Abstract
Polycyclic aromatic hydrocarbons (PAH) are well established carcinogens that are likely to play a role in causing some human cancers. One accepted pathway of PAH metabolic activation is formation of bay region diol epoxides. Some individuals may be particularly susceptible to PAH carcinogenesis because they metabolically activate PAH more effectively than others. We have used measurement of urinary phenanthrene tetraols (Phe-tetraols) as a biomarker of PAH exposure plus metabolic activation, since bay region diol epoxides are hydrolyzed to tetraols. Because of stereoselectivity in Phe metabolism, Phe-(1R,2S,3R,4S)-tetraol (4) results mainly from the bay region diol epoxide pathway and Phe-(1S,2R,3S,4R)-tetraol (7) is formed mainly from the reverse diol epoxide pathway, not generally associated with carcinogenicity. The latter pathway accounts for more than 95% of human urinary Phe-tetraol. In most previous studies, Phe-tetraol was quantified without enantiomeric resolution, using a relatively rapid and practical method, applicable to large studies. It was not clear however whether measurement of overall unresolved Phe-tetraol would accurately represent the bay region diol epoxide metabolic activation pathway. Therefore, in this study we specifically quantified Phe-(1R,2S,3R,4S)-tetraol (4) by supplementing our usual analysis with chiral HPLC separations, and using [13C6]Phe-(1R,2S,3R,4S)-tetraol as internal standard. We then investigated the relationship of urinary levels of 4 to those of Phe-tetraols (4 + 7), quantified without enantiomeric resolution. We applied these methods to urine samples from cigarette smokers and highly PAH-exposed creosote workers. The results were also compared to levels of benzo[a]pyrene-7,8,9,10-tetraol and 1-hydroxypyrene in the same samples. Levels of 4 were highly correlated with those of 4 + 7 (r > 0.9, P<0.0001) in both types of urine samples. Strong correlations of 4 and 4 + 7 with benzo[a]pyrene-7,8,9,10-tetraol and 1-hydroxypyrene were also observed. The results of this study demonstrate therefore that practical and convenient measurement of overall Phe-tetraols (4 + 7) in human urine, without enantiomeric resolution, is an excellent indicator of PAH exposure and metabolism by the bay region diol epoxide metabolic activation pathway.
Keywords: phenanthrene tetraol, benzo[a]pyrene tetraol, 1-hydroxypyrene, polycyclic aromatic hydrocarbon urinary metabolites
Introduction
Polycyclic aromatic hydrocarbons (PAH) are ubiquitous and structurally varied environmental carcinogens formed in the incomplete combustion of organic matter (1,2). They are strongly linked to cancers of the lung and skin resulting from occupational exposures to soots, tars, and other combustion products, and are also considered to be among the major causative agents for lung cancer in smokers (2). One member of the PAH class, benzo[a]pyrene (BaP), is considered carcinogenic to humans by the International Agency for Research on Cancer, and several others are rated as probably or possibly carcinogenic (2). The U.S. government assesses several PAH as “reasonably anticipated to be carcinogenic” to humans (3).
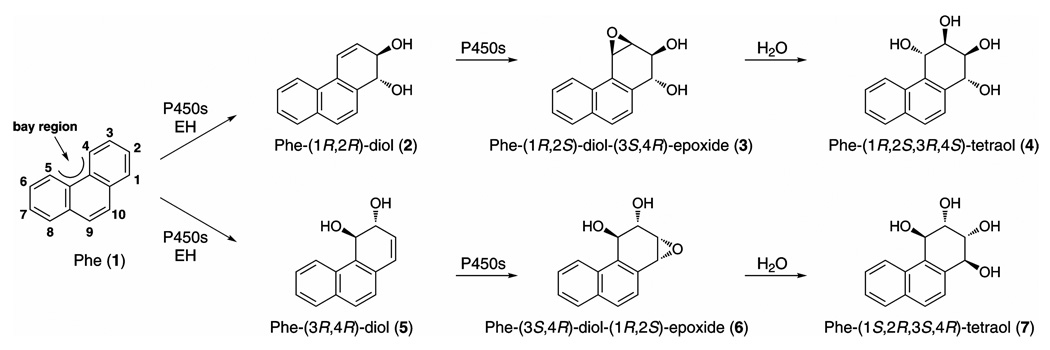
PAH require metabolic activation to exert their carcinogenic effects (4). An important pathway of metabolic activation proceeds through diol epoxide metabolites, illustrated in Scheme 1 for phenanthrene (Phe, 1), the simplest PAH with a bay region, a feature often present in carcinogenic PAH. Phe is generally not considered carcinogenic but its metabolism by the diol epoxide pathway mimics in important ways that of BaP and some other carcinogenic PAH (4–7). The first step is cytochrome P450-catalyzed formation of Phe-1,2-epoxide or Phe-3,4-epoxide. This is followed by epoxide hydrolase (EH)-catalyzed production of Phe-(1R,2R)-diol (2) or Phe-(3R,4R)-diol (5). The next step, also catalyzed mainly by P450s, results in formation of the bay region diol epoxide, Phe-(1R,2S)-diol-(3S,4R)-epoxide (3), or the reverse diol epoxide, Phe-(3S,4R-diol-1R,2S)-epoxide (6). Each of these diol epoxides then undergoes hydrolysis to produce Phe-(1R,2S,3R,4S)-tetraol (4) and Phe-(1S,2R,3S,4R)-tetraol (7), respectively. Because the formation of each metabolite in the diol epoxide pathway is stereoselective, proceeding as illustrated in Scheme 1, tetraol enantiomer 4 results mainly from the bay region diol epoxide pathway while tetraol enantiomer 7 results mainly from the reverse diol epoxide pathway. Bay region diol epoxides are associated with carcinogenicity because in many cases they react easily with DNA to form the same adducts produced metabolically from the parent PAH (4). Furthermore, bay region diol epoxides are more carcinogenic than the parent PAH in some systems (2). In contrast, the reverse diol epoxides are frequently inactive and are not believed to be as important in PAH carcinogenicity (8,9).
Scheme 1. Tetraol formation from Phe.
One persistent and challenging goal of research on PAH has been to identify those individuals who are particularly susceptible to their carcinogenic effects
Various approaches have been used in this research including metabolism of PAH in cultured lymphocytes, genotyping for variants in the enzymes involved in PAH metabolism, and quantitation of PAH-DNA adducts (10–17). The results of these studies are suggestive but have not produced definitive ways of identifying susceptible individuals.
