Abstract
Background: Choline is an essential nutrient for humans, and part of this requirement is met by endogenous synthesis catalyzed by hepatic phosphatidylethanolamine N-methyltransferase (PEMT). PEMT activity is difficult to estimate in humans because it requires a liver biopsy. Previously, we showed that mice that lack functional PEMT have dramatically reduced concentrations of docosahexaenoic acid (DHA; 22:6n−3) in plasma and of liver phosphatidylcholine (PtdCho)—a phospholipid formed by PEMT.
Objective: The objective was to evaluate plasma PtdCho-DHA concentrations as a noninvasive marker of liver PEMT activity in humans.
Design: Plasma PtdCho-DHA concentrations were measured in 72 humans before and after they consumed a low-choline diet, and correlations were analyzed in relation to estrogen status, PEMT polymorphism rs12325817, the ratio of plasma S-adenosylmethionine (AdoMet) to S-adenosylhomocysteine (AdoHcy), and dietary choline intake; all of these factors are associated with changes in liver PEMT activity. PtdCho-DHA and PEMT activity were also measured in human liver specimens.
Results: At baseline, the portion of PtdCho species containing DHA (pmol PtdCho-DHA/nmol PtdCho) was higher in premenopausal women than in men and postmenopausal women (P < 0.01). This ratio was lower in premenopausal women with the rs12325817 polymorphism in the PEMT gene (P < 0.05), and PtdCho-DHA concentration and PEMT activity were lower in human liver samples from women who were homozygous for PEMT rs12325817 (P < 0.05). The ratio of DHA-PtdCho to PtdCho in plasma was directly correlated with the ratio of AdoMet to AdoHcy (P = 0.0001). The portion of PtdCho species containing DHA in plasma was altered in subjects who consumed a low-choline diet.
Conclusion: PtdCho-DHA may be useful as a surrogate marker for in vivo hepatic PEMT activity in humans. This trial was registered at clinicaltrials.gov as NCT00065546.
INTRODUCTION
Humans develop fatty liver, liver damage, and muscle damage when they consume diets low in choline (1, 2). This nutrient is obtained in the diet from a wide variety of foods (3–5) and also can be derived from de novo biosynthesis of phosphatidylcholine (PtdCho) in liver (6). This pathway is catalyzed by phosphatidylethanolamine N-methyltransferase (PEMT), which utilizes S-adenosylmethionine (AdoMet) to sequentially methylate phosphatidylethanolamine to form PtdCho. PtdCho is the most common phospholipid in eukaryotic cellular membranes and can be hydrolyzed by phospholipase D to release free choline (6). Most PEMT activity occurs in the liver (7).
Activity of hepatic PEMT is of significant interest because it can influence the amount of choline that must be obtained from the diet. We previously reported that individuals with a particular single nucleotide polymorphism (SNP) in the PEMT gene are at greater risk of developing liver and muscle dysfunction when they consume a diet low in choline (8). Moreover, because PEMT activity is regulated by estrogen in human and mouse hepatocytes (9–12), the choline requirement is increased for postmenopausal women (because they have lower estrogen concentrations than do premenopausal women) (13). Furthermore, knockout mice with no functional PEMT activity (PEMT −/−) cannot survive without adequate choline provided in the diet (14). Many other factors also can alter PEMT activity, including ethanol (15), certain hypolipidemic drugs (16, 17), consumption of a choline-methionine deficient diet (18, 19), and other SNPs in the PEMT gene (20). In addition to its influence on dietary choline requirements, perturbations of hepatic PEMT activity also alter the normal secretion of VLDLs from liver (21–23) and are associated with the development of hepatosteatosis (20, 24).
PEMT also may have an important role in fatty acid distribution to tissues. The term PtdCho represents a family of phospholipids that differ in fatty acid composition but share a common glycerophosphocholine backbone. PtdCho derived from the PEMT pathway differs from that generated by the cytidine diphosphocholine (CDP)–choline pathway. The CDP-choline pathway forms PtdCho molecules primarily containing medium-chain and saturated fatty acids, whereas PtdCho formed by the PEMT pathway primarily contains long-chain polyunsaturated fatty acids (PUFAs), such as arachidonic acid (20:4n−6) and docosahexaenoic acid (DHA; 22:6n−3) (25). The fact that the phosphatidylethanolamine used by PEMT is rich in DHA, whereas PtdCho synthesized from choline is not, makes PtdCho-DHA a good index of PEMT activity. In addition, we previously showed that PEMT−/− mice have significantly lower DHA species in plasma and liver PtdCho than do wild-type mice—an effect not seen with any other fatty acids (26). In isolated rat hepatocytes, a rapid increase in PtdCho-DHA concentrations occurred after treatment with ethanolamine for 2 h, and this increase was prevented when PEMT was inhibited by 3-deazaadenosine (27), which also suggests that the PtdCho-DHA concentration is a surrogate marker for PEMT activity.
