Abstract
Purpose of review
To review the current scientific literature and recent clinical trials on HIV protease inhibitors (PIs) and their potential role in the pathogenesis of lipodystrophy and metabolic disorders.
Recent findings
HIV PI treatment may affect the normal stimulatory effect of insulin on glucose and fat storage. Further, chronic inflammation from HIV infection and PI treatment trigger cellular homeostatic stress responses with adverse effects on intermediary metabolism. The physiologic outcome is such that total adipocyte storage capacity is decreased, and the remaining adipocytes resist further fat storage. This process leads to a pathologic cycle of lipodystrophy and lipotoxicity, a pro-atherogenic lipid profile, and a clinical phenotype of increased central body fat distribution similar to the metabolic syndrome.
Summary
PIs are a key component of antiretroviral therapy and have dramatically improved the life expectancy of HIV-infected individuals. However, they are also associated with abnormalities in glucose/lipid metabolism and body fat distribution. Further studies are needed to better define the pathogenesis of PI-associated metabolic and body fat changes and their potential treatment.
Keywords: HIV, protease inhibitors, metabolic disorders, lipodystrophy
Introduction
Human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS) were first recognized in 1981 by the US Centers for Disease Control and Prevention1. Since then, the impact of AIDS and/or HIV on demographic, social, and economic conditions has been substantial. According to the World Health Organization, ~60 million people have been infected with HIV and 25 million people worldwide have died of the disease. In 2008, ~33.4 million people were living with HIV/AIDS and ~2.7 million people were newly infected2. HIV/AIDS has thus become a leading cause of mortality worldwide and ranks as one of most important infectious diseases facing civilization in the 21st century. There are two distinct serotypes of human AIDS viruses, HIV type 1 (HIV-1) and type 2 (HIV-2), with HIV-1 accounting for the majority of infection worldwide3. The majority of untreated individuals with HIV infection develop AIDS within 7–10 years.
Although Sub-Saharan Africa remains the region most heavily affected by HIV, accounting for ~67% of HIV infections worldwide, some countries in Eastern Europe (such as Ukraine and the Russian Federation) are experiencing severe and growing national epidemics. In the United States (US), although the incidence of HIV infection has remained relatively stable, new epidemiological patterns have evolved; for instance, rates of new HIV infection in women, minorities, and younger gay men have increased, while the rate of new HIV infection among injecting drug users has fallen.
Treatment of HIV infection: HAART
The successful introduction in 1995 of highly active antiretroviral therapy (HAART), a combination of potent antiretroviral agents, has substantially decreased mortality among HIV-infected patients4, 5. HAART is the standard of care to avoid selection of viral mutations, and the regimen is typically chosen based on the patient's comorbidities, efficacy and tolerability in clinical trials, potential drug interactions, adverse drug effects, and potential long-term complications6. Furthermore, selection of drugs for treatment-naïve and treatment-experienced patients take into account the benefit/risk ratio and the HIV genotype. Current US and international guidelines recommend that treatment-naïve patients receive a combination of two nucleoside reverse-transcriptase inhibitors (NRTI) together with one non-nucleoside reverse-transcriptase inhibitors (NNRTI) or one (ritonavir-boosted) protease inhibitor (PI), and that treatment-experienced patients receive a combination of at least two active ART drugs from different classes based on the viral genotype6–8. Treatment of experienced patients that have failed previous regimens is more complex and might include newer ritonavir-boosted PIs such as darunavir or tipranavir, the new NNRTI etravirine, the CCR5 inhibitor maraviroc or the integrase inhibitor raltegravir. Currently, there are 25 antiretroviral drugs available in six different classes approved for clinical use in the treatment of AIDS9*. New drugs are also constantly in development10, 11*. Over the past two decades the treatment of HIV has advanced considerably, changing HIV from a deadly infection into a manageable complex infection requiring lifelong treatment12–14.
However, HAART is associated with a number of metabolic and anthropometric abnormalities, including dyslipidemia and insulin resistance as well as subcutaneous fat loss (lipoatrophy) and abdominal obesity (lipohypertrophy), all of which may contribute to an increased risk of cardiovascular disease (CVD)15. Furthermore, besides the side effects from HIV treatment, there is recognition that HIV infection by itself, or in combination with genetic and/or environmental factors, may cause metabolic abnormalities due to the dynamic relationship between the virus and the host. In view of this, it is noteworthy that increased mortality and morbidity rates from CVD has been reported among HIV-infected patients16. As the HIV-positive population ages, the combination of cardiovascular risk factors commonly seen in the general population with the presence of HIV infection and its treatment thus pose significant therapeutic and health care challenges.
Protease Inhibitors
PIs were first introduced in 1996 and have resulted in dramatic decline in HIV-related mortality and morbidity; thus, they remain a cornerstone of antiretroviral therapy. Nine PIs are currently available: saquinavir, indinavir, ritonavir, nelfinavir, lopinavir, fosamprenavir, atazanavir, darunavir and tipranavir (Table 1).
Table 1.
| Drug generation | Protease inhibitors | Recommended Dosagea | Absorption tmax (h) | Protein binding (%) | Plasma t1/2 (h) | Metabolism: CYP450 |
|---|---|---|---|---|---|---|
| 1st | Saquinavirb | 1000/r100 mg BID | 1–2 | 97 | 5 | Renal+CYP3A4 |
| Indinavirb | 800/r100 mg BID | 1 | 61 | 1.5–2 | Renal+CYP3A4 | |
| Ritonavir | 600 mg BID | 3 | 98–99 | 3–5 | Renal+CYP3A and 2D6 | |
| Nelfinavir | 1250 mg BID | 3 | ≥98 | 5–6 | Renal+CYP3A and 2C19 | |
|
| ||||||
| 2nd | Lopinavirb | 400/r100 BID or 800/r100 QD | 5 | 98–99 | 5–6 | Renal+CYP3A |
| Fosamprenavirb | 700/r100 mg BID | 2 | 90 | 7–12 | Renal+CYP3A | |
| Atazanavirb | 300/r100 mg QD | 2 | 86 | 7 | Renal+CYP3A | |
|
| ||||||
| 3rd | Darunavirb | 800/r100 mg QD | 1–4 | 94 | 10–15 | Renal+CYP3A |
| Tipranavirb | 500/r200 mg BID | 3 | 99 | 6 | Renal+CYP3A | |
Adapted from: Panel on Antiretroviral Guidelines for Adults and Adolescents
Ritonavir boosted × 1–2; BID, twice a day; TDS, three times a day; QD, once-daily dosing
However, soon after their introduction, safety concerns for lipodystrophy19 and associated metabolic complications such as insulin resistance20 and dyslipidemia21 were raised, and although several studies have established an association between the use of PI therapy and a wide range of adverse effects, the clinical significance of these findings remains unknown22–24. Results from cross-sectional studies have shown that compared to healthy subjects, HIV-positive patients receiving antiretroviral therapy have increased secretion and decreased clearance of VLDL particles25, increased synthesis26 and reduced catabolism of apolipoprotein B27, the protein backbone of atherogenic lipoproteins, and diminished lipoprotein lipase activity28. Hypertriglyceridemia and increased levels of pro-atherogenic remnant lipoproteins have also been noted in HIV-positive patients on HAART21, 29. Furthermore, until recently, a high pill burden for many PIs caused poor adherence. This problem has in part been solved by newer PIs, which have more convenient formulations with fewer pills and once-daily administration.
