Abstract
Objective
To characterize the relationship between insulin sensitivity, assessed by the homeostasis model of insulin (HOMA), and objective measurements of sleep duration in adolescents.
Study design
Cross-sectional analysis from two examinations conducted in the Cleveland Children’s Sleep and Health Cohort (n=387; 43% minorities). Biochemical and anthropometry measurements made in a Clinical Research Unit. Sleep duration measured by actigraphy.
Results
Decreased sleep duration was associated with increased adiposity and minority race. Sleep duration had a quadratic “u-shape” association with HOMA. When adjusted for age, sex, race, preterm status and activity, adolescents who slept 7.75 hours had the lowest predicted HOMA (1.96 [95% CI: 1.82, 2.10]), and adolescents who slept 5.0 hours or 10.5 hours had HOMA indices that were about 20% higher (2.36 [95% CI: 1.94, 2.86] and 2.41 [95% CI: 1.93, 3.01], respectively). After adjusting for adiposity, the association between shorter sleep and HOMA was appreciably attenuated, but the association with longer sleep persisted.
Conclusions
Shorter and longer sleep durations are associated with decreased insulin sensitivity in adolescents. Whereas the association between shorter sleep duration with insulin sensitivity is likely explained by the association between short sleep and obesity, association between longer sleep and insulin sensitivity is independent of obesity.
Keywords: Sleep, insulin resistance, obesity
The incidence of type 2 diabetes (DM-2) has increased significantly over the last few decades.1–3 DM-2 is occurring at an earlier age in adults and has begun to affect adolescents and even children world-wide.1, 4–7 Among US adolescents, the increased incidence of DM-2 has paralleled the rise in adolescent obesity, with such trends particularly evident in minority groups.7, 8 There are likely multiple reasons for these trends. Concurrent with an increase in obesity and DM-2, has been a rising prevalence of insufficient sleep.9 There is strong evidence to support an association between childhood obesity and sleep duration in diverse pediatric populations10–12. Evidence from studies of adult samples also indicates that insufficient sleep is a contributing factor for obesity as well as for altered glucose metabolism and DM-2. 13–24, 26 Conversely, prolonged sleep duration, postulated to be a marker of underlying co-morbidity, also has been identified to be a risk factor for a number of adverse health outcomes, including impaired glucose tolerance in adults.14 Two of the largest studies in adults to reveal the aforementioned findings, the Sleep Heart Health Study and NHANES I, use questionnaires to obtain sleep duration. They reveal that less than 5–6 hours and greater than 9 hours of sleep are associated with diabetes mellitus in adults, independently of sleep apnea.11,29 Thus, there appears to be a “u-shaped” relationship between sleep duration and impaired glucose homeostasis, the basis of which is unclear.
A few studies have examined the relationship between sleep and insulin resistance in pediatric populations, though these studies have generally targeted subject with sleep disordered breathing 25,26. In this report, we examine the relation between insulin sensitivity, as measured by the homeostasis model assessment (HOMA), and objective measures of sleep duration in a community-based cohort of adolescents. We hypothesize that adolescents with shorter and longer sleep duration will have higher HOMA levels compared with adolescents with average sleep duration. By studying a generally healthy, young sample, we posit that inferences on potential causal pathways will be less influenced by unmeasured confounders compared to investigations of older populations with greater co-morbidities.
Methods
This sample was comprised of adolescents participating in the Cleveland Children’s Sleep and Health Study (CCSHS), an ongoing longitudinal cohort study designed to evaluate sleep measures and their health outcomes. As previously described,27–29 the cohort was originally assembled by stratified sampling of full-term and preterm (<37 weeks gestational age) children born between 1988–1993, and was designed to over-represent African-American and preterm children. The first CCSHS examination included 907 children aged 8 to 11 years of age. The second examination (2002–2006; TeenZzz) targeted the subsample of participants, then ages 13 to 16 years, with habitual snoring or sleep apnea, as well as a stratified (race, sex, term status) random sample of the remaining cohort. All 907 subjects that participated in the first CCSHS examination were eligible to participate in the third examination (TREC-Cleveland Cohort) that targeted children ages 16 to 19 years. Recruitment for this last examination is ongoing, and at the time of this report, 404 adolescents had participated. Analyses are based on data from both the early adolescent (TeenZzz) and late adolescent (TREC-cohort) examinations, including data from 168 adolescents who participated in both exams, 149 in the TeenZzz exam only, and 154 in the TREC-cohort exam.
