Abstract
Background: By using the response to protein hydrolysate formula (PHF) as a model system, we discovered the existence of a sensitive period, before 4 mo, when exposure determines the hedonic tone to flavors.
Objective: We aimed to characterize the timing and duration of this sensitive period.
Design: Healthy infants, whose parents had chosen formula feeding, were randomly assigned into 1 of 6 groups at age 0.5 mo: 2 control groups, one fed cow milk–based formula (CMF) and the other fed PHF for 7 mo; 2 groups fed PHF for either 1 or 3 mo beginning at 1.5 mo and CMF otherwise; and 2 groups fed PHF for 1 mo beginning at either 2.5 or 3.5 mo and CMF otherwise. Brief access taste tests were conducted monthly, and complete “meals” of both formulas occurred at the end of the study.
Results: Three months of PHF exposure led to acceptance similar to that at 1 mo of exposure. Although these infants were more accepting than were infants with no exposure, they were less accepting than were infants with 7 mo of exposure, which suggests a dosing effect. The time when flavor experiences began was also significant. Among infants exposed to PHF for 1 mo, those who were first fed PHF at 3.5 mo rejected PHF relative to CMF more than did infants exposed at younger ages.
Conclusion: The general principles observed are likely of broader significance, indicating a fundamental feature of mammalian development and reflecting the importance of familiarizing infants with flavors that their mothers consume and transmit to breast milk. This trial was registered at clinicaltrials.gov as NCT00994747.
See corresponding editorial on page 909
INTRODUCTION
Early nutrition has long-lasting effects on health, programming risks for later obesity, and many diseases (1). Whereas research has focused on the effects of the nutrient quality of the diet or on the long-term effects of early growth, relatively little attention has been paid to another important feature of nutrition: how humans learn to like the flavor of foods. To this end, randomized clinical trials were conducted in which women ate or avoided certain foods at specified times during pregnancy or lactation (2). As in other mammals (3), flavors in amniotic fluid and mother's milk reflected foods eaten by the mother during pregnancy and lactation, respectively, and these early flavor experiences contributed to individual differences in the liking of foods during childhood (2, 4). In general, preferences for flavors are highly influenced by experiences and those occurring early in life are particularly salient (5).
The absence of a robust experimental paradigm, like that used for other sensory systems and other animals, has inhibited progress in understanding whether there are age-related changes in functional plasticity—commonly referred to as sensitive periods—for human flavor programming. To address this gap in knowledge, we have investigated a model system that exploits the naturally occurring flavor variation in infant formulas (6). To adults, extensively hydrolyzed protein hydrolysate formulas (PHFs) are extremely unpalatable compared with cow milk–based formulas (CMFs) because of the distinctive unpleasant flavors of PHFs, including both volatile (odors) and nonvolatile (bitter and sour tastes) components (7). In previous investigations, we identified a “window” of acceptance when young infants readily accept PHF (8). Then, beginning at ≈4 mo of age and continuing through adulthood, its flavor is rejected unless the individual has been exposed to PHF during early life (8, 9). That is, PHF acquires a completely different hedonic tone depending on whether the individual was exposed to this formula during the first few months of life (6). Effects of early exposure on taste and food preferences were particularly persistent and lasted several years (10, 11).
To characterize the sensitive period, we conducted a randomized clinical trial and varied the age at which PHF exposure began and the length of exposure. We evaluated acceptance of this formula when infants were 7.5 mo old and found that infants exposed to PHF for 3 mo, either from 0.5 to 3.5 mo or from 2.5 to 5.5 mo of life, did not differ in PHF acceptance, but both of these groups of infants were more accepting than were infants never exposed to PHF and less accepting than infants exposed for 7 mo (6). The most parsimonious explanation is that acceptance is a function of the absolute amount of exposure. However, this does not account for the finding that most infants aged ≥4 mo strongly reject PHF (9). This suggests that, in addition to quantity of exposure, timing is important. Thus, the present study was designed to determine both the effects of timing and duration of early-life exposure on acceptance of PHF relative to that of CMF.
