCycler MyiQ™2 real-time system (Bio-Rad) in triplicate. Reactions containing 0.1 ng of total mRNA from each sample served as controls for DNA contamination. Primers used in the qPCR reactions are listed in Table S1. Standard curves were generated with serial dilutions of pCH078 for dksA and pCH0116 for dksA2. The cycling conditions were as follows: 1 cycle at 95°C for 3 min., 40 cycles of 95°C for 10 sec. and 58°C for 30 sec. Product uniformity was determine using melt curves. Data was analyzed using iQ™5 optical system software (Bio-Rad).
The other sample was spun down and pellets were frozen at −80°C. Pellets were thawed and cell concentrations were normalized. Gel electrophoresis was carried out using 15% sodium-dodecyl sulfate-polyacrylamide gels. Protein was transferred to an Immobilon-P polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA) and immunoblotted according to standard procedures using rabbit polyclonal antibodies (Harlan Laboratories, Inc. Indianapolis, IN) specific for DksA or DksA2 as primary antibody. Specificity of primary antibodies was confirmed by blotting against whole cell extracts from PAO1, ΔdksA, and Δzur grown in LB. For detection, anti-rabbit IgG (whole molecule) alkaline phosphatase conjugate antibody developed in goat (Sigma-Aldrich, St Louis, MO, USA) with CPD-Star reagent was used. qRT-PCR and Western blot analysis were independently repeated twice.
Reagents for in vitro assays
All general reagents were obtained from Sigma-Aldrich and Fisher (Pittsburgh, PA, USA), NTPs from GE healthcare (Piscataway, NJ, USA), PCR reagents from Gene Choice (Frederick, MD, USA) restriction and modification enzymes from New England Biolabs (Ipswich, MA, USA), and [α32P]-NTPs from Perkin Elmer (Waltham, MA, USA). Oligonucleotides were obtained from Sigma-Aldrich. DNA purification kits were from Promega (Madison, WI, USA) and ppGpp from TriLink BioTechnologies (San Diego, CA, USA).
Protein purification
The zur gene was cloned into the NdeI and HindIII sites of pET28a (Novagen, Darmstadt, Germany). The resulting vector was transformed into E. coli BL21(DE3). Overexpression of His6-Zur was achieved as previously described (Gabriel et al., 2008) and cells were lysed in lysis buffer (20 mM sodium phosphate, 500 mM NaCl, 10 mM imidazole, pH 7.3 and 2 mM DTT) using TEEN B Lysing matrix and a FastPrep-24 (MP Biomedicals, Aurora, OH). Cleared lysate was loaded on a 25 mL Ni Sepharose High Performance column (GE healthcare, Waukesha, WI, USA). After washing with 10 vol of wash buffer (lysis buffer with 20 mM imidazole instead of 10 mM), His6-Zur was eluted with a linear gradient of imidazole from 20 mM to 500 mM. His6-Zur eluted around 250 mM imidazole. His6-Zur containing fractions were combined and stored at −20°C in 50% glycerol.
E. coli DksA and RNAP were purified as described previously (Vassylyeva et al., 2004, Artsimovitch et al., 2000). RNAP holoenzyme was prepared by mixing core RNAP with σ70 in a 1:4 molar ratio and incubating for 30 minutes at 32°C.
P. aeruginosa dksA2 gene was PCR amplified from pCH040 and cloned between the NdeI and HindIII sites of pIA884, a derivate of pET28a (EMD Chemicals, Gibbstown, NJ, USA), which carries N-terminal 10-histidines tag followed by a TEV cleavage site, resulting in a DksA2-His fusion (pIA923). When induced in strain XJB, DksA2 was soluble and constituted ≈20% of total cell protein. Cells were lysed by French Press in a disruption buffer (50 mM Tris-HCl pH 6.9, 150 mM NaCl, 0.1 mM EDTA, 1 mM 2-mercaptoethanol). Cleared lysate was loaded on a Ni-NTA agarose (Qiagen) column. After four consecutive washes with 10 vol of the disruption buffer containing 0, 50, and 100 mM imidazole (pH 7.5), DksA2 was eluted with 250 mM imidazole and loaded on a heparin column (GE healthcare) equilibrated with HepA buffer (50 mM Tris-HCl pH 6.9, 0.1 mM EDTA, 1 mM 2-mercaptoethanol, 5% glycerol). Elution was carried out using 50-1500 mM NaCl gradient. DksA2 was eluted between 40-50 mSi (milli Siemens, units of conductivity). A final purification was done on Resource Q (GE healthcare) equilibrated with HepA, elution was carried out with 75-1500 mM NaCl; DksA2 was eluted at 30 mSi. Purified DksA2 was incubated with the His-tagged TEV protease overnight at room temperature and loaded again on resource Q column to remove the short His-tag, the TEV protease, and the uncleaved protein.