The study protocols and data collection methods were similar for the second and third examinations. Overnight polysomnography (PSG) and physiological and anthropometric assessments were performed using a standardized protocol in a dedicated clinical research unit (CRU) when the adolescent was free from acute illness.28, 30 Within one week of their examination at the CRU, participants were asked to wear a wrist actigraph and complete a daily sleep log for 5–7 consecutive 24-hour periods. Questionnaires were used to obtain demographic and medical information from both adolescents and their parents. The protocol was approved by the University Hospitals of Cleveland Institutional Review Board. Written assent and/or consent was obtained from the study participants and their primary caregivers.
Questionnaires were used to collect demographic data. For analytic purposes, race/ethnicity was based on self- (or parent-) report and coded as Caucasian or Minority. Adolescents were asked to estimate the average amount of time over the past year spent each week on various activities such as walking, running, swimming, and weight training using standardized questionnaires. Activities requiring at least 3 metabolic equivalent task units (METS) were considered to be moderate-vigorous activities. Given the CDC’s recommendation that children engage in physical activity for ≥60 minutes daily and most of that activity should be aerobic (http://www.cdc.gov/physicalactivity/everyone/guidelines/children.html), we categorized average daily amount of moderate-vigorous activity as < 30 minutes, 31–59 minutes, or ≥ 60 minutes. A digital scale (Health-o-meter, Shelton, CT) and a rigid stadiometer (Holtain Ltd, Pembrokeshire, UK) were used to measure weight and height respectively. BMI was calculated by dividing weight in kilograms by height in meters squared and was then converted into sex- and age-adjusted percentiles (http://www.cdc.gov/growthcharts/). Obesity was defined as a BMI ≥30 or BMI ≥95th percentile for age and sex. Waist circumference was collected by using inelastic tape to measure the smallest horizontal circumference between the ribs and iliac crest at the end of a normal expiration.
Sleep-wake estimation was completed with wrist actigraphy (Octagonal Sleep Watch 2.01, Ambulatory Monitoring Inc, Ardsley, NY) and analyzed with the Action-W software and the time-above-threshold algorithm.28 Mean weekday sleep duration was calculated for participants with at least 3 weekdays of actigraphy data to enhance the reliability of this measure. Mean weekend sleep duration was summarized for participants with 1 or 2 weekend nights of actigraphy data. We also calculated the difference between weekend and weekday sleep duration as this is considered to be a marker of insufficient sleep. Participants who slept at least 2 hours longer on weekends than weekdays were categorized as having extended weekend sleep duration. Adolescents who had weekday but not weekend actigraphy data (about 12% of the sample) were coded as unknown extended weekend sleep duration. To identify sleep apnea, polysomnography was performed. 31
After an overnight fast and a PSG study conducted at the CRU, fasting insulin and glucose levels were collected at 8 AM and measured at a central laboratory. Glucose was measured using a colorimetric reflectance spectrophotometric method (Vitros 950IRC). Fasting insulin was measured using the ALPCO Human Insulin ELISA(range: 0.15 – 20 μIU/mL; inter-assay coefficient of variation ranged from 3.9 to 9.5%). The HOMA index, a reliable measure of insulin sensitivity in adults as well as in children,32 was calculated by taking the product of fasting glucose (mg/dL) and fasting insulin (μIU/mL) divided by 405. The HOMA index, which increases with insulin resistance, is a highly specific and sensitive method of estimating basal insulin resistance in epidemiologic studies 32–34. The fasting glucose to fasting insulin ratio (G:I ratio), an alternative measure of insulin resistance, with lower numbers indicative of greater insulin resistance, was calculated by dividing fasting glucose by fasting insulin. A G:I ratio of <7 was selected as a measure of insulin resistance 32.
At the time of this report, 259 and 289 adolescents from the early and late adolescent exams, respectively, had both actigraphy and HOMA data available. After excluding participants with <3 weekdays of actigraphy data (n=37), sleep apnea; (apnea hypopnea index ≥ 5, n=33), diabetes treated with medications (n=5) and serious health conditions (n=1 narcolepsy, n=1 with chronic oral corticosteroid use), the final analytic sample was comprised of 149 participants from the early adolescent examination, 154 participants from the late adolescent exam, and 168 adolescents who participated in both exams, yielding 471 records on 387 individuals.
