Abstract
Background: The optimal feeding (breast milk, formula, or a combination) for infants with cystic fibrosis (CF) is unknown. Recommendations from the CF Foundation are based on limited data.
Objective: We compared growth and pulmonary outcomes between breastfed and formula-fed infants through the age of 2 y.
Design: A total of 103 CF infants born in 1994–2006 and diagnosed through newborn screening in Wisconsin were studied. Breastfed infants were classified by the duration of exclusive breastfeeding (ExBF). Exclusive formula-feeding (ExFM) was classified by the formula's caloric density (ie, standard [0.67 kcal/mL (20 kcal/oz) (ExFM20)] throughout infancy or high density [≥0.74 kcal/mL (22 kcal/oz) (ExFM22+)] for some duration of infancy).
Results: Fifty-three infants (51% of infants) were breastfed and 50 infants (49% of infants) were ExFM. In breastfed infants, the duration of ExBF was <1 mo (53% of infants), 1–1.9 mo (21% of infants), 2–3 mo (17% of infants), and 4–9 mo (9% of infants). In ExFM infants, 23 infants (46%) received a formula with a high caloric density; approximately half (n = 13) of the ExFM infants received the formula by 6 mo of age. Proportionately more infants with pancreatic sufficiency (n = 9) were ExBF ≥1 mo (44% of infants), and none of the infants were ExFM22+, compared with infants with meconium ileus (n = 24; 13% of infants were ExBF ≥1 mo, and 38% of infants were ExFM22+) or pancreatic insufficiency (n = 70; 25% of infants were ExBF ≥1 mo, and 20% of infants were ExFM22+) (P = 0.02). In infants with pancreatic insufficiency, weight z scores declined from birth to 6 mo (P < 0.0001) in infants who were ExBF ≥2 mo, and the number of Pseudomonas aeruginosa infections through the age of 2 y was fewer in breastfed than in ExFM infants (P = 0.003) but did not differ by the duration of ExBF.
Conclusion: For infants with CF, ExBF <2 mo does not compromise growth and is associated with a respiratory benefit.
INTRODUCTION
Cystic fibrosis (CF) is a life-shortening, autosomal-recessive disorder that is characterized by intestinal malabsorption, impaired growth, and lung disease (1). Malnutrition and growth faltering are common (2–5) and even occur in infants diagnosed through newborn screening (NBS) (6). Optimizing nutritional status is critical in children with CF because malnutrition is associated with poor clinical outcomes (7–11).
Research over the past 2 decades has proven that NBS for CF is feasible (12, 13, 23, 24) and leads to unequivocal nutritional benefits (14–16) and possible pulmonary (17–19), cognitive (20), and survival benefits (21, 22). As of 2010, NBS for CF is universal in the United States (25, 26). This creates a new opportunity to diagnose and treat infants with CF within weeks of birth. However, optimal feeding for CF infants (ie, breast milk, formula, or a combination) is unknown. The breastfeeding issue was less relevant before nationwide implementation of NBS, when CF infants were diagnosed at a median age of 8–9 mo (27), which is an age when most infants would no longer be breastfed; now, the breastfeeding issue is of prime importance.
Breastfeeding was historically discouraged for CF infants because of concerns about protein energy malnutrition, which is manifested by hypoproteinemia, hyponatremia, edema, and anemia (28–33). Despite these reports, a 1990 survey showed that 77% of CF centers encouraged breastfeeding, with nearly 37% of CF centers recommending exclusive breastfeeding (ExBF) (34). Similar trends were confirmed by a 2004 survey (35). Breast milk may be nutritionally inadequate in caloric density, protein, essential fatty acids, and sodium to meet the increased requirements of CF infants, especially of CF infants with meconium ileus (MI) or pancreatic insufficiency (PI), who are at greatest risks of poor growth (4, 28–32). However, its antimicrobial constituents may offer protection against respiratory infections (36–41). The new 2009 CF Foundation (CFF) infant care guidelines (42) continued the 2002 recommendation (43) to suggest breast milk as the initial type of feeding for CF infants on the basis of surprisingly little evidence from only one US (35) and 2 European studies (44, 45). Of utmost importance, the CFF guidelines (42) do not specify the exclusiveness or the duration of breastfeeding. Therefore, whether ExBF promotes optimal growth and provides respiratory benefits for CF infants remain to be elucidated.
To our knowledge, no published reports have examined detailed breastfeeding characteristics, such as exclusiveness and duration of breastfeeding, with concurrent comparisons to formula-feeding characteristics, such as the use of a formula with a standard or high caloric density [commonly prescribed to CF infants (42, 43)], and their associations to longitudinal growth and pulmonary outcomes while taking into account MI and PI. The current study was designed to address this gap of knowledge through an investigation in a cohort of infants who were born in 1994–2006 and identified through the Wisconsin Routine CF NBS Program (24) from diagnosis to 2 y of age.
SUBJECTS AND METHODS
Study design
The study population consisted of 103 infants with CF born in 1994–2006 who were diagnosed through the State of Wisconsin's Routine CF NBS Program (24) and cared for at the University of Wisconsin-Madison Pediatric CF Center (Madison, WI) and the Children's Hospital of Wisconsin Pediatric CF Center (Milwaukee, WI). Of the 103 infants, 24 infants had MI. Nine infants had pancreatic sufficiency (PS), which was defined as having at least one mutation known to be associated with PS (46) and not taking pancreatic enzyme replacement therapy. The remaining 70 infants had PI but not MI (noMI-PI). The study protocol was approved by the human subjects committee at the University of Wisconsin-Madison and the Research and Publications Committee/Human Rights Board at the Children's Hospital of Wisconsin.
Collection of feeding, growth, pulmonary, and other relevant clinical data
Information related to feeding during infancy was obtained by an extensive medical record review. This involved reviewing data on breastfeeding (exclusiveness and duration), formula-feeding (type and caloric density), and age at the introduction of solid foods and cow milk from all clinic contacts including visits, phone calls, and e-mails.
Weight and length data from diagnosis through 2 y of age were retrieved from the CFF Patient Registry (47) and verified from medical records. A total of 788 observations were identified; 60% of subjects had 8–10 measurements, and 35% of subjects had 5–7 measurements. Age- and sex-specific z scores for weight and length were computed by using the 2000 Centers for Disease Control and Prevention growth reference data (48).