Because liver tissue is not easily obtained from human subjects, it has been difficult to assess the activity of hepatic PEMT in humans. Considering the physiologic importance of PEMT and its ability to be modulated by many factors, a marker of in vivo hepatic PEMT activity in humans would be useful. Current measures of PEMT activity are quite often performed in vitro by using isolated tissues. In humans, this would require an invasive liver biopsy and would most likely yield inaccurate results because in vitro assays cannot replicate the fluctuating substrate and inhibitor concentrations found in vivo. Therefore, this investigation evaluated the usefulness of assessing plasma PtdCho-DHA concentrations as a surrogate marker of liver PEMT activity in humans.
SUBJECTS AND METHODS
Subjects and diets
Seventy-two healthy volunteers (20 men and 52 women) ranging in age from 18 to 70 y were recruited for this study. Subject enrollment began on 14 March 2001 for the first phase of the study and on 27 June 2007 for the second phase. Of these women, 25 were postmenopausal and 27 were premenopausal. Postmenopausal status was defined as having had the last spontaneous menstrual bleeding >12 mo previously and a follicle-stimulating hormone concentration of >30 IU/L. The details of subject recruitment and the clinical protocol were approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. The subjects were admitted to the University of North Carolina at Chapel Hill Clinical and Translational Research Center where they received specified diets (28) and remained under the constant supervision of study staff for the entire duration of the study.
Initially, all participants received a conventional diet of normal foods containing 550 mg choline/70 kg daily (the current Adequate Intake level; 29), 50 mg betaine/70 kg daily, and 400 dietary folate equivalents (DFE) daily (baseline). After 10 d of this initial baseline diet, the subjects entered a choline-depletion phase and were fed a low-choline diet containing <50 mg choline/70 kg daily (with 6 mg betaine/70 kg daily), as confirmed by chemical analysis of a sample of duplicate food portions (3, 30). All diets were isocaloric, with 30% of energy from fat, 0.8 g high biological value protein/kg body weight, and the remaining energy from carbohydrate. These diets also met or exceeded the Estimated Average Requirement for methionine and cysteine and the dietary reference intake for all vitamins with the exception of folate (some of the subjects received 100 μg DFE/d, and others received 400 μg DFE/d; folate intake did not influence choline-deficiency organ dysfunction; 1, 8, 31). Periodic measurements of urinary choline and betaine concentrations (30) were used to confirm compliance with the dietary restrictions (data not shown). The subjects remained on the depletion diet until they developed organ dysfunction associated with choline deficiency or for 42 d if they did not.
The subjects were deemed to have organ dysfunction associated with choline deficiency if they had an increase in serum creatine phosphokinase activity >5-fold (2), an increase in aspartate aminotransferase >1.5 fold, or an increase in liver fat (measured by magnetic resonance imaging; 1) of >28% while consuming the choline-depletion diet. Aspartate aminotransferase and creatine phosphokinase analyses were performed by the McClendon Clinical Laboratory at University of North Carolina Hospitals (Clinical Laboratory Improvement Act and College of American Pathologists accredited) by using blood drawn by venipuncture. Additional samples of blood were collected. After centrifugation, plasma was removed and stored at −80°C until the day of analysis to measure plasma AdoMet, S-adenosylhomocysteine (AdoHcy), PtdCho, and PtdCho-DHA concentrations.
Liver tissue procurement
Human liver samples from premenopausal women were obtained from the Liver Procurement and Distribution System (University of Minnesota, Minneapolis MN; funded by NIH contract N01 DK92310). Of the 8 donors, 7 had fatty liver (2 of which were cirrhotic as well). Synopses of the tissue donors’ medical histories, including the pathologists’ impression diagnoses concerning liver fat content, were obtained. The specimens were snap-frozen after removal from the organ donors, delivered on dry ice, and stored at –80°C until analyzed.
Analysis of plasma PtdCho fatty acids
Lipids were extracted from duplicate plasma samples, and the livers were pulverized in liquid nitrogen by using the method of Bligh and Dyer (32) in the presence of an internal standard (1,2-diheptadecanoyl PtdCho; Avanti Polar Lipids, Alabaster, AL) and 0.02% butylated hydroxytoluene. Lipid classes were separated by thin-layer chromatography (CHCl3:MeOH:H2O, 65:30:4, vol:vol), and the PtdCho bands that coeluted with authentic standard were collected and saponified, and the fatty acids were trans-methylated by the sequential addition of 4.25% NaOH in CHCl3/MeOH (2:1, vol:vol) and 1 N HCl in saline (33). Fatty acid methyl esters (FAMEs) were separated by gas chromatography by using a BPX-70 capillary column (SGE, Austin, TX) and a split injection system (Agilent 7890A Gas Chromatograph; Agilent Technologies, Santa Clara, CA), with helium as the carrier gas. The injector temperature was 240°C, and the flame ionization detector 280°C. Data were analyzed by using ChemStation Firmware A.01.09 (Agilent). DHA was identified and quantified against authentic standards (NuCheck Prep, Elysian, MN). Data were expressed as pmol PtdCho-DHA/nmol PtdCho.
Determination of plasma PtdCho, AdoMet, and AdoHcy
PtdCho was extracted from plasma by the method of Bligh and Dyer (32). Aqueous and organic compounds were separated, analyzed, and quantified directly by using liquid chromatography/electrospray ionization-isotope dilution mass spectrometry after the addition of internal standards labeled with stable isotopes to correct for recovery (30).
