Abstract
Background: Cranberry juice contains polyphenolic compounds that could improve endothelial function and reduce cardiovascular disease risk.
Objective: The objective was to examine the effects of cranberry juice on vascular function in subjects with coronary artery disease.
Design: We completed an acute pilot study with no placebo (n = 15) and a chronic placebo-controlled crossover study (n = 44) that examined the effects of cranberry juice on vascular function in subjects with coronary artery disease.
Results: In the chronic crossover study, subjects with coronary heart disease consumed a research preparation of double-strength cranberry juice (54% juice, 835 mg total polyphenols, and 94 mg anthocyanins) or a matched placebo beverage (480 mL/d) for 4 wk each with a 2-wk rest period between beverages. Beverage order was randomly assigned, and participants refrained from consuming other flavonoid-containing beverages during the study. Vascular function was measured before and after each beverage, with follow-up testing ≥12 h after consumption of the last beverage. Mean (±SD) carotid-femoral pulse wave velocity, a measure of central aortic stiffness, decreased after cranberry juice (8.3 ± 2.3 to 7.8 ± 2.2 m/s) in contrast with an increase after placebo (8.0 ± 2.0 to 8.4 ± 2.8 m/s) (P = 0.003). Brachial artery flow-mediated dilation, digital pulse amplitude tonometry, blood pressure, and carotid-radial pulse wave velocity did not change. In the uncontrolled pilot study, we observed improved brachial artery flow-mediated dilation (7.7 ± 2.9% to 8.7 ± 3.1%, P = 0.01) and digital pulse amplitude tonometry ratio (0.10 ± 0.12 to 0.23 ± 0.16, P = 0.001) 4 h after consumption of a single 480-mL portion of cranberry juice.
Conclusions: Chronic cranberry juice consumption reduced carotid femoral pulse wave velocity—a clinically relevant measure of arterial stiffness. The uncontrolled pilot study suggested an acute benefit; however, no chronic effect on measures of endothelial vasodilator function was found. This trial was registered at clinicaltrials.gov as NCT00553904.
INTRODUCTION
Epidemiologic studies have shown that consumption of flavonoid-containing foods and beverages is associated with reduced cardiovascular disease risk (1, 2). Whereas the mechanisms accounting for this benefit remain incompletely defined, clinical studies have identified many relevant effects. For example, consumption of grapes, cocoa, and other flavonoid-containing foods may reduce blood pressure (3), platelet aggregation (4), insulin resistance (5), and cholesterol concentrations (6). In vitro and biomarker studies suggest that such foods have antiinflammatory effects (2, 7). Recently, there has been particular interest in the possibility that flavonoids also reduce cardiovascular disease risk by reversing endothelial dysfunction.
Endothelial dysfunction is a key mechanism in the pathogenesis of atherosclerotic vascular disease (8, 9). Risk factors decrease the bioavailability of endothelium-derived nitric oxide and induce a proinflammatory endothelial phenotype that promotes atherosclerosis and arterial stiffness. Risk-reduction therapies and lifestyle changes improve endothelial function (9). Prior studies have shown favorable effects of tea, cocoa, grape juice, and other dietary sources of flavonoids on endothelial function in patients with coronary disease and other risk factors (10–12).
Cranberry juice is a rich source of polyphenolic compounds, particularly anthocyanins (13, 14). Investigators have proposed that cranberry consumption might have protective effects against cardiovascular disease by reducing inflammation and serum lipids (15, 16). The present study was designed to investigate whether consumption of cranberry juice would improve vascular function and reduce inflammation. We used noninvasive methods to examine the effects of cranberry juice on multiple measures of vascular function in different vascular beds, including endothelium-dependent vasodilation and arterial stiffness in patients with coronary artery disease.
SUBJECTS AND METHODS
Study subjects
The study had 2 parts. We first completed an open-label, uncontrolled pilot study to determine the acute effects of cranberry juice consumption on vascular function. We recently reported the anthocyanin bioavailability results from this pilot study in a separate publication (13). We subsequently completed a randomized, double-blind, crossover study that examined vascular function before and after longer-term consumption of cranberry juice and a placebo beverage.
The pilot and crossover studies had the same entry criteria. We enrolled patients with stable coronary artery disease, confirmed by angiography, exercise test, or documented history of myocardial infarction. We excluded patients with body weight >115 kg, congestive heart failure, or acute medical illness. We also excluded potential subjects if they were taking vitamins exceeding 2 times the Recommended Dietary Allowance or food supplements within 1 mo of study. The subjects were asked to stop the consumption of cranberries and cranberry products, wine, grape juice, green or black tea, other dark juices (eg, pomegranate juice), and all dietary supplements 1 wk before beginning the study and thereafter for the duration of the study. All subjects provided written informed consent, and the protocols were approved by the Boston Medical Center Institutional Review Board.