SUBJECTS AND METHODS
Study design
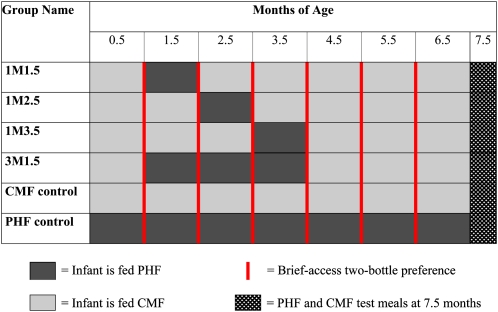
We recruited recently parturient women who had chosen to exclusively formula feed their healthy, full-term newborns CMF (or to predominantly formula feed but breastfeed once or twice a day during the first months of life). When infants were 0.5 mo old, we randomly assigned the mother-infant pairs (43% African American, 17% white, 22% Hispanic, and 17% other/mixed race) into 1 of 6 groups based on minimization to ensure balance among the groups in terms of race-ethnicity and sex. The groups differed in the timing, duration, and type of formula fed during the 7-mo study to test 2 major hypotheses (Figure 1). To test the first hypothesis that acceptance at 7.5 mo is a function of exposure duration, we compared infants exposed to PHF for only 1 mo with infants exposed for 3 mo. To test the second hypothesis that early exposure is more potent and persistent than later exposure, we held the duration of exposure constant at 1 mo but altered its timing. For both hypotheses, treatment groups were compared with control groups of infants with either no PHF exposure or 7 mo of PHF exposure.
FIGURE 1.
Description of the control and treatment groups. The names of the groups refer to the duration [1 or 3 mo (1M or 3M)] and age (1.5, 2.5, or 3.5 mo) at which the infants were fed protein hydrolysate formula (PHF; dark gray boxes) or cow milk–based formula (CMF; light gray boxes). The months refer to the age of the infants at the beginning of each monthly cycle and then the age of the infants when the test meals were conducted. A brief-access, 2-bottle taste test (solid red vertical lines) was conducted at the end of each monthly cycle, and PHF and CMF test meals (dark trellis bars) were conducted at the end of the study (ie, 7.5 mo).
Infants randomly assigned to the control groups were fed either CMF (Enfamil; Mead Johnson Nutritionals; Evansville, IN) or PHF (Nutramigen; Mead Johnson Nutritionals) during the entire 7 mo of the study. The 4 other groups were assigned to be fed PHF for a specified duration (1 or 3 mo) and/or beginning at a specified time (1.5, 2.5, or 3.5 mo of life; Figure 1). We did not include a group whose exposure to PHF began at 4.5 mo because infants at this age and older strongly reject PHF, their mothers are reluctant to continue to attempt to feed it to them, and they consequently withdraw from the study (6). On the basis of our previous study in which the response within each group was normally distributed (6), we determined that a target sample size of 10 to 12 per group would provide 95% power to detect a change at the 5% level. Mothers were not aware of the hypotheses or which of the formulas they were provided to feed their infants. The Office of Regulatory Affairs at the University of Pennsylvania approved the study, and informed consent was obtained from each mother before inclusion.
Monthly procedures: brief access, 2-bottle taste tests
At the start of the study and at the beginning of each 1-mo cycle, mothers came to the Monell Center, where infants were weighed and measured and mothers were queried about feeding practices. At each monthly visit, we conducted a brief-access, 2-bottle taste test that consisted of four 2-min trials in an ABAB design. One of the bottles (A) contained the formula that the infant was fed during the past month, whereas the other bottle (B) contained the formula to which the infant was assigned to consume in the subsequent month. In addition to determining how much formula the infants consumed, we had the mothers rate their infants' enjoyment of each bottle on a scale from 1 (extreme dislike) to 9 (extreme like). These monthly evaluations were performed to document the infants' initial acceptance of the assigned formula, to chart how acceptance changed with exposure, and to obtain accurate information on the introduction of solid foods. The next month's supply of formula was then distributed.
Procedures at the end of study: test meals
Testing was conducted under naturalistic conditions in which infants determined the pacing and duration of feeding, and a variety of validated methods were used to evaluate the infants' hedonic responses independently of caregivers and experimenters (6, 9, 12). At the same time of day, we documented the acceptance of the formulas in a complete meal by videotaping the feeding of PHF on one day and CMF on another day. Mothers continued the feeding until their infants refused the bottle 3 consecutive times. During feeding, mothers refrained from talking or making faces to eliminate any potential influence on their infants' behaviors (13). Immediately after each meal, mothers rated how much they thought their infants liked the formula on the 9-point scale.