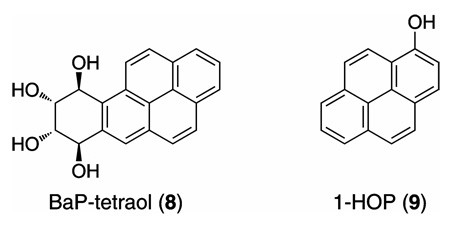
We have suggested that quantitation of Phe-tetraol could provide an index of individual exposure to, plus metabolic activation of, PAH by the critical bay region diol epoxide pathway (18). Quantitation of Phe-tetraol in human urine or blood can be readily accomplished by gas chromatography-negative ion chemical ionization – tandem mass spectrometry (GC-NICI-MS/MS), and is generally easier than quantitation of tetraols derived from carcinogenic PAH such as BaP because human exposure to Phe is far greater than to most carcinogenic PAH, and Phe metabolites are excreted mainly in urine in contrast to those of higher molecular weight PAH such as BaP (18–25). In most studies to date, Phe-tetraol has been quantified as a mixture of enantiomers 4 and 7. We recently discovered, however, that Phe-tetraol analyzed in human urine was comprised of >95% enantiomer 7 resulting from the reverse diol epoxide pathway (26). This raised some questions about the validity of our proposed biomarker since the reverse diol epoxide pathway is not generally associated with carcinogenicity. It is the carcinogenic bay region diol epoxide pathway that we wished to represent by measurement of Phe-tetraol. Therefore, in this study, we have quantified Phe-tetraols (4 + 7) and Phe-(1R,2S,3R,4S)-tetraol (4) in the same urine samples to determine whether the two analytes were correlated. We also examined the correlation of these tetraols to BaP-7,8,9,10-tetraol (BaP-tetraol 8) resulting from BaP metabolism and to 1-hydroxypyrene (1-HOP 9), a widely used biomarker of PAH exposure, in these urine samples. The urine samples were obtained from cigarette smokers and from highly exposed creosote workers.
Materials and Methods
Chemicals
Racemic BaP-7,8,9,10-tetraol (8) and racemic anti-Phe-1,2-diol-3,4-epoxide were obtained from the National Cancer Institute Chemical Carcinogen Reference Standard Repository. Phe-(1R,2S,3R,4S)-tetraol (4) was prepared by hydrolysis of Phe-(1R,2S)-diol-(3S,4R)-epoxide (27). 1-HOP, 1-HOP-glucuronide, and [D9]1-HOP were purchased from Toronto Research Chemicals. 2,7-Dihydroxynaphthalene was obtained from Acros Organics and 2-naphthylmethanol from Sigma-Aldrich. bis-Trimethylsilyltrifluoroacetamide (BSTFA) was procured from Regis Technologies.
Urine Samples
Urine samples from smokers were obtained from ongoing studies at the University of Minnesota, approved by the Institutional Review Board. They were stored at −20°C until analysis. Urine samples from creosote workers were kindly provided by Dr. Mary Wolff (Mt. Sinai Medical Center, New York).
Racemic r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene [Phe-tetraols (4 + 7)]
Briefly, the racemic mixture was prepared by hydrolysis of 5 mg 3 + 6 in 50% aq THF. The reaction mixture was concentrated to dryness on a Speedvac, the residue was dissolved in 1 mL of 50% aq CH3OH, and applied to a Strata-X polymeric reversed phase sorbent (200 mg) cartridge that had been activated with 5 mL of CH3OH and 5 mL of H2O. Phe-tetraols (4 + 7) were eluted with 5 mL of 50% aq CH3OH, concentrated to dryness, dissolved in 1 mL of 30% aq CH3OH, and 100 µL aliquots were injected onto a 25 cm × 4.6 mm, 5 µm Luna C18 HPLC column (Phenomenex) eluted at 1 mL/min with 30% aq CH3OH. Phe-tetraols (4 + 7) eluted at 14 min and the corresponding r-1,t-2,3,4- isomer eluted at 19 min. A stock solution of 4 + 7 was prepared by pooling the collected 14 min peaks, concentrating to dryness, and dissolving in 3 mL of DMSO. The concentration was determined by comparing its peak area, as determined by HPLC on the Luna C18 column with UV detection at 225.9 nm, to a calibration curve constructed by injecting various amounts of 2-naphthylmethanol on the same system with detection at 225.9 nm.
[13C6]Phe-(1R,2S,3R,4S)-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene {[13C6]Phe-(1R,2S,3R,4S)-tetraol ([13C]4)}
[13C6]Phe-(1R,2S)-diol-(3S,4R)-epoxide was prepared from [13C6]Phe-(1R,2R)-diol as previously described (27), and was hydrolyzed to [13C]4 by dissolving it in 50% aq THF and allowing the reaction to proceed for several days at room temperature. [13C]4 was collected from a (R,R) Whelk-O 5/100 Kromasil #780201 (250 mm × 4.6 mm, 5 µm) Pirkle chiral HPLC column (Regis Technologies) essentially as described below. Enantiomer purity was 98.5% as determined by collecting eluant from the Pirkle column at the retention time of each enantiomer and analyzing by GC-NICI-MS/MS. The concentrations of stock solutions of [13C]4 were determined by GC-NICI-MS/MS comparison to known concentrations of Phe-tetraols (4 + 7).
Analysis of Phe-(1R,2S,3R,4S)-tetraol (4) and Phe-tetraols (4 + 7) in urine
Aliquots of urine containing approximately 3 to 15 pmol of Phe-tetraols (4 + 7) were combined with 360 fmol of [13C6]Phe-(1R,2S,3R,4S)-tetraol ([13C]4) and subjected to β-glucuronidase and arylsulfatase treatment, and Strata-X and phenylboronic acid solid-phase extraction cartridge clean up steps as previously described (26). The fraction containing 4 + 7 was dissolved in 100 µL of CH3OH and transferred to 2 insert vials, with 33 µL reserved for analysis of 4 + 7, as previously described (26), and 66 µL for specific analysis of Phe-(1R,2S,3R,4S)-tetraol (4). This latter sample was concentrated to dryness, and the residue dissolved, using sonication, in 20 µL of isopropanol containing 500 ng of 2,7-dihydroxynaphthalene as a UV marker. After dilution with 50 µL of hexane, it was injected on the Pirkle chiral HPLC column. The column was kept at 25 °C, eluted at 1 mL/min with 30% isopropanol in hexane for 8 min, then at 1.7 mL/min with 79% isopropanol in hexane for 7 min, and monitored by UV detection at 230.9 nm. The UV marker eluted at 5.0 min, and Phe-(1R,2S,3R,4S)-tetraol (4) was collected in a 3 min fraction starting 1.8 min before until 1.2 min after the UV marker (retention time 3.2 – 6.2 min). The eluant was collected in 15 mL centrifuge tubes using a fraction collector. The fraction containing 4 was dried on a Speedvac, the residue was dissolved in 1 mL of CH3OH with sonication, transferred to a 2 mL silanized vial, and concentrated to dryness. The residue was dissolved in 100 µL of CH3OH with sonication, transferred to an insert vial, and concentrated to dryness. The residue was then processed a second time on the Pirkle column for isolation of 4. The residue was transferred to an insert vial and concentrated to dryness.