Pulmonary outcome measures included respiratory infections and chest radiography (CXR). Data on 2 pathogens, Pseudomonas aeruginosa and Staphylococcus aureus, which are the most common bacteria detected in cultures of respiratory secretions of young children with CF, were retrieved from medical records from diagnosis to 2 y of age. Of the 70 noMI-PI subjects included in the pulmonary analysis, a total of 552 culture observations were identified; 69% of subjects had 8–10 cultures, and 29% of subjects had 5–7 cultures. CXR films obtained at diagnosis and at 2 y of age were retrieved for scoring on the basis of the Wisconsin system (49, 50) and the Brasfield system (51, 52) by 2 pulmonologists who were unaware of patient identities by using a rigorous protocol that was previously validated (50, 53). Of the 70 noMI-PI subjects, 47 subjects had films at diagnosis (3.0 ± 1.9 mo of age), and 53 subjects had films at 2 y of age (2.0 ± 0.3 y of age). Other clinical data (eg, genotype and age of diagnosis) were retrieved from the CFF Patient Registry.
Classification of infant feeding
Feeding characteristics were first divided into 2 categories of infants who were ever breastfed and infants who were never breastfed [ie, exclusively formula-fed (ExFM)]. In the ever-breastfed group, ExBF was defined as receiving breast milk only without formula or solid foods. Durations of exclusive and partial and total breastfeeding were determined by using the age at clinic contact when the last known instance of breastfeeding was documented. For example, an infant reported as ExBF at 1.9 mo of age, partially breastfed at 2.9 to 8.3 mo of age, and not breastfed at 10.2 mo of age was defined as being ExBF for 1.9 mo and partially breastfed for 6.4 mo (from 1.9 to 8.3 mo of age) with a total duration of breastfeeding of 8.3 mo.
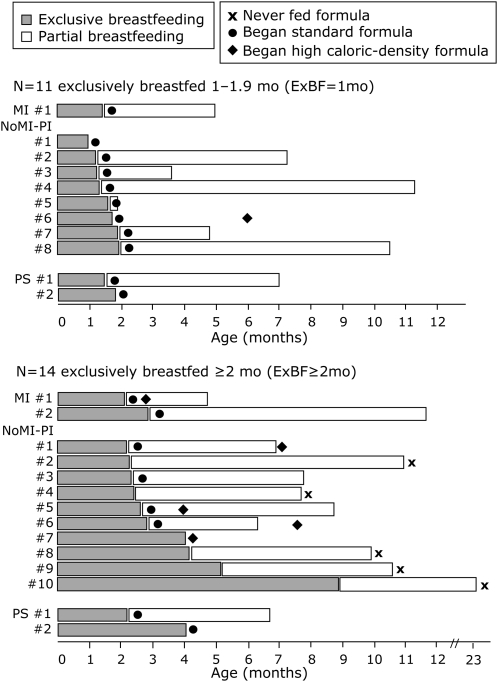
The examination of the ever-breastfed group (n = 53) revealed that more than one-half of these infants (n = 28; 53%) were ExBF <1 mo and very few of these infants (n = 5; 9%) were ExBF ≥3 mo. Therefore, ever-breastfed infants were further divided on the basis of the duration of ExBF into <1 mo (ExBF<1mo group; n = 28), 1–1.9 mo (ExBF=1mo group; n = 11), and ≥2 mo (ExBF≥2mo group; n = 14 (Figure 1). Because of the small sample size, infants who were ExBF ≥ 3mo (n = 5) were not separated from the ExBF≥2 mo group.
FIGURE 1.
Feeding characteristics of cystic fibrosis infants who were exclusively breastfed (ExBF) >1 mo. The standard formula contained 0.67 kcal/mL (20 kcal/oz), and the formula with high caloric density contained ≥0.74 kcal/mL (≥22 kcal/oz). MI, meconium ileus; NoMI-PI, no MI but pancreatic insufficient; PS, pancreatic sufficient.
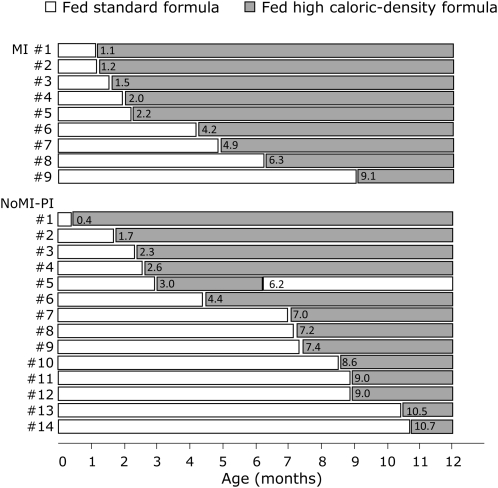
The ExFM group was divided into 2 groups of infants fed formula that contained a standard caloric density of 0.67 kcal/mL (20 kcal/oz) throughout infancy (ExFM20; n = 27) and infants fed formula that contained a high caloric density [≥0.74 kcal/mL (22 kcal/oz)] for some duration of infancy (ExFM22+; n = 23) (Figure 2).
FIGURE 2.
Feeding characteristics of cystic fibrosis infants who were exclusively formula-fed and who received a formula with high caloric density for some duration during infancy. The number within each bar indicates the age of the infant at the introduction of the formula with high caloric density. The standard formula contained 0.67 kcal/mL (20 kcal/oz), and the formula with high caloric density contained ≥0.74 kcal/mL (≥22 kcal/oz). MI, meconium ileus; NoMI-PI, no MI but pancreatic insufficient.
Statistical analyses
SAS (version 9.13, 2001; SAS Institute Inc, Cary, NC) was used for data processing and statistical analyses. Chi-square test (when the sample size was >5 in all subgroups) and Fisher's exact test (when the sample size was <5 in any subgroup) were used to compare differences in proportions, and analysis of variance (ANOVA) was used to compare differences in continuous measures. The median test was used to compare differences in median ages at diagnosis.
All analyses that compared growth and pulmonary outcomes were stratified or conducted separately by phenotype (ie, PS, MI, and no MI-PI) on the basis of the following rationales: 1) PS patients are at lower risk of malnutrition and are able to achieve normal growth (ie, mean weight- and length-for-age at approximately the 50th percentile) with energy intake at 99% of their recommended requirement (15), and 2) MI patients have been shown to experience poorer growth despite a higher energy intake than patients without MI with a similar age at diagnosis and treatment protocol (54).