Acute pilot study
As outlined in Figure 1 (top), the acute study was open-label and involved measurement of vascular function at baseline and 2 and 4 h after juice consumption. Subjects were asked to fast overnight and to refrain from smoking on the morning of the study visit. Participants consumed a single 480-mL portion of double-strength cranberry juice (54% juice compared with the traditional 27% juice). Each serving contained 835 mg total polyphenols, 94 mg anthocyanins, 10 g carbohydrates, and 40 kcal. More detailed information about juice composition was published previously (13, 17, 18). The study beverage was provided by the study sponsor (Ocean Spray Inc, Lakeville, MA). The tests of vascular function in the pilot study included brachial artery flow-mediated dilation and digital pulse amplitude tonometry.
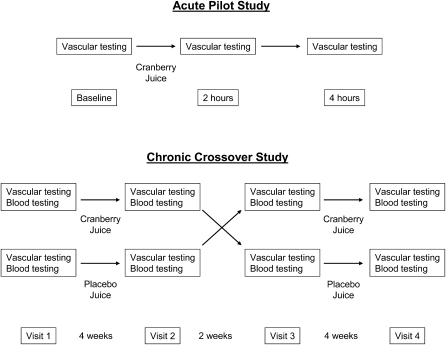
FIGURE 1.
Study design. Upper panel: for the acute pilot study, participants (n = 15) received cranberry juice and vascular testing was repeated 2 and 4 h after juice consumption. Lower panel: for the chronic crossover study, participants were randomly assigned to consume cranberry juice first (upper row; n = 24) or placebo first (lower row; n = 23) and consumed the assigned beverage for 4 wk. After a 2-wk rest period, they then crossed over to the alternate beverage. Two subjects withdrew while consuming cranberry juice because of dyspepsia, and one subject withdrew because of scheduling problems while consuming placebo. A total of 44 subjects completed the study.
Chronic randomized crossover study
As shown in Figure 1 (bottom), subjects in the chronic study consumed double-strength cranberry juice (480 mL/d) and a calorie, taste, and appearance-matched placebo beverage containing no polyphenols (480 mL/d). The subjects consumed each beverage for 4 wk with a 2-wk rest period between beverages. Beverage order was randomized (juice first or placebo first). The subjects returned bottle caps after each beverage consumption period for assessment of compliance. Investigators and participants were blinded to beverage identity until after completion of the data analysis. The beverages were stored at 4°C by a commercial storage company (Millbrook Cold Storage, Inc, Somerville, MA) and were delivered weekly to the participants by a delivery company experienced in clinical trials involving beverages (Inquil Solutions, LLC, Melrose, MA). The subjects consumed the beverage during the day with or without meals, according to their preference. The daily intake of anthocyanins from cranberry juice in the present study (94 mg/d) greatly exceeds the estimated average daily anthocyanin intake (12.5 mg) in the United States (19).
We completed vascular testing and collected blood samples for biochemical analysis during 4 study visits (before and after the 2 beverage-consumption periods). Before each visit, the subjects fasted after midnight and refrained from smoking. The subjects did not take their morning doses of cardiac medications until after completion of the visit (the last dose of daily vasoactive medications were taken 24 h before the study began) and did not consume the study beverage on the morning of the study visits.
Vascular testing
After a 10-min rest period in a recumbent position, we measured arterial blood pressure with an automated physiologic recorder (Dinamap; GE Health Care, Piscataway, NJ) and then assessed endothelium-dependent brachial artery flow-mediated dilation as previously described (20, 21). Briefly, 2-dimensional images and Doppler ultrasound signals were recorded from the brachial artery before and 1 min after induction of reactive hyperemia by 5-min cuff occlusion of the upper arm. We simultaneously measured endothelial function in the fingertip vessels by using digital pulse amplitude tonometry (Endo-PAT; Itamar Medical Ltd, Caesarea, Israel) to evaluate flow-mediated increases in pulse amplitude (22). Endo-PAT results are expressed as the natural logarithm of the ratio of the pulse amplitude recorded 90–120 s after cuff release to the baseline amplitude divided by the hyperemic to baseline ratio in the contralateral control finger (lnPAT ratio) (23). A higher lnPAT ratio reflects better endothelial function.