AdoMet and AdoHcy were measured in a subset of plasma samples from subjects enrolled in 2001–2004 by using an HPLC method (34). Briefly, 500 μL plasma (in duplicate) was deproteinized with trichloroacetic acid, and AdoMet and AdoHcy were separated by HPLC with the use of a C8 column. Fractions were collected and derivatized by using naphthalenedialdehyde and cyanide. The resulting fluorescent isoindoles were separated by HPLC with the use of a C18 column and a fluorescence detector.
PEMT activity assay
Liver homogenates were assayed for PEMT activity by using a modified method of Ridgway and Vance (35). Briefly, activity was assessed by using 50 μg protein in 125 mmol Tris-HCl/L (pH 9.2) and 5 mmol DTT buffer/L (Sigma, St Louis, MO) in the presence of 200 μmol S-adenosyl-l-methionine/L containing 0.5 μCi S-adenosyl- l-[methyl-3H] methionine (55.70 Ci/mmol; GE Health Care, Piscataway, NJ) and 0.4 mmol exogenous phosphatidyldimethylethanolamine/L (P2;Avanti Polar Lipids, Alabaster, AL). The reaction was carried out for 30 min at 37°C and stopped by adding CHCl3:MeOH:1 N HCl (100:50:1, vol:vol). An aliquot of the chloroform phase was applied to a silica gel thin-layer chromatography plate [Si250-PA (19C)-Silica Gel, Baker Inc, Phillipsburg, NJ ] and developed in CHCl3:MeOH: acetic acid:H2O (50:30:5:2, vol:vol). Disintegrations per minute from [3H]-PtdCho were determined in bands that comigrated with authentic standards by using liquid scintillation spectrophotometry (Wallac 1410; Pharmacia LKB Nuclear, Gaithersburg, MD).
Genotyping
SNP analysis was performed as previously described (8, 13). Briefly, genomic DNA was extracted from liver tissues by using TriZol (Invitrogen, Carlsbad, CA) and from peripheral lymphocytes isolated from blood by using PureGene (Gentra Systems, Minneapolis, MN) according to the manufacturer's instructions. DNA sequencing was performed on double-stranded DNA templates obtained from genomic DNA by polymerase chain reaction (PCR) amplification. Sequencing reactions were performed by the University of North Carolina at Chapel Hill Genome Analysis Facility by using a capillary sequencing machine (model 3100; Applied Biosystems, Foster City, CA). Sequence results were interpreted by using the program Sequencher (Gene Code Corp, Ann Arbor, MI). Successful amplification of the 1896–base pair DNA fragment of the PEMT gene was performed by using Takara Ex Taq polymerase (Fisher Scientific, Fair Lawn, NJ) with forward and reverse primers: 5′GAGCACGTGAGCTGTCAGTGCCTTTTG3′ and 5′CCAACCTCCTTCATACAACAGAGGTCC3′, respectively. A 3-step PCR was performed on an Applied Biosystems 2720 Thermal Cycler under the following conditions: 96°C for 2 min, 30 cycles (94°C for 30 s, 60°C for 1 min, and 72°C for 2 min), extend 72°C for 7 min, and soak at 4°C. For the sequence determination of PEMT rs12325817, we used an additional primer, 5′TAGATTGGTCATGGGAGGCTT3′, to verify the sequence in a region containing Alu repeats and a poly-A tail. We also designed forward primers specific to the rs12325817 allele using GeneFisher (http://bibiserv.techfak.uni-bielefeld.de/genefisher) so that the SNP would be located at the 3′-end of the priming sequence, which allows for specific PCR products to be synthesized only if the primer is 100% complementary to its template DNA. We purchased them from Qiagen Operon (Huntsville, AL). The PCR reactions were optimized for each pair of primers, and the products were visualized on a 1.5% agarose gel to determine the genotype.
Statistical analysis
The effect of sex and menopausal status on the plasma PtdCho-DHA/PtdCho ratio was determined by mixed-model analysis of variance by using JMP software (V2; SAS Institute, Cary, NC), followed by pairwise comparisons of individuals with and without the PEMT rs12325817 genotype and by dietary treatment after grouping by sex and menopausal status. For the human liver data, statistical differences in the plasma PtdCho-DHA/PtdCho ratio and PEMT activity were determined by using Student's t test after grouping by genotype. The relation of plasma AdoMet and AdoHcy concentrations to the plasma PtdCho-DHA/PtdCho ratio was determined by linear regression and expressed as the Pearson correlation coefficient. Data are reported as means ± SEs, and all results were considered significant at P < 0.05.