The first 2 min of videotape for each test meal was subjected to frame-by-frame analyses by trained raters unaware of the experimental conditions; the videorecorder for 1 infant malfunctioned during testing. Raters determined the number of times the infants rejected the bottle (eg, turned head away) and the frequency of a variety of facial expressions of distaste (6, 12, 14). Two observers individually scored the videotapes of 30 test meals selected at random; reliability was 92% (P < 0.0001).
Statistical analyses
For the monthly brief-access taste tests, the dependent measures were formula intake and maternal ratings of the infants' enjoyment. Our analyses compared the groups (1M1.5, 1M2.5, and 1M3.5) who transitioned from CMF to PHF at 1.5, 2.5, or 3.5 mo, respectively, to determine whether there were age-related differences in formula acceptance. To this end, we conducted separate repeated-measures analysis of variance (ANOVAs) with formula (CMF and PHF) and time (beginning and end of month) as the within-subject factors and group as the between-subjects factor. Data could not be obtained for one infant in the 1M3.5 group.
For the 7.5-mo test meals, the dependent measures were total intake, frequency of distaste/rejection behaviors, and maternal ratings. For each measure, we calculated a proportional score by dividing the infant's response to the PHF by his or her response to the PHF plus CMF [PHF/(PHF+CMF)], which eliminated absolute differences in responses that may have been due to individual differences (eg, infant size). These measures are hereafter referred to as relative intake, relative maternal ratings, and relative rejection/distaste behaviors. An arcsine transformation was conducted to stabilize the variance of the proportional data before parametric analyses (15). To test the hypothesis that the duration of PHF exposure affected PHF acceptance at 7.5 mo (hypothesis 1), we conducted for each measure separate ANOVAs with a priori contrasts specified to determine whether the 1M1.5 group differed from the 3M1.5 group and whether these groups differed from the control groups. To test the hypothesis that the timing of exposure to PHF affected PHF acceptance at 7.5 mo (hypothesis 2), we conducted another set of ANOVAs with a priori contrasts to determine whether the 1-mo exposure groups (1M1.5, 1M2.5, and 1M3.5) differed from the no-exposure control group (CMF control). The analyses were conducted with procedures in STATISTICA (version 8; StatSoft, Tulsa, OK), and the criterion for statistical significance was P ≤ 0.05. When multiple comparisons were made, a Bonferroni correction of the P value was used. Values that were not significant after the Bonferroni correction are noted in the text and figure legends as trends. All summary statistics are expressed as means ± SEMs.
RESULTS
Subject characteristics
Between late 2006 and 2009, 79 mother-child dyads were enrolled but 10 withdrew because they were unable to complete the study. As shown in Table 1, we found no significant differences between the groups in ages of the mothers and infants, the sex ratio of the infants, infant weights and lengths at the start of the study, age at which the infants were introduced to solid foods, or the parity, income, and education levels of the mothers.
TABLE 1.