Electrophoretic mobility shift assay (EMSA)
A fragment containing the promoter region of dksA2 was amplified by PCR using P. aeruginosa genomic DNA as a template and ZurdksA2EMSAFor and Rev (Table S1). ZurdksA2EMSAFor was prelabeled at the 5′-end with biotin. The PCR fragment was purified using a QIAquick spin column. EMSAs were performed as previously described (Gaballa & Helmann, 1998) except 1.5 ng of biotin-labeled DNA was used as the substrate. After electrophoresis, DNA was transferred to a positively charged nylon membrance (Roche, Indianapolis, IN, USA). Transferred DNA was cross-linked with a Fisher-Scientific FB-UVXL-1000 UV Crosslinker by using the optimal cross-link setting. Biotin-labeled DNA was detected using a Phototope-Star Detection Kit (NEB) following manufacturer’s guidelines. Membranes were exposed to a Kodak x-ray film.
For EMSA competition assays, oligonucleotides (Table S1) corresponding to both strands of the putative Zur-binding site were synthesized. As a control, oligonucelotides corresponding to 42 bp from the PA4577 promoter region were synthesized. Annealing was carried out by mixing equimolar amounts of each complementary oligonucelotide in 1X ligase buffer. The mixture was heated at 98°C for 5 min. then allowed to cool to room temperature.
In vitro transcription
Linear templates for all the in vitro experiments were generated by PCR amplification of the plasmids encoding the test promoters. Sequences of all the plasmids and oligonucleotides will be provided upon request. Plasmid pIA536 was used to amplify a 153-bp fragment containing the rrnB P1 promoter (encompassing positions −60 to +10 relative to the promoter start site). Plasmids pFW11 and pIA349 were used to generate linear templates encoding the lacUV5 and T7A1 promoters, respectively. DksA, DksA2 (0.1-15 μM), ppGpp (50 μM) or Storage buffer (10 mM Tris-HCl pH 7.9, 100 mM NaCl, 1 mM DTT, 0.1 mM EDTA, 50% glycerol) were mixed with RNAP holoenzyme (30 nM), ApC (200 μM),UTP (200 μM), GTP (4 μM) and [α32P]-GTP (10 μCi of 3000 Ci/mmol) in 10 μl of Transcription buffer (20 mM Tris-HCl pH 7.9, 20 mM NaCl, 10 mM MgCl2, 14 mM 2-mercaptoethanol, 0.1 mM EDTA) at 37°C for 15 minutes. The DNA fragment was added and samples were incubated for an additional 15 minutes at 37°C. Reactions were stopped by the addition of an equal volume of Stop buffer (10 M urea, 20 mM EDTA, and 45 mM Tris-borate, pH 8.3). Samples were separated by electrophoresis on 8% polyacrylamide, 7 M urea gels, and dried gels were visualized and quantified by phosphorimaging (ImageQuant software, Molecular Dynamics).
DNAseI footprinting
The end-labeled 153-bp rrnB P1 template was generated by PCR with a non-template strand primer, which has been 5′-labeled with [32P]-γATP and polynucleotide kinase (Epicentre, Madison, WI, USA) prior to PCR. PCR products were gel-purified using a Promega kit. Sequencing reactions were performed using the same labeled primer with a SequiTherm kit (Epicentre). For DNaseI footprinting experiments, wild-type E. coli RNAP holoenzyme (200 nM) was pre-incubated with DksA, DksA2 (2 μM final concentration) or an equal volume of Storage buffer for 15 min at 37°C in GB buffer (10 mM Tris-Acetate pH 7.9, 10 mM MgCl2, 30 mM KCl, 1 mM DTT). The labeled rrnB P1 promoter fragment (10 nM) was added and the reaction was incubated for additional 15 min. Samples were shifted to room temperature (22°C) and treated with 0.002 U of DNaseI (Roche) for 2 min. The reaction was stopped by the addition of an equal volume of Stop buffer. Samples were separated by electrophoresis on 6% polyacrylamide, 7 M urea gels, as described above.