RESULTS
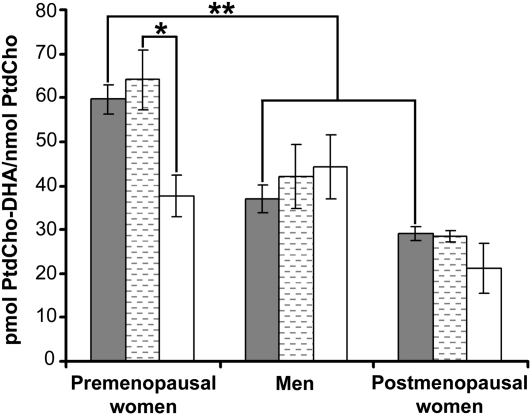
At baseline, plasma PtdCho-DHA/PtdCho ratios in plasma were greater in premenopausal women than in men or postmenopausal women (Figure 1; P < 0.01). This pattern was maintained when subjects were placed on a low-choline diet (data not shown). Because PtdCho concentrations were expected to decrease with choline deficiency, we expressed the data as a concentration ratio so that PtdCho-DHA could be represented by the actual amount of PtdCho present. However, we found that expressing the PtdCho-DHA data as nmol/mL plasma gave similar results (see Supplemental Figure 1 under “Supplemental data” in the online issue). The PEMT gene polymorphism rs12325817 was previously reported to greatly increase the likelihood of developing organ dysfunction when subjects with the variant allele consumed a low-choline diet (8), and we suggested that this indicated diminished capacity to form choline via PEMT activity. In the current study, premenopausal women homozygous for this SNP had a lower plasma PtdCho-DHA/PtdCho ratio in plasma than did premenopausal women without the SNP at baseline (Figure 1; P < 0.05 by t test) and after the low-choline diet (data not shown). The premenopausal women with one allele of the SNP had intermediate values (47 ± 4 pmol PtdCho-DHA/nmol PtdCho; n = 14), which were not statistically different from those with 2 alleles of the SNP. The plasma PtdCho-DHA/PtdCho ratio did not differ significantly between postmenopausal women and men by genotype, including in those with one allele of the SNP.
FIGURE 1.
Mean (±SE) ratios of phosphatidylcholine docosahexaenoic acid to phosphatidylcholine (PtdCho-DHA/PtdCho) concentrations in plasma were highest in premenopausal women with no alleles of the rs12325817 single nucleotide polymorphism (SNP) in the PEMT gene. Plasma was obtained from healthy women and men who consumed a standardized diet that delivered 550 mg choline/70 kg body weight daily for 10 d. At the end of this period, blood was collected and the portion of PtdCho species containing DHA (pmol PtdCho-DHA/nmol PtdCho) was measured in plasma by using gas chromatography as described in Subjects and Methods. DNA was extracted from peripheral lymphocytes and genotyped for the PEMT SNP rs12325817 as described in Subjects and Methods. Filled bars represent all subjects (n = 20–27 per group), hatched bars represent subjects with no alleles of the PEMT SNP (6–8 per group), and open bars represent subjects with 2 alleles of the PEMT SNP (3–5 per group). *,**ANOVA: *P < 0.05, **P < 0.01.
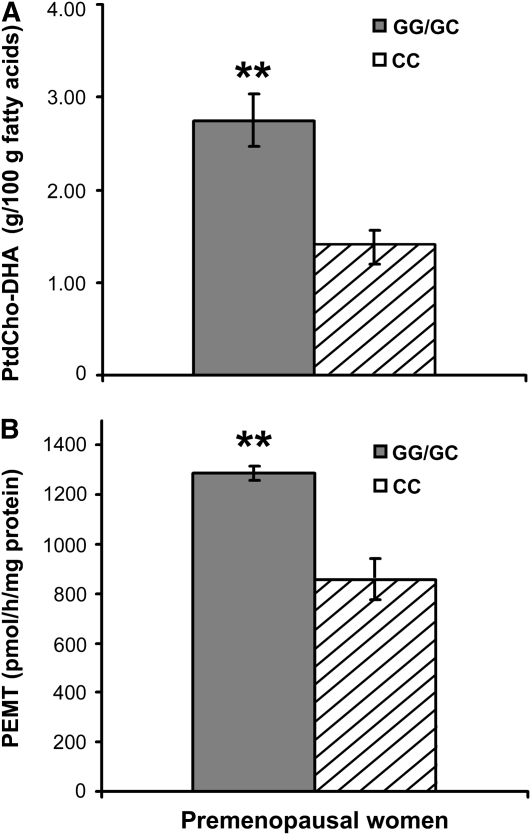
In human livers (n = 8) and plasma (n = 72), ≈3% of PtdCho was present as the DHA species (liver: 2.7 ± 0.2 g/100 g fatty acids; plasma: 3.0 ± 0.1 g/100 g fatty acids). Because cirrhotic livers have fewer hepatocytes and more fibroblasts, we excluded such livers (n = 2) from our analyses. It is interesting to note that the cirrhotic livers (both GC genotype) had a lower PtdCho-DHA content (1.6 ± 0.1 g/100 g fatty acids) than did the other GG/GC samples. Samples from premenopausal women homozygous for the variant allele (CC genotype) had significantly less PtdCho-DHA than did samples from women with the other genotypes (Figure 2A; P = 0.01). We measured PEMT activity in the human livers to determine whether there was a correlation with PtdCho-DHA concentrations. We found that women homozygous for the SNP had less PEMT activity than did the other women (Figure 2B; P = 0.008). There was an insufficient sample size to analyze the GG and GC genotype groups separately.
FIGURE 2.