For analysis by GC-NICI-MS/MS, the samples containing 4 or 4 + 7 were dissolved in 10 µL (38.5 µmol) of BSTFA, allowed to stand at room temp for several days with periodic mixing, and 2 µL analyzed by GC-NICI-MS/MS essentially as described previously on a TSQ Quantum instrument (Thermo Scientific) with a 0.25 mm i.d. × 0.15 µm film thickness × 30 m DB17-MS column (Agilent) (26).
Calibration curves were constructed by mixing a fixed amount of ([13C6]Phe-(1 R,2S,3R,4S)-tetraol (4) with increasing amounts of Phe-tetraols (4 + 7), derivatizing with BSTFA, and analyzing by GC-NICI-MS/MS. The ratio of the areas of the peak corresponding to Phe-tetraols (4 + 7) (m/z 372 → m/z 210) to that corresponding to [13C6]Phe-(1R,2S,3R,4S)-tetraol ([13C6]4) (m/z 378 → m/z 216) was plotted against amount of Phe-tetraols (4 + 7) injected. Amounts of analyte in the urine samples were determined from the ratio of analyte to internal standard, using the calibration curve.
Analysis of 1-HOP and BaP-tetraol in urine
The analysis of 1-HOP in urine was carried out essentially as previously described (28,29) with the following modifications: β-glucuronidase (2.5 × 106 units/g) plus sulfatase (>1 × 105 units/g), from Helix pomatia Type H-1, partially purified solid (Sigma/Aldrich, # G0751) was used, with 1250 units per 1.0 mL urine; the internal standard was [D9]1-HOP (0.25 ng per sample); a 96-well plate vacuum manifold (Varian) was employed; and a Prosphere C18–300 5µ column, 150 × 4.6 (i.d.) mm from Grace Davison Discovery Sciences was used, with elution by 57% aq CH3OH with a 3 min CH3OH washout after each injection.
BaP-tetraol in urine was analyzed as described previously, without resolution of enantiomers (23).
Statistical Analyses
Pearson correlation coefficients and two-sample t-tests were used to analyze the relationships among biomarkers.
Results
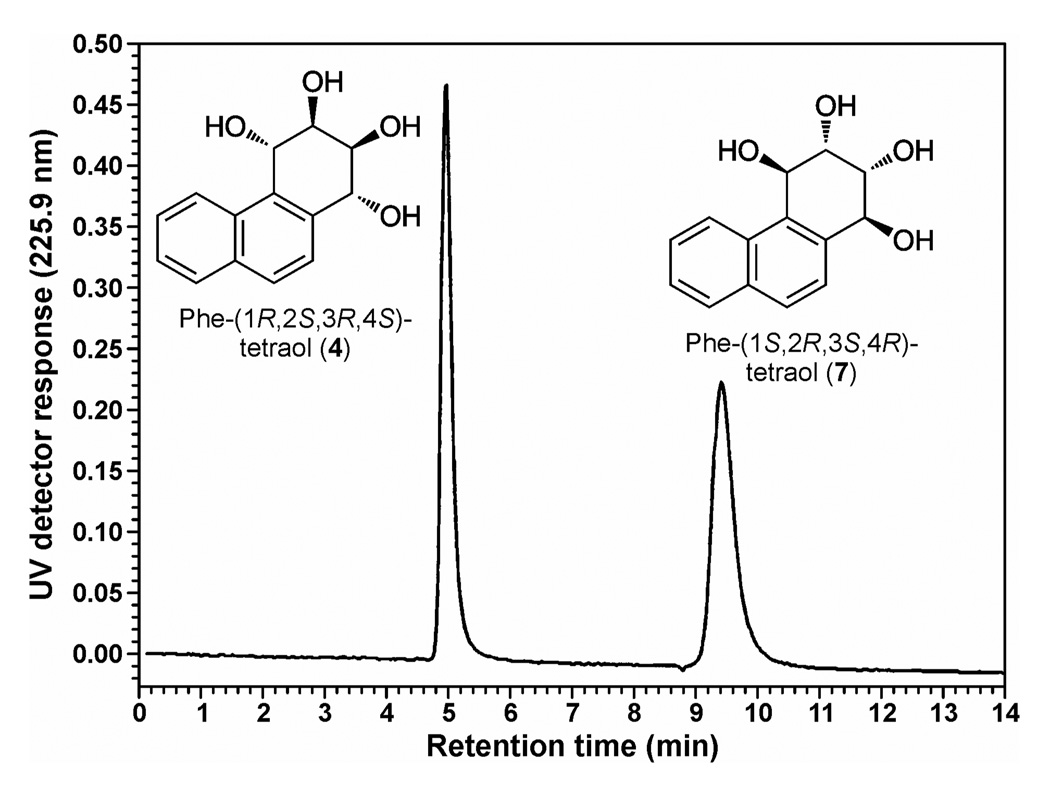
The method which we used for analysis of Phe-(1R,2S,3R,4S)-tetraol (4) depended on the separation of the Phe-tetraol enantiomers 4 and 7 on a Pirkle column, as illustrated in Figure 1. Standard Phe-(1R,2S,3R,4S)-tetraol (4) co-eluted with the first peak on the Pirkle column when racemic Phe-tetraols (4 + 7) were injected. The second peak was therefore Phe-(1S,2R,3S,4R)-tetraol (7). This method was similar to that which we reported previously, but in the study described here we used [13C6]Phe-(1R,2S,3R,4S)-tetraol ([13C6]4) as an internal standard, which allowed quantitation of Phe-(1R,2S,3R,4S)-tetraol (4), while in our previous study we simply established the ratio of the two enantiomers (26). The use of ([13C6]4 as the internal standard also allowed us to quantify Phe-tetraols (4 + 7) in the same samples by reserving an aliquot prior to enantiomer separation on the Pirkle column.
Figure 1.
Separation of Phe-(1R,2S,3R,4S)-tetraol (4) and Phe-(1S,2R,3S,4R)-tetraol (7) on a Pirkle HPLC column as described in Materials and Methods. When Phe-(1R,2S,3R,4S)-tetraol (4) is collected from a urine sample its UV absorption could not be seen; the fraction encompassing its retention time was collected using the co-eluting compound 2,7-dihydroxynaphthalene as a marker.