In the chronic study, but not in the acute study, we also assessed stiffness of the central aorta and conduit arteries in the upper extremity by measuring carotid-femoral and carotid-radial pulse wave velocity, respectively, using an applanation tonometry device (Complior SP; Artech Medical, Pantin, France). Briefly, the device simultaneously records arterial pressure waveforms from the carotid and femoral arteries and from the carotid and radial arteries. We measured the distance from each recording point to the sternal notching using a customized caliper, and the device calculated pulse wave velocity (m/s) (24). The CV for repeated measurements of carotid femoral pulse wave velocity 4 wk apart without intervention was 11%. Finally, in the chronic study, we used vascular ultrasound to measure the brachial artery diameter before and 3 min after a 0.4-mg sublingual dose of nitroglycerin. Nitroglycerin was omitted if the patient had a history of prior adverse reaction to nitroglycerin or a systolic blood pressure <100 mm Hg.
Biochemical analyses
Serum total and HDL-cholesterol, triglyceride, insulin, and glucose concentrations were analyzed in the Boston Medical Center Clinical Chemistry Laboratory. LDL cholesterol was calculated by using the Friedewald formula (25). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as [(fasting insulin μIU/mL) × (fasting glucose mmol/L)]/22.5. Serum C-reactive protein concentrations were measured at the Children's Hospital (Boston, MA) by using a high-sensitivity nephelometric method (26). Soluble intercellular adhesion molecule-1 (ICAM-1) was measured by using an enzyme-linked immunosorbent assay (Bender MedSystems GmbH, Vienna, Austria) (27).
Statistical analysis
For the acute, open-label pilot study, we used repeated-measures analysis of variance with simple within-subject comparison to relate the measures of vascular function 2 and 4 h after cranberry juice consumption to the baseline values (SPSS, IBM Inc, Somers, NY.). In these analyses, we used the Bonferroni correction to account for multiple comparisons.
For the chronic, randomized, placebo-controlled, crossover study, we compared the clinical characteristics of the 2 treatment groups (cranberry juice first and placebo first) by using the unpaired t test or the chi-square test for continuous and categorical variables, respectively. Each subject had measurements of vascular function and the biochemical markers at 4 time points (before and after each beverage). We evaluated the effect of the 2 beverages on each endpoint using a general linear model for correlated data with PROC MIXED, an unstructured covariance matrix, and standard residual maximum likelihood estimation (SAS Institute Inc, Cary, NC). C-reactive protein concentrations were assessed after log transformation because they lacked a normal distribution. We considered the effect of cranberry juice to be different from placebo if the treatment (cranberry juice or placebo) by follow-up (before beverage or after beverage) interaction had a P value <0.05. We assessed potential carryover effects by testing the 3-factor interaction: treatment, by follow-up, by treatment period (first beverage or second beverage). Data are presented as means ± SDs.
On the basis of prior data from our laboratory, the study was designed to provide 88% power (α = 0.05) to detect a 1% change in the prespecified primary endpoint of brachial artery flow-mediated dilation (eg, from 6.0% to 7.0%) with a sample size of 44 participants and a crossover design.
RESULTS
Acute pilot study
We enrolled 15 subjects with coronary artery disease into the pilot study (mean ± SD age: 62 ± 8 y, 13% female). As expected, there was a high prevalence of risk factors, including hypertension (53%), history of cigarette smoking (67%), and diabetes mellitus (33%). Fasting glucose was 103 ± 36 mg/dL and LDL cholesterol was 82 ± 10 mg/dL. The participants were taking cardiovascular drugs, including lipid-lowering therapy (100%), angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapy (60%), and aspirin (100%).
The vascular function testing results are shown in Table 1. As shown, there were significant improvements in brachial artery flow-mediated dilation 4 h after cranberry juice and in the lnPAT ratio 2 and 4 h after acute juice consumption. A modest decrease in resting brachial artery blood flow was found, but no effects on hyperemic flow, baseline brachial diameter, blood pressure, or heart rate were found.
TABLE 1.