Mean (±SE) phosphatidylcholine docosahexaenoic acid (PtdCho-DHA) concentrations and phosphatidylethanolamine N-methyltransferase (PEMT) activity in the liver were lower in premenopausal women who were homozygous for the rs12325817 single nucleotide polymorphism (SNP) in the PEMT gene. Snap-frozen human liver samples were obtained from the Liver Tissue Procurement and Distribution System (Minneapolis, MN). Genomic DNA was extracted and genotyped for PEMT rs12325817. A: Hepatic lipids were extracted, and the portion of PtdCho species containing DHA was measured by gas chromatography. B: Another aliquot of liver was homogenized, and PEMT activity was measured as described in Subjects and Methods. Filled bars represent subjects with 1 or 2 of the major G alleles, hatched bars represent subjects who were homozygous for the SNP. n = 3 per group. **Significantly different from women with the CC genotype, P ≤ 0.01 (t test).
We previously reported that some subjects, when placed on a low-choline diet, developed signs of organ dysfunction, whereas others did not (1). Plasma PtdCho concentration decreased in premenopausal women and men fed a low-choline diet (Table 1; P < 0.05 by paired t test). However, only the men who developed organ dysfunction (n = 14) had significantly lower plasma PtdCho concentrations (1615 ± 70 nmol/mL) than their baseline values (1950 ± 68 nmol/mL; P = 0.0003 by paired t test). The postmenopausal group did not differ regardless of diet or depletion status.
TABLE 1.
Plasma phosphatidylcholine concentrations in subjects after consumption of a low-choline diet1
| Group | Baseline diet | Low-choline diet |
| nmol/mL | nmol/mL | |
| Premenopausal women (n = 27) | 1742 ± 47 | 1629 ± 522 |
| Men (n = 20) | 1842 ± 63 | 1574 ± 582 |
| Postmenopausal women (n = 25) | 1997 ± 82 | 1906 ± 69 |
All values are means ± SEs. Healthy volunteers consumed a standardized diet that delivered 550 mg choline/70 kg body weight daily for 10 d (baseline), followed by a diet containing <50 mg choline/70 kg body weight daily (low choline) until they developed liver or muscle dysfunction (determined as described in Subjects and Methods) or for 42 d. Blood was collected at the end of each diet phase, and plasma phosphatidylcholine concentrations were measured by mass spectrometry as described in Subjects and Methods.
Significantly different from the baseline diet, P < 0.05 (paired t test).
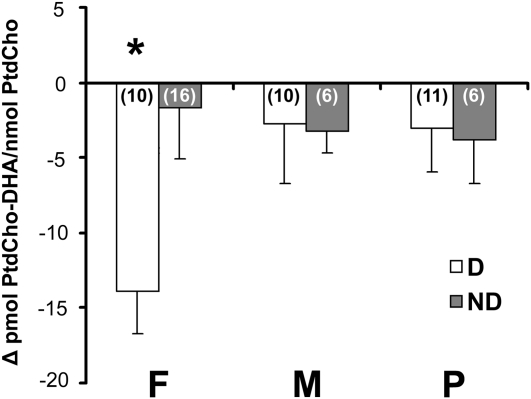
A low-choline diet lowered plasma PtdCho-DHA/PtdCho ratios in premenopausal women (Figure 3). Of these women, those who developed organ dysfunction with the low-choline diet had a 24% decrease in the plasma PtdCho-DHA/total PtdCho ratio from their basal concentration (−14 pmol DHA/nmol PtdCho) compared with 3% (−2 pmol DHA/nmol PtdCho) in those who did not develop organ dysfunction with the deficient diet (Figure 3; P = 0.023, t test). Men and postmenopausal women did not experience a change in the plasma PtdCho-DHA/total PtdCho ratio when they consumed the deficient diet.
FIGURE 3.
Mean (±SE) ratios of phosphatidylcholine docosahexaenoic acid to phosphatidylcholine (PtdCho-DHA/PtdCho) concentrations in plasma in healthy premenopausal women (F), men (M), and postmenopausal women (P) after consumption of a standardized diet that delivered 550 mg choline/70 kg body weight daily for 10 d and after consumption of a diet containing <50 mg choline/70 kg body weight daily for ≤42 d or until they developed liver or muscle dysfunction (D) as described in Subjects and Methods. Subjects who developed signs of organ dysfunction while consuming the low-choline diet had decreased plasma PtdCho-DHA/PtdCho ratios. Some subjects did not develop organ dysfunction within 42 d of consuming the low-choline diet (ND). Blood was collected at the end of each diet phase, and the portion of PtdCho species containing DHA was measured in plasma by using gas chromatography as described in Subjects and Methods. Data are expressed as the difference between the low-choline and baseline values. n in parentheses. *Significantly different from ND subjects with the same estrogen status, P < 0.05 (t test).
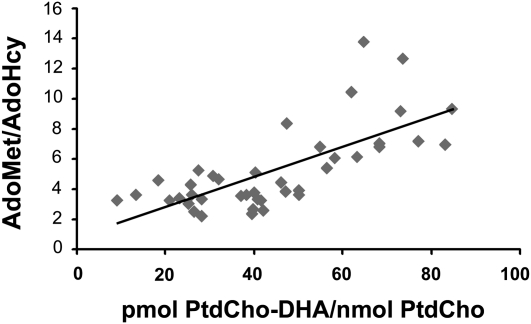
AdoMet is a substrate for PEMT, and AdoHcy (a product of PEMT) inhibits the enzyme. While consuming the low-choline diet, subjects with the lowest AdoMet/AdoHcy ratios also had the lowest plasma PtdCho-DHA/PtdCho as determined by linear regression (Figure 4; r = 0.716, P = 0.0001).