PIs prevent cleavage of viral polyproteins after viral budding and thereby inhibit the ability of virus particles to infect new host cells30, 31; they have no effect on cells already harboring integrated proviral DNA. Most PIs are only moderately absorbed in the gastrointestinal (GI) tract and absorption is increased when PIs are taken with food, except fosamprenavir, for which the fasting state is recommended. PIs distribute into most body compartments, and diffusion through anatomical barriers is usually moderate, with only indinavir penetrating the blood-brain and blood-testis barriers in therapeutic concentrations32, 33. Plasma protein binding is primarily to α1-acid glycoprotein and albumin, and all the PIs, except indinavir, are highly protein-bound (Table 1). PIs are primarily metabolized through the cytochrome P450 (CYP) system in the liver (major) and small intestine (minor)34–36. Furthermore, beyond being substrates for CYP isozymes, the PIs are also inhibitors of CYP3A4, causing variable elimination half-lives for the drugs, ranging from 1–2 hours for saquinavir to 10–15 hours for darunavir (Table 1). As the most potent CYP3A4 inhibitor, ritonavir inhibits CYP450-mediated metabolism in the small intestine and liver, and ritonavir-boosted PIs show increased plasma minimum concentrations (Cmin), maximum concentrations (Cmax), and plasma half-lives (t1/2). Thus, lower doses of ritonavir are used in combination with other PIs, except for nelfinavir, which is metabolized by CYP 2C19. Table 1 shows the list of all available PIs and current treatment guidelines for drug-naïve individuals. Hepatic metabolism is the primary route of biotransformation for PIs, which may therefore potentiate drug-drug interactions with this class of agents as well17. During last 3–4 years, many clinical trials among treatment-naïve populations such as KLEAN37, CASTLE38 and ARTEMIS39 have expanded the ritonavir-boosted PI treatment options (Table 2). Furthermore, two third generation PI agents – tipranavir and darunavir – have been approved and are important therapeutic options for treatment-experienced patients (Table 1).
Table 2.
Protease inhibitor clinical trials in drug-naïve HIV patients.
| Trial | Authors | Drugs | Patients (in each arm) | Outcome |
|---|---|---|---|---|
| MaxCmin240 | Dragsted et al | SQV/r vs. LPV/r | 161/133 | Better antiretroviral effects, lower virological failure and treatment discontinuation rates of LPV/r compared with SQV/r |
| M97–72041 | Murphy et al | LPV/r | 100 | 59% patients (ITT) had <50 copies/ml plasma HIV-1 RNA through 7 years |
| KLEAN37 | Eron et al | fAPV/r vs. LPV/r | 434/444 | Similar antiretroviral efficacy, safety, tolerability, and emergence of resistance of fAPV compared to LPV/r |
| CASTLE38 | Molina et al | ATV/r vs. LPV/r | 440/443 | Similar antiretroviral efficacy, less GI toxicity, and better lipid and safety profiles with ATV/r |
| SWAN42 | Gatell et al | ATV/r vs. other PI | 278/141 | Better virologic suppression, a comparable safety profile, and improved lipid parameters with ATV/r |
| RESIST-343 | Hicks et al | TPV/r vs. other PI | 746/737 | Better virological and immunological responses over 48 weeks with TPV/r compared to control PI |
| POWER 1–244 | Clotet et al | DRV/r vs. other PI | 131/124 | Favorable safety and tolerability, better efficacy at 48 weeks with DRV/r compared to control PI |
| ARTEMIS39 | Ortiz et al | DRV/r vs. LPV/r | 343/346 | Non-inferiority of DRV/r vs. LPV/r at 48 weeks, with a more favorable safety profile |
| TITAN45 | Madruga et al | DRV/r vs. LPV/r | 298/297 | Non-inferiority of DRV/r monotherapy vs. LPV/r |
| MONET46* | Arribas et al | DRV/r monotherapy vs. triple | 127/128 | Non-inferiority of DRV/r monotherapy vs. triple ART |
SQV/r, Saquinavir/ritonavir; LPV/r, Lopinavir/ritonavir; fAPV/r, Fosamprenavir/ritonavir; ATV/r, Atazanavir/ritonavir; TPV/r, Tipranavir/ritonavir; DRV/r, Darunavir/ritonavir; ITT, intention-to-treat, ART, antiretroviral treatment.
Trials: MaxCmin2, open-label, randomized, multicenter, comparative trial evaluating the safety and efficacy of lopinavir/ritonavir vs. saquinavir/ritonavir; M97–720, open-label, follow-up of prospective, randomized, multicenter trial evaluating the efficacy and tolerability of lopinavir/ritonavir in combination with stavudine and lamivudine; KLEAN (Kaletra versus Lexiva with Epivir and Abacavir in ART-Naive patients), open-label, randomized, non-inferiority study comparing fosamprenavir/ritonavir vs. lopinavir/ritonavir; CASTLE, open-label, randomized, multicenter, non-inferiority study evaluating the safety and efficacy of atazanavir/ritonavir vs. lopinavir/ritonavir; SWAN (Switch to Another Protease Inhibitor), open-label, comparative trial evaluating the safety and efficacy of atazanavir/ritonavir vs. comparator PI; RESIST-3 (Randomized Evaluation of Strategic Intervention in Multidrug Resistant Patients With Tipranavir), open-label, randomized, multinational, phase III study evaluating the efficacy of tipranavir/ritonavis vs. other PI; POWER 1–2 (Performance of TMC114/r When Evaluated in Treatment-Experienced Patients with PI Resistance) open-label, randomized, multinational, phase III study evaluating the efficacy and safety of darunavir/ritonavir vs. currently available PIs; ARTEMIS (AntiRetroviral Therapy with TMC114 ExaMined In naive Subjects) open-label, randomized, comparative trial evaluating the efficacy and safety of darunavir/ritonavir vs. lopinavir/ritonavir; TITAN (TMC114/r In Treatment-experienced pAtients Naïve to lopinavir) open-label, randomized, international, comparative trial evaluating the safety and efficacy of darunavir/ritonavir vs. lopinavir/ritonavir; MONET (Montreal Ottawa New Emerging Team), open-label, randomized, phase III non-inferiority study evaluating safety and efficacy of darunavir/ritonavir (monotherapy) vs. two nucleoside analogues and darunavir/ritonavir (triple therapy arm).