Statistical Analysis
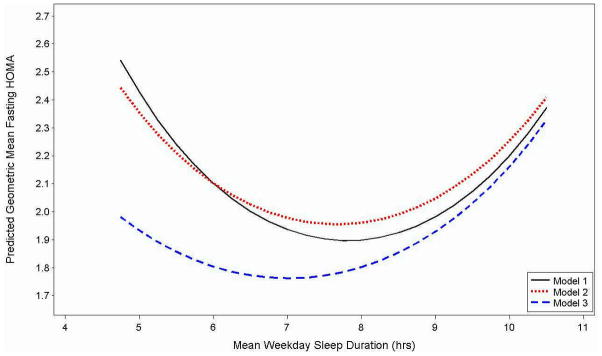
Subject characteristics, sleep duration and measures of insulin resistance were summarized using means and standard deviations for normally distributed measures, medians and the inter-quartile range for non-normally distributed variables, and frequencies and proportions for categorical variables. Weekday sleep duration, the primary exposure, was categorized for descriptive purposes and modeled as a continuous variable in regression analyses. Because there is no consensus on sleep duration thresholds that define long and short sleep in adolescents, for descriptive analyses, the ~15th and ~85th percentiles of the sample distribution of mean weekday sleep duration were used to define shorter (≥6.50 hrs), intermediate (6.51 – 8.74 hrs) and longer (≥8.75 hours) sleep duration. HOMA was right-skewed and therefore was transformed using the natural logarithm to achieve approximate normality. To examine bivariate associations and test for linear (e.g., age) or quadratic (e.g, mean weekday sleep duration) trends while accounting for the correlations among the 168 adolescents who participated in both exams, repeated measures models with a compound symmetric covariance structure and robust variance estimate, and generalized estimating equations with an exchangeable correlation structure and robust variance estimate were fitted. Omnibus p-values of any group difference are reported for categorical variables with more than 2 levels. To further assess the relationship between mean sleep duration and HOMA, three sets of repeated measures models were examined. The initial model was adjusted for age only (Model 1), and subsequent models were adjusted for subject characteristics: age, sex, race, average daily amount of moderate/vigorous activity and preterm history [Model 2]). Model 3 was then additionally adjusted for waist circumference (which, of all the adiposity measures, was most strongly associated with HOMA levels) to examine the marginal effect of obesity. Squared terms were centered to the sample mean to reduce co-linearity. In secondary analyses, the regression models were refitted with extended weekday sleep duration as an additional covariate to examine whether extended weekend sleep duration was associated with HOMA levels or confounded the association between weekday sleep duration and HOMA levels. The results of the repeated measures models are summarized via regression coefficients (betas) and 95% confidence intervals (95% CI) on the ln-scale in the tables, and back-transformed adjusted predicted geometric means are provided in the Figure. SAS version 9.2 (SAS Institute Inc., Cary, NC) was used to analyze the data.
Figure 1.
Predicted Geometric Mean HOMA Levels As A Function of Mean Weekday Sleep Duration from Repeated Measures Analyses
Model 1. Adjusted for Age; Model 2: Adjusted for Subject Characteristics (Age, Sex, Race, Preterm Status, Moderate/Vigorous Daily Activity); Model 3. Adjusted for Subject Characteristics and Obesity (Waist Circumference)
Results
Sample characteristics for the analytic sample and stratified by mean sleep duration category are shown in Table I. Consistent with the sampling strategy, approximately one half of the sample was male and had been preterm, and 42.7% were racial minority race (predominantly African-American). Nearly 20% of the sample was obese and approximately 26% had a G:I ratio of < 7. Mean weekday sleep duration was 7.63 ± 1.08 hours, and was similar for participants in the early adolescent exam (7.70 ± 1.03 hours) and the late adolescent exam (7.56 ± 1.13 hours) (results not shown).
Table 1.
Sample Characteristics Overall and by Mean Sleep Duration Categories
| Subject Characteristics | All Subjects (n=471) | Mean Sleep Duration Categories |
||
|---|---|---|---|---|
| Shorter ≤6.5 hrs (n=61) | Intermediate 6.6 – 8.74 hrs (n=338) | Longer ≥ 8.75 hrs (n=72) | ||
| Age | 15.7 ± 2.1 | 16.1 ± 2.0 | 15.6 ± 2.1 | 15.8 ± 2.2 |
| Male Sex | 232 (49.3%) | 32 (52.5%) | 171 (50.6%) | 29 (40.3%) |
| Minority race | 201 (42.7%) | 36 (59.0%) | 137 (40.5%) | 28 (38.9%) |
| Preterm | 232 (49.3%) | 30 (49.2%) | 164 (48.5%) | 38 (52.8%) |
| Moderate/ vigorous daily activity | ||||