Promoter complex stability assay
Linear 92- and 249-bp DNA fragments containing the lacUV5 (from −60 to +23) and T7 A1 (from −86 to +29) promoters (10 nM) were incubated with RNAP holoenzyme (30 nM) and DksA, DksA2 (1 μM each) or SB for 15 minutes at 37°C. For lacUV5, the assay was carried out in 100 μl of 40 mM Tris-Cl pH 7.9, 200 mM NaCl, 10 mM MgCl2, 1 mM DTT. The T7A1 assay was carried out in 100 μl TGA buffer (40 mM Tris-Acetate, pH 7.9, 20 mM Na-Acetate, 2 mM Mg-Acetate, 5% glycerol, 1 mM DTT and 0.1 mM EDTA). Heparin (10 μg/ml) was added to sequester any free RNAP at time 0. Thereafter, reaction aliquots were withdrawn at various times and combined with nucleotide substrates. ApU (100 μM), ATP (500 μM), UTP (8 μM) and [α32P]-UTP (10 μCi of 3000 Ci/mmol) were used with lacUV5; ApU (100 μM), CTP (8 μM) and [α32P]-CTP (10 μCi of 3000 Ci/mmol) - with T7A1. Following a15-min incubation at 37°C, reactions were quenched by the addition of an equal volume of Stop buffer. Samples were separated by electrophoresis on 8% polyacrylamide, 7 M urea gels, as described above.
ICP-MS analysis
Both DksA and DksA2 were dialyzed against 20 mM Tris-Cl and 100 mM NaCl. 0.285 μM (5 mg/l) final concentration of each protein was analyzed with ICP-MS at the Wisconsin State Laboratory of Hygiene Trace Element research group with an ELEMENT2 sector field ICP-MS (Thermo Scientific). Buffer was used as a blank.
Supplementary Material
Acknowledgements
We are grateful to Dr. Richard Gourse for providing RLG8124, Dr. Shouguang Jin for providing P. aeruginosa PAO1 and pEX18Tc, Dr. Herbert Schweizer for providing pHERD20T, Dr. Ian K. Blaby for PAO1 genomic DNA, Dr. Kalyani Mondal for assistance with antibody production and Dr. Ruth Saecker for help with the ICP MS analysis and discussions. This study was supported by the National Science Foundation grant MCB-0949569 (to I.A.), by the National Institutes of Health grant R01 GM70641-01 (to V. de C.-L), and by a grant from the Russian Academy of Sciences (program “Molecular and Cellular Biology”) (to D.A.R).
References
- Aberg A, Shingler V, Balsalobre C. Regulation of the fimB promoter: a case of differential regulation by ppGpp and DksA in vivo. Mol Microbiol. 2008;67:1223–1241. doi: 10.1111/j.1365-2958.2008.06115.x. [DOI] [PubMed] [Google Scholar]
- Akanuma G, Nanamiya H, Natori Y, Nomura N, Kawamura F. Liberation of zinc-containing L31 (RpmE) from ribosomes by its paralogous gene product, YtiA, in Bacillus subtilis. J Bacteriol. 2006;188:2715–2720. doi: 10.1128/JB.188.7.2715-2720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola S, Pasquali P, Pistoia C, Petrucci P, Petrarca P, Rotilio G, Battistoni A. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect Immun. 2007;75:5867–5876. doi: 10.1128/IAI.00559-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. J Proteome Res. 2006;5:3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- Artsimovitch I, Svetlov V, Anthony L, Burgess R, Landick R. RNA polymerases from Bacillus subtilis and Escherichia coli differ in recognition of regulatory signals in vitro. J Bacteriol. 2000;182:6027–6035. doi: 10.1128/jb.182.21.6027-6035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker M, Gourse R. Regulation of rRNA transcription correlates with nucleoside triphosphate sensing. J Bacteriol. 2001;183:6315–6323. doi: 10.1128/JB.183.21.6315-6323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belogurov G, Mooney R, Svetlov V, Landick R, Artsimovitch I. Functional specialization of transcription elongation factors. EMBO J. 2009;28:112–122. doi: 10.1038/emboj.2008.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2009;37:D26–31. doi: 10.1093/nar/gkn723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe D, Morby A. Zn(II) metabolism in prokaryotes. FEMS Microbiol Rev. 2003;27:291–311. doi: 10.1016/S0168-6445(03)00041-X. [DOI] [PubMed] [Google Scholar]