In general, PIs show an acceptable safety profile. The most common acute adverse effects are GI intolerance, such as nausea, vomiting, diarrhea, and bloating, which vary in intensity among different PIs. Recurrent or chronic diarrhea is the most common adverse effect, but it rarely occurs with indinavir, atazanavir, or fosamprenavir47. The incidence of diarrhea is 15–20% in patients treated with lopinavir/ritonavir48. Newer PIs, such as atazanavir/ritonavir (evaluated in the CASTLE study38) and darunavir/ritonavir (evaluated in the pooled data analysis of the POWER 1 and POWER 2 trials44) have shown a reduced incidence of such events. The PI-associated dyslipidemic pattern described above has most commonly been reported among patients receiving old PIs, such as indinavir, nelfinavir, and ritonavir49. The newer PI atazanavir is specifically less likely to induce lipid abnormalities compared with other PIs50. For example, atazanavir/ritonavir demonstrated a better lipid profile than lopinavir/ritonavir in a 96-week study51. Nephrolithiasis is also a unique adverse effect of indinavir, occurring in as many as 12.4% of patients52. Furthermore, both atazanavir and indinavir are associated with isolated indirect hyperbilirubinemia (Gilbert's syndrome)53. Other morphological abnormalities (lipodystrophy - fat atrophy and fat deposition) and metabolic disturbances (hyperglycaemia and hyperlipidemia) are outlined below.
PI-Associated Body Fat Abnormalities
Changes in body fat distribution – often referred to as HIV/HAART-associated lipodystrophy – are common in HIV-infected individuals and typically start to occur after 6–12 months of PI therapy. Importantly, these body fat changes include both lipoatrophy and lipohypertrophy19, 22, 54. Lipoatrophy denotes a decrease in adipose tissue volume, and is an HIV-specific change that occurs with HIV/HAART therapy affecting all subcutaneous adipose tissue depots55; the least amount of subcutaneous fat loss occurs in the upper trunk, contributing to the characteristic “buffalo hump” in HIV-treated patients55. Alternatively, lipohypertrophy denotes an increase in adipose tissue volume, and most commonly occurs in visceral adipose tissue (VAT) and adipose tissue depots in the upper trunk, particularly in the breast and dorsocervical fat pads55. Lipohypertrophy, particularly of VAT, may occur concomitantly with lipoatrophy of subcutaneous fat depots56–58, suggesting that the two processes are not linked and differentially regulated. Moreover, the HIV drug factors that play a role in lipoatrophy do not appear to contribute to lipohypertrophy; rather, lipohypertrophy is associated with effective viral suppression, restored health, and weight gain56–58.
This abnormal HIV/HAART-associated redistribution of fat has important clinical implications. First, the physical manifestations of lipodystrophy (i.e., the “buffalo hump” and truncal obesity with facial/limb wasting) may affect adherence to an otherwise successful HAART regimen in a body-image conscious individual, resulting in poor self-esteem, immunological and clinical deterioration, and non-adherence with treatment regimens that could lead to potential viral mutations and drug resistance59, 60. Second, increased VAT, even in the absence of HIV infection, is associated with systemic inflammation, which may negatively affect not only a patient's response to HAART, but also accelerate their lifetime risk of CVD and other adverse events15, 61, 62. Third, and of profound metabolic importance, the circulation of free fatty acids (FFAs) and triglycerides (TGs) in the bloodstream during the fat redistribution process may increase the possibility of “ectopic” fat deposition (i.e., the deposition of fat in non-adipose tissues such as the liver, skeletal muscle, heart, and pancreas).
The evaluation of ectopic lipid deposition in organ dysfunction is an area of active investigation, and the literature on this topic has recently been reviewed63, 64**. To summarize the major findings: (i) ectopic intracellular lipid deposition in the skeletal muscle (intramyocellular lipid [IMCL]) is associated with insulin resistance and inflammatory processes65, 66; (ii) ectopic intracellular lipid deposition in the liver (intrahepatocellular lipid [IHCL]) is associated with non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), insulin resistance, and inflammatory processes67, 68**; (iii) ectopic lipid deposition in the heart (myocardial lipid) is associated with cardiovascular dysfunction and heart failure69, 70; and (iv) ectopic lipid deposition in the pancreas (pancreatic lipid) is associated with beta-cell dysfunction and altered insulin secretion71, 72*. Fat redistribution during HIV/HAART may also promote insulin resistance through altered secretion of adipokines (including adiponectin, leptin, plasminogen activator inhibitor-1 [PAI-1], resistin, tumor necrosis factor-alpha [TNF-α], and other inflammatory markers including interleukins 6 [IL-6], 8 [IL-8], and 10 [IL-10], and macrophage chemotactic protein-1 [MCP-1]), which act as both paracrine factors in adipose tissue and endocrine factors affecting both systemic glucose and lipid metabolism73. Overweight HIV patients with increased VAT treated with PIs are at particularly high risk for disordered glucose metabolism74. Moreover, the expansion of VAT that occurs during HIV/HAART therapy is associated with macrophage infiltration, decreased adiponectin secretion, and the release of inflammatory factors73, 75, all of which are associated with insulin resistance and its associated metabolic traits.