| < 30 min | 223 (47.3%) | 30 (49.2%) | 155 (45.9%) | 38 (52.8%) |
| 30 to 59 min | 81 (17.2%) | 9 (14.7%) | 60 (17.7%) | 12 (16.7%) |
| ≥ 60 min | 167 (35.5%) | 22 (36.1%) | 123 (36.4%) | 22 (30.5%) |
| Extended weekend sleep duration | ||||
| No | 347 (73.2%) | 26 (42.6%) | 252 (74.6%) | 67 (93.1%) |
| Yes | 70 (14.9%) | 23 (37.7%) | 47 (13.9%) | 0 (0%) |
| Unknown | 56 (11.9%) | 12 (19.7%) | 39 (11.5%) | 5 (6.9%) |
| Adiposity | ||||
| Waist Circumference (cm) | 74.8 ± 11.8 | 76.5 ± 12.9 | 74.7 ± 11.5 | 73.4 ± 12.0 |
| Obesity (BMI ≥30 or BMI ≥ 95th percentile) | 88 (18.7%) | 13 (21.3%) | 64 (18.9%) | 11 (15.3%) |
| BMI percentile | 70.4 (45.7, 92.1) | 74.4 (42.7, 89.8) | 68.7 (46.4, 92.4) | 66.9 (36.0, 90.5) |
| Insulin & Glucose | ||||
| HOMA | 1.95 (1.37, 2.82) | 1.96 (1.30, 3.29) | 1.95 (1.37, 2.71) | 1.99 (1.43, 2.89) |
| Fasting Insulin (uIU/mL) | 8.9 (6.6, 12.7) | 8.7 (6.3, 13.9) | 8.9 (6.6, 12.3) | 9.1 (6.8, 12.8) |
| Fasting Glucose (mg/dL) | 88.0 ± 7.7 | 87.9 ± 7.1 | 88.1 ± 7.6 | 88.0 ± 8.7 |
| G:I Ratio * | 9.9 (6.9, 13.2) | 9.6 (6.1, 13.4) | 9.9 (7.0, 13.2) | 9.9 (6.8, 13.0) |
| G:I Ratio < 7 | 122 (25.9%) | 17 (27.9%) | 84 (24.9%) | 21 (29.2%) |
N=471 observations on n=387 people; N (column %) shown for categorical variables; median (IQR) or mean ± SD presented for continuous variables
G:I ratio = fasting glucose / fasting insulin
Examination of subject characteristics by sleep duration category showed that the proportion of minority and obese adolescents was highest among shorter sleepers and lowest among longer sleepers (Table I). Similarly, BMI percentile and waist circumference were highest among shorter sleepers and lowest among longer sleepers. As expected, the proportion of adolescents with extended weekend sleep duration was highest among those who slept 6.5 hours or less on weekdays. The proportion of males was lowest among adolescents with longer sleep duration. HOMA, insulin and glucose levels were similar for the 3 sleep duration groups. The proportion of participants with a G:I ratio < 7 was lowest among the intermediate sleep group.
Bivariate associations between subject characteristics and HOMA quartiles are shown in Table II. Although minority race was positively associated with HOMA quartiles, age and average daily amount of moderate-vigorous exercise were negatively associated with HOMA quartiles. Waist circumference, BMI percentile and the prevalence of obesity increased in a quadratic fashion with HOMA quartiles. In these unadjusted analyses, mean weekday sleep duration was similar across HOMA quartiles.
Table 2.
Sample Characteristics by HOMA Quartiles
| Subject Characteristics | HOMA Quartiles |
||||
|---|---|---|---|---|---|
| Quartile 1 0.341 –1.366 (n=118) |
Quartile 2 1.367 – 1.949 (n=117) |
Quartile 3 1.950 – 2.773 (n=118) |
Quartile 4 2.817 – 20.677(n=118) |
p-value* | |
| Weekday Sleep Duration | 7.63 ± 1.02 | 7.64 ± 1.06 | 7.62 ± 1.00 | 7.63 ± 1.24 | 0.96 |
| Age | 16.4 ± 1.9 | 16.1 ± 2.1 | 15.2 ± 2.1 | 15.0 ± 2.0 | <0.001 |
| Male Sex | 65 (55.1%) | 49 (41.9%) | 59 (50.0%) | 59 (50.0%) | 0.37 |
| Minority Race | 35 (29.7%) | 41 (35.0%) | 57 (48.3%) | 68 (57.6%) | <0.001 |
| Preterm Status | 49 (41.5%) | 58 (49.6%) | 58 (49.2%) | 67 (56.8%) | 0.22 |
| Moderate/vigorous daily activity | |||||
| < 30 min | 40 (33.9%) | 49 (41.9%) | 57 (48.3%) | 77 (65.2%) | <0.001 |
| 30–59 min | 20 (16.9%) | 22 (18.8%) | 25 (21.2%) | 14 (11.9%) | |
| 60 min | 58 (49.2%) | 46 (39.3%) | 36 (30.5%) | 27 (22.9%) | |
| Extended weekend sleep duration | 15 (12.7%) | 20 (17.1%) | 16 (13.6%) | 19 (16.1%) | 0.82 |
| Adiposity | |||||
| Waist Circumference (cm) | 70.0 ± 7.4 | 70.3 ± 7.5 | 74.6 ± 11.1 | 84.1 ± 13.7 | <0.001 |
| Obese (BMI ≥ 30 or BMI ≥ 95th) | 1 (0.9%) | 6 (5.1%) | 22 (18.6%) | 59 (50.0%) | <0.001 |
| BMI Percentile | 51.6 (31.3, 68.7) | 58.5 (39.8, 80.9) | 71.3 (47.1, 90.9) | 95.0 (85.3, 98.2) | <0.001 |
N=471 observations on n=387 people; N (column %) shown for categorical variables; median (IQR) or mean ± SD presented for continuous variables
Quadratic trend test for mean weekday sleep duration, BMI percentile and waist circumference; linear trend test shown for age; omnibus test of any group difference shown for categorical variables.
