Abstract
Background: Polyunsaturated fatty acids and fish may influence bone health.
Objective: We aimed to examine associations between dietary polyunsaturated fatty acid and fish intakes and hip bone mineral density (BMD) at baseline (1988–1989; n = 854) and changes 4 y later in adults (n = 623) with a mean age of 75 y in the Framingham Osteoporosis Study.
Design: BMD measures were regressed on energy-adjusted quartiles of fatty acid intakes [n−3 (omega-3): α-linolenic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and EPA+ DHA; n−6 (omega-6): linoleic acid (LA) and arachidonic acid (AA); and n−6:n−3 ratio] and on categorized fish intakes, with adjustment for covariates. Effect modification by EPA+DHA intake was tested for n−6 exposures.
Results: High intakes (≥3 servings/wk) of fish relative to lower intakes were associated with maintenance of femoral neck BMD (FN-BMD) in men (dark fish + tuna, dark fish, and tuna) and in women (dark fish) (P < 0.05). Significant interactions between AA and EPA+DHA intakes were observed cross-sectionally in women and longitudinally in men. In women with EPA+DHA intakes at or above the median, those with the highest AA intakes had a higher mean baseline FN-BMD than did those with the lowest intakes (quartile 4 compared with quartile 1: P = 0.03, P for trend = 0.02). In men with the lowest EPA+DHA intakes (quartile 1), those with the highest intakes of AA (quartile 4) lost more FN-BMD than did men with the lowest intakes of AA (quartile 1; P = 0.04). LA intake tended to be associated with FN-BMD loss in women (P for trend < 0.06).
Conclusions: Fish consumption may protect against bone loss. The protective effects of a high AA intake may be dependent on the amount of EPA+DHA intake.
INTRODUCTION
The n−3 and n−6 polyunsaturated fatty acids (PUFAs) have been suggested to influence bone health through several mechanisms, including opposing effects on inflammatory cytokines (1, 2), modulation of prostaglandin E2 (PGE2) production (3, 4), enhancement of calcium transport, and reducing urinary calcium excretion (5, 6). Fatty acids (n−3 and n−6) of various chain lengths and their derivatives have also been shown to serve as ligands for peroxisome proliferator–activator receptor-α and -γ (7), which have been found to inhibit the function of nuclear transcription factor κB (NF-κB) (8, 9) and to be involved in the differentiation of mesenchymal stem cells to adipocytes or osteoblasts (10–12), respectively. Long-chain PUFAs also serve as precursors in the production of pro-resolving lipid mediators, including lipoxins synthesized from arachidonic acid (AA), and E-series and D-series resolvins synthesized from eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), respectively (13, 14). Lipoxins and resolvins appear to have a myriad of effects that promote the resolution of inflammation, and both classes of lipid mediators have been found to reduce bone loss induced by periodontitis in animal models (15–17).
Several animal studies support a protective relation between n−3 fatty acids or a lower n−6 to n−3 ratio and bone health (18–24). For example, Watkins et al (19) showed that male rats randomized to a diet with a low n−6:n−3 ratio had a significantly lower ex vivo PGE2 production and a significantly greater activity of serum alkaline phosphatase, a marker of bone formation, than did rats randomized to diets with a higher n−6:n−3 ratio. Watkins and Seifert (24) showed further that female ovariectomized rats fed a diet with a 5:1 n−6:n−3 ratio lost significantly less femur and tibia bone mineral content over 12 wk than did rats fed a diet with a 10:1 ratio, regardless of the amount of n−6 fatty acids. Others, however, have found either no effect (25, 26) or detrimental effects when supplemented with high intakes of long-chain n−3 fatty acids (27, 28). Growing rabbits fed a diet supplemented with vitamin E and fish oil (10 g/100 g diet) for 40 d were shown to have significantly shorter tibias than controls (27). Poulsen and Kruger (28) found that ovariectomized rats supplemented with EPA (1.0 g/kg body weight) for 9 wk had a significantly lower BMD than did both ovariectomized controls and sham controls, but no difference in BMD was found between ovariectomized rats supplemented with EPA at a lower dose (0.1 g/kg body weight) and ovariectomized controls.
Human studies of the relation between n−3 and n−6 fatty acid intakes and bone are limited. In a controlled feeding study with a crossover design, subjects randomly assigned to a diet high in α-linolenic acid (ALA) had significantly lower serum N-telopeptide concentrations, a marker of bone resorption, than did subjects randomly assigned to an average American diet low in ALA (29). Protective effects with fish oil or n−3 fatty acid supplementation in postmenopausal women have been observed (30, 31). However, Bassey et al (32) observed no significant differences in BMD or in markers of bone turnover in 85 pre- and postmenopausal women given a supplement containing fish oil in addition to evening primrose oil and calcium for 12 mo compared with calcium alone. Serum n−3 fatty acids, particularly DHA, were associated with total-body BMD and accrual of BMD of the total body and spine over 4 y in young men aged <18 y at baseline (33). In the present study, we examined associations between PUFAs and fish intakes and hip BMD at baseline and 4-y changes in hip BMD in older adult women and men in the Framingham Osteoporosis Study.
SUBJECTS AND METHODS
Subjects
Subjects were drawn from the Framingham Heart Study, which began in 1948 with the goal of identifying risk factors for cardiovascular disease. At its inception, 5209 women and men aged 28–62 y were recruited from a random sample of two-thirds of the households in Framingham, MA, to participate in the initial examination and were examined biennially thereafter for >50 y (34). In 1988, the Framingham Osteoporosis Study was initiated as an ancillary study to the Framingham Heart Study, and 1164 of the 1402 surviving members of the original cohort had a femur BMD measurement taken at the 20th biennial examination. This study used data from participants of the original cohort at exam 20 (1988–1989) who had femoral neck (FN) or trochanter BMD measured at exam 20 for cross-sectional analyses and FN or trochanter BMD measured at exam 22 for longitudinal analyses. At baseline (exam 20), we excluded subjects with missing BMD measurements at the FN or trochanter sites (n = 64). We further excluded subjects with missing or incomplete food-frequency questionnaire (FFQ) (based on the criteria of >12 food items left blank or energy intakes <600 or >4000 kcal/d) data at exam 20 (n = 159 for missing FFQ and n = 77 for invalid FFQ) and missing covariate information for body mass index (BMI; n = 6), smoking status (n = 2), and physical activity (n = 2). The final baseline sample used for cross-sectional analyses comprised 530 women and 324 men with complete BMD, FFQ, and covariate information at exam 20. The final sample used for longitudinal analyses comprised 397 women and 225 men with complete BMD measures at exams 20 and 22 in addition to complete FFQ and covariate information at exam 20. This study was approved by the Institutional Review Boards for Human Research at Boston University; Hebrew Rehabilitation Center, Institute for Aging Research; and Tufts University.