Subject and demographic characteristics1
| Group name |
|||||||
| 1M1.5 (n = 11) | 1M2.5 (n = 11) | 1M3.5 (n = 11) | 3M1.5 (n = 11) | CMF control (n = 13) | PHF control (n = 12) | P2 | |
| Characteristics of infants | |||||||
| Sex (% female) | 45.5 | 45.5 | 45.5 | 45.5 | 46.2 | 41.7 | 1.00 |
| Ethnicity (%) | 0.91 | ||||||
| African American | 36.4 | 54.5 | 45.4 | 45.4 | 38.5 | 41.7 | |
| White | 9.1 | 18.2 | 18.2 | 18.2 | 15.4 | 25.0 | |
| Hispanic | 18.2 | 27.3 | 18.2 | 27.3 | 30.8 | 8.3 | |
| Other/more than one | 36.4 | 0.0 | 18.2 | 9.1 | 15.4 | 25.0 | |
| Birth weight (kg) | 3.1 ± 0.13 | 3.4 ± 0.1 | 3.4 ± 0.1 | 3.3 ± 0.1 | 3.4 ± 0.1 | 3.4 ± 0.1 | 0.65 |
| Birth length (cm) | 50.8 ± 0.8 | 50.2 ± 0.8 | 50.2 ± 0.8 | 51.0 ± 0.8 | 51.6 ± 0.7 | 50.7 ± 0.8 | 0.79 |
| Age at introduction (mo)4 | |||||||
| Cereal | 3.7 ± 0.5 | 3.4 ± 0.5 | 3.5 ± 0.5 | 4.9 ± 0.5 | 4.2 ± 0.5 | 4.4 ± 0.5 | 0.32 |
| Fruit | 5.2 ± 0.4 | 4.8 ± 0.4 | 5.4 ± 0.4 | 5.0 ± 0.4 | 5.4 ± 0.4 | 4.7 ± 0.4 | 0.75 |
| Vegetables | 5.2 ± 0.4 | 5.7 ± 0.4 | 5.9 ± 0.4 | 5.8 ± 0.4 | 6.0 ± 0.4 | 5.4 ± 0.4 | 0.77 |
| Characteristics of mothers | |||||||
| Age (y) | 25.2 ± 2.0 | 28.0 ± 2.0 | 22.8 ± 2.0 | 26.8 ± 2.0 | 26.4 ± 1.8 | 27.0 ± 1.9 | 0.51 |
| Parity (% multiparous) | 45.5 | 54.6 | 54.6 | 45.5 | 61.5 | 16.7 | 0.45 |
| Education (% college) | 36.4 | 63.6 | 45.5 | 27.3 | 23.1 | 50.0 | 0.36 |
The names of the groups refer to the duration [1 or 3 mo (1M or 3M)] and age (1.5, 2.5, or 3.5 mo) at which the infants were fed protein hydrolysate formula (PHF) or refer to the control groups who were fed cow milk–based formula (CMF) or PHF for 7 mo.
P values obtained from Pearson chi-square analyses or one-factor ANOVAs with group as the between-subjects factor.
Mean ± SEM (all such values).
Those infants who had not been exposed to cereal (n = 6), fruit (n = 3), or vegetables (n = 8) by the 7.5-mo visit were excluded from these analyses.
Acceptance at monthly visits: brief-access, 2-bottle taste tests
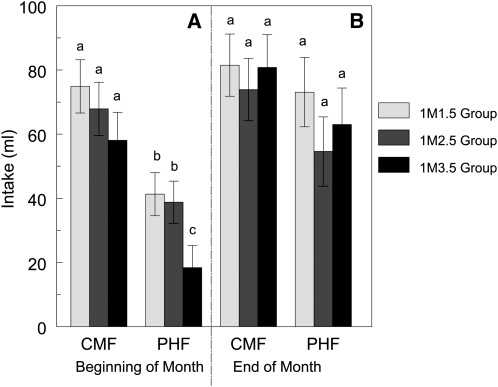
We found no significant effect of group on acceptance of the formula that infants were fed during the previous month throughout the 7-mo study, which suggests compliance with the study procedures. To determine whether there were age-related differences in initial PHF acceptance, we focused on the three 1-mo exposure groups. Specifically, our analyses focused on the infants' first brief access test that included PHF (this occurred at the beginning of month 1.5 for the 1M1.5 group, month 2.5 for the 1M2.5 group, and month 3.5 for the 1M3.5 group) and the brief access test that occurred at the end of the month of PHF exposure. As shown in Figure 2A, infants consumed significantly less PHF than CMF (the formula assigned to all of these infants during the previous month) at the beginning of the first month of exposure (P < 0.0001). Those infants who first experienced PHF at 3.5 mo (1M3.5 group) consumed significantly less PHF during their initial taste test than did infants in the 1M1.5 and 1M2.5 groups (Figure 2A), and there was a trend for the 1M3.5 group mothers to rate that their infants enjoyed the PHF less than the other 2 groups (P = 0.06).
FIGURE 2.