The results of the repeated measures analyses, modeling sleep duration as a continuous measure, show that weekday sleep duration has a quadratic “u-shape” association with HOMA (Table III and Figure). In age-adjusted analyses, the statistical model estimated that adolescents who slept approximately 7.75 hours to have the lowest predicted HOMA levels (1.90 [95% CI: 1.77, 2.03]). Adolescents at the tails of the distribution, with short sleep duration (5.0 hours) or long sleep duration (10.5 hours) had HOMA levels that were about 25% higher (Model 1: 2.43 [95% CI: 1.97, 3.00] and 2.37 [95% CI: 1.91, 2.95], respectively). Similar results were observed after additionally adjusting for sex, race, preterm status and daily moderate-vigorous activity (Model 2), indicating that the association between sleep duration and HOMA is not appreciably confounded by these subject characteristics; the lowest predicted HOMA levels (1.96 [95% CI: 1.82, 2.10]) were among adolescents who slept approximately 7.75 hours, and HOMA levels were about 20% higher among those who slept 5.0 hours (2.36 [95% CI: 1.94, 2.86]) or 10.5 hours (2.41 [95% CI: 1.93, 3.01]). However, additional adjustment for waist circumference (Model 3) attenuated the association between HOMA level and sleep duration at shorter and intermediate sleep durations, but not at longer sleep durations. For example, compared with adolescents who slept 7.75 hours, those who slept 5.0 hrs had adjusted HOMA levels that were estimated to be only 8% higher, and those who slept 10.5 hrs had adjusted HOMA levels that were 30% higher (1.78 [95% CI: 1.67, 1.91], 1.93 [95% CI: 1.62, 2.30] and 2.33 [95% CI: 1.97, 2.76], for adolescents who slept 7.75, 5.0 and 10.5 hours, respectively).
Table 3.
Association Between Mean Weekday Sleep Duration and Ln HOMA
| Model 1: Adjusted for Age | Model 2: Adjusted for Subject Characteristics | Model 3: Adjusted for Subject Characteristics+ Waist circumference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | p-value | Beta | 95% CI | p-value | Beta | 95% CI | p-value | |
| Intercept | 1.982 | 1.624, 2.339 | <0.001 | 1.500 | 1.086, 1.913 | <0.001 | 2.182 | 1.828, 2.536 | <0.001 |
| Sleep Duration | −0.012 | −0.059, 0.035 | 0.62 | −0.002 | −0.049, 0.045 | 0.93 | 0.028 | −0.011, 0.068 | 0.16 |
| Sleep Duration 2 | 0.031 | 0.006, 0.057 | 0.02 | 0.026 | 0.002, 0.050 | 0.03 | 0.023 | 0.003, 0.043 | 0.03 |
| Age (per 1 yr increase) | −0.084 | −0.107, −0.062 | <0.001 | −0.067 | −0.091, −0.044 | <0.001 | −0.100 | −0.121, −0.080 | <0.001 |
| Male Sex | −0.001 | −0.111, 0.111 | 0.99 | −0.137 | −0.230, −0.044 | 0.004 | |||
| Minority race | 0.126 | 0.004, 0.249 | 0.04 | 0.129 | 0.034, 0.224 | 0.008 | |||
| Preterm | 0.054 | −0.055, 0.162 | 0.33 | 0.045 | −0.045, 0.136 | 0.33 | |||
| Activity † | |||||||||
| < 30 min | 0.270 | 0.154, 0.386 | 0.192 | 0.098, 0.286 | |||||
| 30–59 min | 0.109 | −0.027, 0.244 | <0.001 | 0.075 | −0.038, 0.187 | <0.001 | |||
| ≥ 60 min | -ref- | -ref- | -- | -- | |||||
| Waist (per 5 cm increase) | 0.166 | 0.140, 0.192 | <0.001 | ||||||
| Waist 2 | −0.009 | −0.015, −0.002 | 0.007 | ||||||
Sleep duration and waist circumference are centered to the sample mean (7.63 and 74.75, respectively).