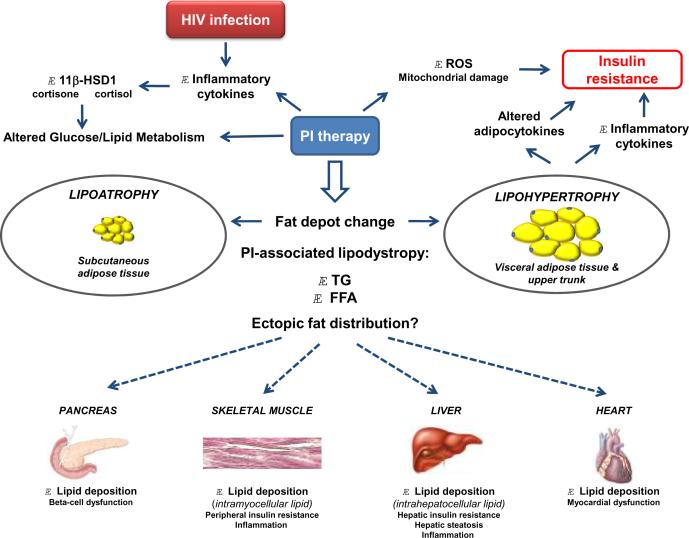
The etiology of HIV/HAART-associated lipodystrophy is most likely multifactorial in nature (Figure 1). HIV infection itself causes dysregulation of cytokines (such as TNF-α, IL-1, and IL-6) that affect both lipid/glucose metabolism and insulin sensitivity23, and the HIV-1 virus encodes several proteins (such as Vpr and Tat) that change the activity of the glucocorticoid receptor in target tissues (such as fat and liver), causing glucocorticoid hypersensitivity and insulin resistance76, 77. In addition, the secretion of inflammatory cytokines – either in response to HIV infection and/or HAART – increase expression of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), thus increasing the intracellular conversion of inactive cortisone to active cortisol; in adipose tissue, this would cause increased lipolysis and release of FFAs which could then be deposited in ectopic tissues78. PIs in particular also have many other effects connecting them to altered metabolism and the pathogenesis of lipodystrophy. First, as noted above, PIs inhibit degradation of apolipoprotein B and affect the secretion of apolipoprotein B-containing lipoprotein particles from the liver79. Second, PIs inhibit insulin signaling pathways by reducing insulin-induced phosphorylation of insulin-receptor substrate (IRS) 1 and protein kinase B (PKB, also termed Akt)80. Third, PIs affect cellular levels of peroxisome proliferator-activating receptor (PPAR) γ and CCAAT/enhancer-binding protein (C/EBP) α, both of which are important in preadipocyte differentiation into mature adipocytes, as well as sterol regulatory element binding protein 1 (SREB-1), which regulates gene expression of enzymes involved in cholesterol, fatty acid, and glucose metabolism81, 82. Fourth, PIs suppress the function of the glucose transporter GLUT-4, diminishing insulin-stimulated glucose uptake83. Fifth, PIs stimulate the production of reactive oxygen species84, which can damage important intracellular organelles; mitochondrial dysfunction may then promote fatty infiltration in liver and muscle, further exacerbating insulin resistance85.
Figure 1. A schema for the development of HIV/PI-associated lipodystrophy and its associated adverse effects.
Abbreviations: 11β-HSD1, 11β-hydroxysteroid dehydrogenase type 1; FFA, free fatty acids; HIV, human immunodeficiency virus; PI, protease inhibitor; ROS, reactive oxygen species; TG, triglyceride.
In addition to adults, children also experience HIV/HAART-associated metabolic complications and lipodystrophy60, 86, particularly when exposed to PI therapy87. The abnormalities in fat distribution can be especially distressing to a child, leading to low self-esteem and embarrassment87. Furthermore, PIs increase the risk of developing diabetes with age88, putting children on PI therapy at especially high risk. Thus, given that the development of these HIV/HAART-associated conditions in children may have long-lasting social and health implications, further study of the side effects of PI therapy in the pediatric population is warranted.
Management strategies for the morphologic changes associated with HIV/HAART-induced lipodystrophy have been recently reviewed89, 90. Both pioglitazone91 and pravastatin92have shown some promise for the treatment of lipoatrophy; however, to date, no pharmacological agent has been shown to definitely improve HIV/HAART-associated lipoatrophic changes. Alternatively, interventions that have been shown to be efficacious for HIV/HAART-associated lipohypertrophy include metformin93, 94, recombinant human growth hormone (rGH)95*, 96, tesamorelin (a growth hormone-releasing factor)97, 98, and diet and exercise99–102. Reconstructive surgical interventions to correct the physical abnormalities associated with HIV/HAART-associated lipodystrophy have also been studied103, 104, and may be a reasonable option in appropriate surgical candidates; however, their long-term efficacy have not been established. Given that the clinical and laboratory data suggest that the underlying biological mechanisms of lipoatrophy and lipohypertrophy differ, no single agent/intervention is likely to manage all aspects of HIV/HAART-associated lipodystrophy. Rather, each entity should be considered and treated separately.
Conclusions
PIs are a key component of antiretroviral therapy and have dramatically improved the life expectancy of HIV-infected individuals. However, they are also associated with abnormalities in glucose/lipid metabolism and body fat distribution. Further studies are needed to better define the pathogenesis of PI-associated metabolic and body fat changes and their potential treatment.
Acknowledgements
Supported by grants HL 65938 and 62705 (PI: L Berglund) from the National Heart, Lung, and Blood Institute. This work was supported by the UC Davis Clinical and Translational Research Center (RR024146). We are grateful to Dr. David Asmuth for valuable discussions and suggestions.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pneumocystis pneumonia - Los Angeles. Mmwr. 1981;30(21):250–252. [PubMed] [Google Scholar]
- 2.UNAIDS. WHO . AIDS epidemic update: December 2009. Joint UN Programme on HIV/AIDS, World Health Organization; Geneva: 2009. [Google Scholar]
- 3.Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287(5453):607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- 4.Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, Sax PE, Weinstein MC, Freedberg KA. The survival benefits of AIDS treatment in the United States. The Journal of infectious diseases. 2006;194(1):11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 5.Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, Thiebaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 6.Hammer SM, Eron JJ, Jr., Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. Jama. 2008;300(5):555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services [DHSS] Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. DHSS; Bethesda (MD): Dec 1, 2009. Available online from URL: http://aidsinfo.nih.gov/Guidelines/GuidelineDetail.aspx?MenuItem=Guidelines&Search=Off&GuidelineID=7&ClassID=1. 2009. [Google Scholar]
- 8.Rockstroh JK, Bhagani S, Benhamou Y, Bruno R, Mauss S, Peters L, Puoti M, Soriano V, Tural C. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV medicine. 2008;9(2):82–88. doi: 10.1111/j.1468-1293.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 9*.De Clercq E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int J Antimicrob Agents. 2009;33(4):307–320. doi: 10.1016/j.ijantimicag.2008.10.010. [DOI] [PubMed] [Google Scholar]; A review of all 25 anti-HIV compounds formally approved for clinical use in the treatment of AIDS.
- 10.HIV drugs in development. Available online from URL: http://www.thebody.com/index/treat/newdrugs.html. 2010.
- 11**.Hughes CA, Robinson L, Tseng A, MacArthur RD. New antiretroviral drugs: a review of the efficacy, safety, pharmacokinetics, and resistance profile of tipranavir, darunavir, etravirine, rilpivirine, maraviroc, and raltegravir. Expert Opin Pharmacother. 2009;10(15):2445–2466. doi: 10.1517/14656560903176446. [DOI] [PubMed] [Google Scholar]; A review of the efficacy, safety, pharmacokinetics, and resistance profile of new antiretroviral drugs.