Mean (±SEM) amount of cow milk–based formula (CMF) and protein hydrolysate formula (PHF) consumed during the first brief-access, 2-bottle acceptance taste test in which infants were exposed to PHF at the beginning of the month (A) and then again after the first month of exposure (end of the month; B). The names of the groups refer to the duration [1 mo (1M)] and age (1.5, 2.5, or 3.5 mo) at which the infants were fed PHF: 1M1.5 (n = 11), 1M2.5 (n = 11), and 1M3.5 (n = 10). Although all 3 groups consumed significantly less PHF than CMF on their first day of exposure to PHF (P < 0.0001), the 1M3.5 group consumed significantly less PHF than did the other 2 groups (P < 0.05). By the end of the month, PHF acceptance had increased significantly (P < 0.0001) and was nearly as great as CMF acceptance (B). Although the end-of-month test indicated a significant main effect of formula (P = 0.04), none of the post hoc tests indicated significant differences in CMF and PHF intakes for any of the groups. Bars with different superscript letters are significantly different, P < 0.05.
When compared with PHF intake at the beginning of the month, PHF intake significantly increased at the end of 1 mo of exposure for each of the 3 groups (P < 0.0001) and was nearly as great as CMF acceptance (Figure 2B). Although there was a significant main effect of formula for brief-access test data collected at the end of the month (P = 0.04), none of the post hoc tests indicated significant differences between CMF and PHF intakes for any of the groups. Further analyses indicated that the relative increase in PHF acceptance from the beginning to the end of the month was similar for the 3 groups.
Acceptance at 7.5 mo: entire formula meals
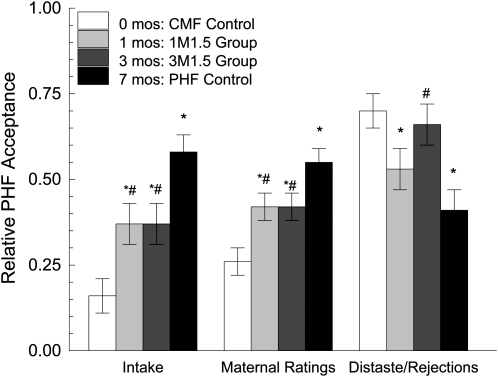
The study was designed to test 2 hypotheses related to the duration and timing of the sensitive period. The first hypothesis focused on the 1M1.5, 3M1.5, and control groups to determine whether 1 mo differed from 0, 3, and 7 mo of exposure in relative PHF acceptance. As can be seen in Figure 3, there were significant differences based on the duration of exposure in relative intake (P < 0.001), maternal perceptions (P < 0.001), and rejection/distaste behaviors (P = 0.002). The relative PHF intake by infants in the 1M1.5 group was similar to that of the 3M1.5 group (Figure 3); PHF acceptance of these 2 groups was greater than that of the no-exposure CMF control group but less than that of the full-exposure PHF control group. In general, patterns of maternal perceptions and frequency of distaste/rejection behaviors were consistent with the differences in intake that were based on duration of exposure (Figure 3).
FIGURE 3.
Mean (±SEM) relative responses to protein hydrolysate formula (PHF/[PHF+cow milk–based formula (CMF)]) at the 7.5-mo test meals, based on the duration of exposure to PHF. Separate ANOVAs were conducted with a priori contrasts specified to determine whether 1 mo (1M1.5 group; n = 11), 3 mo (1M3.5 group; n = 11), and 7 mo (PHF Control; n = 12) of exposure differed from one another and whether these groups differed from the no-exposure control group (CMF Control; n = 13). Significant differences in relative intake (P < 0.001), maternal perceptions (P < 0.001), and rejection or distaste behaviors (P = 0.002) were observed. *Significantly different from the CMF Control, P < 0.0167. #Significantly different from the PHF Control, P < 0.0167. There were no significant differences between the 1M1.5 and 1M3.5 groups for any measure.