Most of the participants (89%, or 213 of 238) who did not have BMD measures taken at the baseline exam were previously reported to be nursing home residents or required examination within their home, which excluded these subjects because of the lack of machine portability (35). These subjects were reported to be slightly older (average age: 78.1 ± 6.88 y compared with 76.1 ± 5.19 y) and consistent of more women (66.2%) than men (61.5%) (35). No significant differences in sex, educational level, smoking status, or BMI were found between participants with and without complete BMD measures and dietary data in previous investigations of this cohort (36). As expected, participants with complete data were younger and more physically active than were those without complete data (36). In this cohort, it has been reported that participants who did not return for clinic visits had a lower BMD than did those who did have follow-up measures (37).
BMD measurements
BMD (g/cm2) of the right proximal hip (FN and trochanter) was measured at baseline (exam 20) by using a dual-photon absorptiometer (DP3; Lunar Radiation, Madison, WI) and again at the 4-y follow-up (exam 22) by using dual-energy X-ray absorptiometry (DPX-L; Lunar Radiation) as previously described (35, 38). At baseline (exam 20), the CV determined from a phantom of the hip was 4.15% (FN) and 1.49% (trochanter). The CV for baseline measurements was also determined in young normal controls (n = 3) and was found to be 2.65% (FN) and 2.80% (trochanter) (35). At follow-up (exam 22), a bone phantom was measured monthly over the follow-up data collection period, which indicated no machine drift over time. The CV for follow-up measurements was also determined in a group of cohort participants (n = 21), measured twice, and was found to be 1.7% (FN) and 2.5% (trochanter) (38). Cross-calibration performed between the 2 machines showed a small, consistent shift in hip BMD values; therefore, published correction equations were used to adjust exam 20 BMD values to make them more comparable with exam 22 values (39).
Dietary assessment
Usual dietary intake during the previous 12 mo was assessed with a self-administered 126-item semiquantitative Willett FFQ, which was previously validated for several nutrients, including total PUFAs and linoleic acid (LA) (40–43), and most recently for EPA, DHA, and fish intakes in elderly subjects (44). Participants were mailed the FFQ before their baseline exam visit and were asked to report their frequency of consumption of each food item before returning the completed FFQ at the exam visit. Questionnaires in which subjects reported energy intakes <600 and >4000 kcal (<2.51 and <16.74 MJ) per day or left >12 food items blank were considered invalid and were excluded from the analysis (n = 77). The FFQ identifies nutrient intakes from both food and vitamin and mineral supplements, as described in detail elsewhere (41). Briefly, nutrient intakes are calculated by multiplying the nutrient composition for a specified portion size by a weight, representing the frequency of use. After invalid FFQs were excluded for blank responses, remaining blank food items were considered “never used” (40). Use of n−3 supplements was identified by the FFQ, but few participants reported supplement use (n = 2 for women and n = 2 for men). The n−6:n−3 ratio was derived by dividing the total n−6 fatty acid intake by the total n−3 fatty acid intake. Total fish intake (3–5-oz servings/wk, or ≈85–142 g/wk) was defined as the sum of canned tuna, dark fish (mackerel, salmon, sardines, bluefish, and swordfish), white fish, and shellfish as reported by subjects on the FFQ. Fish intakes were categorized separately for women and men as low (<1 serving/wk), moderate (≥1 serving/wk, but <3 servings/wk), and high (≥3 servings/wk). These fish intake cutoffs were used to account for the distribution of fish intake observed in this cohort and to ensure that the American Heart Association recommendation of consuming 2 servings of fish per week would be included in the moderate fish intake category.
Covariate measurements
Covariates included factors known to affect BMD: age (y), BMI (kg/m2), height at exam 1 (cm), dietary and supplemental intakes of calcium (mg/d) and vitamin D (IU/d), energy intake (kcal/d), alcohol intake, physical activity, smoking status, and estrogen use for women (38). Protein intake (g/d) was also considered as a confounder in the statistical models because it has been identified as a possible nutritional risk factor for osteoporosis (38) and because dietary sources of n−3 fatty acids, namely fish and seafood, are also rich sources of protein. We also considered vitamin K (μg/d) and fruit and vegetables (servings/d) as confounders for n−6 fatty acid exposures, because both vitamin K (45) and fruit and vegetable intakes (36) have been found to be associated with BMD and because vitamin K is a fat-soluble vitamin found in leafy green vegetables usually consumed with salad dressings, which are a rich source of the n−6 fatty acid LA. One serving of fruit and vegetables was considered equivalent to 1 cup raw leafy vegetables (30 g), 0.5 cups cut-up raw or cooked vegetable (91 g), 0.5 cups fruit or vegetable juice (124 g), 1 medium whole fruit (138 g), 0.25 cups dried fruit (36 g), or 0.5 cups fresh, frozen, or canned fruit (115 g). Usual dietary intakes of calcium, vitamin D, alcohol, energy, protein, and vitamin K were assessed with the FFQ described previously. Intakes of calcium, vitamin D, and vitamin K were calculated as intakes from both food and supplement sources. For alcohol intake, subjects were classified as nondrinkers, moderate drinkers (<13.2 g/d for women and <26.4 g/d for men), or heavy drinkers (≥13.2 g/d for women and ≥26.4 g/d) based on the Dietary Guidelines for Americans, which recommend a maximum of 1 drink/d for women and 2 drinks/d for men (46). Physical activity was measured by using the Framingham physical activity index (PAI), which represents the sum of the number of hours per day spent engaged in activity, weighted according to amount of activity as follows: sleep (1.0), sedentary (1.1), slight (1.5), moderate (2.4), and heavy (5.0) (47). PAI from exam 19 (1986–1987) was used for subjects with a missing PAI at exam 20. Smoking status was determined based on questionnaire responses and was defined as nonsmoker, past smoker, or current smoker (smoked regularly in the past year). BMI (kg/m2) was calculated from weight measurements taken at exam 20 and height measurements taken at exam 1 (1948–1949). Because BMI is a measure of relative weight designed to be independent of height, both BMI and height at exam 1 were included in the statistical models to adjust for ponderosity and body stature, which may be related to dietary intake and BMD (48). For women, estrogen use was defined as current use (use of estrogen for >1 y) compared with never or past use. Never or past users were categorized together because it has been shown that beneficial effects on bone are not retained with past estrogen use (49).
Statistical analysis
SAS statistical software (version 9.1; SAS Institute, Cary, NC) was used to perform all statistical analyses. P < 0.05 (2-sided) was considered statistically significant in all analyses. We tested for effect modification by sex by first including an interaction term in the analysis of the combined sample. Measures of baseline BMD and 4-y change in BMD at the FN and trochanter were regressed onto quartiles of energy-adjusted essential fatty acid intakes separately for women and men by using the general linear model procedure in SAS, with adjustment for covariates and multiple comparisons with Dunnett's adjustment. For n−3 fatty acid intakes, these exposures were ALA, EPA, DHA, and EPA+DHA. For n−6 fatty acid intakes, these exposures were LA and AA. We also examined the n−6:n−3 ratio as an exposure and intakes of total fish, dark fish + tuna, dark fish, and tuna. We also performed a test for linear trend across the quartiles by creating a continuous variable set equal to the median energy-adjusted fatty acid value for each quartile and regressing BMD measures on this continuous variable, adjusting for covariates.