- 12.Opravil M, Ledergerber B, Furrer H, Hirschel B, Imhof A, Gallant S, Wagels T, Bernasconi E, Meienberg F, Rickenbach M, Weber R. Clinical efficacy of early initiation of HAART in patients with asymptomatic HIV infection and CD4 cell count > 350 × 10(6) /l. AIDS (London, England) 2002;16(10):1371–1381. doi: 10.1097/00002030-200207050-00009. [DOI] [PubMed] [Google Scholar]
- 13.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, Costagliola D, D'Arminio Monforte A, de Wolf F, Reiss P, Lundgren JD, Justice AC, Staszewski S, Leport C, Hogg RS, Sabin CA, Gill MJ, Salzberger B, Sterne JA. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 14.Chene G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, Dabis F, Lundgren J, D'Arminio Monforte A, de Wolf F, Hogg R, Reiss P, Justice A, Leport C, Staszewski S, Gill J, Fatkenheuer G, Egger ME. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362(9385):679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 15.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 16.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, Vaeth M, Obel N. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146(2):87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Montero JV, Barreiro P, Soriano V. HIV protease inhibitors: recent clinical trials and recommendations on use. Expert Opin Pharmacother. 2009;10(10):1615–1629. doi: 10.1517/14656560902980202. [DOI] [PubMed] [Google Scholar]
- 18.Bazzoli C, Jullien V, Le Tiec C, Rey E, Mentre F, Taburet AM. Intracellular Pharmacokinetics of Antiretroviral Drugs in HIV-Infected Patients, and their Correlation with Drug Action. Clin Pharmacokinet. 2010;49(1):17–45. doi: 10.2165/11318110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351(9119):1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 20.Walli R, Herfort O, Michl GM, Demant T, Jager H, Dieterle C, Bogner JR, Landgraf R, Goebel FD. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS (London, England) 1998;12(15):F167–173. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Periard D, Telenti A, Sudre P, Cheseaux JJ, Halfon P, Reymond MJ, Marcovina SM, Glauser MP, Nicod P, Darioli R, Mooser V. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100(7):700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 22.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(7):F51–58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Kino T, Mirani M, Alesci S, Chrousos GP. AIDS-related lipodystrophy/insulin resistance syndrome. Horm Metab Res. 2003;35(3):129–136. doi: 10.1055/s-2003-39072. [DOI] [PubMed] [Google Scholar]
- 24.Barbaro G. Metabolic and cardiovascular complications of highly active antiretroviral therapy for HIV infection. Current HIV research. 2006;4(1):79–85. doi: 10.2174/157016206775197664. [DOI] [PubMed] [Google Scholar]
- 25.Reeds DN, Mittendorfer B, Patterson BW, Powderly WG, Yarasheski KE, Klein S. Alterations in lipid kinetics in men with HIV-dyslipidemia. Am J Physiol Endocrinol Metab. 2003;285(3):E490–497. doi: 10.1152/ajpendo.00118.2003. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz M, Michl GM, Walli R, Bogner J, Bedynek A, Seidel D, Goebel FD, Demant T. Alterations of apolipoprotein B metabolism in HIV-infected patients with antiretroviral combination therapy. Journal of acquired immune deficiency syndromes (1999) 2001;26(3):225–235. doi: 10.1097/00042560-200103010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Shahmanesh M, Das S, Stolinski M, Shojaee-Moradie F, Jackson NC, Jefferson W, Cramb R, Nightingale P, Umpleby AM. Antiretroviral treatment reduces very-low-density lipoprotein and intermediate-density lipoprotein apolipoprotein B fractional catabolic rate in human immunodeficiency virus-infected patients with mild dyslipidemia. J Clin Endocrinol Metab. 2005;90(2):755–760. doi: 10.1210/jc.2004-1273. [DOI] [PubMed] [Google Scholar]
- 28.Baril L, Beucler I, Valantin MA, Bruckert E, Bonnefont-Rousselot D, Coutellier A, Caumes E, Katlama C, Bricaire F. Low lipolytic enzyme activity in patients with severe hypertriglyceridemia on highly active antiretroviral therapy. AIDS (London, England) 2001;15(3):415–417. doi: 10.1097/00002030-200102160-00016. [DOI] [PubMed] [Google Scholar]
- 29.Anuurad E, Thomas-Geevarghese A, Devaraj S, Albu J, Minolfo R, El-Sadr WM, Lu G, Karmally W, Berglund L. Increased lipoprotein remnant cholesterol levels in HIV-positive patients during antiretroviral therapy. Atherosclerosis. 2008;198(1):192–197. doi: 10.1016/j.atherosclerosis.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338(18):1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 31.Rathbun RC, Lockhart SM, Stephens JR. Current HIV treatment guidelines--an overview. Curr Pharm Des. 2006;12(9):1045–1063. doi: 10.2174/138161206776055840. [DOI] [PubMed] [Google Scholar]
- 32.Antinori A, Perno CF, Giancola ML, Forbici F, Ippolito G, Hoetelmans RM, Piscitelli SC. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis. 2005;41(12):1787–1793. doi: 10.1086/498310. [DOI] [PubMed] [Google Scholar]
- 33.Taylor S, Back DJ, Drake SM, Workman J, Reynolds H, Gibbons SE, White DJ, Pillay D. Antiretroviral drug concentrations in semen of HIV-infected men: differential penetration of indinavir, ritonavir and saquinavir. J Antimicrob Chemother. 2001;48(3):351–354. doi: 10.1093/jac/48.3.351. [DOI] [PubMed] [Google Scholar]
- 34.Fitzsimmons ME, Collins JM. Selective biotransformation of the human immunodeficiency virus protease inhibitor saquinavir by human small-intestinal cytochrome P4503A4: potential contribution to high first-pass metabolism. Drug Metab Dispos. 1997;25(2):256–266. [PubMed] [Google Scholar]
- 35.Denissen JF, Grabowski BA, Johnson MK, Buko AM, Kempf DJ, Thomas SB, Surber BW. Metabolism and disposition of the HIV-1 protease inhibitor ritonavir (ABT-538) in rats, dogs, and humans. Drug Metab Dispos. 1997;25(4):489–501. [PubMed] [Google Scholar]
- 36.Chiba M, Hensleigh M, Lin JH. Hepatic and intestinal metabolism of indinavir, an HIV protease inhibitor, in rat and human microsomes. Major role of CYP3A. Biochem Pharmacol. 1997;53(8):1187–1195. doi: 10.1016/s0006-2952(97)00100-7. [DOI] [PubMed] [Google Scholar]
- 37.Eron J, Jr., Yeni P, Gathe J, Jr., Estrada V, DeJesus E, Staszewski S, Lackey P, Katlama C, Young B, Yau L, Sutherland-Phillips D, Wannamaker P, Vavro C, Patel L, Yeo J, Shaefer M. The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: a randomised non-inferiority trial. Lancet. 2006;368(9534):476–482. doi: 10.1016/S0140-6736(06)69155-1. [DOI] [PubMed] [Google Scholar]
- 38.Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, Moyle G, Mancini M, Percival L, Yang R, Thiry A, McGrath D. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372(9639):646–655. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 39.Ortiz R, Dejesus E, Khanlou H, Voronin E, van Lunzen J, Andrade-Villanueva J, Fourie J, De Meyer S, De Pauw M, Lefebvre E, Vangeneugden T, Spinosa-Guzman S. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS (London, England) 2008;22(12):1389–1397. doi: 10.1097/QAD.0b013e32830285fb. [DOI] [PubMed] [Google Scholar]