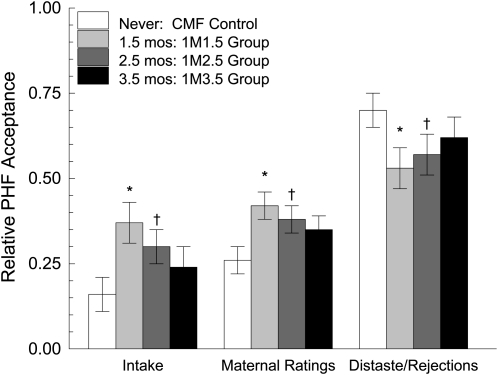
The analyses related to the second hypothesis focused on the 1-mo exposure groups to determine the effects of timing and whether these groups differed from each other and the control group. We found significant differences based on the timing of exposure in relative intake (P = 0.02), maternal perceptions (P = 0.01), and rejection/distaste behaviors (P = 0.04). As can be seen in Figure 4, infants who started feeding PHF before 3.5 mo consumed relatively more PHF than did the CMF control group. However, if feeding the PHF began when infants were 3.5 mo, they were no different from the control group. In general, patterns of maternal perceptions and the frequency of distaste/rejection behaviors were consistent with this timing-related difference in intake (Figure 4). The profound experience-related differences in facial reactivity while feeding PHF are illustrated in Figure 5. All of these findings were unchanged when we statistically controlled for the number of daily breastfeeds some infants received during the early months of the study. The data for each of the measures obtained at the 7.5-mo test meals are shown in Table 2. Statistical analyses conducted for each of the 2 hypotheses tested can be found above as well as in the figure legends.
FIGURE 4.
Mean (±SEM) relative responses to protein hydrolysate formula (PHF/[PHF+cow milk–based formula (CMF)]) at the 7.5-mo test meals, based on the timing of exposure to PHF. Separate ANOVAs were conducted with a priori contrasts to determine whether the 1-mo exposure groups [1M1.5 (n = 11), 1M2.5 (n = 11), and 1M3.5 (n = 11)] differed from the no-exposure control group (CMF control; n = 13). The names of the groups refer to the duration [1 mo (1M)] and age (1.5, 2.5, or 3.5 mo) at which the infants were fed PHF or to the control group who was fed CMF. Significant differences in relative intake (P = 0.02), maternal perceptions (P = 0.01), and rejection/distaste behaviors (P = 0.04) were observed. *Significantly different from the CMF Control, P < 0.0167. †Trend in differences from the CMF Control.
FIGURE 5.
Infants' typical facial responses while consuming protein hydrolysate formula (PHF) when tested at 7.5 mo of age. A: Infant from the cow milk–based formula (CMF) control group (fed CMF for 7 mo). B: Infant from the PHF control group (fed PHF for 7 mo).
TABLE 2.
Acceptance of cow milk–based formula (CMF) and protein hydrolysate formula (PHF) during the 7.5-mo test meals1
| Group name |
||||||
| 1M1.5 (n = 11) | 1M2.5 (n = 11) | 1M3.5 (n = 11) | 3M1.5 (n = 11) | CMF control (n = 13) | PHF control (n = 12) | |
| Intake (mL) | ||||||
| CMF | 156.2 ± 28.32 | 171.5 ± 28.3 | 163.1 ± 28.3 | 211.5 ± 28.3 | 180.6 ± 26.0 | 113.4 ± 27.1 |
| PHF | 93.5 ± 27.2 | 110.3 ± 27.2 | 74.7 ± 27.2 | 130.3 ± 27.2 | 36.1 ± 25.0 | 159.9 ± 26.0 |
| Relative response3 | 0.37 ± 0.06 | 0.30 ± 0.06 | 0.24 ± 0.06 | 0.37 ± 0.06 | 0.16 ± 0.05 | 0.58 ± 0.05 |
| Maternal ratings of infants' enjoyment of formula | ||||||
| CMF | 7.7 ± 0.5 | 7.7 ± 0.5 | 8.0 ± 0.5 | 7.7 ± 0.5 | 7.9 ± 0.4 | 6.3 ± 0.4 |
| PHF | 5.8 ± 0.7 | 5.5 ± 0.7 | 4.6 ± 0.7 | 5.9 ± 0.7 | 3.1 ± 0.6 | 7.4 ± 0.6 |
| Relative response | 0.42 ± 0.04 | 0.38 ± 0.04 | 0.35 ± 0.04 | 0.42 ± 0.04 | 0.26 ± 0.04 | 0.55 ± 0.04 |
| Number of distaste/rejection behaviors during first 2 min of meal | ||||||
| CMF | 5.1 ± 1.5 | 5.3 ± 1.64 | 3.5 ± 1.5 | 2.6 ± 1.5 | 2.3 ± 1.4 | 5.0 ± 1.4 |
| PHF | 5.0 ± 1.4 | 5.8 ± 1.44 | 5.6 ± 1.4 | 5.2 ± 1.4 | 8.1 ± 1.3 | 3.2 ± 1.3 |
| Relative response | 0.53 ± 0.06 | 0.57 ± 0.064 | 0.62 ± 0.06 | 0.66 ± 0.06 | 0.70 ± 0.05 | 0.41 ± 0.05 |
The names of the groups refer to the duration [1 or 3 mo (1M or 3M)] and age (1.5, 2.5, or 3.5 mo) at which the infants were fed PHF or CMF. See Figures 3 and 4 for the statistical findings based on the analyses conducted to test the 2 hypotheses regarding timing and duration of exposure.