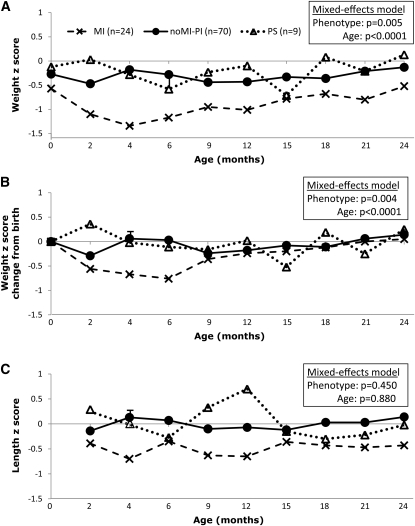
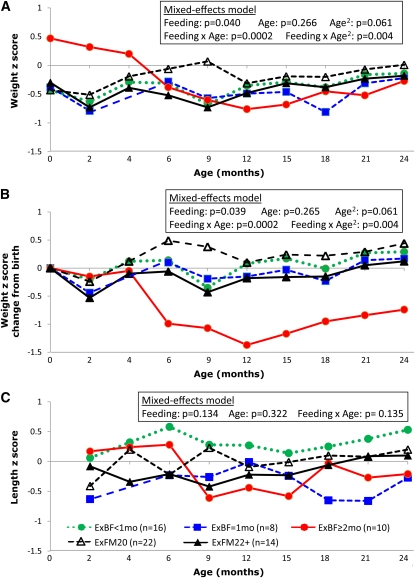
Within each phenotype (Figure 3) and feeding (Figure 4) group, growth in relation to age was assessed by regression analysis. To compare differences in longitudinal growth patterns through 2 y of age in phenotype (Figure 3) and feeding (Figure 4) groups, mixed-effects models with repeated measures were performed with adjustment for sex, birth weight z score, CF center, and age. Potential biases because of missing data were evaluated by comparing the percentages of patients with missing data, which did not differ significantly between feeding groups or by age. No statistical analysis was performed to compare growth outcomes among the 5 feeding groups within the MI and PS subgroups because sample sizes were too small (<3 in 3 of the 5 feeding groups for MI and in all feeding groups for PS).
FIGURE 3.
Growth of cystic fibrosis infants by phenotype. A: Weight z scores. B: Weight gain from birth expressed by the change of weight z scores from birth. C: Length z scores. Mixed-effects models with repeated measures were performed to compare differences between phenotypes with adjustment for sex, birth weight z score, cystic fibrosis center, and age. The phenotype-by-age interaction was not significant (P > 0.50 for all panels); therefore, only main effects were included in the mixed-effects models. MI, meconium ileus; noMI-PI, no MI but pancreatic insufficient; PS, pancreatic sufficient.
FIGURE 4.
Growth by feeding groups in the subgroup of cystic fibrosis infants with pancreatic insufficiency but not meconium ileus. A: Weight z scores. B: Weight gain from birth expressed by the change of weight z scores from birth. C: Length z scores. Mixed-effects models with repeated measures were performed to compare differences in feeding groups while adjusting for sex, birth weight z score, cystic fibrosis center, and age. The effect of age was nonlinear in panels A and B; therefore, a quadratic term for age and a feeding group by quadratic age interaction were included in the mixed-effects models. ExBF, exclusive breastfeeding; ExBF=1mo, ExBF for 1–1.9 mo; ExFM20, exclusively fed a formula that contained a standard caloric density of 0.67 kcal/mL (equivalent to 20 kcal/oz); ExFM22+, exclusively formula-fed and received a formula with a high caloric density [≥0.74 kcal/mL (≥22 kcal/oz)] for some duration during infancy.
Statistical analysis for P. aeruginosa and S. aureus infections was based on the number of positive cultures during the first 2 y of life within each individual patient. The rationale for this approach was that these infections were more likely to be transient in very young children with CF. Therefore, the traditional approach of first-positive or cross-sectional prevalence at each age was less meaningful in our study population. The number of infections was categorized into 3 categories of 0, 1, and ≥2 infections and analyzed by Fisher's exact test. CXR scores were compared by ANOVA and included sex and center as covariates followed by multiple comparisons with Bonferroni correction.
RESULTS
Baseline characteristics
Baseline characteristics in PS, MI, and noMI-PI infants are compared in Table 1. No significant differences were shown for sex and median age of diagnosis. By definition, no PS infants were homozygous for the F508del mutation, and no significant difference was observed in the F508del status between MI and noMI-PI infants (P = 0.17).
TABLE 1.
Characteristics of the study population1
| MI (n = 24) | NoMI-PI (n = 70) | PS (n = 9) | P | |
| Sex [n (%)] | 0.202 | |||
| M | 9 (38) | 39 (56) | 6 (67) | — |
| F | 15 (62) | 31 (44) | 3 (33) | — |
| Birth weight (g) | 3120 ± 5323 | 3310 ± 466 | 3413 ± 430 | 0.174 |
| Birth weight z score | −0.57 ± 1.08 | −0.27 ± 0.89 | −0.12 ± 0.79 | 0.314 |
| Median age at diagnosis (wk) | 1.1 | 1.7 | 2.6 | 0.155 |
| Genotype [n (%)] | <0.0016 | |||
| F508del/F508del | 14 (58) | 30 (43) | 0 | — |
| F508del/other | 9 (38) | 36 (52) | 8 (89) | — |
| Other/other | 1 (4) | 4 (6) | 1 (11) | — |
| Breast or formula feeding [n (%)] | 0.0456 | |||
| ExBF <1 mo | 10 (42) | 16 (23) | 2 (22) | — |
| ExBF = 1 mo | 1 (4) | 8 (11) | 2 (22) | — |
| ExBF ≥2 mo | 2 (8) | 10 (14) | 2 (22) | — |
| ExFM20 | 2 (8) | 22 (31) | 3 (33) | — |
| ExFM22+ | 9 (38) | 14 (20) | 0 | — |
MI, meconium ileus; NoMI-PI, no MI but pancreatic insufficient; PS, pancreatic sufficient; ExBF, exclusive breastfeeding; ExBF = 1 mo, ExBF for 1–1.9 mo; ExFM20, exclusively fed formula containing standard caloric density of 0.67 kcal/mL (equivalent to 20 kcal/oz); ExFM22+, exclusively formula-fed and received high caloric density formula [≥0.74 kcal/mL (≥22 kcal/oz)] for some duration during infancy.