Pilot study results: acute effects of cranberry juice consumption1
| Vascular function variable | Baseline | 2 h | 4 h | P2 |
| Systolic BP (mm Hg) | 131 ± 15 | 133 ± 17 | 134 ± 13 | 0.274 |
| Diastolic BP (mm Hg) | 77 ± 9 | 79 ± 9 | 78 ± 8 | 0.503 |
| Heart rate (beats/min) | 62 ± 9 | 58 ± 9 | 59 ± 9 | 0.066 |
| Baseline brachial diameter (mm) | 4.23 ± 1.03 | 4.23 ± 1.04 | 4.18 ± 1.03 | 0.15 |
| Flow-mediated dilation (%) | 7.7 ± 2.9 | 7.3 ± 2.8 | 8.7 ± 3.1* | 0.003 |
| Flow-mediated dilation (mm) | 0.33 ± 0.16 | 0.31 ± 0.15 | 0.36 ± 0.16* | 0.008 |
| Baseline flow (mL/min) | 180 ± 94 | 143 ± 70 | 141 ± 87* | 0.03 |
| Hyperemic flow (mL/min) | 1276 ± 632 | 1227 ± 564 | 1275 ± 696 | 0.51 |
| lnPAT ratio | 0.10 ± 0.12 | 0.22 ± 0.14* | 0.23 ± 0.16* | 0.006 |
All values are means ± SDs. BP, blood pressure; PAT, pulse amplitude tonometry measured in the finger. *Significantly different from baseline, P < 0.05 [with Bonferroni correction; simple within-subject comparison in SPSS (SPSS Inc, Chicago, IL) (n = 15), except for natural log of the PAT for the lnPAT ratio (n = 14)].
Overall P value for repeated-measures ANOVA.
Chronic randomized crossover study: study subjects
A total of 47 participants qualified for the study and began beverage consumption. One subject withdrew for scheduling reasons, and 2 subjects withdrew because of dyspepsia while consuming cranberry juice. The clinical characteristics of the 44 subjects who completed the study are displayed in Table 2. As shown, the juice first and placebo groups were similar. Participants were middle-aged, predominantly white, and overweight, and there was a high prevalence of risk factors. Nearly all were receiving risk-reduction therapy and had relatively low lipid concentrations but high fasting glucose concentrations.
TABLE 2.
Baseline clinical characteristics: randomized crossover study1
| Characteristic | Juice first (n = 22) | Placebo first (n = 22) | P2 |
| Age (y) | 61 ± 113 | 63 ± 9 | 0.44 |
| Male sex [n (%)] | 15 (68) | 15 (68) | 1.00 |
| Black race [n (%)] | 4 (18) | 6 (27) | 0.47 |
| History of hypertension [n (%)] | 17 (77) | 14 (64) | 0.32 |
| History of diabetes mellitus [n (%)] | 12 (55) | 8 (36) | 0.23 |
| History of smoking [n (%)] | 15 (68) | 15 (68) | 1.00 |
| Lipid-lowering therapy [n (%)] | 21 (95) | 21 (95) | 1.00 |
| Aspirin therapy [n (%)] | 17 (77) | 18 (82) | 0.71 |
| Clopidogrel therapy [n (%)] | 8 (36) | 5 (23) | 0.32 |
| ACE inhibitor or ARB therapy [n (%)] | 11 (50) | 11 (50) | 1.00 |
| BMI (kg/m2) | 30 ± 5 | 29 ± 4 | 0.65 |
| Total cholesterol (mg/dL) | 161 ± 46 | 152 ± 34 | 0.45 |
| LDL cholesterol (mg/dL) | 92 ± 38 | 84 ± 30 | 0.45 |
| HDL cholesterol (mg/dL) | 40 ± 11 | 43 ± 9 | 0.40 |
| Triglycerides (mg/dL) | 144 ± 56 | 126 ± 59 | 0.29 |
| Fasting glucose (g/dL) | 130 ± 47 | 115 ± 30 | 0.22 |
| Fasting insulin (μIU/dL) | 9.1 ± 5.9 | 9.4 ± 8.1 | 0.92 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
P value for unpaired t test or chi-square test as appropriate.
Mean ± SD (all such values).
Chronic vascular function results
We sought to determine whether long-term cranberry juice would have a cumulative effect on vascular function. Participants consumed each beverage for 4 wk with the last consumption on the day before the study. As shown in Table 3, we observed no cumulative effect of juice consumption on brachial artery flow-mediated dilation—the prespecified primary endpoint. We also observed no effects on blood pressure, baseline or hyperemic flow, nitroglycerin-mediated dilation, or lnPAT ratio.
TABLE 3.