Mean ± SEM (all such values).
Relative responses are calculated as PHF/(CMF+PHF).
One infant in this group was not videotaped because of equipment malfunction.
DISCUSSION
Before any exposure to PHF, infants as young as 1.5 mo reject its flavor, as evidenced during the brief-access taste tests. This indicated that infants can clearly detect the flavor differences between PHF and CMF, favoring their familiar CMF. The rejection was greatest in those infants who first tasted PHF when they were 3.5 mo old—a finding consistent with our published data (6, 8) and clinical reports (16). The finding that the mothers of these infants had a tendency to rate that their infants enjoyed PHF less than did the other groups suggests that the rejection was sensory-based. Although PHF acceptance increased equally in all groups by the end of their first month of exposure, those first exposed to PHF at 3.5 mo were less accepting of this formula during the test meals several months later, when they were 7.5 mo of age. Taken together with the finding that it is difficult to introduce PHF to infants aged ≥4 mo (9), these data suggest that the “window” for early acceptance and long-term influences is beginning to close at ≈3.5 mo. Furthermore, because those exposed to PHF at 3.5 mo had the most recent exposure to PHF, we conclude that the recency of the exposure per se does not appear to be as important as when the exposure began.
The present study provides new evidence that characterizes the sensitive period in early development when hedonic responses to flavors are established. Among infants whose feeding of PHF began at 1.5 mo, those fed for 1 mo were as accepting of the formula (as determined by intake, facial reactivity, and maternal perceptions) as were those fed for 3 mo. In other words, flavor experience of a relatively brief occurrence, at least 1 mo before the infant is 3.5 mo, is sufficient to maintain acceptance. That is, exposure prevents the shift in hedonic tone from acceptance to rejection of the PHF flavor that typically occurs in nonexposed infants at 4 mo of age. The finding that both groups were less accepting than were infants who were exposed to PHF for the entire 7 mo indicates that there is a dosing effect as well. We caution that one cannot assume that there is only one sensitive period in flavor programming, any more than there is only one sensitive period for auditory (17) or visual (18) learning. The model system that we identified allows us to explore the ability to change behavior based on experiences during only one period. Like other senses (19, 20), windows of plasticity in flavor learning may not shut abruptly, and some level of plasticity is probably retained as the child ages.
The neural substrates underlying the observed developmental transition in human flavor learning remain unknown (21). We suggest that knowledge about the sensitive periods for other senses, particularly vision (22), may shed light on aspects of flavor learning not yet explored. Before scientific knowledge of the timing of the sensitive period of visual development, physicians rarely removed cataracts in children younger than 6 mo, and this resulted in a lifetime of poor vision (22, 23). Equipped with knowledge of the timing of this sensitive period, particularly the deleterious effects of monocular deprivation during the first 3 mo of life, physicians now often remove cataracts during the first weeks of life, with significantly improved outcomes. Whether similar long-term effects occur if infants are deprived of food flavors during early life is an important area of future research.
Although our model system examined the flavors of hydrolyzed formulas, the general principles observed are likely of much broader significance, which indicates a fundamental feature of human development. We suggest that the adaptive reason for this age-related plasticity reflects the importance of infants becoming familiar with and accepting the flavors that their mothers consume and transmit to breast milk (2). It is these flavors that they most likely will confront during weaning and that reflect the culinary traditions of their families.
Acknowledgments
We acknowledge the expert technical assistance of Lauren Yourshaw and Myrline Gillot, a recipient of a special supplement from this NIH grant under the Research Supplements to Promote Diversity in Health-Related Research Program.