Activity = moderate/vigorous daily activity
To examine whether extended weekend sleep duration was associated with HOMA levels or if it confounded the association between weekday sleep duration and HOMA levels, secondary analyses included extended weekday sleep duration as an additional covariate in the regression models. Extended weekend sleep duration was not significantly associated with HOMA levels in unadjusted or adjusted regression analyses. Moreover, adjusting for extended weekend sleep duration did not appreciably change the point estimates or standard errors for weekday sleep duration, indicating that it did not confound the association between weekday sleep duration and HOMA levels (results not shown).
Discussion
This study evaluated the relationship between an objective measure of habitual sleep duration (using actigraphy) and an index of insulin sensitivity in an adolescent population. In this sample, a birth cohort from an urban Mid-Western area assembled to over-represent minorities and preterm children, we observed shorter sleep duration to be associated with minority race and indices of adiposity. As expected, increased HOMA levels were associated with adiposity, decreased physical activity and minority race, as well as with younger age. A quadratic association between sleep duration and HOMA levels was observed, with adolescents with shorter and longer sleep having HOMA levels that were on average 20% higher than levels in adolescents with intermediate sleep durations in models adjusted for age, sex, race, preterm status and daily moderate/vigorous activity. After additionally adjusting for waist circumference, the relationship with shorter sleep was appreciably attenuated, and the association with longer sleep was essentially unchanged. These analyses are consistent with the following: 1) shorter sleep and longer sleep are both associated with elevations in HOMA levels in generally healthy adolescents; 2) the association between shorter sleep duration with HOMA levels is to a large extent mediated through the association between short sleep and central obesity (i.e., as measured by waist circumference); and 3) the association between longer sleep and HOMA levels appears to be through pathways largely independent of obesity.
One prior study of 40 obese children, the majority of who also had sleep apnea, reported that children with shorter sleep duration as measured on a single overnight sleep study had evidence of insulin resistance.35 However, this study examined neither habitual sleep as measured in the child’s usual environment, children across a range of BMI, nor the impact of longer sleep on insulin levels.
The finding of a u-shaped relation between sleep duration and HOMA levels in our adolescent sample is consistent with a number of adult studies. A cross-sectional analysis from the Sleep Heart Health Study found that sleep duration less than 6 or more than 9 hours was associated with diabetes and impaired glucose tolerance independently of sleep apnea and body habitus.14 In the Nurses’ Health Study, adult women sleeping less than five or more than 9 hours per night were reported to be at increased risk of physician-diagnosed diabetes.13 Analysis of the longitudinal National Health and Nutrition Examination Survey (NHANES I) also found that subjects sleeping less than 5 or more than 9 hours were significantly more likely to develop incident diabetes, a temporal relation that was attenuated after considering obesity and hypertension.36 The FIN-D2D cross-sectional study also identified a u-shaped curve between sleep duration and type 2 diabetes in middle aged women,37 and the Massachusetts Male Aging Study found that short sleep duration (≤5 or 6 hours) doubled the odds and long sleep ( ≥8 hours) tripled the risk of developing diabetes in men free of diabetes at baseline.38 Finally, Nakajima et al. found a u-shaped relation between HbA1c and sleep duration in a rural Japanese community.39 However, in most of the studies of adult populations, concerns have been raised regarding the influences of residual confounding, particularly regarding the influence of mood disorders, sleep apnea, and other chronic illnesses as contributing factors to associations with longer sleep. Although it is also possible that residual confounding may have influenced our findings, the low prevalence of significant health and psychiatric conditions in a community sample of adolescents, our ability to exclude individuals with sleep apnea through direct measurements, and adjustment for physical activity, make it less likely that residual confounding was the explanation for our findings. Thus, these results, underscore the importance of considering variations in sleep duration at both extremes in influencing insulin sensitivity, and demonstrate associations even in a young, relatively healthy population. They also highlight the need for research to better define levels of “optimal” sleep duration, which varies across the age-developmental period, and also may vary within individuals of any given age.