- Branny P, Pearson J, Pesci E, Köhler T, Iglewski B, Van Delden C. Inhibition of quorum sensing by a Pseudomonas aeruginosa dksA homologue. J Bacteriol. 2001;183:1531–1539. doi: 10.1128/JB.183.5.1531-1539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H, Dennis P. Feedback control of ribosome function in Escherichia coli. Biochimie. 2008;90:493–499. doi: 10.1016/j.biochi.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Brown L, Gentry D, Elliott T, Cashel M. DksA affects ppGpp induction of RpoS at a translational level. J Bacteriol. 2002;184:4455–4465. doi: 10.1128/JB.184.16.4455-4465.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R, Anthony L. How sigma docks to RNA polymerase and what sigma does. Curr Opin Microbiol. 2001;4:126–131. doi: 10.1016/s1369-5274(00)00177-6. [DOI] [PubMed] [Google Scholar]
- Caldwell C, Chen Y, Goetzmann H, Hao Y, Borchers M, Hassett D, Young L, Mavrodi D, Thomashow L, Lau G. Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. Am J Pathol. 2009;175:2473–2488. doi: 10.2353/ajpath.2009.090166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoy S, Jara M, Busquets N, Pérez De Rozas A, Badiola I, Barbé J. Role of the high-affinity zinc uptake ZnuABC system in Salmonella enterica serovar typhimurium virulence. Infect Immun. 2002;70:4721–4725. doi: 10.1128/IAI.70.8.4721-4725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kumar A, Schweizer H. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods. 2006;64:391–397. doi: 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Choi K, Schweizer H. An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol. 2005;5:30. doi: 10.1186/1471-2180-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clohessy P, Golden B. Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand J Immunol. 1995;42:551–556. doi: 10.1111/j.1365-3083.1995.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Dahiya I, Stevenson R. The ZnuABC operon is important for Yersinia ruckeri infections of rainbow trout, Oncorhynchus mykiss (Walbaum) J Fish Dis. 2010;33:331–340. doi: 10.1111/j.1365-2761.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Dalebroux Z, Yagi B, Sahr T, Buchrieser C, Swanson M. Distinct roles of ppGpp and DksA in Legionella pneumophila differentiation. Mol Microbiol. 2010;76:200–219. doi: 10.1111/j.1365-2958.2010.07094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Kakuda T, DiRita V. A Campylobacter jejuni znuA orthologue is essential for growth in low-zinc environments and chick colonization. J Bacteriol. 2009;191:1631–1640. doi: 10.1128/JB.01394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell H, Garvey M, Cormican M, Laffey J, Rowan N. Investigation of critical inter-related factors affecting the efficacy of pulsed light for inactivating clinically relevant bacterial pathogens. J Appl Microbiol. 2010;108:1494–1508. doi: 10.1111/j.1365-2672.2009.04545.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. An alternating least squares approach to inferring phylogenies from pairwise distances. Syst Biol. 1997;46:101–111. doi: 10.1093/sysbio/46.1.101. [DOI] [PubMed] [Google Scholar]
- Gaballa A, Helmann JD. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J Bacteriol. 1998;180:5815–5821. doi: 10.1128/jb.180.22.5815-5821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S, Helmann J. Contributions of Zur-controlled ribosomal proteins to growth under zinc starvation conditions. J Bacteriol. 2009;191:6116–6122. doi: 10.1128/JB.00802-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SE, Miyagi F, Gaballa A, Helmann JD. Regulation of the Bacillus subtilis yciC gene and insights into the DNA-binding specificity of the zinc-sensing metalloregulator Zur. J Bacteriol. 2008;190:3482–3488. doi: 10.1128/JB.01978-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Hunt S, Stokes S, Bramall N, Bunch J, Cox A, McLeod C, Poole R. Severe zinc depletion of Escherichia coli: roles for high affinity zinc binding by ZinT, zinc transport and zinc-independent proteins. J Biol Chem. 2009;284:18377–18389. doi: 10.1074/jbc.M109.001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, MacGregor G, Noble D, Imrie M, Dewar M, Boyd A, Innes J, Porteous D, Greening A. Sputum proteomics in inflammatory and suppurative respiratory diseases. Am J Respir Crit Care Med. 2008;178:444–452. doi: 10.1164/rccm.200703-409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L, Belin D, Carson M, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas CE, Rodionov DA, Kropat J, Malasarn D, Merchant SS, de Crécy-Lagard V. A subset of the diverse COG0523 family of putative metal chaperones is linked to zinc homeostasis in all kingdoms of life. BMC Genomics. 