All models were first adjusted for age, BMI, height at exam 1, dietary and supplemental intakes of calcium and vitamin D, physical activity, smoking status (nonsmoker, past smoker, or current smoker), alcohol intake (no intake, moderate intake, or heavy intake), baseline BMD (longitudinal analyses only), energy intake (kcal/d), and estrogen use for women (current compared with never or past use). For fish intake models, vitamin D from supplemental and dietary sources was considered separately. Models were then adjusted, separately, for protein intake, total fat intake, and vitamin K intake and fruit and vegetable intake (for the n−6 exposures only). Protein intake was previously found to be associated with BMD (50) and may be associated with fatty acid intakes. We adjusted for total fat intake (residually energy-adjusted and as an absolute amount in g/d + energy intake, separately in the model) to ensure that the relations observed between essential fatty acid intakes and bone were independent of total fat intake. In addition to PUFAs, other components of total fat may be related to bone. Saturated fat intake has been inversely associated with hip BMD (51), whereas monounsaturated fat intake was found to be positively associated with BMD (52). Because PUFA intake was correlated with monounsaturated fat intake (r = 0.65) and saturated fat intake (r = 0.39), and because total fat intake was highly correlated with monounsaturated fat intake (r = 0.97) and saturated fat intake (r = 0.93), we were unable to assess the effect of substituting essential fatty acid intakes for these specific types of other dietary fats. Vitamin K intake and fruit and vegetable intakes were adjusted, separately, in n−6 models only, because both vitamin K and fruit and vegetables have been found to have beneficial effects on bone and may be associated with n−6 fatty acid intake through consumption of salad dressings (36, 45).
To adjust for energy intake in the fatty acid exposure models, we used the residual-energy-adjustment method in which the energy-yielding nutrients (essential fatty acid intakes and protein intake) were regressed on total energy intake to obtain nutrient intake residuals. These residuals were then used to create quartiles of essential fatty acid intakes before the regression models were performed. Use of this method of energy adjustment provides a measure of nutrient intake that is not associated with energy intake (53).
We tested for effect modification by EPA+DHA intake for the n−6 fatty acid exposures to examine whether associations between n−6 fatty acid intakes and BMD differ according to intakes of the long-chain n−3 fatty acids. The EPA+DHA variable was categorized (0,1) as below or at or above the median value (0.145 g/d for men and 0.140 g/d for women) in addition to being analyzed as a continuous variable and quartiles.
Dietary essential fatty acid exposure variables and calcium and vitamin K intakes were positively skewed. A natural logarithmic transformation was applied to intakes of ALA, EPA, DHA, EPA+DHA, calcium, and vitamin K, whereas a square root transformation was applied to intakes of LA and AA before all of the statistical analyses were performed to achieve normality of the nutrient intakes and energy-adjusted nutrient intake residuals. Transforming skewed nutrient intake exposures has been recommended to improve heteroscedasticity, which occurs as a result of greater variation in nutrient intakes at higher energy intakes (54).
To ensure that the findings of the longitudinal analyses were not biased as a result of loss to follow-up, we performed our baseline analyses with both a “full” baseline sample and a “restricted” baseline sample, which comprised only those participants who were available at the 4-y follow-up.
RESULTS
An interaction between sex and essential fatty acid intake was found for ALA at the trochanter (P = 0.05), and interactions for ALA at the FN, AA at the trochanter, and the n−6:n−3 ratio at the trochanter were nearly significant (P < 0.1) in cross-sectional analyses. An interaction for EPA at the FN was also nearly significant (P < 0.1) in longitudinal analyses; thus, analyses were conducted separately for women and men. No associations between fatty acid intakes and BMD status at the trochanter site were observed in either cross-sectional or longitudinal analyses, and associations between fish intake and BMD at the trochanter site were similar to associations observed at the FN; thus, only results for the FN site are described below to simplify the reporting of results.
Subject characteristics
Mean (±SD) values and percentages for subject characteristics at baseline (exam 20) are presented in Table 1. Proportionately more women (39%) than men (11%) reported n−3 ALA intakes that met or exceeded the current Adequate Intake values of 1.1 g/d for women and 1.6 g/d for men, according to Dietary Reference Intakes (46). The percentages of women and men reporting n−6 LA intakes above the upper limit of the Acceptable Macronutrient Distribution Range (AMDR) of 10 g/d were 42% and 46%, respectively. The AMDR is a range of intakes that, when consumed in excess of the upper limit, is associated with an increasing risk of chronic diseases (46). Average total fish intakes (2.2 and 2.1 servings/wk for women and men, respectively) concurred with current recommendations of the American Heart Association to eat fish 2 times/wk (55). The type of fish consumed was mainly tuna (36% of total fish intake for women and 31% for men) and white and other fish (38% for both women and men), whereas dark fish accounted for 12% (women) and 17% (men). The average percentage change in FN-BMD was −3.5% for women and −1.5% for men, which represented an average annualized percentage changes of −0.88% and −0.38%, respectively. The average percentage change in trochanter BMD was −3.4% for women and 0.4% for men, which represented average annualized percentage changes of −0.85% and 0.1%, respectively.
TABLE 1.