Shorter sleep has been associated with altered secretion of appetite-regulating hormones and appetitive behaviors, potentially leading to increased adiposity and secondary insulin resistance.24 Sleep deprivation also causes an increase in counter-regulatory hormones such as cortisol, as well as an increase in sympathetic nervous system activity, both of which can contribute to insulin resistance.15, 21, 23, 40 In our study, short sleep was strongly associated with indices of adiposity, and after adjusting for waist circumference, the association between shorter sleep and HOMA was attenuated. This suggests that in our healthy adolescent sample, reduced sleep most likely influenced HOMA levels predominantly through effects on weight. Increased adiposity may increase insulin resistance through augmented release of tumor necrosis factor-alpha, interleukin-6,41–43 or free fatty acids,44 or through decreased release of adiponectin, an insulin sensitizing agent.45
It has been postulated that longer sleep time may be an early symptom or reflection of poorer physical health due to a chronic health condition or lack of exercise. In our study, although self-reported average daily amount of moderate-vigorous activity was negatively associated with HOMA levels, including activity levels in statistical models did not alter the findings. Nonetheless, self-reported levels likely overestimate activity. Further research into a potential reciprocal association between sleep duration and activity, and the joint effects of both exposures on endocrine function are needed. Others have speculated that longer sleep may be a surrogate for a variety of unmeasured health and psychiatric problems.46 Although we cannot exclude this as an explanation for our findings, our sample was young, recruited from a community rather than a clinic, and we took care to exclude children with known serious health problems from this analysis. Other potential mechanisms explaining the association with longer sleep may relate to the influences of sleep on cerebral and systemic glucose utilization47 or to the effects of counter-regulatory hormones released during sleep.48
Our primary outcome in this study was based on HOMA levels, which were reported to be a reliable measure of insulin resistance in children and adolescence32. Similar results were obtained using fasting insulin, which also has been used as a marker for insulin resistance in children with sleep disorders. 49 Another index, the G:I ratio, was highly correlated with HOMA (Spearman correlations r=−0.94).
Limitations to this study include that it is cross-sectional analysis and therefore neither cause and effect nor temporal relations can be ascertained. Detailed metabolic data were not available to assess the potential contributions of hepatic and peripheral glucose homeostasis. To increase statistical power, data from two examinations were used and analyzed with appropriate statistical models for correlated measurements. Additionally, comparisons of those included and excluded (116 observations on 114 subjects excluded due to missing exposure or outcome data) from the analyses showed that the latter were on average 1.3 years older (17.0 ± 1.8 vs. 15.7 ± 2.1 years) and 1.6 kg/m2 heavier (BMI 25.2 ± 6.5 vs 23.6 ± 5.4), but the groups were similar in terms of sex and race. Adjusted analyses were based on modeling sleep duration as a continuous measurement and definitions of “shorter” and “longer” sleep were not intended to identify strict thresholds associated with health or disease. Study strengths include evaluation of a racially diverse, community-based sample of adolescents in their natural environments, use of objective measurements of habitual sleep duration, standardized collection of outcomes, and consideration of several potential confounders, including physical activity. Additionally, subjects did not have sleep apnea and other co-morbidities such as diabetes and cardiovascular disease which could have confounded the associations.
Given the high prevalence of insufficient sleep in adolescents, which is especially high in ethnic minorities at risk for diabetes and other health problems, the current findings provide further support for efforts to address sleep behaviors in adolescents as part of routine health care. Randomized controlled trials and experimental studies are needed to better understand causal relations, as well as to identify what the “optimal” sleep patterns and durations are for individuals of given ages.
Acknowledgments
Supported by NIH grants (NIH HL07567, HL60957, UL1-RR024989 and 1U54CA116867).
We are grateful for the ongoing participation and support of the children and families in the Cleveland Children’s Sleep and Health Cohort. We also are indebted to the expert assistance of our research assistants: Joan Aylor, Kathryn Clark, Jennifer Frame, and Heather Rosebrock.
Abbreviations
- HOMA
Homeostasis model assessment
- DM-2
Type 2 Diabetes mellitus
- CCSHS
Children’s Sleep and Health Study
- PSG
polysomnography
- METS
metabolic equivalent task units
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–54. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 2.Duncan GE. Prevalence of diabetes and impaired fasting glucose levels among US adolescents: National Health and Nutrition Examination Survey, 1999–2002. Arch Pediatr Adolesc Med. 2006;160:523–8. doi: 10.1001/archpedi.160.5.523. [DOI] [PubMed] [Google Scholar]
- 3.Association AD. Type 2 diabetes in children and adolescents: consensus conference report; 2000. [Google Scholar]