2009;10:470. doi: 10.1186/1471-2164-10-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T, Karkhoff-Schweizer R, Kutchma A, Schweizer H. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- Jude F, Köhler T, Branny P, Perron K, Mayer M, Comte R, van Delden C. Posttranscriptional control of quorum-sensing-dependent virulence genes by DksA in Pseudomonas aeruginosa. J Bacteriol. 2003;185:3558–3566. doi: 10.1128/JB.185.12.3558-3566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P, Craig E. Identification and characterization of a new Escherichia coli gene that is a dosage-dependent suppressor of a dnaK deletion mutation. J Bacteriol. 1990;172:2055–2064. doi: 10.1128/jb.172.4.2055-2064.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie T, Skaar E. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Watanabe K, Shirahata T, Watarai M. Zinc uptake system (znuA locus) of Brucella abortus is essential for intracellular survival and virulence in mice. J Vet Med Sci. 2004;66:1059–1063. doi: 10.1292/jvms.66.1059. [DOI] [PubMed] [Google Scholar]
- Kurachi M. Studies on the biosynthesis of pyocyanine. Bull Inst Chem Res. 1958;36:188–196. [Google Scholar]
- Laptenko O, Kim S, Lee J, Starodubtseva M, Cava F, Berenguer J, Kong X, Borukhov S. pH-dependent conformational switch activates the inhibitor of transcription elongation. EMBO J. 2006;25:2131–2141. doi: 10.1038/sj.emboj.7601094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laptenko O, Lee J, Lomakin I, Borukhov S. Transcript cleavage factors GreA and GreB act as transient catalytic components of RNA polymerase. EMBO J. 2003;22:6322–6334. doi: 10.1093/emboj/cdg610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Helmann J. Functional specialization within the Fur family of metalloregulators. Biometals. 2007;20:485–499. doi: 10.1007/s10534-006-9070-7. [DOI] [PubMed] [Google Scholar]
- Magnusson L, Farewell A, Nyström T. ppGpp: a global regulator in Escherichia coli. Trends Microbiol. 2005;13:236–242. doi: 10.1016/j.tim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Magnusson L, Gummesson B, Joksimović P, Farewell A, Nyström T. Identical, independent, and opposing roles of ppGpp and DksA in Escherichia coli. J Bacteriol. 2007;189:5193–5202. doi: 10.1128/JB.00330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova K, Ponomarev V, Koonin E. Two C or not two C: recurrent disruption of Zn-ribbons, gene duplication, lineage-specific gene loss, and horizontal gene transfer in evolution of bacterial ribosomal proteins. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-9-research0033. RESEARCH 0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson J, Chitsaz F, Derbyshire M, DeWeese-Scott C, Fong J, Geer L, Geer R, Gonzales N, Gwadz M, He S, Hurwitz D, Jackson J, Ke Z, Lanczycki C, Liebert C, Liu C, Lu F, Lu S, Marchler G, Mullokandov M, Song J, Tasneem A, Thanki N, Yamashita R, Zhang D, Zhang N, Bryant S. CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res. 2009;37:D205–210. doi: 10.1093/nar/gkn845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1972. [Google Scholar]
- Natori Y, Nanamiya H, Akanuma G, Kosono S, Kudo T, Ochi K, Kawamura F. A fail-safe system for the ribosome under zinc-limiting conditions in Bacillus subtilis. Mol Microbiol. 2007;63:294–307. doi: 10.1111/j.1365-2958.2006.05513.x. [DOI] [PubMed] [Google Scholar]