Subject characteristics at baseline (exam 20)1
| Women (n = 530) | Men (n = 324) | |
| Descriptive variables | ||
| Age (y) | 75.3 ± 4.8 (67–93)2 | 75.1 ± 4.9 (68–91) |
| BMI (kg/m2) | 26.3 ± 4.9 | 27.1 ± 4.0 |
| Physical activity index | 33.3 ± 5.1 (26–58) | 34.0 ± 6.3 (25–63) |
| Smoking status (%) | ||
| Past smoker | 44.0 | 64.5 |
| Current smoker | 11.5 | 9.3 |
| Estrogen use (%) | 4.7 | NA |
| Dietary variables | ||
| ALA intake (g/d) | 1.03 ± 0.47 | 1.05 ± 0.43 |
| EPA intake (g/d) | 0.06 ± 0.07 | 0.06 ± 0.07 |
| DHA intake (g/d) | 0.14 ± 0.12 | 0.14 ± 0.13 |
| LA intake (g/d) | 9.96 ± 4.90 | 10.83 ± 5.36 |
| AA intake (g/d) | 0.10 ± 0.05 | 0.11 ± 0.05 |
| n−6:n−3 ratio | 8.22 ± 2.89 | 9.01 ± 4.09 |
| Energy intake (kcal/d) | 1674 ± 553 | 1871 ± 612 |
| Protein intake (g/d) | 67 ± 23.6 | 69 ± 23.0 |
| Alcohol use (%) | ||
| Moderate | 34.2 | 45.4 |
| Heavy | 17.4 | 19.8 |
| Calcium intake (mg/d)3 | 823 ± 441 | 767 ± 395 |
| Vitamin D intake (IU/d)3 | 334 ± 261 | 326 ± 282 |
| Vitamin K intake (μg/d)3 | 164 ± 119 | 142 ± 97 |
| Fruit and vegetable intake (servings/d) | 5.5 ± 2.7 | 4.8 ± 2.4 |
| Fish variables (servings/wk)4 | ||
| Tuna | 0.81 ± 0.97 | 0.65 ± 0.85 |
| Dark fish | 0.27 ± 0.61 | 0.35 ± 0.79 |
| White and other fish | 0.84 ± 0.99 | 0.79 ± 0.98 |
| Shellfish | 0.30 ± 0.55 | 0.27 ± 0.41 |
| Total fish | 2.23 ± 1.95 | 2.07 ± 1.83 |
| Baseline BMD (g/cm2) | ||
| Femoral neck | 0.722 ± 0.11 | 0.880 ± 0.15 |
| Trochanter | 0.623 ± 0.12 | 0.837 ± 0.14 |
| 4-y change in BMD (g/cm2) | ||
| Femoral neck | −0.025 ± 0.054 | −0.013 ± 0.063 |
| Trochanter | −0.021 ± 0.062 | 0.003 ± 0.087 |
Data are based on the full baseline data set, which consists of subjects with femoral neck and trochanter bone mineral density (BMD) measurements at baseline and valid food-frequency questionnaire and complete covariate data. ALA, α-linolenic acid; LA, linoleic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; NA, not applicable.
Mean ± SD; range in parentheses (all such values).
Intake from diet plus supplements.
1 fish serving = 85–142 g/wk.
Cross-sectional associations between essential fatty acid intakes and BMD
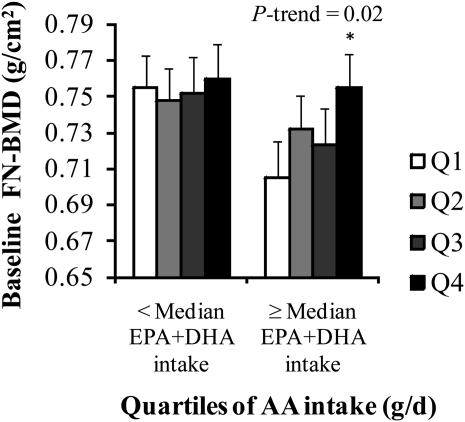
No significant cross-sectional associations were observed for intakes of the individual essential fatty acid intakes in women or men (P > 0.05, data not shown). However, a significant interaction was observed between AA intake and EPA+DHA intake (below or at or above the median) at the FN in women (P = 0.03). This interaction was nearly significant when AA and EPA+DHA intakes were analyzed as continuous variables (P = 0.06), but was not significant when AA and EPA+DHA intakes were analyzed as quartiles (P = 0.19). We therefore performed stratified analyses by EPA+DHA intake. In women with intakes of EPA+DHA at or above the median, those with the highest intakes of AA had a higher mean baseline FN-BMD than did those with the lowest intakes (Q4 compared with Q1: P = 0.03, P for trend = 0.02) (Figure 1), whereas no association with AA was observed in those with intakes of EPA+DHA below the median. After adjustment for protein intake, this association became nonsignificant (Q4 compared with Q1: P > 0.1, P for trend > 0.1), but mean BMD values were not substantially changed and the association between AA intake and FN-BMD remained in a protective direction. Energy-adjusted AA intake (g/d) tended to be lower in women with EPA+DHA intakes below the median than in women with EPA+DHA intakes at or above the median (Q1: 0.048 compared with 0.070; Q2: 0.073 compared with 0.097; Q3: 0.094 compared with 0.118; and Q4: 0.135 compared with 0.161). However, the magnitudes of the difference in energy-adjusted AA intakes between quartiles were similar (Q1 to Q2: 0.025 compared with 0.027; Q2 to Q3: 0.021 compared with 0.021; and Q3 to Q4: 0.041 compared with 0.043).
FIGURE 1.
Mean (±SEM) femoral neck bone mineral density (FN-BMD) regressed onto energy-adjusted quartiles (Q) of arachidonic acid (AA) intake in women stratified by intake of eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) (below or at or above the median; n = 530). Data were adjusted for age, BMI, height at exam 1, dietary and supplemental intakes of calcium, dietary and supplemental intakes of vitamin D, physical activity, smoking status, alcohol intake, energy intake, and estrogen use. Energy was adjusted by using the residual method. Adjustment for multiple comparisons was performed by using Dunnett's adjustment. Natural log transformation was applied to EPA+DHA intakes; square root transformation was applied to AA intakes. Median EPA+DHA intake = 0.14 g/d. P for interaction = 0.03. *Significantly different from Q1, P < 0.05.
No significant interaction was observed between EPA+DHA intake and LA in women (P > 0.05), nor were any significant interactions observed between EPA+DHA intake and either AA or LA intake in men in the cross-sectional analysis (P > 0.05). Adjustment for vitamin K and fruit and vegetable intake in n−6 models did not appreciably alter any adjusted mean BMD values or significance values. The repeat baseline analyses of the “restricted” baseline sample consisted of only those participants who were available at the 4-y follow-up (n = 397 women and 225 men) and did not appreciably alter the direction of the associations between essential fatty acid intakes and BMD or significance values; thus, we presented only the results of analyses using the “full” baseline sample (n = 530 women and 324 men).
Longitudinal associations between essential fatty acid intakes and 4-y change in BMD
The main effects of longitudinal associations are shown in Table 2. Women with the greatest intakes of LA tended to lose more FN-BMD over 4 y than did those with the lowest intakes (P for trend = 0.06). No significant associations were found between intakes of the other essential fatty acids and 4-y changes in FN-BMD in women. Also, no significant interactions longitudinally were observed with EPA+DHA intake for LA or AA in women (P > 0.05). In men, those with greater intakes of EPA, DHA, and EPA+DHA lost significantly less FN-BMD over 4 y than did those with lower EPA, DHA, and EPA+DHA intakes (P for trend < 0.05). Men with greater intakes of AA also tended to lose less FN-BMD than did those with lower AA intakes (P for trend = 0.06). Adjustment for protein intake did not substantially attenuate the associations between the long-chain n−3 fatty acids and bone loss (P for trend remained < 0.05 for these exposures), but did attenuate the significance of the association between AA intake and bone loss (P for trend > 0.1).
TABLE 2.