- 4.Shaw J. Epidemiology of childhood type 2 diabetes and obesity. Pediatr Diabetes. 2007;8 (Suppl 9):7–15. doi: 10.1111/j.1399-5448.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- 5.Kitagawa T, Owada M, Urakami T, Tajima N. Epidemiology of type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in Japanese children. Diabetes Res Clin Pract. 1994;24 (Suppl):S7–13. doi: 10.1016/0168-8227(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 6.Hsia Y, Neubert AC, Rani F, Viner RM, Hindmarsh PC, Wong IC. An increase in the prevalence of type 1 and 2 diabetes in children and adolescents: results from prescription data from a UK general practice database. Br J Clin Pharmacol. 2009;67:242–9. doi: 10.1111/j.1365-2125.2008.03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverstein JH, Rosenbloom AL. Type 2 diabetes in children. Curr Diab Rep. 2001;1:19–27. doi: 10.1007/s11892-001-0006-x. [DOI] [PubMed] [Google Scholar]
- 8.Nadeau K, Dabelea D. Epidemiology of type 2 diabetes in children and adolescents. Endocr Res. 2008;33:35–58. doi: 10.1080/07435800802080138. [DOI] [PubMed] [Google Scholar]
- 9.Perceived insufficient rest or sleep among adults - United States, 2008. Mmwr. 2009;58:1175–9. [PubMed] [Google Scholar]
- 10.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis Obesity (Silver Spring) 2008;16:265–74. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 12.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 15.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–7. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 18.Meisinger C, Heier M, Loewel H. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48:235–41. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson PM, Roost M, Engstrom G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27:2464–9. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 20.Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 22.Schmid SM, Hallschmid M, Jauch-Chara K, Bandorf N, Born J, Schultes B. Sleep loss alters basal metabolic hormone secretion and modulates the dynamic counterregulatory response to hypoglycemia. J Clin Endocrinol Metab. 2007;92:3044–51. doi: 10.1210/jc.2006-2788. [DOI] [PubMed] [Google Scholar]
- 23.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 24.Van Cauter E, Holmback U, Knutson K, et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67 (Suppl 1):2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- 25.Kaditis AG, Alexopoulos EI, Damani E, et al. Obstructive sleep-disordered breathing and fasting insulin levels in nonobese children. Pediatr Pulmonol. 2005;40:515–23. doi: 10.1002/ppul.20306. [DOI] [PubMed] [Google Scholar]
- 26.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177:1142–9. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 28.Johnson NLKH, Rosen CL, Storfer-Isser A, Cartar LN, Ancoli-Israel S, Emancipator JL, Kibler AM, Redline S. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: a comparison of three data modes Sleep. 2007;30:899–905. doi: 10.1093/sleep/30.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176:401–8. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 32.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115:e500–3. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 34.Hermans MP, Levy JC, Morris RJ, Turner RC. Comparison of insulin sensitivity tests across a range of glucose tolerance from normal to diabetes. Diabetologia. 1999;42:678–87. doi: 10.1007/s001250051215. [DOI] [PubMed] [Google Scholar]
- 35.Flint J, Kothare SV, Zihlif M, et al. Association between inadequate sleep and insulin resistance in obese children. J Pediatr. 2007;150:364–9. doi: 10.1016/j.jpeds.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 36.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuomilehto H, Peltonen M, Partinen M, et al. Sleep duration is associated with an increased risk for the prevalence of type 2 diabetes in middle-aged women - The FIN-D2D survey. Sleep medicine. 2008;9:221–7. doi: 10.1016/j.sleep.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 39.Nakajima H, Kaneita Y, Yokoyama E, et al. Association between sleep duration and hemoglobin A1c level. Sleep medicine. 2008;9:745–52. doi: 10.1016/j.sleep.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Plat L, Leproult R, L’Hermite-Baleriaux M, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84:3082–92. doi: 10.1210/jcem.84.9.5978. [DOI] [PubMed] [Google Scholar]
- 41.Bastard JP, Maachi M, Van Nhieu JT, et al. Adipose tissue IL-6 content correlates ith resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084–9. doi: 10.1210/jcem.87.5.8450. [DOI] [PubMed] [Google Scholar]
- 42.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Lagathu C, Bastard JP, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem Biophys Res Commun. 2003;311:372–9. doi: 10.1016/j.bbrc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. European journal of endocrinology / European Federation of Endocrine Societies. 2008;159 (Suppl 1):S67–74. doi: 10.1530/EJE-08-0245. [DOI] [PubMed] [Google Scholar]
- 45.Gil-Campos M, Canete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clinical nutrition (Edinburgh, Scotland) 2004;23:963–74. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Bliwise DL, Young TB. The parable of parabola: what the U-shaped curve can and cannot tell us about sleep. Sleep. 2007;30:1614–5. doi: 10.1093/sleep/30.12.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyle PJ, Scott JC, Krentz AJ, Nagy RJ, Comstock E, Hoffman C. Diminished brain glucose metabolism is a significant determinant for falling rates of systemic glucose utilization during sleep in normal humans. The Journal of clinical investigation. 1994;93:529–35. doi: 10.1172/JCI117003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moller N, Jorgensen JO, Schmitz O, et al. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. The American journal of physiology. 1990;258:E86–91. doi: 10.1152/ajpendo.1990.258.1.E86. [DOI] [PubMed] [Google Scholar]
- 49.de la Eva RC, Baur LA, Donaghue KC, Waters KA. Metabolic correlates with obstructive sleep apnea in obese subjects. J Pediatr. 2002;140:654–9. doi: 10.1067/mpd.2002.123765. [DOI] [PubMed] [Google Scholar]