- Novichkov P, Laikova O, Novichkova E, Gelfand M, Arkin A, Dubchak I, Rodionov D. RegPrecise: a database of curated genomic inferences of transcriptional regulatory interactions in prokaryotes. Nucleic Acids Res. 2010;38:D111–118. doi: 10.1093/nar/gkp894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outten C, O’Halloran T. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang HY, Cohoon M, de Crécy-Lagard V, Diaz N, Disz T, Edwards R, Fonstein M, Frank ED, Gerdes S, Glass EM, Goesmann A, Hanson A, Iwata-Reuyl D, Jensen R, Jamshidi N, Krause L, Kubal M, Larsen N, Linke B, McHardy AC, Meyer F, Neuweger H, Olsen G, Olson R, Osterman A, Portnoy V, Pusch GD, Rodionov DA, Rückert C, Steiner J, Stevens R, Thiele I, Vassieva O, Ye Y, Zagnitko O, Vonstein V. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 2005;33:5691–5702. doi: 10.1093/nar/gki866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer K, Mashburn L, Singh P, Whiteley M. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol. 2005;187:5267–5277. doi: 10.1128/JB.187.15.5267-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panina EM, Mironov AA, Gelfand MS. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc Natl Acad Sci U S A. 2003;100:9912–9917. doi: 10.1073/pnas.1733691100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer S, Hantke K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol. 1998;28:1199–1210. doi: 10.1046/j.1365-2958.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- Patzer S, Hantke K. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J Biol Chem. 2000;275:24321–24332. doi: 10.1074/jbc.M001775200. [DOI] [PubMed] [Google Scholar]
- Paul B, Barker M, Ross W, Schneider D, Webb C, Foster J, Gourse R. DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell. 2004;118:311–322. doi: 10.1016/j.cell.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Paul B, Berkmen M, Gourse R. DksA potentiates direct activation of amino acid promoters by ppGpp. Proc Natl Acad Sci U S A. 2005;102:7823–7828. doi: 10.1073/pnas.0501170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perederina A, Svetlov V, Vassylyeva M, Tahirov T, Yokoyama S, Artsimovitch I, Vassylyev D. Regulation through the secondary channel--structural framework for ppGpp-DksA synergism during transcription. Cell. 2004;118:297–309. doi: 10.1016/j.cell.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Perron K, Comte R, van Delden C. DksA represses ribosomal gene transcription in Pseudomonas aeruginosa by interacting with RNA polymerase on ribosomal promoters. Mol Microbiol. 2005;56:1087–1102. doi: 10.1111/j.1365-2958.2005.04597.x. [DOI] [PubMed] [Google Scholar]
- Petrarca P, Ammendola S, Pasquali P, Battistoni A. The Zur-regulated ZinT protein is an auxiliary component of the high-affinity ZnuABC zinc transporter that facilitates metal recruitment during severe zinc shortage. J Bacteriol. 2010;192:1553–1564. doi: 10.1128/JB.01310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- Potrykus K, Vinella D, Murphy H, Szalewska-Palasz A, D’Ari R, Cashel M. Antagonistic regulation of Escherichia coli ribosomal RNA rrnB P1 promoter activity by GreA and DksA. J Biol Chem. 2006;281:15238–15248. doi: 10.1074/jbc.M601531200. [DOI] [PubMed] [Google Scholar]
- Qiu D, Damron F, Mima T, Schweizer H, Yu H. PBAD-based shuttle vectors for functional analysis of toxic and highly regulated genes in Pseudomonas and Burkholderia spp. and other bacteria. Appl Environ Microbiol. 2008;74:7422–7426. doi: 10.1128/AEM.01369-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S, Villers C, Lee J, Ross W, Gourse R. Allosteric control of Escherichia coli rRNA promoter complexes by DksA. Genes Dev. 2009;23:236–248. doi: 10.1101/gad.1745409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell D. Molecular Cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2001. [Google Scholar]
- Sankaran B, Bonnett SA, Shah K, Gabriel S, Reddy R, Schimmel P, Rodionov DA, de Crécy-Lagard V, Helmann JD, Iwata-Reuyl D, Swairjo MA. Zinc-independent folate biosynthesis: genetic, biochemical, and structural investigations reveal new metal dependence for GTP cyclohydrolase IB. J Bacteriol. 2009;191:6936–6949. doi: 10.1128/JB.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D, Gaal T, Gourse R. NTP-sensing by rRNA promoters in Escherichia coli is direct. Proc Natl Acad Sci U S A. 2002;99:8602–8607. doi: 10.1073/pnas.132285199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J, Jochmann N, Rodionov D, Tauch A. The Zur regulon of Corynebacterium glutamicum ATCC 13032. BMC Genomics. 2010;11:12. doi: 10.1186/1471-2164-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H, Hoang T. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- Srivatsan A, Wang J. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr Opin Microbiol. 2008;11:100–105. doi: 10.1016/j.mib.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Stebbins C, Borukhov S, Orlova M, Polyakov A, Goldfarb A, Darst S. Crystal structure of the GreA transcript cleavage factor from Escherichia coli. Nature. 1995;373:636–640. doi: 10.1038/373636a0. [DOI] [PubMed] [Google Scholar]