FIGURE 4.
Ratios of phosphatidylcholine docosahexaenoic acid to phosphatidylcholine (PtdCho-DHA/PtdCho) concentrations in plasma correlated with ratios of plasma S-adenosylmethionine (AdoMet) to S-adenosylhomocysteine (AdoHcy). Healthy volunteers consumed a standardized diet that delivered 550 mg choline/70 kg body weight daily for 10 d and then consumed a diet containing <50 mg choline/70 kg body weight daily for ≤42 d or until they developed liver or muscle dysfunction as described in Subjects and Methods. At the end of this period, blood was collected and the portion of PtdCho species containing DHA was measured in plasma by using gas chromatography as described in Subjects and Methods. Plasma AdoMet and AdoHcy concentrations were measured at the end of the low-choline phase of the study as described in Subjects and Methods. The correlation was assessed by using linear regression analysis (P = 0.0001, r = 0.716). n = 41.
DISCUSSION
We found that plasma PtdCho-DHA/PtdCho ratios in humans varied in a manner that was consistent with this measure being a good surrogate marker for in vivo PEMT activity in liver. PEMT activity is induced by estrogen (11, 12) in premenopausal women, and it is expected to be lower in men and postmenopausal women (who have low estrogen concentrations). We observed that premenopausal women had the highest plasma PtdCho-DHA/PtdCho ratios compared with men and postmenopausal women.
PEMT uses AdoMet as a methyl donor and is maximally active at ≈200 μmol AdoMet/L. PEMT is inhibited by AdoHcy (36, 37); thus, PEMT activity should be proportional to the AdoMet/AdoHcy ratio. We found that the AdoMet/AdoHcy ratio measured in plasma was correlated with PtdCho-DHA/PtdCho ratios in plasma.
The PEMT SNP rs12325817 marks a haplotype for which the variant allele is associated with decreased induction of gene expression by estrogen (12). Premenopausal women with one or more variant alleles for this SNP are more likely to develop organ dysfunction when they consumed a low-choline diet (8) because of their reduced capacity for endogenous synthesis of PtdCho via PEMT. These women also have diminished plasma PtdCho-DHA/PtdCho ratios compared with those without this SNP. In addition, livers from women homozygous for the variant allele had less PtdCho-DHA and lower PEMT activity than did women with the major allele. We also observed that cirrhotic livers had less PtdCho-DHA—a finding we had previously noted between normal and cirrhotic livers from men (SH Zeisel and K-A da Costa, unpublished observations, 2006) and which is consistent with the published report that plasma long-chain fatty acids are lower in nonalcoholic steatohepatitis patients than in controls (38).
Whereas the plasma PtdCho-DHA/PtdCho ratio at baseline correlated well with the differences in PEMT activity expected from estrogen status and genetic polymorphisms, this ratio did not increase with consumption of the low-choline diet. Indeed, we report that in premenopausal women, a low-choline diet was associated with a decrease in the plasma PtdCho-DHA/PtdCho ratio in subjects who developed organ dysfunction. This was surprising because rats fed a choline-methionine deficient diet for 3 wk had increased production of PEMT RNA, increased PEMT protein, and increased PEMT activity (measured in liver homogenates by using an in vitro assay in which AdoMet was added to saturating concentrations; 19). However, another study (18), which administered [1,2-14C]ethanolamine to rats, determined that in vivo PEMT activity decreased in animals fed a diet deficient in choline and methionine. This decrease in activity was attributed to the limited availability of the methyl group donor, AdoMet, coupled with an elevation in AdoHcy concentrations that occurs during choline deficiency (39). We suggest that the study using an in vitro assay for PEMT activity gave a different result because it did not accurately recreate the substrate/inhibitor concentration balance that was present in vivo. It is likely that, despite an increase in PEMT protein, PEMT-catalyzed formation of PtdCho decreases during choline deficiency. It makes sense that this effect of a low-choline diet is most apparent in women with estrogen, because men and postmenopausal women have little estrogen-induced PEMT activity that can be lost when substrates become limiting.
When evaluating plasma PtdCho-DHA/PtdCho ratios as a surrogate marker for PEMT activity, one factor that should be considered is the subject's diet. The fatty acids in plasma phospholipids are responsive to changes in dietary fatty acids, particularly n−3 fatty acids (40), and specifically, supplementation of the diet with DHA increased serum phospholipid DHA (41). Diet was not a factor in our comparisons because all of the individuals in our study were maintained on the same defined diets. However, fat intake varies widely in free-living individuals, and this may alter PtdCho-DHA concentrations independently of PEMT activity. Therefore, it is important to consider dietary intake of DHA in studies in which plasma PtdCho-DHA/PtdCho ratios are being used as a surrogate for PEMT activity. Additional studies using DHA supplementation would be helpful in determining the extent to which dietary intake of DHA affects plasma PtdCho-DHA/PtdCho ratios and its usefulness as a marker for hepatic PEMT activity.