- 40.Dragsted UB, Gerstoft J, Youle M, Fox Z, Losso M, Benetucci J, Jayaweera DT, Rieger A, Bruun JN, Castagna A, Gazzard B, Walmsley S, Hill A, Lundgren JD. A randomized trial to evaluate lopinavir/ritonavir versus saquinavir/ritonavir in HIV-1-infected patients: the MaxCmin2 trial. Antiviral therapy. 2005;10(6):735–743. [PubMed] [Google Scholar]
- 41.Murphy RL, da Silva BA, Hicks CB, Eron JJ, Gulick RM, Thompson MA, McMillan F, King MS, Hanna GJ, Brun SC. Seven-year efficacy of a lopinavir/ritonavir-based regimen in antiretroviral-naive HIV-1-infected patients. HIV clinical trials. 2008;9(1):1–10. doi: 10.1310/hct0901-1. [DOI] [PubMed] [Google Scholar]
- 42.Gatell J, Salmon-Ceron D, Lazzarin A, Van Wijngaerden E, Antunes F, Leen C, Horban A, Wirtz V, Odeshoo L, Van den Dungen M, Gruber C, Ledesma E. Efficacy and safety of atazanavir-based highly active antiretroviral therapy in patients with virologic suppression switched from a stable, boosted or unboosted protease inhibitor treatment regimen: the SWAN Study (AI424-097) 48-week results. Clin Infect Dis. 2007;44(11):1484–1492. doi: 10.1086/517497. [DOI] [PubMed] [Google Scholar]
- 43.Hicks CB, Cahn P, Cooper DA, Walmsley SL, Katlama C, Clotet B, Lazzarin A, Johnson MA, Neubacher D, Mayers D, Valdez H. Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment-experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials. Lancet. 2006;368(9534):466–475. doi: 10.1016/S0140-6736(06)69154-X. [DOI] [PubMed] [Google Scholar]
- 44.Clotet B, Bellos N, Molina JM, Cooper D, Goffard JC, Lazzarin A, Wohrmann A, Katlama C, Wilkin T, Haubrich R, Cohen C, Farthing C, Jayaweera D, Markowitz M, Ruane P, Spinosa-Guzman S, Lefebvre E. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369(9568):1169–1178. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 45.Madruga JV, Berger D, McMurchie M, Suter F, Banhegyi D, Ruxrungtham K, Norris D, Lefebvre E, de Bethune MP, Tomaka F, De Pauw M, Vangeneugden T, Spinosa-Guzman S. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007;370(9581):49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 46*.Arribas JR, Horban A, Gerstoft J, Fatkenheuer G, Nelson M, Clumeck N, Pulido F, Hill A, van Delft Y, Stark T, Moecklinghoff C. The MONET trial: darunavir/ritonavir with or without nucleoside analogues, for patients with HIV RNA below 50 copies/ml. AIDS (London, England) 2010;24(2):223–230. doi: 10.1097/QAD.0b013e3283348944. [DOI] [PubMed] [Google Scholar]; Results from ongoing, randomized controlled, open-label, phase III, non-inferiority study evaluating safety and eficacy of darunavir/ritonavir (monotherapy) versus two nucleoside analogues and darunavir/ritonavir (triple therapy arm) therapy.
- 47.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356(9239):1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 48.Walmsley S, Bernstein B, King M, Arribas J, Beall G, Ruane P, Johnson M, Johnson D, Lalonde R, Japour A, Brun S, Sun E. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346(26):2039–2046. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 49.Fontas E, van Leth F, Sabin CA, Friis-Moller N, Rickenbach M, d'Arminio Monforte A, Kirk O, Dupon M, Morfeldt L, Mateu S, Petoumenos K, El-Sadr W, de Wit S, Lundgren JD, Pradier C, Reiss P. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? The Journal of infectious diseases. 2004;189(6):1056–1074. doi: 10.1086/381783. [DOI] [PubMed] [Google Scholar]
- 50.Dube MP, Stein JH, Aberg JA, Fichtenbaum CJ, Gerber JG, Tashima KT, Henry WK, Currier JS, Sprecher D, Glesby MJ. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37(5):613–627. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]
- 51.Johnson M, Grinsztejn B, Rodriguez C, Coco J, DeJesus E, Lazzarin A, Lichtenstein K, Rightmire A, Sankoh S, Wilber R. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS (London, England) 2005;19(7):685–694. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 52.Reiter WJ, Schon-Pernerstorfer H, Dorfinger K, Hofbauer J, Marberger M. Frequency of urolithiasis in individuals seropositive for human immunodeficiency virus treated with indinavir is higher than previously assumed. J Urol. 1999;161(4):1082–1084. [PubMed] [Google Scholar]
- 53.Sulkowski MS. Drug-induced liver injury associated with antiretroviral therapy that includes HIV-1 protease inhibitors. Clin Infect Dis. 2004;38(Suppl 2):S90–97. doi: 10.1086/381444. [DOI] [PubMed] [Google Scholar]
- 54.Safrin S, Grunfeld C. Fat distribution and metabolic changes in patients with HIV infection. AIDS. 1999;13(18):2493–2505. doi: 10.1097/00002030-199912240-00002. [DOI] [PubMed] [Google Scholar]
- 55.Grunfeld C, Kotler DP, Arnett DK, Falutz JM, Haffner SM, Hruz P, Masur H, Meigs JB, Mulligan K, Reiss P, Samaras K. Contribution of metabolic and anthropometric abnormalities to cardiovascular disease risk factors. Circulation. 2008;118(2):e20–28. doi: 10.1161/CIRCULATIONAHA.107.189623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, Saag M, Scherzer R, Shlipak M, Tien P. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40(2):121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyaki K, Hara A, Naito M, Naito T, Nakayama T. Two new criteria of the metabolic syndrome: prevalence and the association with brachial-ankle pulse wave velocity in Japanese male workers. J Occup Health. 2006;48(2):134–140. doi: 10.1539/joh.48.134. [DOI] [PubMed] [Google Scholar]
- 58.Mulligan K, Parker RA, Komarow L, Grinspoon SK, Tebas P, Robbins GK, Roubenoff R, Dube MP. Mixed patterns of changes in central and peripheral fat following initiation of antiretroviral therapy in a randomized trial. J Acquir Immune Defic Syndr. 2006;41(5):590–597. doi: 10.1097/01.qai.0000214811.72916.67. [DOI] [PubMed] [Google Scholar]
- 59.Power R, Tate HL, McGill SM, Taylor C. A qualitative study of the psychosocial implications of lipodystrophy syndrome on HIV positive individuals. Sex Transm Infect. 2003;79(2):137–141. doi: 10.1136/sti.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Desai N, Mullen P, Mathur M. Lipodystrophy in pediatric HIV. Indian J Pediatr. 2008;75(4):351–354. doi: 10.1007/s12098-008-0037-2. [DOI] [PubMed] [Google Scholar]
- 61.Despres JP. Health consequences of visceral obesity. Ann Med. 2001;33(8):534–541. doi: 10.3109/07853890108995963. [DOI] [PubMed] [Google Scholar]
- 62.Forouhi NG, Sattar N, McKeigue PM. Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. Int J Obes Relat Metab Disord. 2001;25(9):1327–1331. doi: 10.1038/sj.ijo.0801723. [DOI] [PubMed] [Google Scholar]
- 63.Lettner A, Roden M. Ectopic fat and insulin resistance. Curr Diab Rep. 2008;8(3):185–191. doi: 10.1007/s11892-008-0032-z. [DOI] [PubMed] [Google Scholar]
- 64**.Szendroedi J, Roden M. Ectopic lipids and organ function. Curr Opin Lipidol. 2009;20(1):50–56. doi: 10.1097/mol.0b013e328321b3a8. [DOI] [PubMed] [Google Scholar]; A summary of recent studies describing potential mechanisms by which ectopic lipid deposition affects organ function.