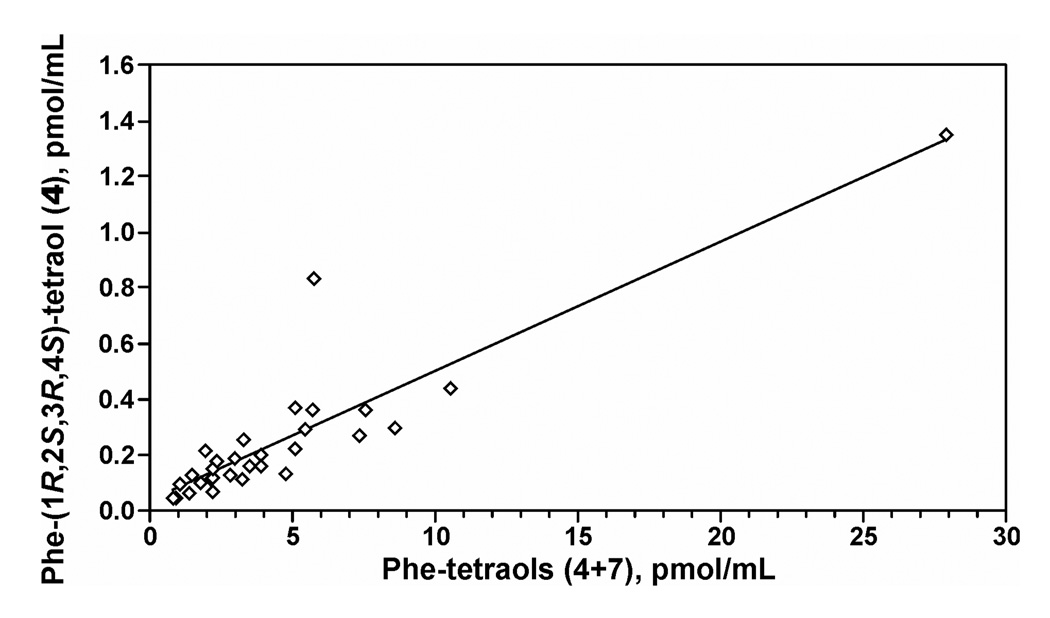
The results of the analyses of 4 and 4 + 7 in urine samples from 30 smokers are summarized in Table 1. Mean levels (± S.D.) of 4 and 4 + 7 were 0.246 ± 0.261 pmol/mL urine and 4.60 ± 5.01 pmol/mL urine, respectively. Concentrations of 4 and 4 + 7 in smokers’ urine were highly correlated (r = 0.90, P<0.0001), as illustrated in Figure 2 and summarized in Table 2.
Table 1.
Results of analyses of 4 urinary PAH biomarkers in 30 smokers
| Subject Number |
Gender | Cigarettes Per Day |
Phe-tetraols (4 + 7) pmol/mL |
Phe (1R,2S,3R,4S)- tetraol (4) pmol/mL |
1-HOP (9) pmol/mL |
BaP-tetraol (8) fmol/mL |
|---|---|---|---|---|---|---|
| 1 | F | 14 | 5.73 | 0.363 | 7.07 | 1.31 |
| 2 | F | 12 | 8.57 | 0.295 | 1.75 | 0.615 |
| 3 | M | 15 | 5.09 | 0.367 | 0.84 | 0.413 |
| 4 | M | 16 | 10.6 | 0.440 | 3.75 | 0.729 |
| 5 | F | 12 | 27.9 | 1.35 | 16.1 | 1.20 |
| 6 | M | 20 | 2.82 | 0.123 | 0.92 | 0.500 |
| 7 | M | 20 | 2.36 | 0.173 | 0.72 | 0.512 |
| 8 | M | 30 | 2.17 | 0.107 | 1.30 | 0.603 |
| 9 | F | 15 | 0.943 | 0.0420 | 0.70 | 0.606 |
| 10 | M | 10 | 1.51 | 0.127 | 1.37 | 0.363 |
| 11 | F | 20 | 2.99 | 0.188 | 2.58 | 0.551 |
| 12 | M | 24 | 3.91 | 0.200 | 2.21 | 0.429 |
| 13 | M | 20 | 5.47 | 0.291 | 3.11 | 0.624 |
| 14 | M | 20 | 1.95 | 0.214 | 2.33 | 0.619 |
| 15 | M | 25 | 4.80 | 0.131 | 1.55 | 0.468 |
| 16 | F | 20 | 2.23 | 0.0633 | 0.77 | 0.400 |
| 17 | M | 8 | 2.21 | 0.114 | 1.23 | 0.557 |
| 18 | M | 40 | 3.30 | 0.253 | 2.70 | 0.927 |
| 19 | F | 20 | 1.80 | 0.0980 | 1.75 | 0.278 |
| 20 | M | 30 | 5.08 | 0.220 | 1.98 | 0.382 |
| 21 | F | 20 | 7.57 | 0.361 | 6.62 | 0.364 |
| 22 | F | 20 | 3.48 | 0.159 | 2.96 | 0.644 |
| 23 | F | 20 | 0.802 | 0.0423 | 0.41 | 0.288 |
| 24 | M | 10 | 1.06 | 0.0915 | 0.74 | 0.286 |
| 25 | M | 40 | 3.24 | 0.109 | 1.73 | 0.448 |
| 26 | M | 12 | 1.37 | 0.0628 | 0.45 | 0.267 |
| 27 | M | 20 | 2.23 | 0.146 | 1.77 | 0.331 |
| 28 | M | 10 | 3.89 | 0.158 | 3.63 | 0.605 |
| 29 | M | 18 | 7.34 | 0.269 | 1.89 | 0.686 |
| 30 | F | 15 | 5.76 | 0.833 | 22.5 | 1.61 |
| Mean ± S.D. | 19 ± 7.9 | 4.60 ± 5.01 | 0.246 ± 0.261 | 3.25 ± 4.71 | 0.587 ± 0.311 | |
Figure 2.
Relationship of levels of Phe-(1R,2S,3R,4S)-tetraol (4) to Phe-tetraols (4 + 7) in smokers’ urine.
Table 2.
Pearson correlation coefficients (r) among the biomarkers.