Four-year change in femoral neck bone mineral density (FN-BMD) by quartile (Q) of essential fatty acid intake1
| Essential fatty acid intake |
|||||
| Q1 | Q2 | Q3 | Q4 | P for trend | |
| g/cm2 | |||||
| Women | |||||
| ALA | −0.021 ± 0.008 | −0.018 ± 0.008 | −0.027 ± 0.008 | −0.029 ± 0.008 | 0.18 |
| EPA | −0.026 ± 0.008 | −0.021 ± 0.008 | −0.019 ± 0.008 | −0.032 ± 0.008 | 0.55 |
| DHA | −0.026 ± 0.008 | −0.023 ± 0.008 | −0.030 ± 0.008 | −0.022 ± 0.008 | 0.85 |
| EPA+DHA | −0.025 ± 0.008 | −0.024 ± 0.008 | −0.023 ± 0.008 | −0.027 ± 0.008 | 0.85 |
| LA | −0.020 ± 0.008 | −0.023 ± 0.008 | −0.016 ± 0.008 | −0.038 ± 0.008† | 0.06 |
| AA | −0.025 ± 0.008 | −0.025 ± 0.008 | −0.028 ± 0.008 | −0.023 ± 0.008 | 0.82 |
| n−6:n−3 | −0.021 ± 0.008 | −0.027 ± 0.008 | −0.025 ± 0.008 | −0.029 ± 0.008 | 0.35 |
| Men | |||||
| ALA | −0.015 ± 0.009 | −0.028 ± 0.010 | −0.028 ± 0.010 | −0.025 ± 0.010 | 0.43 |
| EPA | −0.042 ± 0.010 | −0.016 ± 0.010† | −0.021 ± 0.009 | −0.013 ± 0.010† | 0.02 |
| DHA | −0.040 ± 0.009 | −0.022 ± 0.010 | −0.018 ± 0.009 | −0.009 ± 0.010* | 0.03 |
| EPA+DHA | −0.043 ± 0.010 | −0.018 ± 0.010 | −0.019 ± 0.009† | −0.011 ± 0.010* | 0.01 |
| LA | −0.028 ± 0.009 | −0.019 ± 0.009 | −0.018 ± 0.010 | −0.029 ± 0.010 | 0.92 |
| AA | −0.028 ± 0.010 | −0.035 ± 0.010 | −0.025 ± 0.009 | −0.006 ± 0.010 | 0.06 |
| n−6:n−3 | −0.020 ± 0.010 | −0.024 ± 0.010 | −0.018 ± 0.010 | −0.030 ± 0.009 | 0.47 |
All values are means ± SEMs. Data were regressed onto quartiles of energy-adjusted essential fatty acid intakes and adjusted for age, BMI, height at examination 1, dietary and supplemental intakes of calcium, dietary and supplemental intakes of vitamin D, physical activity, smoking status, alcohol intake, energy intake, baseline BMD, energy intake, and estrogen use in women (n = 397 for women, n = 225 for men). Energy was adjusted by using the residual method. Natural log transformation was applied to α-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), EPA+DHA, and n−6:n−3 ratio; square root transformation was applied to linoleic acid (LA) and arachidonic acid (AA). *,†Significantly different from Q1: *P < 0.05, †P < 0.1.
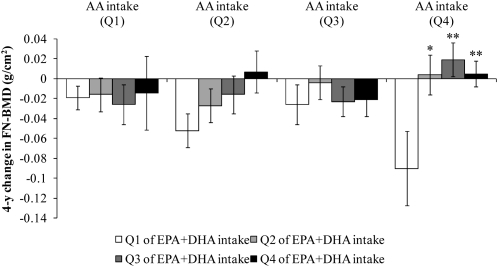
The interaction between AA intake and EPA+DHA intake (below or at or above the median) was not significant (P > 0.05), but was significant when AA and EPA+DHA intakes were analyzed as continuous variables (P = 0.01) and as quartiles (P = 0.04). The interaction remained significant after adjustment for protein intake (P = 0.01). We therefore cross-classified men according to energy-adjusted quartiles of EPA+DHA and AA intake and regressed the 4-y change in FN-BMD measures onto these cross-classified quartiles, adjusted for covariates (Figures 2 and 3). Men with the highest intakes of EPA+DHA generally lost less FN-BMD than did men with the lowest intakes of EPA+DHA, at each amount of AA intake. These associations were not significant (P > 0.05), with the exception of an association observed within quartile 4 of AA intake. In men with the highest intake of AA (Q4), those with the lowest intakes of EPA+DHA (Q1) lost significantly more FN-BMD than did men with higher intakes of EPA+DHA (Q1 compared with Q2: P = 0.02; Q1 compared with Q3: P < 0.01; Q1 compared with Q4: P < 0.01).
FIGURE 2.
Mean (±SEM) 4-y change in femoral neck bone mineral density (FN-BMD) regressed onto energy-adjusted quartiles (Q) of eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) intake, stratified by quartile of arachidonic acid (AA) intake in men (n = 225). Data were adjusted for age, BMI, height at exam 1, dietary and supplemental intakes of calcium, dietary and supplemental intakes of vitamin D, physical activity, smoking status, alcohol intake, and energy intake. Energy was adjusted by using the residual method. Natural log transformation was applied to EPA+DHA intakes; square root transformation was applied to AA intakes. P for interaction = 0.04. *,**Significantly different from Q1: *P < 0.05, **P < 0.01.
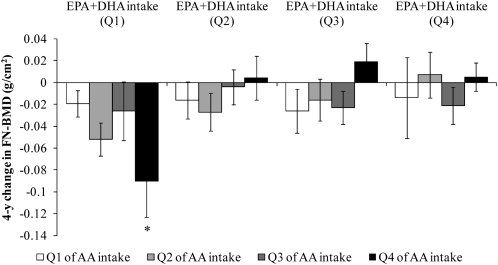
FIGURE 3.
Mean (±SEM) 4-y change in femoral neck bone mineral density (FN-BMD) regressed onto energy-adjusted quartiles (Q) of arachidonic acid (AA) intake, stratified by quartile of eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) intake in men (n = 225). Data were adjusted for age, BMI, height at exam 1, dietary and supplemental intakes of calcium, dietary and supplemental intakes of vitamin D, physical activity, smoking status, alcohol intake, and energy intake. Energy was adjusted by using the residual method. Natural log transformation was applied to EPA+DHA intakes; square root transformation was applied to AA intakes. P for interaction = 0.04. *Significantly different from Q1 of AA intake, P < 0.05.
The protective effect of high AA intake appeared dependent on the amount of EPA+DHA intake. In men with the lowest EPA+DHA intakes (Q1), those with the highest intakes of AA (Q4) lost significantly more FN-BMD than did men with the lowest intakes of AA (Q1) (P = 0.04). In contrast, associations between AA intake and FN-BMD within quartiles 2, 3, and 4 of EPA+DHA intakes were in a protective direction, but were not significant within these quartiles (P > 0.05).
No evidence of an interaction between EPA+DHA intake and LA intake in men was found (P > 0.05), and no significant associations was found between intakes of LA or the n−6:n−3 ratio and 4-y change in FN-BMD in men. Further adjustment for total fat and for vitamin K and fruit and vegetable intake in n−6 models did not appreciably alter the adjusted BMD values or significance values.