Chronic effects of cranberry juice on vascular function1
| Vascular function variable | Beforejuice | Afterjuice | Before placebo | After placebo | P2 |
| Systolic blood pressure (mm Hg) | 131 ± 16 | 133 ± 18 | 133 ± 17 | 135 ± 18 | 0.44 |
| Diastolic blood pressure (mm Hg) | 72 ± 9 | 73 ± 9 | 74 ± 8 | 74 ± 9 | 0.94 |
| Flow-mediated dilation (%) | 6.3 ± 4.4 | 6.7 ± 4.4 | 6.5 ± 4.0 | 6.6 ± 3.9 | 0.69 |
| Baseline diameter (mm) | 4.58 ± 0.70 | 4.55 ± 0.68 | 4.53 ± 0.70 | 4.53 ± 0.65 | 0.64 |
| Dilation to nitroglycerin (%) | 8.7 ± 5.0 | 9.5 ± 4.9 | 11 ± 4.8 | 10 ± 5.2 | 0.21 |
| Baseline flow (mL/min) | 176 ± 74 | 195 ± 82 | 187 ± 65 | 184 ± 70 | 0.09 |
| Hyperemic flow (mL/min) | 1106 ± 388 | 1198 ± 377 | 1086 ± 350 | 1146 ± 380 | 0.56 |
| lnPAT ratio | 0.40 ± 0.36 | 0.36 ± 0.34 | 0.29 ± 0.29 | 0.33 ± 0.30 | 0.33 |
| Cartoid-radial PWV (m/s) | 8.2 ± 1.9 | 7.9 ± 1.6 | 8.1 ± 1.3 | 7.9 ± 1.4 | 0.47 |
| Cartoid-femoral PWV (m/s) | 8.3 ± 2.3 | 7.8 ± 2.2 | 8.0 ± 2.0 | 8.4 ± 2.8 | 0.003 |
All values are means ± SDs; n = 44. PAT, pulse amplitude tonometry measured in the finger; PWV, pulse wave velocity measured by applanation tonometry.
P for treatment by follow-up interaction as determined by using a general linear model for correlated data with PROC MIXED, an unstructured covariance matrix, and standard residual maximum likelihood estimation in SAS (SAS Institute Inc, Cary, NC).
As shown in Table 3, a favorable effect of cranberry juice consumption compared with placebo consumption on arterial stiffness was found. Carotid-femoral pulse wave velocity decreased by 0.5 m/s (6%) after cranberry juice consumption and increased by 0.4 m/s after placebo consumption. This favorable effect of juice compared with placebo was statistically significant. No effect of beverage consumption on carotid-radial pulse wave velocity was found.
Chronic biochemical marker results
The effects of beverage consumption on blood biochemical markers are shown in Table 4. No effects of beverage consumption on serum lipids were observed, except for a 1-mg/dL reduction in HDL cholesterol compared with a 1-mg/dL (2%) increase after placebo. No significant effects of beverage consumption on blood glucose, insulin, or markers of inflammation were found.
TABLE 4.
Effects of beverage consumption on the biochemical profile1
| Blood marker | Beforejuice | Afterjuice | Before placebo | After placebo | P2 |
| Total cholesterol (mg/dL) | 158 ± 39 | 156 ± 40 | 157 ± 42 | 157 ± 41 | 0.61 |
| LDL cholesterol (mg/dL) | 89 ± 33 | 89 ± 33 | 89 ± 36 | 88 ± 33 | 0.89 |
| HDL cholesterol (mg/dL) | 43 ± 10 | 42 ± 9 | 41 ± 9 | 42 ± 9 | 0.04 |
| Triglycerides (mg/dL) | 130 ± 57 | 128 ± 51 | 136 ± 56 | 137 ± 65 | 0.86 |
| Glucose (mg/dL) | 127 ± 49 | 124 ± 48 | 126 ± 42 | 130 ± 53 | 0.36 |
| Insulin (μIU/dL) | 10 ± 8 | 9 ± 8 | 10 ± 7 | 10 ± 8 | 0.22 |
| HOMA-IR (arbitrary units) | 3.3 ± 3.1 | 3.2 ± 4.5 | 3.1 ± 2.8 | 3.5 ± 3.7 | 0.24 |
| C-reactive protein (mg/L) | 3.1 ± 5.5 | 2.5 ± 3.4 | 5.2 ± 9.9 | 2.7 ± 3.9 | 0.39 |
| Soluble ICAM-1 (ng/mL) | 245 ± 78 | 246 ± 77 | 256 ± 80 | 250 ± 82 | 0.15 |
All values are means ± SDs; n = 44. HOMA-IR, homeostasis model assessment of insulin resistance; ICAM-1, intercellular adhesion molecule-1.
P for treatment by follow-up interaction as determined by using a general linear model for correlated data with PROC MIXED, an unstructured covariance matrix, and standard residual maximum likelihood estimation in SAS (SAS Institute Inc, Cary, NC).
Chronic per protocol analysis
We assessed compliance with the study protocol as the percentage of expected bottle caps that were returned. Average compliance was 94 ± 8% for cranberry juice (range: 68–100%). We completed a per protocol analysis that excluded the 2 participants who did not participate in the compliance assessment and the 2 participants who had <80% compliance. The results were similar, with a significant reduction in carotid-femoral pulse wave velocity after cranberry juice and no other significant effects on the other variables (data not shown).