Calculated by using the chi-square test.
Mean ± SD (all such values).
Calculated by using one-factor ANOVA.
Calculated by using the Median test.
Calculated by using Fisher's exact test.
Feeding characteristics of breastfed infants
Approximately one-half of infants with CF (n = 53; 51%) were breastfed. Of these 53 infants, the duration of ExBF ranged from <1 mo (n = 28; 53%), 1–2 mo (n = 11; 21%), 2–3 mo (n = 9; 17%), 4–6 mo (n = 4; 7%), and 8.9 mo (n = 1; 2%). Of the 28 infants in the ExBF<1mo group, more than one-half of infants (n = 15; 54%) transitioned to ExFM before 2 mo of age. The remaining 13 infants (46%) continued partial breastfeeding beyond 2 mo of age [n = 2 (2–3 mo of age), n = 4 (3–6 mo of age), n = 4 (6–9 mo of age), and n = 3 (9–13 mo of age)].
Of the 25 infants who were ExBF >1 mo, the duration of partial breastfeeding also varied greatly, as detailed in Figure 1. Specifically, 5 infants (20%) transitioned directly to formula without partial breastfeeding, 1 infant (4%) received partial breastfeeding until 2 mo of age, 4 infants (16%) received partial breastfeeding until 3–6 mo of age, 8 infants (32%) received partial breastfeeding until 6–9 mo of age, 6 infants (24%) received partial breastfeeding until 9–12 mo of age, and 1 infant (4%) received partial breastfeeding until 23 mo of age (Figure 1).
Feeding characteristics of exclusively formula-fed infants
Approximately one-half of infants with CF were ExFM (n = 50; 49%). Of these 50 infants, 27 infants (54%) were fed formula with a standard caloric density (20 kcal/oz) at all times (Table 1). The remaining 23 infants (46%) received a formula with a higher caloric density (22–27 kcal/oz) as early as the neonatal period (age: 0.4 mo) to as late as near the end of the first year (age: 10.7 mo), as detailed in Figure 2. Overall, of 23 infants in the ExFM22+ group, 6 infants (26%) received the formula with a high caloric density by 2 mo of age, 13 infants (57%) received the formula with a high caloric density by 6 mo of age, and 20 infants (87%) received the formula with a high caloric density by 9 mo of age (Figure 2).
Association between phenotype and growth
As shown in Figure 3A, the longitudinal pattern of weight z scores from birth to 2 y of age differed significantly between the MI, NoMI-PI, and PS groups (P = 0.005). These differences were primarily due to MI infants, who showed consistently lower weight and length z scores than did NoMI-PI and PS infants. Weight gain from birth, as indicated by the change in weight z scores from birth (ΔWtZBR) (Figure 3B) also differed significantly among the 3 phenotypes through 2 y of age (P = 0.004). The longitudinal pattern of length z scores (Figure 3C) appeared to be lower in MI than in non-MI PI and PS infants, but this difference was not significant (P = 0.450).
Association between infant feeding and growth in noMI-PI infants
As shown in Figure 4A, birth weight tended to be higher in the ExBF≥2mo group than in the other 4 feeding groups, but these differences were not significant (P = 0.065 for absolute birth weight and P = 0.079 for birth weight z score, respectively). The apparent relation between weight z score and age differed in the 5 feeding groups. Specifically, within the ExBF≥2 mo group, the weight z score declined rapidly from birth to 6 mo of age (P < 0.0001), stabilized during 6–12 mo of age, and slightly increased during the second year of life. In contrast, within each of the other 4 feeding groups, weight z scores fluctuated but did not differ significantly with age throughout the first 2 y of life (P values ranged from 0.20 to 0.89 in the regression analysis). This apparent difference was supported by significant interactions between the feeding group and age in the mixed-effects model (Figure 4A). In addition to the effects of the feeding group and age, the mixed-effects model also showed that the birth weight z score was a significant predictor of the weight z score pattern through 2 y of age (P = 0.022), whereas the effect of sex (P = 0.34) and center (P = 0.67) were not significant.
Given these observations, weight gain from birth as reflected by the ΔWtZBR, which is an indicator that we showed affected growth and pulmonary outcomes through 6 y of age (55, 56), was further examined. As shown in Figure 4B, in the ExBF≥2mo group, ΔWtZBR decreased substantially from birth to 6 mo of age; this pattern was not observed in the other groups. ΔWtZBR increased in the second year of life in the ExBF≥2mo group, but did not recover to the level at birth. In addition, 4 of the 10 infants in the ExBF≥2mo group began receiving the formula with high caloric density between ages 4–9 mo (Figure 1), but weight z scores in 3 out of the 4 infants remained low by the end of infancy.
It was noted that 3 infants in the ExBF≥2mo group were large for gestational age (LGA), with birth weights >4000 g (with corresponding z scores of 1.4–2.3; all above the 90th percentile), therefore, 3 alternative analyses were performed. First, ΔWtZBR values in the 3 LGA infants were redefined by assuming a birth weight z score of 1.0 because it may have been unrealistic to expect these 3 infants to have maintained postnatal weights at >90th percentiles through 2 y of age. Second, the 3 LGA infants were excluded from the analysis. The decline in weight z scores and ΔWtZBR from birth to 2 y of age observed in the ExBF≥2mo remained significant (P < 0.001 and P < 0.01 from regression analysis). Third, the absolute weight gain (in g/d) during the first year of life was examined. Weight gain was significantly lower in the ExBF≥2mo group between 2–4 mo of age (19 g/d) and 4–6 mo of age (10 g/d) than in the other 4 groups (26 g/d between 2–4 mo of age and 21 g/d between 4–6 mo of age) (P = 0.008 and P = 0.012, respectively, from ANOVA).
Except for the ExBF≥2mo group, no significant differences were observed in the other 4 feeding groups in the pattern of weight z scores or weight gain throughout the first 2 y of life (Figure 4, A and B). Also, no significant differences in length z scores during the first 2 y of life were shown in all 5 feeding groups (Figure 4C). We were unable to evaluate the change in length z scores from birth because birth length data were not documented in medical records for the majority of the study population. The age at introduction of solid foods (5.4 ± 2.1 mo of age) did not differ significantly between the feeding groups and did not change the comparisons of weight z scores among the feeding groups shown in Figure 4B.