Currently, there is no accurate method to measure in vivo PEMT activity in humans. Methods using radiolabeled ethanolamine are inappropriate for use in humans. Liver biopsy, an invasive procedure, could yield liver tissue with which to determine PEMT activity; however, measurement outside the body would require the addition of exogenous substrates (such as AdoMet). If these added substrates are not reflective of the in vivo substrate concentrations, this approach would yield an inaccurate measurement of PEMT activity. Thus, a surrogate marker for PEMT activity in vivo, such as plasma PtdCho-DHA/PtdCho ratios, would be of help in identifying humans with functionally important SNPs in the PEMT gene. The rs12325817 SNP examined in this study, which is located in the promoter region of the gene, is very common—≈70% of our subjects had one or more variant alleles for this polymorphism (8). There may be many more functionally important SNPs in PEMT because the gene is highly polymorphic; 98 SNPs were found in a group of only 48 Japanese subjects (42). Identifying other functionally important SNPs in PEMT by measuring plasma PtdCho-DHA/total PtdCho ratios could help us to refine our understanding of functional SNPs, which may influence dietary choline requirements in humans.
Supplementary Material
Acknowledgments
We thank Elena Pop and Audrey McLean-Pottinger for their help with the PtdCho-DHA analyses; Jiannan Song for the PEMT assays; Conrad Wagner for performing the AdoMet and AdoHcy assays; Olga Kozyreva and Hye Mee Hwang for their assistance with genotyping; Marjorie Busby, Beth MacIntosh, and the nutrition research staff at the University of North Carolina at Chapel Hill Clinical and Translational Research Center for designing and preparing the research diets used in this study; and the Solae Company, which provided the lecithin used to formulate the diets.
The authors' responsibilities were as follows—KAdC: was responsible for sample collection and processing, supervised the genotyping and PtdCho-DHA and choline analyses, and participated in the data and statistical analyses and writing of the manuscript; LMS: conducted the biochemical and data analyses and assisted with the writing of the manuscript; LMF: participated in the supervision of the human study; and SHZ: participated in the statistical analyses and data interpretation, provided major input in the writing of the manuscript, and was responsible for the conceptualization, implementation, and design of the human study. None of the authors had a financial conflict of interest in relation to this study. SHZ received grant support from Mead Johnson Nutritionals and the Egg Nutrition Research Center for studies other than those described in this article and serves on an advisory board for Solae.
REFERENCES
- 1.Fischer LM, daCosta K, Kwock L, et al. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr 2007;85:1275–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Costa KA, Badea M, Fischer LM, Zeisel SH. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr 2004;80:163–70 [DOI] [PubMed] [Google Scholar]
- 3.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 2003;133:1302–7 [DOI] [PubMed] [Google Scholar]
- 4.Zeisel SH, Mar M-H, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 2003;133:2918–9 [DOI] [PubMed] [Google Scholar]
- 5.Fischer LM, Scearce JA, Mar MH, et al. Ad libitum choline intake in healthy individuals meets or exceeds the proposed adequate intake level. J Nutr 2005;135:826–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 2006;26:229–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vance DE, Walkey CJ, Cui Z. Phosphatidylethanolamine N-methyltransferase from liver. Biochim Biophys Acta 1997;1348:142–50 [DOI] [PubMed] [Google Scholar]
- 8.da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J 2006;20:1336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young DL. Estradiol- and testosterone-induced alterations in phosphatidylcholine and triglyceride synthesis in hepatic endoplasmic reticulum. J Lipid Res 1971;12:590–5 [PubMed] [Google Scholar]
- 10.Vigo C, Vance DE. Effect of diethylstilboestrol on phosphatidylcholine biosynthesis in the liver of roosters. Biochem Soc Trans 1981;9:98–9 [DOI] [PubMed] [Google Scholar]
- 11.Resseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J 2007;21:2622–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resseguie ME, da Costa KA, Galanko JA, Patel M, Davis IJ, Zeisel SH. Aberrant estrogen regulation of PEMT results in choline deficiency-associated liver dysfunction. J Biol Chem 2011;286:1649–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer LM, da Costa KA, Kwock L, Galanko J, Zeisel SH. Dietary choline requirements of women: effects of estrogen and genetic variation. Am J Clin Nutr 2010;92(5):1113–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waite KA, Cabilio NR, Vance DE. Choline deficiency-induced liver damage is reversible in Pemt(−/−) mice. J Nutr 2002;132:68–71 [DOI] [PubMed] [Google Scholar]
- 15.Furtado VC, Takiya CM, Braulio VB. Phosphatidylethanolamine N-methyltransferase activity is increased in rat intestinal brush-border membrane by chronic ethanol ingestion. Alcohol Alcohol 2002;37:561–5 [DOI] [PubMed] [Google Scholar]