DISCUSSION
The crossover study was designed to investigate whether chronic consumption of cranberry juice has a cumulative beneficial effect on vascular function. For this reason, vascular function was measured in the morning ≥12 h after the last beverage consumption. We observed no effect of cranberry juice on measures of endothelial vasodilator function, including flow-mediated dilation and lnPAT ratio. In addition, no effects on serum total and LDL cholesterol, insulin, glucose, or markers of inflammation were observed. However, a significant reduction in carotid-femoral pulse wave velocity, reflecting decreased aortic stiffness, was found. We emphasize that the research beverage contained twice the amount of cranberry juice in commercially available cranberry juice and that the amount of anthocyanins consumed during the study greatly exceeded the average daily intake in the United States.
Carotid-femoral pulse wave velocity is emerging as an important measure of vascular function that relates to cardiovascular disease risk. It predicted cardiovascular events in the Framingham Heart Study after adjustment for other cardiovascular disease risk factors (28) and was a stronger predictor than other measures of arterial stiffness, including the augmentation index and central pulse pressure. A variety of drugs (29, 30) and lifestyle interventions (31, 32) have been shown to reduce carotid femoral pulse wave velocity in patients with hypertension and other risk factors. In contrast, carotid-radial pulse wave velocity, which was unaffected by cranberry juice in our study, relates poorly to risk factors, does not predict events, and responds poorly to interventions (28, 30).
Arterial stiffness is determined by a variety of factors, including dynamic factors such as endothelium-derived vasodilators, blood pressure, and sympathetic tone (33, 34). Intrinsic properties of the arterial wall—including wall thickness, relative collagen and elastin content, and the degree of calcification—also influence stiffness. Additional studies will be needed to determine how cranberry juice reduces central aortic stiffness, but our finding of improved pulse wave velocity without a change in endothelial function may be consistent with an effect at the level of the arterial wall or a change in sympathetic tone.
A few prior studies examined the effects of other polyphenol-containing foods on arterial stiffness. For example, flavonoids in green and black tea appear to blunt caffeine-induced increases in pulse wave velocity (35). Isoflavone supplementation for 6 wk reduced carotid-femoral pulse wave velocity without improving flow-mediated dilation in older healthy men and postmenopausal women (36). The effect size with isoflavone treatment was similar to the reduction (≈6%) in pulse wave velocity observed in the present study, but was less than the 10–15% reduction produced by the drug ramipril (37). Overall, our study suggests that chronic cranberry juice consumption may have a meaningful effect on a clinically relevant measure of vascular function and is consistent with prior work.
The current study showed no beneficial effect of chronic cranberry juice consumption on flow-mediated vasodilation of the brachial artery or small vessels in the finger. These results appear to contrast with the results of prior studies, which have shown favorable long-term effects of tea, grape juice, and cocoa consumption on brachial artery flow-mediated dilation (10–12). Differences in the study design, time of last beverage consumption, and population characteristics may account for these apparently discrepant results. For example, nearly all of the patients in the current study were receiving risk-reduction therapy and had well-controlled lipid values. The discrepant results might also reflect differences in the polyphenolic content of the various beverages.
In contrast with the chronic study, our open-label pilot study showed a favorable acute effect on brachial artery flow-mediated dilation. No acute effects on baseline brachial diameter or hyperemic flow were observed, which suggests that these results were not attributable to a change in resting arterial geometry or the stimulus for vasodilation. We also observed an improved lnPAT ratio 2 and 4 h after juice consumption. Although limited by the lack of a placebo group, these findings suggest improved endothelial function in the conduit brachial artery and small vessels in the finger after acute consumption of cranberry juice.
Several prior studies have shown an acute benefit, but no chronic benefit, of similar interventions on endothelial function. For example, our research group showed improved endothelial function after acute, but not after chronic, administration of epigallocatechin gallate (38). The discrepancy between acute and chronic results is most likely attributable to the kinetics of cranberry anthocyanins and/or other polyphenols. We recently reported that cranberry anthocyanins are rapidly cleared from the plasma after an oral dose of cranberry juice with maximum bioavailability between 1 and 3 h (13). In the current study, subjects last consumed cranberry juice on the day before the follow-up study visits. Brachial artery flow-mediated dilation and the lnPAT ratio are vascular responses that largely depend on nitric oxide production and change rapidly over a period of minutes to hours after interventions (20, 22). An acute improvement in these measures of vascular function may not persist for 12 h or more after the last dose of cranberry juice. On the other hand, an effect of cranberry juice on the composition of the arterial wall could conceivably persist for a longer period of time. Additional studies are required to gain a mechanistic understanding of these effects on vascular function.