Association between infant feeding and pulmonary outcomes in noMI-PI infants
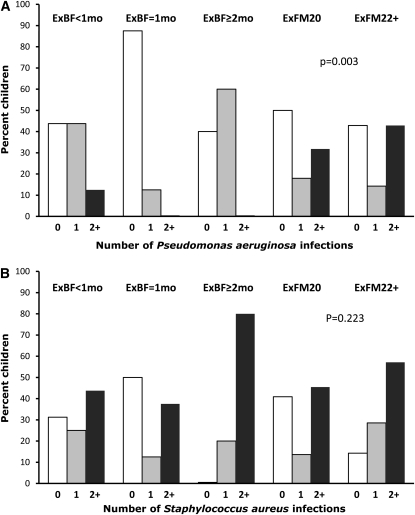
As shown in Figure 5A, infants in the ExBF=1mo group had the fewest number of P. aeruginosa infections (almost 90% never colonized with P. aeruginosa, and none of the infants had ≥2 positive P. aeruginosa infections) followed by the ExBF≥2mo group (40% never colonized with P. aeruginosa, and none of the infants had ≥2 positive P. aeruginosa infections) and the ExBF<1mo group (44% never colonized with P. aeruginosa, 44% had one positive P. aeruginosa infection, and 13% had ≥2 positive P. aeruginosa infections) compared with the number of P. aeruginosa infections in the 2 ExFM groups (43–50% never colonized with P. aeruginosa, 14–18% had one positive P. aeruginosa infection, and 32–43% had ≥2 positive P. aeruginosa infections) [P = 0.026 for comparison of 5 groups and P = 0.003 for comparison of breastfed (ie, with the 3 ExBF subgroups combined) with formula-fed infants]. S. aureus infections were more frequently present than P. aeruginosa infections (Figure 5B), but no significant differences were observed between breastfed and formula-fed infants (P = 0.22 in 5 feeding groups; P = 0.95 between breastfed and formula-fed infants).
FIGURE 5.
Respiratory infections by feeding groups in the subgroup of cystic fibrosis infants with pancreatic insufficiency but not meconium ileus. A: Number of Pseudomonas aeruginosa infections through 2 y of age. B: Number of Staphylococcus aureus infections. P values indicate significance as assessed by Fisher's exact test. ExBF, exclusive breastfeeding; ExBF=1mo, ExBF for 1–1.9 mo; ExFM20, exclusively fed a formula that contained a standard caloric density of 0.67 kcal/mL (equivalent to 20 kcal/oz); ExFM22+, exclusively formula-fed and received a formula with a high caloric density [≥0.74 kcal/mL (≥22 kcal/oz)] for some duration during infancy.
CXR scores at diagnosis, which were all in the normal range, did not differ significantly the 5 feeding groups (Table 2). At 2 y of age, Wisconsin CXR scores were significantly different between the 5 feeding groups (P = 0.015), with the ExBF=1mo group having the best score, which was significantly better than the score for the ExBF≥2mo group. Consistent trends were observed in Brasfield CXR scores, but P did not reach significance (P = 0.077). CXR scores at 2 y of age indicated mild lung disease.
TABLE 2.
Wisconsin and Brasfield chest radiographic scores for comparisons of 5 feeding groups1
| Chest radiographic score |
||||
| Wisconsin score |
Brasfield score |
|||
| Feeding group | At diagnosis | At age 2 y | At diagnosis | At age 2 y |
| ExBF <1 mo | 1.2 ± 0.9 | 4.5 ± 2.4a,b | 23.3 ± 0.7 | 20.9 ± 1.3 |
| ExBF = 1 mo | 0.9 ± 1.2 | 2.0 ± 1.2b | 23.9 ± 1.1 | 22.5 ± 1.8 |
| ExBF ≥2 mo | 0.9 ± 0.7 | 5.7 ± 2.3a | 23.8 ± 1.0 | 20.6 ± 0.8 |
| ExFM20 | 1.4 ± 1.4 | 3.4 ± 1.7a,b | 23.3 ± 1.3 | 21.3 ± 1.3 |
| ExFM22+ | 1.9 ± 1.9 | 4.1 ± 2.8a,b | 22.8 ± 2.1 | 21.1 ± 1.6 |
| P | 0.43 | 0.015 | 0.24 | 0.077 |
All values are means ± SDs. ExBF, exclusive breastfeeding; ExBF = 1 mo, ExBF for 1–1.9 mo; ExFM20, exclusively fed formula containing standard caloric density of 0.67 kcal/mL (equivalent to 20 kcal/oz); ExFM22+, exclusively formula-fed and received high caloric density formula [≥0.74 kcal/mL (≥22 kcal/oz)] for some duration during infancy. Wisconsin scores range from 0 (best) to 100 (worst), and Brasfield scores ranged from 25 (best) to 0 (worst). Values followed by the same superscript letter were not significantly different. P values were calculated by using the chi-square test.
DISCUSSION
To our knowledge, this study presented the first comprehensive analysis of breastfeeding and its association to growth and pulmonary outcomes in CF infants diagnosed during early infancy through a routine NBS program in the United States. In this study population, breastfeeding was prevalent (≈50%), but ExBF was short and less common (<25% of infants beyond the neonatal period). By 2 mo of age, two-thirds of all CF infants were ExFM, another 20% of infants were partially breastfed, and only 13% of infants remained ExBF. By 3 mo of age, almost all infants discontinued ExBF. Of the infants ExFM from birth, about one-half received the formula with high caloric density during infancy, with the highest rate in MI infants (>80%), one-half that rate (40%) in noMI-PI infants, and none in PS infants. In addition, a quarter of ExFM infants began receiving the formula with high caloric density by 2 mo of age.
The most important finding from our study was that noMI-PI infants who were ExBF <2 mo achieved adequate weight gain and experienced fewer P. aeruginosa infections during the first 2 y of life than did infants who were exclusively formula fed. In contrast, ExBF ≥2 mo was associated with a reduced weight gain without an additional respiratory benefit. Although the effect of the reduced weight gain observed in the ExBF≥2mo group may have been compensated for by the group's somewhat higher birth weight z score, which requires further investigation, Wisconsin CXR scores at 2 y of age were significantly worse in the ExBF≥2mo group than in the ExBF=1mo group. This observation is worrisome in light of our findings that noMI-PI infants who did not recover their birth weight z scores by 2 y of age had difficulty achieving further catch-up growth between 2 and 6 y of age and had a worse pulmonary status at 6 y of age than did infants who recovered their birth weight z scores before 2 y of age (56).