- 16.Nishimaki-Mogami T, Suzuki K, Okochi E, Takahashi A. Bezafibrate and clofibric acid are novel inhibitors of phosphatidylcholine synthesis via the methylation of phosphatidylethanolamine. Biochim Biophys Acta 1996;1304:11–20 [DOI] [PubMed] [Google Scholar]
- 17.Nishimaki-Mogami T, Suzuki T, Takahashi A. The role of phosphatidylethanolamine methylation in the secretion of very low density lipoproteins by cultured rat hepatocytes: rapid inhibition of phosphatidylethanolamine methylation by bezafibrate increases the density of apolipoprotein B48-containing lipoproteins. Biochim Biophys Acta 1996;1304:21–31 [DOI] [PubMed] [Google Scholar]
- 18.Hoffman DR, Haning JA, Cornatzer WE. Effects of a methyl-deficient diet on rat liver phosphatidylcholine biosynthesis. Can J Biochem 1981;59:543–50 [DOI] [PubMed] [Google Scholar]
- 19.Cui Z, Vance DE. Expression of phosphatidylethanolamine N-methyltransferase-2 is markedly enhanced in long term choline-deficient rats. J Biol Chem 1996;271:2839–43 [DOI] [PubMed] [Google Scholar]
- 20.Song J, da Costa KA, Fischer LM, et al. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J 2005;19:1266–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noga AA, Vance DE. A gender-specific role for phosphatidylethanolamine N-methyltransferase-derived phosphatidylcholine in the regulation of plasma high density and very low density lipoproteins in mice. J Biol Chem 2003;278:21851–9 [DOI] [PubMed] [Google Scholar]
- 22.Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J Biol Chem 2002;277:42358–65 [DOI] [PubMed] [Google Scholar]
- 23.Vance JE. Secretion of VLDL, but not HDL, by rat hepatocytes is inhibited by the ethanolamine analogue N-monomethylethanolamine. J Lipid Res 1991;32:1971–82 [PubMed] [Google Scholar]
- 24.Lieber CS. Alcoholic liver injury: pathogenesis and therapy in 2001. Pathol Biol (Paris) 2001;49:738–52 [DOI] [PubMed] [Google Scholar]
- 25.DeLong CJ, Shen YJ, Thomas MJ, Cui Z. Molecular distinction of phosphatidylcholine synthesis between the CDP- choline pathway and phosphatidylethanolamine methylation pathway. J Biol Chem 1999;274:29683–8 [DOI] [PubMed] [Google Scholar]
- 26.Watkins SM, Zhu X, Zeisel SH. Phosphatidylethanolamine-N-methyltransferase activity and dietary choline regulate liver-plasma lipid flux and essential fatty acid metabolism in mice. J Nutr 2003;133:3386–91 [DOI] [PubMed] [Google Scholar]
- 27.Samborski RW, Ridgway ND, Vance DE. Metabolism of molecular species of phosphatidylethanolamine and phosphatidylcholine in rat hepatocytes during prolonged inhibition of phosphatidylethanolamine N-methyltransferase. J Lipid Res 1993;34:125–37 [PubMed] [Google Scholar]
- 28.Busby MG, Fischer L, Da Costa KA, Thompson D, Mar MH, Zeisel SH. Choline- and betaine-defined diets for use in clinical research and for the management of trimethylaminuria. J Am Diet Assoc 2004;104:1836–45 [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine, National Academy of Sciences Choline. Dietary Reference Intakes for folate, thiamin, riboflavin, niacin, vitamin B12, pantothenic acid, biotin, and choline. Washington DC: National Academy Press, 1998:390–422 [PubMed] [Google Scholar]
- 30.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem 2002;74:4734–40 [DOI] [PubMed] [Google Scholar]
- 31.Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci USA 2005;102:16025–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–7 [DOI] [PubMed] [Google Scholar]
- 33.Tacconi M, Wurtman RJ. Rat brain phosphatidyl-N,N-dimethylethanolamine is rich in polyunsaturated fatty acids. J Neurochem 1985;45:805–9 [DOI] [PubMed] [Google Scholar]
- 34.Capdevila A, Wagner C. Measurement of plasma S-adenosylmethionine and S-adenosylhomocysteine as their fluorescent isoindoles. Anal Biochem 1998;264:180–4 [DOI] [PubMed] [Google Scholar]
- 35.Ridgway ND, Vance DE. Kinetic mechanism of phosphatidylethanolamine N-methyltransferase. J Biol Chem 1988;263:16864–71 [PubMed] [Google Scholar]
- 36.Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL. Is it time to reevaluate methyl balance in humans?. Am J Clin Nutr 2006;83:5–10 [DOI] [PubMed] [Google Scholar]
- 37.Shields DJ, Altarejos JY, Wang X, Agellon LB, Vance DE. Molecular dissection of the S-adenosylmethionine-binding site of phosphatidylethanolamine N-methyltransferase. J Biol Chem 2003;278:35826–36 [DOI] [PubMed] [Google Scholar]
- 38.Kalhan SC, Guo L, Edmison J, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2010;60:404–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeisel SH, Zola T, daCosta K, Pomfret EA. Effect of choline deficiency on S-adenosylmethionine and methionine concentrations in rat liver. Biochem J 1989;259:725–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spector AA. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J Mol Neurosci 2001;16:159–65, discussion 215–21 [DOI] [PubMed] [Google Scholar]
- 41.Grimsgaard S, Bonaa KH, Hansen JB, Nordoy A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am J Clin Nutr 1997;66:649–59 [DOI] [PubMed] [Google Scholar]
- 42.Saito S, Iida A, Sekine A, et al. Identification of 197 genetic variations in six human methyltransferase genes in the Japanese population. J Hum Genet 2001;46:529–37 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.