| Phe-Tetraols (4 + 7) |
Phe-(1R,2S,3R,4S)- tetraol (4) |
1-HOP (9) | BaP-tetraol (8) | ||
|---|---|---|---|---|---|
| Phe-Tetraols (4 + 7) | 0.98a | 0.86a | 0.77a | creosote workers | |
| Phe-(1R,2S,3R,4S)-tetraol (4) | 0.90a | 0.81a | 0.71a | ||
| 1-HOP (9) | 0.60b | 0.86a | 0.83a | ||
| BaP-tetraol (8) | 0.50c | 0.72a | 0.82a | ||
| smokers |
|||||
P < 0.0001
P = 0.0005
P = 0.005
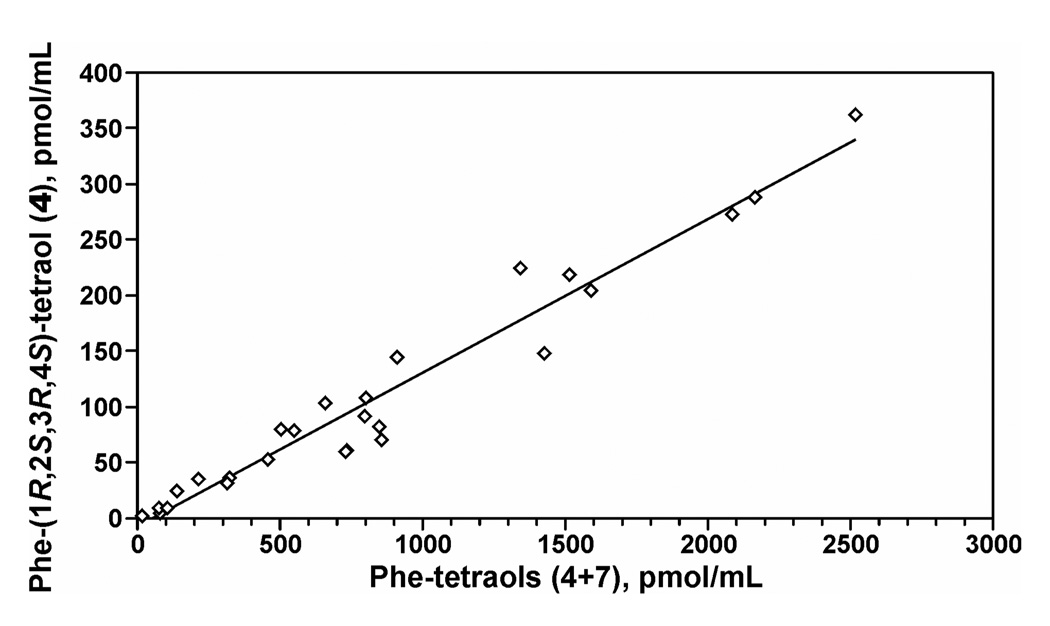
The results of the analyses of 4 and 4 + 7 in urine samples from 26 creosote workers are summarized in Table 3. Mean levels (± S.D.) of 4 and 4 + 7 were 108 ± 97.9 pmol/mL urine and 837 ± 692 pmol/mL urine, respectively. Concentrations of 4 and 4 + 7 in creosote workers’ urine were highly correlated (r = 0.98, P<0.0001), as illustrated in Figure 3 and summarized in Table 2.
Table 3.
Results of analyses of 4 urinary PAH biomarkers in 26 creosote workers
| Subject Number |
Phe-tetraols (4 + 7) pmol/mL |
Phe (1R,2S,3R,4S) tetraol (4) pmol/mL |
1-HOP (9) pmol/mL |
BaP-tetraol (8) fmol/mL |
|---|---|---|---|---|
| 1 | 77.9 | 4.23 | 16.1 | 2.32 |
| 2 | 214 | 34.5 | 185 | 3.75 |
| 3 | 457 | 52.8 | 61.8 | 4.03 |
| 4 | 1430 | 148 | 495 | 25.8 |
| 5 | 138 | 24.0 | 158 | 5.66 |
| 6 | 76.7 | 8.38 | 19.9 | 1.26 |
| 7 | 1590 | 205 | 475 | 19.3 |
| 8 | 18.1 | 2.21 | 5.02 | 1.68 |
| 9 | 2090 | 273 | 794 | 18.3 |
| 10 | 800 | 107 | 279 | 8.34 |
| 11 | 550 | 78.1 | 251 | 14.2 |
| 12 | 660 | 103 | 141 | 8.00 |
| 13 | 321 | 36.0 | 454 | 12.7 |
| 14 | 734 | 60.4 | 449 | 27.0 |
| 15 | 503 | 79.6 | 235 | 15.4 |
| 16 | 1510 | 219 | 260 | 33.2 |
| 17 | 798 | 91.9 | 352 | 22.7 |
| 18 | 2520 | 362 | 842 | 44.7 |
| 19 | 1340 | 225 | 509 | 24.4 |
| 20 | 2160 | 289 | 802 | 44.9 |
| 21 | 314 | 31.6 | 232 | 8.05 |
| 22 | 730 | 59.2 | 524 | 40.5 |
| 23 | 849 | 81.6 | 557 | 32.4 |
| 24 | 855 | 69.7 | 262 | 12.4 |
| 25 | 104 | 8.92 | 19.5 | 2.62 |
| 26 | 910 | 145 | 455 | 13.5 |
| Mean ± S.D. | 837 ± 692 | 108 ± 97.9 | 340 ± 245 | 17.2 ± 13.5 |
Eighteen of these values are averages of measurements from urine collections on 2 separate days. The remaining 8, samples numbers 2, 17, and 21–26 are single values.
Figure 3.
Relationship of levels of Phe-(1R,2S,3R,4S)-tetraol (4) to Phe-tetraols (4 + 7) in creosote workers’ urine.
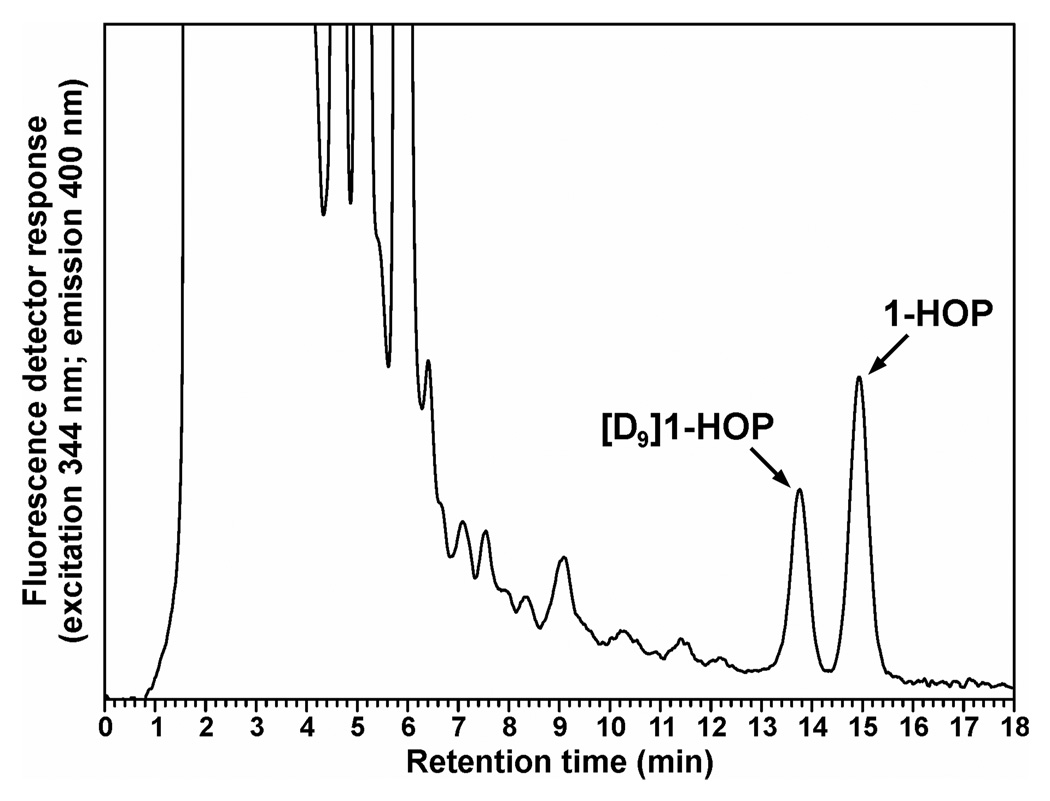
For the analysis of 1-HOP in the urine of smokers, we modified our published 96-well plate semi-automated method. Based on the study by Chetiyanukornkul et al. (29), we used [D9]1-HOP as internal standard, rather than 1-hydroxybenz[a]anthracene. Baseline resolution of [D9]1-HOP and 1-HOP was achieved, as illustrated in Figure 4. We also investigated the effect of the β-glucuronidase/sulfatase enzyme on potentially interfering flurorescent peaks in the HPLC analysis and found that partially purified β-glucuronidase type H-1 with sulfatase activity, extracted from H. pomatia, gave a HPLC baseline with no interference. The efficiency of this enzyme was established by testing its ability to catalyze the hydrolysis of standard 1-HOP-glucuronide.