Our observation that fewer P. aeruginosa infections in the first 2 y of life were associated with breastfeeding supplements findings from 2 previous studies. Colombo et al (45) studied 106 children with CF in Italy and reported that breastfeeding ≥4 mo was associated with a fewer number of infections during the first 3 y of life and better lung function from a single measure at 5–18 y of age, whereas Parker et al (35) surveyed 33 US CF Centers and reported that infants who were ExBF ≥6 mo had fewer courses of intravenous antibiotics over the 2 y preceding the study (at 1–25 y of age) than did infants who were ExFM. However, both studies were retrospective, primarily obtained feeding data from older patients or through parental recall, and were unclear regarding adjustments of confounding factors. Therefore, to our knowledge, our study provided the first evidence of the benefits of breastfeeding on P. aeruginosa infections and CXR scores during the first 2 y of life in infants with CF.
The differential growth patterns by feeding groups observed in our study differed from those in 2 previous studies (44, 45) that were used to support the CFF guidelines (42). Holiday et al (44) studied 88 infants with CF diagnosed through NBS in Australia and reported that breastfeeding, with or without supplemental formula feeding, led to similar weight and length z scores than did exclusive formula feeding in the first 2 y of life. In the study by Colombo et al (45), no differences in growth during the first year of life were observed when formula-fed infants were compared with infants who were breastfed for 1–4 or ≥4 mo.
Several factors may explain the disparate findings between our study and previous studies (44, 45). First, our study separated infants with MI and PS, which are the 2 CF phenotypes that are known to be strong determinants, with opposite effects, on growth in CF children (15, 54). If we had combined MI and PS infants with noMI-PI infants, the differences in growth between the ExBF≥2mo group and the other groups would have been masked because fewer MI infants but more PS infants were ExBF ≥2 mo. Second, our study focused on examining the effect of ExBF with defined durations during the first 3 mo of life. If we simply compared ever-breastfed to never-breastfed infants, no differences would have been observed in weight patterns. Third, our analysis assessed longitudinal growth and adjusted for birth weight, which is an important predictor of early childhood growth, whereas the other studies (44, 45) only performed univariate comparisons at each time point, and included few time points during the first 1–2 y of life.
The classification of infant feeding into various groups by the exclusiveness and duration of breastfeeding and caloric density of the formula allowed us to uncover new associations between feeding and growth outcomes. However, whether breast milk as the sole source of an infant's diet is inadequate for CF infants is difficult to prove because the adequacy largely depends on the degree of malabsorption in individual infants, which is determined by the severity of PI and the appropriateness of the pancreatic enzyme-replacement therapy, in particular the lipase dose per feeding. In our study, data from medical records were lacking with regard to the degree of residual pancreatic function in individual CF infants and were also insufficient to determine the lipase dose per feeding because the amount of breast milk consumed in a feeding was not documented.
Another possibility for the poorer weight gain associated with the ExBF≥2mo group observed in our study is that infants in this group appeared to have had adequate growth during early infancy and, therefore, may have been encouraged to exclusively breastfeed. However, growth faltering occurred, and it appeared that catch-up growth was difficult to achieve, as evidenced by the extended low weight z scores through 2 y of age observed in the ExBF≥2mo group.
In our study, noMI-PI infants who received the formula with a standard caloric density grew similarly to infants who received the formula with a high caloric density. This observation may have represented a reverse causality (ie, infants fed the formula with the standard caloric density throughout infancy may have been the infants who had milder CF and, thus, would not need to be transitioned to the formula with the higher caloric density. With regard to MI infants, although we were not able to assess the effect of breast or formula feeding on growth because of small sample sizes, their persistently poor weight status, as we previously observed in a different cohort of MI infants (54), poses an even greater concern with ExBF than noMI-PI infants.
Our study has several limitations. First, the duration of ExBF was underestimated by design because we chose the most conservative approach to calculate the duration of breastfeeding. Second, because of the high variability, small sample size, and limited data from medical records, we were unable to perform a formal statistical analysis to evaluate the effect of other feeding characteristics on growth and pulmonary outcomes, such as the duration and amount of partial breastfeeding, duration and amount of supplemental formula with a high caloric density in breastfed infants, and the type and amount of solid foods. To overcome these drawbacks, a carefully designed prospective observational study (because it is not feasible to randomize breastfeeding in infants with CF at birth) is needed to systematically capture data on the complete feeding history and to assess potential confounding from environmental factors such as parental education, socioeconomic status, daycare, and smoking exposure (57–59).
In conclusion, the major clinical implication of our study is that it provided, to our knowledge, new evidence that, compared with ExFM, ExBF <2 mo was associated with adequate growth and protected against P. aeruginosa infections during the first 2 y of life in CF infants who had pancreatic insufficiency, whereas the benefits of ExBF >2 mo in this at-risk population are unclear. Nevertheless, prospective studies with a larger sample size, a longer duration of follow-up, and a comprehensive data collection that includes potential confounding factors are needed to confirm our findings, further evaluate potential risks of ExBF >2 mo on growth faltering and their long-term effects (ie, whether attenuated growth persists or catch-up growth occurs after 2 y of age), and investigate whether the respiratory infection benefit associated with breastfeeding leads to better pulmonary function later in life.
Acknowledgments
We thank Preston W Campbell from the CFF for providing registry data.
The authors’ responsibilities were as follows—SAJ, SMS, and HJL: contributed to the design of the study, interpretation of results, and writing of the manuscript; SAJ, ZZ, and HJL: contributed to the analysis of data; SAJ and GSW: contributed to the collection of data; BMT and PMF: scored chest radiographic films; MJR: offered clinical interpretation and coordinated enrollment in the Wisconsin Routine CF NBS Study; and HL and TM: facilitated data collection at the Milwaukee site. None of the authors reported a conflict of interest.