Regarding the effects of chronic cranberry juice consumption on blood markers, we observed a modest 2% reduction in HDL cholesterol. This apparently unfavorable effect differs from the study by Ruel et al (39), who observed an increase in HDL cholesterol after supplementation with cranberry juice in obese subjects. Those investigators also observed a reduction in soluble intercellular adhesion molecule-1 (40) which did not change in the current study. The prior studies involved longer treatment periods (12 wk) and different subject characteristics, which might explain the apparent discrepancies.
Our study had several limitations. Most importantly, the pilot study of acute cranberry consumption had no control group and was performed to gain preliminary data and to confirm the bioavailability of cranberry polyphenols before embarking on the chronic study. The findings must therefore be viewed with caution. Cranberry juice was consumed for a relatively short period of time, and it remains unknown whether the favorable effects on arterial stiffness would persist with longer treatment or have any effect on cardiovascular disease risk. Our study provides no insight into the mechanism of benefit. We cannot exclude the possibility that concomitant medications might have affected the vascular function results. Finally, our study involved withholding other polyphenol-containing foods and beverages throughout the study period. This aspect of the study design might explain the apparent increase in arterial stiffness after placebo consumption and could reflect a lack of steady state in regard to flavonoid status. The study limitations were balanced by the placebo-controlled crossover study design, relatively large sample size, and examination of emerging markers of arterial stiffness.
In conclusion, the current study showed no chronic effect of cranberry juice on the primary endpoint of brachial artery flow-mediated dilation. Compliance with the study protocol was excellent, and the study was well powered to detect a change in flow-mediated dilation comparable with that observed after consumption of other flavonoid-containing beverages. Thus, it is unlikely that we missed a clinically meaningful chronic effect of cranberry on flow-mediated dilation. We did observe a highly significant effect of cranberry juice on stiffness of the central aorta, which is increasingly recognized as an important measure of vascular function with relevance to cardiovascular disease. Additional studies are needed to define the mechanisms and clinical implications of these vascular effects. Overall, our results may provide further support for the American Heart Association recommendation that cardiovascular disease risk may be reduced by a diet rich in fruit and vegetables, including cranberries.
Acknowledgments
The authors’ responsibilities were as follows—JAV: conceived the study hypothesis, secured funding, and worked with MH and MAK to design the study and implement the study procedures; MMD, MH, NMH, SMS, WBC, MT, MAK, and PEM: were involved in the collection of data; and MMD, NMH, NW, JP, and JAV: conducted the analyses and drafted the manuscript. All authors critically reviewed and had input into manuscript for important intellectual content. None of the authors had a personal or financial conflict of interest. Employees of Ocean Spray Inc reviewed and agreed to fund the study as designed by the investigators; however, they had no input into the conduct of the study or the data analysis and interpretation.
REFERENCES
- 1.Erdman JW, Jr, Balentine D, Arab L, et al. Flavonoids and heart health: Proceedings of the ILSI North America Flavonoids Workshop, May 31-June 1, 2005, Washington, DC. J Nutr 2007;137:718S–37S [DOI] [PubMed] [Google Scholar]
- 2.Dohadwala MM, Vita JA. Grapes and cardiovascular disease. J Nutr 2009;139:1788S–93S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taubert D, Roesen R, Lehmann C, Jung N, Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA 2007;298:49–60 [DOI] [PubMed] [Google Scholar]
- 4.Freedman JE, Parker C, III, Li L, et al. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation 2001;103:2792–8 [DOI] [PubMed] [Google Scholar]
- 5.Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr 2005;81:611–4 [DOI] [PubMed] [Google Scholar]
- 6.Davies MJ, Judd JT, Baer DJ, et al. Black tea consumption reduces total and LDL cholesterol in mildly hypercholesterolemic adults. J Nutr 2003;133:3298S–302S [DOI] [PubMed] [Google Scholar]
- 7.Sies H, Schewe T, Heiss C, Kelm M. Cocoa polyphenols and inflammatory mediators. Am J Clin Nutr 2005;81:304S–12S [DOI] [PubMed] [Google Scholar]
- 8.Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord 2010;11:61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 2003;42:1149–60 [DOI] [PubMed] [Google Scholar]
- 10.Duffy SJ, Keaney JF, Jr, Holbrook M, et al. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation 2001;104:151–6 [DOI] [PubMed] [Google Scholar]