Associations between fish intake and BMD
Cross-sectionally, both women and men with high fish intakes (≥3 servings/wk) had a greater mean baseline FN-BMD than did those with moderate (≥1 serving/wk, but <3 servings/wk), or low (<1 serving/wk) fish intakes, but the associations were not significant (P > 0.05, data not shown).
Protective associations were observed longitudinally between fish intakes and change in BMD over 4 y in women and men (Figures 4 and 5). Women with high intakes of dark fish, on average, did not lose bone at the FN over 4 y compared with women with moderate (P < 0.01) or low (P = 0.02) intakes of dark fish who lost FN-BMD over 4 y. The addition of protein intake or remaining fish intake to the statistical models did not attenuate this association. Women with high intakes of total fish, dark fish + tuna, and tuna also lost less FN-BMD than did women with moderate or low fish intakes, but the associations were not significant (P > 0.05).
FIGURE 4.
Mean (±SEM) 4-y change in femoral neck bone mineral density (FN-BMD) regressed onto amount of fish intake in women (n = 397). Data were adjusted for age, BMI, height at exam 1, dietary and supplemental intakes of calcium, dietary and supplemental intakes of vitamin D, physical activity, smoking status, alcohol intake, energy intake, estrogen use in women, and baseline BMD. Adjustment for multiple comparisons was performed by using Dunnett's adjustment. *,**Significantly different from the high fish intake group: *P < 0.05, **P < 0.01. Low fish intake = <1 serving/wk, moderate fish intake = ≥1 but <3 servings/wk, and high fish intake = ≥3 servings/wk.
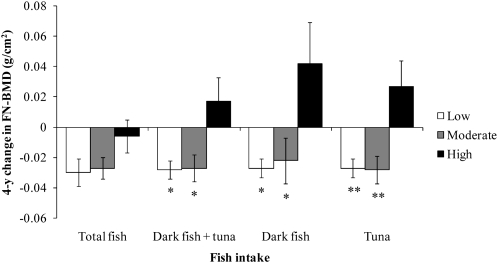
FIGURE 5.
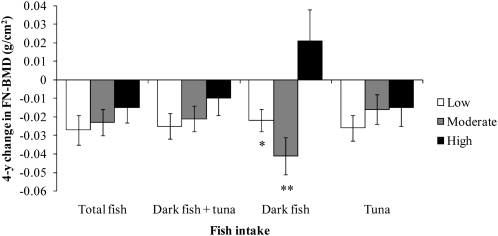
Mean (±SEM) 4-y change in femoral neck bone mineral density (FN-BMD) regressed onto amount of fish intake in men (n = 225). Data were adjusted for age, BMI, height at exam 1, dietary and supplemental intakes of calcium, dietary and supplemental intakes of vitamin D, physical activity, smoking status, alcohol intake, energy intake, and baseline BMD. Adjustment for multiple comparisons was performed by using Dunnett's adjustment. *,**Significantly different from the high fish intake group: *P < 0.05, **P < 0.01. Low fish intake = <1 serving/wk, moderate fish intake = ≥1 but <3 servings/wk, and high fish intake = ≥3 servings/wk.
In men, intakes of dark fish + tuna, dark fish, and tuna were inversely associated with bone loss at the FN over 4 y (P < 0.05 for dark fish + tuna and dark fish, P < 0.01 for tuna). Adjustment for protein intake or remaining fish intake did not attenuate these associations. Men with high intakes of total fish lost less FN-BMD than did men with moderate or low intakes of total fish, but these associations were not significant (P > 0.05).
DISCUSSION
In this study of associations between dietary intakes of PUFAs and fish and hip BMD, we observed significant protective associations between high intakes of fish (≥3 servings/wk) and 4-y bone loss at the FN in women (for dark fish) and men (for dark fish + tuna, dark fish, and tuna). In addition, we observed a significant interaction between intakes of EPA+DHA and AA in women, such that AA was protective in women with high EPA+DHA intakes, but not in women with low intakes. Longitudinally, the main effects of EPA, DHA, and AA were protective overall in men, but a significant interaction between intakes of EPA+DHA and AA was observed, such that AA was detrimental in men with the lowest intake of EPA+DHA.
Cold water fatty fish are rich sources of the metabolically active long-chain n−3 fatty acids EPA and DHA, which have been well documented to exert protective effects on bone through many mechanisms (reviewed in references 1–4). Fish are a good source of protein, but the protective effect of fish intake that we observed appeared to be independent of protein intake. Although not commonly recognized, fish and seafood are also sources of n−6 fatty acids, and AA in particular may exert protective effects on bone (7–9, 56–58). Fish samples obtained from the east and west coast of the United States were recently documented to contain both LA and AA in varying amounts, depending on fish type (59). For example, in addition to high concentrations of n−3 fatty acids, farmed Atlantic salmon contained higher concentrations of n−6 fatty acids than did other species of salmon, such as sock-eye, Coho, and Copper River (59). In contrast, tuna contained low concentrations of total PUFAs relative to other fish, with slightly higher concentrations of n−6 fatty acids than n−3 fatty acids. In our study, the type of fish contributing to total fish intake varied. In women and men, white fish and tuna each contributed ≈30–40% of total fish intake, whereas dark fatty fish and shellfish each contributed ≈10–20% of total fish intake. The combination of n−3 fatty acids and the smaller amounts of n−6 fatty acids found in fish may partly explain why we observed a protective effect of high intakes of dark fish on bone loss in women, but no association between the individual long-chain fatty acids and bone loss in women. Current recommendations by the National Osteoporosis Foundation do not explicitly recommend fish consumption to increase the intake of long-chain PUFAs, but rather recommend the consumption of certain fish types to increase the intake of calcium and vitamin D (60).
We observed protective effects of AA at baseline in women with high EPA+DHA intakes, and longitudinally in men, but AA was detrimental in men with the lowest intake of EPA+DHA. Together, these results suggest that the protective effects of AA may be dependent on EPA+DHA intakes. AA may exert protective effects on bone by suppressing NF-kB activation (7–9, 56) or by generating lipoxins involved in resolving inflammation. A derivative of AA, 12d-PGJ2, has also been shown to stimulate collagen synthesis in human osteoblast cells (57, 58). Dietary supplementation with AA has been shown to elevate bone mass in piglets (26, 61), and maternal umbilical cord AA has been related to bone mass in healthy full-term infants in humans (62). However, findings by Watkins et al (19), in which the AA:EPA ratio of dietary interventions was positively associated with ex vivo PGE2 production but negatively associated with serum biomarkers of bone formation in male rats, suggest that overproduction of PGE2 related to AA intake and the resulting stimulation of osteoclast activity could have detrimental effects on bone modeling and remodeling. From the results of our study, it could be hypothesized that high EPA+DHA intakes may prevent the overproduction of PGE2, such that the protective effects of AA can be observed.