- Stover C, Pham X, Erwin A, Mizoguchi S, Warrener P, Hickey M, Brinkman F, Hufnagle W, Kowalik D, Lagrou M, Garber R, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody L, Coulter S, Folger K, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong G, Wu Z, Paulsen I, Reizer J, Saier M, Hancock R, Lory S, Olson M. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Symersky J, Perederina A, Vassylyeva M, Svetlov V, Artsimovitch I, Vassylyev D. Regulation through the RNA polymerase secondary channel. Structural and functional variability of the coiled-coil transcription factors. J Biol Chem. 2006;281:1309–1312. doi: 10.1074/jbc.C500405200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehranchi A, Blankschien M, Zhang Y, Halliday J, Srivatsan A, Peng J, Herman C, Wang J. The transcription factor DksA prevents conflicts between DNA replication and transcription machinery. Cell. 2010;141:595–605. doi: 10.1016/j.cell.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautinger B, Jaktaji R, Rusakova E, Lloyd R. RNA polymerase modulators and DNA repair activities resolve conflicts between DNA replication and transcription. Mol Cell. 2005;19:247–258. doi: 10.1016/j.molcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Vassylyeva M, Perederina A, Svetlov V, Yokoyama S, Artsimovitch I, Vassylyev D. Cloning, expression, purification, crystallization and initial crystallographic analysis of transcription factor DksA from Escherichia coli. Acta Crystallogr D Biol Crystallogr. 2004;60:1611–1613. doi: 10.1107/S0907444904015033. [DOI] [PubMed] [Google Scholar]
- Vassylyeva M, Svetlov V, Dearborn A, Klyuyev S, Artsimovitch I, Vassylyev D. The carboxy-terminal coiled-coil of the RNA polymerase beta’-subunit is the main binding site for Gre factors. EMBO Rep. 2007;8:1038–1043. doi: 10.1038/sj.embor.7401079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viducic D, Ono T, Murakami K, Susilowati H, Kayama S, Hirota K, Miyake Y. Functional analysis of spoT, relA and dksA genes on quinolone tolerance in Pseudomonas aeruginosa under nongrowing condition. Microbiol Immunol. 2006;50:349–357. doi: 10.1111/j.1348-0421.2006.tb03793.x. [DOI] [PubMed] [Google Scholar]
- Winsor G, Van Rossum T, Lo R, Khaira B, Whiteside M, Hancock R, Brinkman F. Pseudomonas Genome Database: facilitating user-friendly, comprehensive comparisons of microbial genomes. Nucleic Acids Res. 2009;37:D483–488. doi: 10.1093/nar/gkn861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Becker T, Walters N, Pascual D. Deletion of znuA virulence factor attenuates Brucella abortus and confers protection against wild-type challenge. Infect Immun. 2006;74:3874–3879. doi: 10.1128/IAI.01957-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenkin N, Yuzenkova Y, Severinov K. Transcript-assisted transcriptional proofreading. Science. 2006;313:518–520. doi: 10.1126/science.1127422. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jin D. The rpoB mutants destabilizing initiation complexes at stringently controlled promoters behave like “stringent” RNA polymerases in Escherichia coli. Proc Natl Acad Sci U S A. 1998;95:2908–2913. doi: 10.1073/pnas.95.6.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łyzen R, Kochanowska M, Wegrzyn G, Szalewska-Palasz A. Transcription from bacteriophage lambda pR promoter is regulated independently and antagonistically by DksA and ppGpp. Nucleic Acids Res. 2009;37:6655–6664. doi: 10.1093/nar/gkp676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.