Figure 4.
Analysis by HPLC with fluorescence detection of 1-HOP in a smoker’s urine, using [D9]1-HOP as internal standard. Conditions were modified from our previous method (28) and those of Chetiyanukornkul et al (29) as described in Materials and Methods.
Levels of 1-HOP found in the urine of smokers and creosote workers are summarized in Table 1 and Table 3. Mean levels (± S.D.) were 3.25 ± 4.71 pmol/mL in smokers and 340 ± 245 pmol/mL in creosote workers. Levels of 1-HOP were significantly correlated with all of the other biomarkers in both smokers and creosote workers (all r values > 0.8, except for Phe-tetraols (4 + 7) in smokers, r = 0.60), as shown in Table 2.
Levels of BaP-tetraol found in the urine of smokers and creosote workers are summarized in Table 1 and Table 3. Most of the smokers’ urine data are from a previous study and are included here for comparison (23). Mean levels (± S.D.) were 0.586 ± 0.311 fmol/mL in smokers and 17.2 ± 13.5 fmol/mL in creosote workers. Levels of BaP-tetraol were significantly correlated with the other biomarkers (all r values >0.7, except for Phe-tetraols (4 + 7) in smokers, r = 0.50), as shown in Table 2.
There were significant correlations among all biomarkers (P<0.0001) when the data from the smokers and creosote workers were combined. Correlations of Phe-tetraols (4 + 7) with 4, 1-HOP, and BaP-tetraol were r = 0.95, 0.75, and 0.65, respectively. Enantiomer 4 was correlated with 1-HOP (r = 0.84) and BaP-tetraol (r = 0.72), and 1-HOP was correlated with BaP-tetraol (r = 0.83).
Discussion
The results of this study demonstrate a clear and strong correlation between overall urinary levels of Phe-tetraols (4 + 7) and Phe-(1R,2S,3R,4S)-tetraol (4) in smokers and creosote workers exposed to differing levels of Phe, and mainly by different routes of exposure. These results are important with respect to the validity of Phe-tetraols (4 + 7) as a biomarker of the PAH bay region diol epoxide metabolic activation pathway. Phe-tetraols (4 + 7), quantified in most of our studies to date, and easier to measure than Phe-tetraol enantiomer 4 because separation on a chiral HPLC column is not required, is the preferred biomarker from an operational point of view, but is actually measuring mainly (>95%) formation of reverse diol epoxide 6, and reverse diol epoxides are not generally considered to be carcinogenic. Our results show that measurement of Phe-tetraols (4 + 7) is an excellent surrogate for the bay region diol epoxide pathway. This result was not necessarily predictable because several enzymes including cytochrome P450s 1A1, 1B1, and 1A2 as well as epoxide hydrolases, all of which catalyze reactions with considerable stereoselectivity, are involved in both pathways, but their individual contributions to the reverse diol epoxide or bay region diol epoxide pathways have not been fully established and in some cases are different. For example, P450 1A2 is a better catalyst of Phe-3,4-diol formation than P450 1B1, while P450 1A1 produces little if any of this metabolite (5,30,31). P450 1A2 is also a better catalyst of Phe-1,2-diol formation than both P450 1A1 and 1B1, which have about equal activities for production of this metabolite (5,30,31). We conclude that measurement of Phe-tetraols (4 + 7) is an excellent biomarker for both the bay region diol epoxide and reverse diol epoxide pathways of PAH metabolism.
The high exposure group in this study was comprised of dock workers who used creosote. Creosote is a distillate of coal tar that is used as a wood preservative. It is composed primarily of PAH, particularly low-molecular weight compounds such as naphthalene, acenaphthene and Phe (2). Exposure to PAH in creosote may occur potentially via inhalation and skin contact. Based on published data for urinary 1-HOP and air levels of PAH, as well as intervention studies in which the skin was protected, the major route of PAH uptake in creosote workers is clearly through the skin, with only a relatively minor contribution of inhalation (32–34). A number of previous studies have quantified 1-HOP in the urine of creosote workers, with a range of values from about 1 – 85 µmol 1-HOP per mol creatinine (2). Our mean value of 44 µmol/mol creatinine (based on excretion of 1.3 g creatinine and 1.5 L of urine per day) is quite consistent with these published data.
Biomarker levels in our study were consistently far higher in creosote workers than in smokers. Creosote worker values for Phe-tetraols (4 + 7), Phe-(1R,2S,3R,4S)-tetraol (4), 1-HOP, and BaP-tetraol were 182, 430, 105, and 29 times, respectively, greater than the values for smokers. The relatively high amount of 4 in creosote workers’ urine (430 times that in smokers) was 12.8% as great as 4 + 7 compared to 5.4% of 4 + 7 in smokers. This suggested that the bay region diol epoxide pathway was induced by the higher exposure in creosote workers, but the percentages of enantiomer 4 were not statistically different in the two groups (P = 0.89), and further research is required. BaP-tetraol levels were only 29 times higher in creosote workers than in smokers, which is probably a reflection of the fact that higher molecular weight PAH such as BaP are relatively less abundant in creosote than lower molecular weight PAH (2).
Levels of Phe-tetraols (4 + 7), 1-HOP, and BaP-tetraol are generally 2–3 times higher in smokers than in non-smokers (19,23,35). The major route of exposure to PAH in non-occupationally exposed non-smokers is believed to be diet, with some contribution from inhalation of airborne PAH (2). The excess PAH exposure in smokers compared to non-smokers results from inhalation. Thus, based on data in the literature, the smokers in this study were exposed mainly by inhalation and ingestion whereas the creosote workers were exposed mostly by dermal contact. These different routes of exposure appear to have had little effect on the biomarker correlations.