The authors' responsibilities were as follows—JAM: conception and design of the study and oversight of study execution, data collection, data analyses, and personnel; LDL and SMC: data collection and videotape analyses; JAM: drafting and editing of the manuscript; and JAM, GKB, LDL, and SMC: critical review of the manuscript. All authors reviewed and approved the final version of the report. None of the authors had any personal or financial conflicts of interest.
REFERENCES
- 1.Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes (Lond) 2008;32(suppl 7):S62–71 [DOI] [PubMed] [Google Scholar]
- 2.Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and postnatal flavor learning by human infants. Pediatrics 2001;107:E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mennella JA. The chemical senses and the development of flavor preferences in humans Hale TW, Hartmann PE, eds. Textbook on human lactation Amarillo, TX: Hale Publishing, 2007:403–14 [Google Scholar]
- 4.Forestell CA, Mennella JA. Early determinants of fruit and vegetable acceptance. Pediatrics 2007;120:1247–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoshuk LM, Beauchamp GK. Chemical senses. Annu Rev Psychol 1994;45:419–49 [DOI] [PubMed] [Google Scholar]
- 6.Mennella JA, Griffin CE, Beauchamp GK. Flavor programming during infancy. Pediatrics 2004;113:840–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mennella JA, Beauchamp GK. Understanding the origin of flavor preferences. Chem Senses 2005;30(Suppl 1):i242–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mennella JA, Beauchamp GK. Development and bad taste. Pediatr Asthma Allergy Immunol 1998;12:161–3 [Google Scholar]
- 9.Mennella JA, Beauchamp GK. Developmental changes in the acceptance of protein hydrolysate formula. J Dev Behav Pediatr 1996;17:386–91 [DOI] [PubMed] [Google Scholar]
- 10.Mennella JA, Beauchamp GK. Flavor experiences during formula feeding are related to preferences during childhood. Early Hum Dev 2002;68:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liem DG, Mennella JA. Sweet and sour preferences during childhood: role of early experiences. Dev Psychobiol 2002;41:388–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mennella JA, Forestell CA, Morgan LK, Beauchamp GK. Early milk feeding influences taste acceptance and liking during infancy. Am J Clin Nutr 2009;90(suppl):780S–788S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunnar MR, Stone C. The effects of positive maternal affect on infant responses to pleasant, ambiguous and fear-provoking toys. Child Dev 1984;55:1231–6 [Google Scholar]
- 14.Ekman P, Freisen WV, Hagar JC. Facial action coding system. The Manual on CD-ROM. Salt Lake City, UT: Research Nexus Division of Network Information Research, 2002 [Google Scholar]
- 15.Winer BJ. Statistical principles in experimental design. New York, NY: McGraw-Hill Book Company, 1972 [Google Scholar]
- 16.Halken S, Host A, Hansen LG, Osterballe O. Preventive effect of feeding high-risk infants a casein hydrolysate formula or an ultrafiltrated whey hydrolysate formula. A prospective, randomized, comparative clinical study. Pediatr Allergy Immunol 1993;4:173–81 [DOI] [PubMed] [Google Scholar]
- 17.Ruben RJ. A time frame of critical/sensitive periods of language development. Acta Otolaryngol 1997;117:202–5 [DOI] [PubMed] [Google Scholar]
- 18.Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev Psychobiol 2005;46:163–83 [DOI] [PubMed] [Google Scholar]
- 19.Huttenlocher PR. Neural plasticity: the effects of the environment on the development of the cerebral cortex. Cambridge, MA: Harvard University Press, 2002 [Google Scholar]
- 20.Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear 2002;23:532–9 [DOI] [PubMed] [Google Scholar]
- 21.Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neurosci Biobehav Rev 2010;34:835–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olitsky SE, Nelson BA, Brooks S. The sensitive period of visual development in humans. J Pediatr Ophthalmol Strabismus 2002;39:69–72 [DOI] [PubMed] [Google Scholar]
- 23.Beller R, Hoyt CS, Marg E, Odom JV. Good visual function after neonatal surgery for congenital monocular cataracts. Am J Ophthalmol 1981;91:559–65 [DOI] [PubMed] [Google Scholar]