- 65.Machann J, Haring H, Schick F, Stumvoll M. Intramyocellular lipids and insulin resistance. Diabetes Obes Metab. 2004;6(4):239–248. doi: 10.1111/j.1462-8902.2004.00339.x. [DOI] [PubMed] [Google Scholar]
- 66.Roden M. Muscle triglycerides and mitochondrial function: possible mechanisms for the development of type 2 diabetes. Int J Obes (Lond) 2005;29(Suppl 2):S111–115. doi: 10.1038/sj.ijo.0803102. [DOI] [PubMed] [Google Scholar]
- 67.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119(5 Suppl 1):S10–16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68**.Tessari P, Coracina A, Cosma A, Tiengo A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2009;19(4):291–302. doi: 10.1016/j.numecd.2008.12.015. [DOI] [PubMed] [Google Scholar]; An overview of the biochemical steps of fat regulation in the liver and the alterations in hepatic lipid metabolism that occur in the pathogenesis of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis.
- 69.Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D'Ambrosia G, Arbique D, Vongpatanasin W, Unger R, Victor RG. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49(3):417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 70.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18(14):1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 71.Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, Schindhelm RK, Mari A, Heine RJ, Diamant M. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30(11):2916–2921. doi: 10.2337/dc07-0326. [DOI] [PubMed] [Google Scholar]
- 72*.Lingvay I, Esser V, Legendre JL, Price AL, Wertz KM, Adams-Huet B, Zhang S, Unger RH, Szczepaniak LS. Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab. 2009;94(10):4070–4076. doi: 10.1210/jc.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study showing that magnetic resonance spectroscopy (MRS) is a non-invasive and quantitative method that can be used to reliably measure pancreatic triglyceride content in vivo.
- 73.Lara-Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin North Am. 2008;37(4):841–856. doi: 10.1016/j.ecl.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blass SC, Ellinger S, Vogel M, Ingiliz P, Spengler U, Stehle P, von Ruecker A, Rockstroh JK. Overweight HIV patients with abdominal fat distribution treated with protease inhibitors are at high risk for abnormalities in glucose metabolism - a reason for glycemic control. Eur J Med Res. 2008;13(5):209–214. [PubMed] [Google Scholar]
- 75.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kino T, Gragerov A, Slobodskaya O, Tsopanomichalou M, Chrousos GP, Pavlakis GN. Human immunodeficiency virus type 1 (HIV-1) accessory protein Vpr induces transcription of the HIV-1 and glucocorticoid-responsive promoters by binding directly to p300/CBP coactivators. J Virol. 2002;76(19):9724–9734. doi: 10.1128/JVI.76.19.9724-9734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kino T, Slobodskaya O, Pavlakis GN, Chrousos GP. Nuclear receptor coactivator p160 proteins enhance the HIV-1 long terminal repeat promoter by bridging promoter-bound factors and the Tat-P-TEFb complex. J Biol Chem. 2002;277(4):2396–2405. doi: 10.1074/jbc.M106312200. [DOI] [PubMed] [Google Scholar]
- 78.Tomlinson JW, Moore J, Cooper MS, Bujalska I, Shahmanesh M, Burt C, Strain A, Hewison M, Stewart PM. Regulation of expression of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue: tissue-specific induction by cytokines. Endocrinology. 2001;142(5):1982–1989. doi: 10.1210/endo.142.5.8168. [DOI] [PubMed] [Google Scholar]
- 79.Liang JS, Distler O, Cooper DA, Jamil H, Deckelbaum RJ, Ginsberg HN, Sturley SL. HIV protease inhibitors protect apolipoprotein B from degradation by the proteasome: a potential mechanism for protease inhibitor-induced hyperlipidemia. Nat Med. 2001;7(12):1327–1331. doi: 10.1038/nm1201-1327. [DOI] [PubMed] [Google Scholar]
- 80.Schutt M, Meier M, Meyer M, Klein J, Aries SP, Klein HH. The HIV-1 protease inhibitor indinavir impairs insulin signalling in HepG2 hepatoma cells. Diabetologia. 2000;43(9):1145–1148. doi: 10.1007/s001250051505. [DOI] [PubMed] [Google Scholar]
- 81.Dowell P, Flexner C, Kwiterovich PO, Lane MD. Suppression of preadipocyte differentiation and promotion of adipocyte death by HIV protease inhibitors. J Biol Chem. 2000;275(52):41325–41332. doi: 10.1074/jbc.M006474200. [DOI] [PubMed] [Google Scholar]
- 82.Nguyen AT, Gagnon A, Angel JB, Sorisky A. Ritonavir increases the level of active ADD-1/SREBP-1 protein during adipogenesis. AIDS. 2000;14(16):2467–2473. doi: 10.1097/00002030-200011100-00007. [DOI] [PubMed] [Google Scholar]
- 83.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275(27):20251–20254. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 84.Ben-Romano R, Rudich A, Etzion S, Potashnik R, Kagan E, Greenbaum U, Bashan N. Nelfinavir induces adipocyte insulin resistance through the induction of oxidative stress: differential protective effect of antioxidant agents. Antivir Ther. 2006;11(8):1051–1060. [PubMed] [Google Scholar]
- 85.Shikuma CM, Day LJ, Gerschenson M. Insulin resistance in the HIV-infected population: the potential role of mitochondrial dysfunction. Curr Drug Targets Infect Disord. 2005;5(3):255–262. doi: 10.2174/1568005054880163. [DOI] [PubMed] [Google Scholar]