The high biomarker levels in creosote workers raise the question of the relationship of this exposure to cancer. This has been examined in a number of studies that were recently summarized and evaluated. These have shown increases in non-melanoma skin cancer and lung cancer, but not consistently. Overall, a working group of the International Agency for Research on Cancer concluded that there is limited evidence in humans for the carcinogenicity of creosotes (2). Since the major route of PAH exposure in creosote workers is through the skin, whereas in smokers it is by inhalation, caution is necessary in drawing any conclusions with respect to relative biomarker levels and comparative cancer risk in these two groups.
Levels of Phe-tetraols (4 + 7) and 1-HOP were highly correlated in both groups. This is consistent with previous studies (18,36). Phe and pyrene have similar molecular weights and are always components of PAH mixtures. While the metabolism of pyrene to 1-HOP involves only P450s, metabolism of Phe to Phe-tetraols involves both P450s and epoxide hydrolase. As exposure markers, Phe-tetraols (4 + 7) and 1-HOP appear to be virtually interchangeable. Both biomarkers are affected by individual differences in metabolism. Phe-tetraols (4 + 7) may be superior to 1-HOP in assessing metabolic differences as related to the diol epoxide metabolic activation pathway.
In summary, the results of this study demonstrate that urinary levels of Phe-(1R,2S,3R,4S)-tetraol (4), the enantiomer resulting from the bay region diol epoxide pathway of Phe metabolism, are highly correlated with levels of Phe-tetraols (4 + 7), which are more readily quantified. Therefore, measurement of Phe-tetraols (4 + 7) in human urine provides an accurate assessment of an individual’s capacity to metabolize Phe by the bay region diol epoxide pathway. Furthermore, urinary levels of both Phe-(1R,2S,3R,4S)-tetraol (4) and Phe-tetraols (4 + 7) were correlated with those of BaP-tetraol and 1-HOP in urine, even when arising from different PAH exposure circumstances.
Acknowledgements
We thank Ryan Shanley, Biostatistics and Informatics Shared Resource, for statistical analyses, and Peter Villalta, Analytical Biochemistry Shared Resource, for assistance with mass spectrometry. These Masonic Cancer Center shared resources are supported in part by Cancer Center Support Grant CA-777598. We thank Bob Carlson for editorial assistance.
Funding Support. This study was supported by grant no. CA-92025 from the National Cancer Institute.
References
- 1.Dipple A, Moschel RC, Bigger CAH. Polynuclear aromatic hydrocarbon. In: Searle CE, editor. Chemical Carcinogens, Second Edition, ACS Monograph 182. vol. 1. Washington, D.C.: American Chemical Society; 1984. pp. 41–163. [Google Scholar]
- 2.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. v. 92. Lyon, FR: IARC; 2010. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposure; pp. 35–818. [PMC free article] [PubMed] [Google Scholar]
- 3.U.S.Department of Health and Human Services. Report on Carcinogens. 11th Edition. Research Triangle Park, N.C.; 2004. pp. III-220–III-222. [Google Scholar]
- 4.Luch A, Baird WM. Metabolic activation and detoxification of polycyclic aromatic hydrocarbon. In: Luch A, editor. The carcinogenic effects of polycyclic aromatic hydrocarbons. London: Imperial College Press; 2005. pp. 19–96. [Google Scholar]
- 5.Shou M, Korzekwa KR, Krausz KW, Crespi CL, Gonzalez FJ, Gelboin HV. Regio- and stereo-selective metabolism of phenanthrene by twelve cDNA-expressed human, rodent, and rabbit cytochromes P-45. Cancer Lett. 1994;83:305–313. doi: 10.1016/0304-3835(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 6.Nordqvist M, Thakker DR, Vyas KP, Yagi H, Levin W, Ryan DE, Thomas PE, Conney AH, Jerina DM. Metabolism of chrysene and phenanthrene to bay-region diol epoxides by rat liver enzymes. Mol. Pharmacol. 1981;19:168–178. [PubMed] [Google Scholar]
- 7.Thakker DR, Yagi H, Levin W, Wood AW, Conney AH, Jerina DM. Polycyclic aromatic hydrocarbons: metabolic activation to ultimate carcinogen. In: Anders MW, editor. Bioactivation of Foreign Compounds. New York: Academic Press, Inc.; 1985. pp. 177–242. [Google Scholar]
- 8.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G.H.A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 9.Glatt H, Wameling C, Elsberg S, Thomas H, Marquardt H, Hewer A, Phillips DH, Oesch F, Seidel A. Genotoxicity characteristics of reverse diol-epoxides of chrysene. Carcinogenesis. 1993;14:11–19. doi: 10.1093/carcin/14.1.11. [DOI] [PubMed] [Google Scholar]
- 10.Welch RM, Harrison YE, Conney AH, Poppers PJ, Finster M. Cigarette smoking: stimulatory effect on metabolism of 3,4-benzpyrene by enzymes in human placenta. Science. 1968;160:541–542. doi: 10.1126/science.160.3827.541. [DOI] [PubMed] [Google Scholar]
- 11.Kellermann G, Shaw CR, Luyten-Kellerman M. Aryl hydrocarbon hydroxylase inducibility and bronchogenic carcinoma. N. Engl. J. Med. 1973;289:934–937. doi: 10.1056/NEJM197311012891802. [DOI] [PubMed] [Google Scholar]
- 12.Harris CC, Autrup H, Connor R, Barrett LA, McDowell EM, Trump BF. Interindividual variation in binding of benzo[a]pyrene to DNA in cultured human bronchi. Science. 1976;194:1067–1069. doi: 10.1126/science.982061. [DOI] [PubMed] [Google Scholar]
- 13.Sabadie N, Richter-Reichhelm HB, Saracci R, Mohr U, Bartsch H. Inter-individual differences in oxidative benzo(a)pyrene metabolism by normal and tumorous surgical lung specimens from 105 lung cancer patients. Int. J. Cancer. 1981;27:417–425. doi: 10.1002/ijc.2910270402. [DOI] [PubMed] [Google Scholar]
- 14.Nebert DW. Drug-metabolizing enzymes, polymorphisms and interindividual response to environmental toxicants. Clin. Chem. Lab. Med. 2000;38:857–861. doi: 10.1515/CCLM.2000.124. [DOI] [PubMed] [Google Scholar]
- 15.Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol., Biomarkers & Prev. 2000;9:3–28. [PubMed] [Google Scholar]