- 11.Schroeter H, Heiss C, Balzer J, et al. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 2006;103:1024–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation 1999;100:1050–5 [DOI] [PubMed] [Google Scholar]
- 13.Milbury PE, Vita JA, Blumberg JB. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J Nutr 2010;140:1099–104 [DOI] [PubMed] [Google Scholar]
- 14.Vinson JA, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem 2001;49:5315–21 [DOI] [PubMed] [Google Scholar]
- 15.Vattem DA, Ghaedian R, Shetty K. Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry. Asia Pac J Clin Nutr 2005;14:120–30 [PubMed] [Google Scholar]
- 16.Ruel G, Couillard C. Evidences of the cardioprotective potential of fruits: the case of cranberries. Mol Nutr Food Res 2007;51:692–701 [DOI] [PubMed] [Google Scholar]
- 17.Zhang K, Zuo Y. GC-MS determination of flavonoids and phenolic and benzoic acids in human plasma after consumption of cranberry juice. J Agric Food Chem 2004;52:222–7 [DOI] [PubMed] [Google Scholar]
- 18.Zafriri D, Ofek I, Adar R, Pocino M, Sharon N. Inhibitory activity of cranberry juice on adherence of type 1 and type P fimbriated Escherichia coli to eucaryotic cells. Antimicrob Agents Chemother 1989;33:92–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem 2006;54:4069–75 [DOI] [PubMed] [Google Scholar]
- 20.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol 2002;359:186–200 [DOI] [PubMed] [Google Scholar]
- 21.McMackin CJ, Vita JA. Update on nitric oxide-dependent vasodilation in human subjects. Methods Enzymol 2005;396:541–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med 2009;19:6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008;117:2467–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asmar R, Topouchian J, Pannier B, Benetos A, Safar M. Pulse wave velocity as endpoint in large-scale intervention trial. The Complior study. Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study. J Hypertens 2001;19:813–8 [DOI] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 26.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–9 [DOI] [PubMed] [Google Scholar]
- 27.Keaney JF, Jr, Lipinska I, Larson MG, et al. Clinical correlates, heritability, and genetic linkage of circulating CD40 ligand in the Framingham Offspring Study. Am Heart J 2008;156:1003–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010;121(4):505–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell GF, Dunlap ME, Warnica W, et al. Long-term trandolapril treatment is associated with reduced aortic stiffness: the prevention of events with angiotensin-converting enzyme inhibition hemodynamic substudy. Hypertension 2007;49:1271–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orr JS, Dengo AL, Rivero JM, Davy KP. Arterial destiffening with atorvastatin in overweight and obese middle-aged and older adults. Hypertension 2009;54:763–8 [DOI] [PubMed] [Google Scholar]
- 31.Dengo AL, Dennis EA, Orr JS, et al. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension 2010;55:855–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philippou E, Bovill-Taylor C, Rajkumar C, et al. Preliminary report: the effect of a 6-month dietary glycemic index manipulation in addition to healthy eating advice and weight loss on arterial compliance and 24-hour ambulatory blood pressure in men: a pilot study. Metabolism 2009;58:1703–8 [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson IB, MacCallum H, Cockcroft JR, Webb DJ. Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br J Clin Pharmacol 2002;53:189–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell GF. Arterial stiffness and wave reflection: biomarkers of cardiovascular risk. Artery Res 2009;3:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlachopoulos C, Alexopoulos N, Dima I, Aznaouridis K, Andreadou I, Stefanadis C. Acute effect of black and green tea on aortic stiffness and wave reflections. J Am Coll Nutr 2006;25:216–23 [DOI] [PubMed] [Google Scholar]
- 36.Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ. Isoflavones reduce arterial stiffness: a placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol 2003;23:1066–71 [DOI] [PubMed] [Google Scholar]
- 37.Ahimastos AA, Natoli AK, Lawler A, Blombery PA, Kingwell BA. Ramipril reduces large-artery stiffness in peripheral arterial disease and promotes elastogenic remodeling in cell culture. Hypertension 2005;45:1194–9 [DOI] [PubMed] [Google Scholar]
- 38.Widlansky ME, Hamburg NM, Anter E, et al. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J Am Coll Nutr 2007;26:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruel G, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Favourable impact of low-calorie cranberry juice consumption on plasma HDL-cholesterol concentrations in men. Br J Nutr 2006;96:357–64 [DOI] [PubMed] [Google Scholar]
- 40.Ruel G, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Low-calorie cranberry juice supplementation reduces plasma oxidized LDL and cell adhesion molecule concentrations in men. Br J Nutr 2008;99:352–9 [DOI] [PubMed] [Google Scholar]