We further observed that higher intakes of LA, relative to lower intakes, tended to be associated with greater bone loss in women, but not in men. Although dietary LA can serve as a precursor in the biosynthesis of AA, this conversion is generally regarded as limited (63). Detrimental effects of LA may be independent of effects on the production of AA because LA itself has been shown to activate NF-κB (64, 65). Studies of the effect of n−6 fatty acids on bone in humans are sparse. Weiss et al (66) observed that increasing ratios of total n−6 to n−3 fatty acids and of LA to ALA were associated with lower hip BMD in 1532 women and men enrolled in the Rancho Bernardo Study. Macdonald et al (67) found that an increased intake of PUFAs was associated with bone loss at the FN in 891 women, but this study did not differentiate between types of PUFAs. Controversy remains regarding the usefulness of the dietary n−6:n−3 ratio (reviewed in reference 68). We observed no significant associations with the n−6:n−3 ratio in either women or men, but our findings suggest a detrimental effect of LA intake on BMD in women.
No significant associations were observed at the trochanter in either the baseline or the longitudinal analyses in women or men. This finding may appear curious, particularly because the percentage change in FN-BMD and trochanter BMD was similar in women (−3.46% and −3.37%, respectively). However, no significant longitudinal associations were observed at the FN in women, which is consistent with no longitudinal associations being observed at the trochanter. Several longitudinal associations were observed in men at the FN, but the percentage change in men was substantially greater for FN-BMD (−1.5%) than for trochanter BMD (0.4%). Because the average percentage change of 0.4% was small (and positive) at the trochanter, it is not likely that our study had sufficient power to detect the corresponding small effect sizes that would be present at the trochanter in men, if such associations did exist.
Our study had some limitations. Dietary exposures were obtained from an FFQ, which obtains dietary information in a semiquantitative manner. This instrument is useful for ranking subjects to distinguish one another according to intake, rather than estimating precise absolute intakes. However, validation of this FFQ with subcutaneous fat as the comparison measure showed reasonable correlations of 0.50 for total PUFA, 0.47 for EPA, and 0.48 for LA when these nutrients were expressed as a percentage of total fat (43). A recent validation of this FFQ to estimate EPA, DHA, and fish intakes against plasma phospholipid fatty acid profiles also showed reasonable correlations (44). In addition, it has been suggested that the AA content of certain fish estimated from dietary assessment methods that determine fatty acid composition of foods from the US Department of Agriculture National Nutrition Database, such as in this study, may overestimate AA intake and lead to erroneous conclusions regarding relations between AA intake and disease status (59). However, because we have also observed a protective effect of plasma phosphatidylcholine AA concentrations in relation to BMD and fracture risk in analyses conducted subsequent to this study (EK Farina, DP Kiel, R Roubenoff, EJ Schaefer, LA Cupples, KL Tucker, unpublished observations, 2011), it is unlikely that the protective effects of AA on BMD we observed with high intakes of EPA+DHA are due to gross inaccuracies in AA estimation.
Taken together, these results support the hypothesis of a protective effect of fish intake, particularly dark fish intake, and long-chain n−3 fatty acids on loss of FN-BMD in the elderly. Interactive effects between EPA+DHA and AA intakes suggest that protective effects of AA are dependent on high EPA+DHA intakes. Additional work is needed to clarify the interactive effects between the long-chain PUFAs and to determine whether dietary intakes of long-chain PUFAs and fish differentially affect osteoporotic fracture risk in women and men.
Acknowledgments
The authors' responsibilities were as follows—EKF, KLT, and DPK: designed the study; EKF: analyzed the data, wrote the manuscript, and was responsible for the final content; KLT: provided study oversight; KLT, DPK, RR, and EJS: provided essential materials and critical revision of the manuscript; and LAC: provided essential materials. None of the authors had a conflict of interest.
REFERENCES
- 1.Kettler DB. Can manipulation of the ratios of essential fatty acids slow the rapid rate of postmenopausal bone loss? Altern Med Rev 2001;6:61–77 [PubMed] [Google Scholar]
- 2.Albertazzi P, Coupland K. Polyunsaturated fatty acids. Is there a role in postmenopausal osteoporosis prevention? Maturitas 2002;42:13–22 [DOI] [PubMed] [Google Scholar]
- 3.Watkins BA, Lippman HE, Le Bouteiller L, Li Y, Seifert MF. Bioactive fatty acids: role in bone biology and bone cell function. Prog Lipid Res 2001;40:125–48 [DOI] [PubMed] [Google Scholar]
- 4.Watkins BA, Li Y, Lippman HE, Feng S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot Essent Fatty Acids 2003;68:387–98 [DOI] [PubMed] [Google Scholar]
- 5.Coetzer H, Claassen N, van Papendorp DH, Kruger MC. Calcium transport by isolated brush border and basolateral membrane vesicles: role of essential fatty acid supplementation. Prostaglandins Leukot Essent Fatty Acids 1994;50:257–66 [DOI] [PubMed] [Google Scholar]
- 6.Baggio B, Budakovic A, Nassuato MA, et al. Plasma phospholipid arachidonic acid content and calcium metabolism in idiopathic calcium nephrolithiasis. Kidney Int 2000;58:1278–84 [DOI] [PubMed] [Google Scholar]
- 7.Krey G, Braissant O, L'Horset F, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol 1997;11:779–91 [DOI] [PubMed] [Google Scholar]
- 8.Delerive P, De Bosscher K, Besnard S, et al. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem 1999;274:32048–54 [DOI] [PubMed] [Google Scholar]
- 9.Delerive P, De Bosscher K, Vanden Berghe W, Fruchart JC, Haegeman G, Staels B. DNA binding-independent induction of IkappaBalpha gene transcription by PPARalpha. Mol Endocrinol 2002;16:1029–39 [DOI] [PubMed] [Google Scholar]
- 10.Lecka-Czernik B. PPARs in bone: the role in bone cell differentiation and regulation of energy metabolism. Curr Osteoporos Rep 2010;8:84–90 [DOI] [PubMed] [Google Scholar]
- 11.Barak Y, Nelson MC, Ong ES, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 1999;4:585–95 [DOI] [PubMed] [Google Scholar]
- 12.Rosen ED, Sarraf P, Troy AE, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 1999;4:611–7 [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 2002;196:1025–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 2003;278:14677–87 [DOI] [PubMed] [Google Scholar]
- 15.Serhan CN, Jain A, Marleau S, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol 2003;171:6856–65 [DOI] [PubMed] [Google Scholar]