- 86.Farley J, Gona P, Crain M, Cervia J, Oleske J, Seage G, Lindsey J. Prevalence of elevated cholesterol and associated risk factors among perinatally HIV-infected children (4–19 years old) in Pediatric AIDS Clinical Trials Group 219C. J Acquir Immune Defic Syndr. 2005;38(4):480–487. doi: 10.1097/01.qai.0000139397.30612.96. [DOI] [PubMed] [Google Scholar]
- 87.Temple ME, Koranyi KI, Nahata MC. Lipodystrophy in HIV-infected pediatric patients receiving protease inhibitors. Ann Pharmacother. 2003;37(9):1214–1218. doi: 10.1345/aph.1A444. [DOI] [PubMed] [Google Scholar]
- 88.Salehian B, Bilas J, Bazargan M, Abbasian M. Prevalence and incidence of diabetes in HIV-infected minority patients on protease inhibitors. J Natl Med Assoc. 2005;97(8):1088–1092. [PMC free article] [PubMed] [Google Scholar]
- 89.del Mar Gutierrez M, Mateo G, Domingo P. Strategies in the treatment of HIV-1-associated adipose redistribution syndromes. Expert Opin Pharmacother. 2007;8(12):1871–1884. doi: 10.1517/14656566.8.12.1871. [DOI] [PubMed] [Google Scholar]
- 90.Wohl DA, Brown TT. Management of morphologic changes associated with antiretroviral use in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;49(Suppl 2):S93–S100. doi: 10.1097/QAI.0b013e318186521a. [DOI] [PubMed] [Google Scholar]
- 91.Slama L, Lanoy E, Valantin MA, Bastard JP, Chermak A, Boutekatjirt A, William-Faltaos D, Billaud E, Molina JM, Capeau J, Costagliola D, Rozenbaum W. Effect of pioglitazone on HIV-1-related lipodystrophy: a randomized double-blind placebo-controlled trial (ANRS 113) Antivir Ther. 2008;13(1):67–76. [PubMed] [Google Scholar]
- 92.Mallon PW, Miller J, Kovacic JC, Kent-Hughes J, Norris R, Samaras K, Feneley MP, Cooper DA, Carr A. Effect of pravastatin on body composition and markers of cardiovascular disease in HIV-infected men--a randomized, placebo-controlled study. AIDS. 2006;20(7):1003–1010. doi: 10.1097/01.aids.0000222072.37749.5a. [DOI] [PubMed] [Google Scholar]
- 93.Kohli R, Shevitz A, Gorbach S, Wanke C. A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med. 2007;8(7):420–426. doi: 10.1111/j.1468-1293.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- 94.Saint-Marc T, Touraine JL. Effects of metformin on insulin resistance and central adiposity in patients receiving effective protease inhibitor therapy. AIDS. 1999;13(8):1000–1002. doi: 10.1097/00002030-199905280-00023. [DOI] [PubMed] [Google Scholar]
- 95*.Nass R, Thorner MO. Does low-dose growth hormone therapy improve the body composition of patients infected with HIV? Nat Clin Pract Endocrinol Metab. 2009;5(3):142–143. doi: 10.1038/ncpendmet1064. [DOI] [PubMed] [Google Scholar]; A Practice Point commentary discussing the results of an 18-month, randomized, double-blind, placebo-controlled study of low-dose growth hormone (GH) therapy in 56 HIV-positive adults with lipodystrophy and relative GH deficiency. Additional strategies potentially useful in the treatment of lipodystrophy in HIV-positive individuals are also described.
- 96.Grunfeld C, Thompson M, Brown SJ, Richmond G, Lee D, Muurahainen N, Kotler DP. Recombinant human growth hormone to treat HIV-associated adipose redistribution syndrome: 12 week induction and 24-week maintenance therapy. J Acquir Immune Defic Syndr. 2007;45(3):286–297. doi: 10.1097/QAI.0b013e3180691145. [DOI] [PubMed] [Google Scholar]
- 97.Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, Berger D, Brown S, Richmond G, Fessel J, Turner R, Grinspoon S. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med. 2007;357(23):2359–2370. doi: 10.1056/NEJMoa072375. [DOI] [PubMed] [Google Scholar]
- 98.Falutz J, Allas S, Mamputu JC, Potvin D, Kotler D, Somero M, Berger D, Brown S, Richmond G, Fessel J, Turner R, Grinspoon S. Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. AIDS. 2008;22(14):1719–1728. doi: 10.1097/QAD.0b013e32830a5058. [DOI] [PubMed] [Google Scholar]
- 99.Driscoll SD, Meininger GE, Lareau MT, Dolan SE, Killilea KM, Hadigan CM, Lloyd-Jones DM, Klibanski A, Frontera WR, Grinspoon SK. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV-infected patients. AIDS. 2004;18(3):465–473. doi: 10.1097/00002030-200402200-00013. [DOI] [PubMed] [Google Scholar]
- 100.Driscoll SD, Meininger GE, Ljungquist K, Hadigan C, Torriani M, Klibanski A, Frontera WR, Grinspoon S. Differential effects of metformin and exercise on muscle adiposity and metabolic indices in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2004;89(5):2171–2178. doi: 10.1210/jc.2003-031858. [DOI] [PubMed] [Google Scholar]
- 101.Engelson ES, Agin D, Kenya S, Werber-Zion G, Luty B, Albu JB, Kotler DP. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism. 2006;55(10):1327–1336. doi: 10.1016/j.metabol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 102.Florindo AA, de Oliveira Latorre Mdo R, Jaime PC, Segurado AA. Leisure time physical activity prevents accumulation of central fat in HIV/AIDS subjects on highly active antiretroviral therapy. Int J STD AIDS. 2007;18(10):692–696. doi: 10.1258/095646207782193795. [DOI] [PubMed] [Google Scholar]
- 103.Moyle GJ. Plastic surgical approaches for HIV-associated lipoatrophy. Curr HIV/AIDS Rep. 2005;2(3):127–131. doi: 10.1007/s11904-005-0005-7. [DOI] [PubMed] [Google Scholar]
- 104.Hultman CS, McPhail LE, Donaldson JH, Wohl DA. Surgical management of HIV-associated lipodystrophy: role of ultrasonic-assisted liposuction and suction-assisted lipectomy in the treatment of lipohypertrophy. Ann Plast Surg. 2007;58(3):255–263. doi: 10.1097/01.sap.0000248128.33465.83. [DOI] [PubMed] [Google Scholar]