REFERENCES
- 1.Boat TF, Acton JD. Chapter 400: cystic fibrosis. : Kliegman RM, Berhrman RE, Jenson HB, Stanton BF, Nelson textbook of pediatrics. 18th ed Philadelphia, PA: Saunders Elsevier, 2007:1803–16 [Google Scholar]
- 2.Lai HC, Kosorok MR, Sondel SA, et al. Growth status in children with cystic fibrosis based on the national cystic fibrosis patient registry data: evaluation of various criteria used to identify malnutrition. J Pediatr 1998;132:478–85 [DOI] [PubMed] [Google Scholar]
- 3.McNaughton SA, Stormont DA, Shepherd RW, Francis PW, Dean B. Growth failure in cystic fibrosis. J Paediatr Child Health 1999;35:86–92 [DOI] [PubMed] [Google Scholar]
- 4.Lai HJ, Farrell PM. Chapter 42: N. utrition and cystic fibrosis : Coulston AM, Rock CL, Monsen ER. Nutrition in the prevention and treatment of disease. 2nd ed. San Diego, CA: Academic Press, 2008:787–804 [Google Scholar]
- 5.Cystic Fibrosis Foundation Cystic Fibrosis Foundation patient registry annual data report 2008. Bethesda, MD: Cystic Fibrosis Foundation, 2009 [Google Scholar]
- 6.Marcus MS, Sondel SA, Farrell PM, et al. Nutritional status of infants with cystic fibrosis associated with early diagnosis and intervention. Am J Clin Nutr 1991;54:578–85 [DOI] [PubMed] [Google Scholar]
- 7.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med 1992;326:1187–91 [DOI] [PubMed] [Google Scholar]
- 8.Nir M, Lanng S, Johansen HK, Koch C. Long-term survival and nutritional data in patients with cystic fibrosis treated in a Danish centre. Thorax 1996;51:1023–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the cystic fibrosis foundation national CF patient registry. J Pediatr 2000;137:374–80 [DOI] [PubMed] [Google Scholar]
- 10.Beker LT, Russek-Cohen E, Fink RJ. Stature as a prognostic factor in cystic fibrosis survival. J Am Diet Assoc 2001;101:438–42 [DOI] [PubMed] [Google Scholar]
- 11.Konstan MW, Butler SM, Wohl ME, et al. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr 2003;142:624–30 [DOI] [PubMed] [Google Scholar]
- 12.Hammond KB, Abman SH, Sokol RJ, Accurso FJ. Efficacy of statewide neonatal screening for cystic fibrosis by assay of trypsinogen concentrations. N Engl J Med 1991;325:769–74 [DOI] [PubMed] [Google Scholar]
- 13.Gregg RG, Simantel A, Farrell PM, et al. Newborn screening for cystic fibrosis in Wisconsin: comparison of biochemical and molecular methods. Pediatrics 1997;99:819–24 [DOI] [PubMed] [Google Scholar]
- 14.Farrell PM, Kosorok MR, Laxova A, et al. Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. N Engl J Med 1997;337:963–9 [DOI] [PubMed] [Google Scholar]
- 15.Farrell PM, Kosorok MR, Rock MJ, et al. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Pediatrics 2001;107:1–13 [DOI] [PubMed] [Google Scholar]
- 16.Farrell PM, Lai HJ, Li Z, et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: Enough is enough! J Pediatr 2005;147:S30–6 [DOI] [PubMed] [Google Scholar]
- 17.Merelle ME, Schouten JP, Gerritsen J, Dankert-Roelse JE. Influence of neonatal screening and centralized treatment on long-term clinical outcome and survival of CF patients. Eur Respir J 2001;18:306–15 [DOI] [PubMed] [Google Scholar]
- 18.Lai HJ, Cheng Y, Cho H, Kosorok MR, Farrell PM. Association between initial disease presentation, lung disease outcomes and survival in patients with cystic fibrosis. Am J Epidemiol 2004;159:537–46 [DOI] [PubMed] [Google Scholar]
- 19.McKay KO, Waters DL, Gaskin KJ. The influence of newborn screening for cystic fibrosis on pulmonary outcomes in New South Wales. J Pediatr 2005;147:S47–50 [DOI] [PubMed] [Google Scholar]
- 20.Koscik RL, Farrell PM, Kosorok MR, et al. Cognitive function of children with cystic fibrosis: deleterious effect of early malnutrition. Pediatrics 2004;113:1549–58 [DOI] [PubMed] [Google Scholar]
- 21.Doull IJ, Ryley HC, Weller P, Goodchild MC. Cystic fibrosis-related deaths in infancy and the effect of newborn screening. Pediatr Pulmonol 2001;31:363–6 [DOI] [PubMed] [Google Scholar]
- 22.Grosse SD, Rosenfeld M, Devine OJ, Lai HJ, Farrell PM. Potential impact of newborn screening for cystic fibrosis on child survival: a systematic review and analysis. J Pediatr 2006;149:362–6 [DOI] [PubMed] [Google Scholar]
- 23.Fost N, Farrell PM. A prospective randomized trial of early diagnosis and treatment of cystic fibrosis: a unique ethical dilemma. Clin Res 1989;37:495–500 [PubMed] [Google Scholar]
- 24.Rock MJ, Hoffman G, Laessig RH, Kopish GJ, Litsheim TJ, Farrell PM. Newborn screening for cystic fibrosis in Wisconsin: nine-year experience with routine trypsinogen/DNA testing. J Pediatr 2005;147:S73–7 [DOI] [PubMed] [Google Scholar]
- 25.Grosse SD, Boyle CA, Botkin JR, et al. Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. MMWR Recomm Rep 2004;53:1–36 [PubMed] [Google Scholar]
- 26.Groose MK, Reynolds R, Li Z, Farrell PM. Opportunities for quality improvement in cystic fibrosis newborn screening. J Cyst Fibros 2010;9:284–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Accurso FJ, Sontag MK, Wagener JS. Complications associated with symptomatic diagnosis in infants with cystic fibrosis. J Pediatr 2005;147:S37–41 [DOI] [PubMed] [Google Scholar]
- 28.Fleisher DS, DiGeorge AM, Barness LA, Cornfeld D. Hypoproteinemia and edema in infants with cystic fibrosis of the pancreas. J Pediatr 1964;64:341–8 [DOI] [PubMed] [Google Scholar]
- 29.Lee PA, Roloff DW, Howatt WF. Hypoproteinemia and anemia in infants with cystic fibrosis: a presenting symptom complex often misdiagnosed. JAMA 1974;228:585–8 [PubMed] [Google Scholar]