- 16.Hung RJ, Boffetta P, Brockmoller J, Butkiewicz D, Cascorbi I, Clapper ML, Garte S, Haugen A, Hirvonen A, Anttila S, Kalina I, Le Marchand L, London SJ, Rannug A, Romkes M, Salagovic J, Schoket B, Gaspari L, Taioli E. CYP1A1 and GSTM1 genetic polymorphisms and lung cancer risk in Caucasian non-smokers: a pooled analysis. Carcinogenesis. 2003;24:875–882. doi: 10.1093/carcin/bgg026. [DOI] [PubMed] [Google Scholar]
- 17.Carlsten C, Sagoo GS, Frodsham AJ, Burke W, Higgins JP. Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: a literature-based systematic HuGE review and meta-analysis. Am. J. Epidemiol. 2008;167:759–774. doi: 10.1093/aje/kwm383. [DOI] [PubMed] [Google Scholar]
- 18.Hecht SS, Chen M, Yagi H, Jerina DM, Carmella SG. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: a potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiol., Biomarkers & Prev. 2003;12:1501–1508. [PubMed] [Google Scholar]
- 19.Hecht SS, Chen M, Yoder A, Jensen J, Hatsukami D, Le C, Carmella SG. Longitudinal study of urinary phenanthrene metabolite ratios: effect of smoking on the diol epoxide pathway. Cancer Epidemiol., Biomarkers & Prev. 2005;14:2969–2974. doi: 10.1158/1055-9965.EPI-05-0396. [DOI] [PubMed] [Google Scholar]
- 20.Hecht SS, Carmella SG, Yoder A, Chen M, Li Z, Le C, Jensen J, Hatsukami DK. Comparison of polymorphisms in genes involved in polycyclic aromatic hydrocarbon metabolism with urinary phenanthrene metabolite ratios in smokers. Cancer Epidemiol., Biomarkers & Prev. 2006;15:1805–1811. doi: 10.1158/1055-9965.EPI-06-0173. [DOI] [PubMed] [Google Scholar]
- 21.Church TR, Anderson KE, Le C, Zhang Y, Kampa DM, Benoit AR, Yoder AR, Carmella SG, Hecht SS. Temporal stability of urinary and plasma biomarkers of tobacco smoke exposure among cigarette smokers. Biomarkers. 2010;15:345–352. doi: 10.3109/13547501003753881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmella SG, Yoder A, Hecht SS. Combined analysis of r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in smokers' plasma. Cancer Epidemiol., Biomarkers & Prev. 2006;15:1490–1494. doi: 10.1158/1055-9965.EPI-06-0199. [DOI] [PubMed] [Google Scholar]
- 23.Zhong Y, Carmella SG, Hochalter JB, Balbo S, Hecht SS. Analysis of r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene in human urine: a biomarker for directly assessing carcinogenic polycyclic aromatic hydrocarbon exposure plus metabolic activation. Chem. Res. Toxicol. epub ahead of print, 4-Nov-2010. 2010 doi: 10.1021/tx100287n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotin P, Falk HL, Busser R. Distribution, retention, and elimination of C14-3,4-benzpyrene after administration to mice and rats. J. Natl. Cancer Inst. 1959;23:541–555. [Google Scholar]
- 25.Chu I, Ng KM, Benoit FM, Moir D. Comparative metabolism of phenanthrene in the rat and guinea pig. J. Environ. Sci. Health, Part B. 1992;27:729–749. doi: 10.1080/03601239209372809. [DOI] [PubMed] [Google Scholar]
- 26.Hecht SS, Carmella SG, Villalta PW, Hochalter JB. Analysis of phenanthrene and benzo[a]pyrene tetraol enantiomers in human urine: relevance to the bay region diol epoxide hypothesis of benzo[a]pyrene carcinogenesis and to biomarker studies. Chem. Res. Toxicol. 2010;23:900–908. doi: 10.1021/tx9004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hecht SS, Villalta PW, Hochalter JB. Analysis of phenanthrene diol epoxide mercapturic acid detoxification products in human urine: relevance to molecular epidemiology studies of glutathione-S-transferase polymorphisms. Carcinogenesis. 2008;29:937–943. doi: 10.1093/carcin/bgn015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmella SG, Le K, Hecht SS. Improved method for determination of 1-hydroxypyrene in human urine. Cancer Epidemiol., Biomarkers & Prev. 2004;13:1261–1264. [PubMed] [Google Scholar]
- 29.Chetiyanukornkul T, Toriba A, Kizu R, Makino T, Nakazawa H, Hayakaw K. Determination of 1-hydroxypyrene in human urine by high-performance liquid chromatography with fluorescence detection using a deuterated internal standard. J. Chromatogr., A. 2002;961:107–112. doi: 10.1016/s0021-9673(02)00363-1. [DOI] [PubMed] [Google Scholar]
- 30.Schober W, Pusch G, Oeder S, Reindl H, Behrendt H, Buters JT. Metabolic activation of phenanthrene by human and mouse cytochromes P450 and pharmacokinetics in CYP1A2 knockout mice. Chem. -Biol. Interact. 2010;183:57–66. doi: 10.1016/j.cbi.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Jacob J, Doehmer J, Grimmer G, Soballa V, Raab G, Seidel A, Greim H. Metabolism of phenanthrene, benz[a]anthracene, benz[a]pyrene, chrysene and benzo[c]phenanthrene by eight cDNA-expressed human and rate cytochromes P450. Polycyclic Aromat. Compd. 1996;10:1–9. [Google Scholar]
- 32.Van Rooij JG, van Lieshout EM, Bodelier-Bade MM, Jongeneelen FJ. Effect of the reduction of skin contamination on the internal dose of creosote workers exposed to polycyclic aromatic hydrocarbons. Scand. J. Work, Environ. Health. 1993;19:200–207. doi: 10.5271/sjweh.1322. [DOI] [PubMed] [Google Scholar]
- 33.Elovaara E, Heikkila P, Pyy L, Mutanen P, Riihimaki V. Significance of dermal and respiratory uptake in creosote workers: exposure to polycyclic aromatic hydrocarbons and urinary excretion of 1-hydroxypyrene. Occup. Environ. Med. 1995;52:196–203. doi: 10.1136/oem.52.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heikkila P, Luotamo M, Pyy L, Riihimaki V. Urinary 1-naphthol and 1-pyrenol as indicators of exposure to coal tar products. Int. Arch. Occup. Environ. Health. 1995;67:211–217. doi: 10.1007/BF00626355. [DOI] [PubMed] [Google Scholar]
- 35.Hecht SS, Yuan J-M, Hatsukami DK. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem. Res. Toxicol. 2010;23:1001–1008. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JY, Hecht SS, Mukherjee S, Carmella SG, Rodrigues EG, Christiani DC. A urinary metabolite of phenanthrene as a biomarker of polycyclic aromatic hydrocarbon metabolic activation in workers exposed to residual oil fly ash. Cancer Epidemiol., Biomarkers & Prev. 2005;14:687–692. doi: 10.1158/1055-9965.EPI-04-0428. [DOI] [PubMed] [Google Scholar]