- 30.Nielsen OH, Larsen BF. The incidence of anemia, hypoproteinemia, and edema in infants as presenting symptoms of cystic fibrosis: a retrospective survey of the frequency of this symptom complex in 130 patients with cystic fibrosis. J Pediatr Gastroenterol Nutr 1982;1:355–9 [DOI] [PubMed] [Google Scholar]
- 31.Sokol RJ, Reardon MC, Accurso FJ, et al. Fat-soluble vitamin status during the first year of life in infants with cystic fibrosis identified by screening of newborns. Am J Clin Nutr 1989;50:1064–71 [DOI] [PubMed] [Google Scholar]
- 32.Bronstein MN, Sokol RJ, Abman SH, et al. Pancreatic insufficiency, growth, and nutrition in infants identified by newborn screening as having cystic fibrosis. J Pediatr 1992;120:533–40 [DOI] [PubMed] [Google Scholar]
- 33.Fustik S, Jacovska T, Spirevska L, Koceva S. Protein-energy malnutrition as the first manifestation of cystic fibrosis in infancy. Pediatr Int 2009;51:678–83 [DOI] [PubMed] [Google Scholar]
- 34.Luder E, Kattan M, Tanzer-Torres G, Bonforte RJ. Current recommendations for breast-feeding in cystic fibrosis centers. Am J Dis Child 1990;144:1153–6 [DOI] [PubMed] [Google Scholar]
- 35.Parker EM, O'Sullivan BP, Shea JC, Regan MM, Freedman SD. Survey of breast-feeding practices and outcomes in the cystic fibrosis population. Pediatr Pulmonol 2004;37:362–7 [DOI] [PubMed] [Google Scholar]
- 36.Garofalo RP, Goldman AS. Expression of functional immunomodulatory and anti-inflammatory factors in human milk. Clin Perinatol 1999;26:361–77 [PubMed] [Google Scholar]
- 37.Magi B, Ietta F, Romagnoli R, et al. Presence of macrophage migration inhibitory factor in human milk: evidence in the aqueous phase and milk fat globules. Pediatr Res 2002;51:619–24 [DOI] [PubMed] [Google Scholar]
- 38.Wright AL, Holberg CJ, Martinez FD, Morgan WJ, Taussig LM. Breast feeding and lower respiratory tract illness in the first year of life. Group Health Medical Associates. BMJ 1989;299:946–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beaudry M, Dufour R, Marcoux S. Relation between infant feeding and infections during the first six months of life. J Pediatr 1995;126:191–7 [DOI] [PubMed] [Google Scholar]
- 40.Heinig MJ. Host defense benefits of breastfeeding for the infant. Effect of breastfeeding duration and exclusivity. Pediatr Clin North Am 2001;48:105–23 [DOI] [PubMed] [Google Scholar]
- 41.Oddy WH, Sly PD, de Klerk NH, et al. Breast feeding and respiratory morbidity in infancy: a birth cohort study. Arch Dis Child 2003;88:224–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borowitz D, Robinson KA, Rosenfeld M, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr 2009;155:S73–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr 2002;35:246–59 [DOI] [PubMed] [Google Scholar]
- 44.Holliday KE, Allen JR, Waters DL, Gruca MA, Thompson SM, Gaskin KJ. Growth of human milk-fed and formula-fed infants with cystic fibrosis. J Pediatr 1991;118:77–9 [DOI] [PubMed] [Google Scholar]
- 45.Colombo C, Costantini D, Zazzeron L, et al. Benefits of breastfeeding in cystic fibrosis: a single-centre follow-up survey. Acta Paediatr 2007;96:1228–32 [DOI] [PubMed] [Google Scholar]
- 46.Castellani C, Cuppens H, Macek M, Jr, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros 2008;7:179–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr 1993;122:1–9 [DOI] [PubMed] [Google Scholar]
- 48.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 2002;246:1–190 [PubMed] [Google Scholar]
- 49.Weatherly MR, Palmer CG, Peters ME, et al. Wisconsin cystic fibrosis chest radiograph scoring system. Pediatrics 1993;91:488–95 [PubMed] [Google Scholar]
- 50.Koscik RE, Kosorok MR, Farrell PM, et al. Wisconsin cystic fibrosis chest radiograph scoring system: validation and standardization for application to longitudinal studies. Pediatr Pulmonol 2000;29:457–67 [DOI] [PubMed] [Google Scholar]
- 51.Brasfield D, Hicks G, Soong S, Tiller RE. The chest roentgenogram in cystic fibrosis: a new scoring system. Pediatrics 1979;63:24–9 [PubMed] [Google Scholar]
- 52.Brasfield D, Hicks G, Soong S, Peters J, Tiller R. Evaluation of scoring system of the chest radiograph in cystic fibrosis: a collaborative study. AJR Am J Roentgenol 1980;134:1195–8 [DOI] [PubMed] [Google Scholar]
- 53.Farrell PM, Li Z, Kosorok MR, et al. Bronchopulmonary disease in children with cystic fibrosis after early or delayed diagnosis. Am J Respir Crit Care Med 2003;168:1100–8 [DOI] [PubMed] [Google Scholar]
- 54.Lai HC, Kosorok MR, Laxova A, Davis LA, FitzSimmon SC, Farrell PM. Nutritional status of patients with cystic fibrosis with meconium ileus: a comparison with patients without meconium ileus and diagnosed early through neonatal screening. Pediatrics 2000;105:53–61 [DOI] [PubMed] [Google Scholar]
- 55.Shoff SM, Ahn H, Davis LA, Lai HJ, Wisconsin CF. Neonatal Screening Group. Temporal associations between energy intake, plasma linoleic acid and growth improvement in response to treatment initiation after diagnosis of cystic fibrosis. Pediatrics 2006;117:391–400 [DOI] [PubMed] [Google Scholar]
- 56.Lai HJ, Shoff SM. Farrell PM, Wisconsin CF Neonatal Screening Group. Recovery of birth weight z-score within two years of diagnosis is positively associated with pulmonary status at age six years in children with cystic fibrosis. Pediatrics 2009;123:714–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thulier D. Breastfeeding in America: a history of influencing factors. J Hum Lact 2009;25:85–94 [DOI] [PubMed] [Google Scholar]
- 58.Stein RT, Holberg CJ, Sherrill D, et al. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson Children's Respiratory Study. Am J Epidemiol 1999;149:1030–7 [DOI] [PubMed] [Google Scholar]
- 59.Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med 2000;343:538–43 [DOI] [PubMed] [Google Scholar]