- 16.Hasturk H, Kantarci A, Ohira T, et al. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J 2006;20:401–3 [DOI] [PubMed] [Google Scholar]
- 17.Herrera BS, Ohira T, Gao L, et al. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br J Pharmacol 2008;155:1214–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlemmer CK, Coetzer H, Claassen N, Kruger MC. Oestrogen and essential fatty acid supplementation corrects bone loss due to ovariectomy in the female Sprague Dawley rat. Prostaglandins Leukot Essent Fatty Acids 1999;61:381–90 [DOI] [PubMed] [Google Scholar]
- 19.Watkins BA, Li Y, Allen KG, Hoffmann WE, Seifert MF. Dietary ratio of (n−6)/(n−3) polyunsaturated fatty acids alters the fatty acid composition of bone compartments and biomarkers of bone formation in rats. J Nutr 2000;130:2274–84 [DOI] [PubMed] [Google Scholar]
- 20.Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G. Dietary n−3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res 2003;18:1206–16 [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Tamaki H, Ishimaru T, et al. Inhibition of osteoporosis due to restricted food intake by the fish oils DHA and EPA and perilla oil in the rat. Biosci Biotechnol Biochem 2004;68:2613–5 [DOI] [PubMed] [Google Scholar]
- 22.Reinwald S, Li Y, Moriguchi T, Salem N, Jr, Watkins BA. Repletion with (n−3) fatty acids reverses bone structural deficits in (n−3)-deficient rats. J Nutr 2004;134:388–94 [DOI] [PubMed] [Google Scholar]
- 23.Shen CL, Yeh JK, Rasty J, Li Y, Watkins BA. Protective effect of dietary long-chain n−3 polyunsaturated fatty acids on bone loss in gonad-intact middle-aged male rats. Br J Nutr 2006;95:462–8 [DOI] [PubMed] [Google Scholar]
- 24.Watkins BA, Li Y, Seifert MF. Dietary ratio of n−6/n−3 PUFAs and docosahexaenoic acid: actions on bone mineral and serum biomarkers in ovariectomized rats. J Nutr Biochem 2006;17:282–9 [DOI] [PubMed] [Google Scholar]
- 25.Mollard RC, Gillam ME, Wood TM, Taylor CG, Weiler HA. (n−3) fatty acids reduce the release of prostaglandin E2 from bone but do not affect bone mass in obese (fa/fa) and lean Zucker rats. J Nutr 2005;135:499–504 [DOI] [PubMed] [Google Scholar]
- 26.Weiler HA. Dietary supplementation of arachidonic acid is associated with higher whole body weight and bone mineral density in growing pigs. Pediatr Res 2000;47:692–7 [DOI] [PubMed] [Google Scholar]
- 27.Judex S, Wohl GR, Wolff RB, Leng W, Gillis AM, Zernicke RF. Dietary fish oil supplementation adversely affects cortical bone morphology and biomechanics in growing rabbits. Calcif Tissue Int 2000;66:443–8 [DOI] [PubMed] [Google Scholar]
- 28.Poulsen RC, Kruger MC. Detrimental effect of eicosapentaenoic acid supplementation on bone following ovariectomy in rats. Prostaglandins Leukot Essent Fatty Acids 2006;75:419–27 [DOI] [PubMed] [Google Scholar]
- 29.Griel AE, Kris-Etherton PM, Hilpert KF, Zhao G, West SG, Corwin RL. An increase in dietary n−3 fatty acids decreases a marker of bone resorption in humans. Nutr J 2007;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruger MC, Coetzer H, de Winter R, Gericke G, van Papendorp DH. Calcium, gamma-linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging (Milano) 1998;10:385–94 [DOI] [PubMed] [Google Scholar]
- 31.Terano T. Effect of omega 3 polyunsaturated fatty acid ingestion on bone metabolism and osteoporosis. World Rev Nutr Diet 2001;88:141–7 [DOI] [PubMed] [Google Scholar]
- 32.Bassey EJ, Littlewood JJ, Rothwell MC, Pye DW. Lack of effect of supplementation with essential fatty acids on bone mineral density in healthy pre- and postmenopausal women: two randomized controlled trials of Efacal v. calcium alone. Br J Nutr 2000;83:629–35 [DOI] [PubMed] [Google Scholar]
- 33.Hogstrom M, Nordstrom P, Nordstrom A. n−3 Fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: the NO2 Study. Am J Clin Nutr 2007;85:803–7 [DOI] [PubMed] [Google Scholar]
- 34.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 1951;41:279–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hannan MT, Felson DT, Anderson JJ. Bone mineral density in elderly men and women: results from the Framingham osteoporosis study. J Bone Miner Res 1992;7:547–53 [DOI] [PubMed] [Google Scholar]
- 36.Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr 1999;69:727–36 [DOI] [PubMed] [Google Scholar]
- 37.McLean RR, Hannan MT, Epstein BE, et al. Elderly cohort study subjects unable to return for follow-up have lower bone mass than those who can return. Am J Epidemiol 2000;151:689–92 [DOI] [PubMed] [Google Scholar]
- 38.Hannan MT, Felson DT, Dawson-Hughes B, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 2000;15:710–20 [DOI] [PubMed] [Google Scholar]
- 39.Kiel DP, Mercier CA, Dawson-Hughes B, Cali C, Hannan MT, Anderson JJ. The effects of analytic software and scan analysis technique on the comparison of dual X-ray absorptiometry with dual photon absorptiometry of the hip in the elderly. J Bone Miner Res 1995;10:1130–6 [DOI] [PubMed] [Google Scholar]
- 40.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 41.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26, discussion 1127–36 [DOI] [PubMed] [Google Scholar]
- 42.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr 1993;57:182–9 [DOI] [PubMed] [Google Scholar]
- 43.Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol 1992;135:418–27 [DOI] [PubMed] [Google Scholar]
- 44.Arsenault LN, Matthan N, Scott TM, et al. Validity of estimated dietary eicosapentaenoic acid and docosahexaenoic acid intakes determined by interviewer-administered food frequency questionnaire among older adults with mild-to-moderate cognitive impairment or dementia. Am J Epidemiol 2009;170:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Booth SL, Broe KE, Gagnon DR, et al. Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr 2003;77:512–6 [DOI] [PubMed] [Google Scholar]
- 46.Panel on Macronutrients, Panel on the Definition of Dietary Fiber, Subcommittee on Upper Reference Levels of Nutrients, Subcommittee on Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academies Press, 2002/2005 [Google Scholar]
- 47.Shearman AM, Ordovas JM, Cupples LA, et al. Evidence for a gene influencing the TG/HDL-C ratio on chromosome 7q32.3-qter: a genome-wide scan in the Framingham study. Hum Mol Genet 2000;9:1315–20 [DOI] [PubMed] [Google Scholar]
- 48.Tucker KL, Chen H, Hannan MT, et al. Bone mineral density and dietary patterns in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr 2002;76:245–52 [DOI] [PubMed] [Google Scholar]
- 49.Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med 1995;122:9–16 [DOI] [PubMed] [Google Scholar]
- 50.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 2000;15:2504–12 [DOI] [PubMed] [Google Scholar]
- 51.Corwin RL, Hartman TJ, Maczuga SA, Graubard BI. Dietary saturated fat intake is inversely associated with bone density in humans: analysis of NHANES III. J Nutr 2006;136:159–65 [DOI] [PubMed] [Google Scholar]
- 52.Trichopoulou A, Georgiou E, Bassiakos Y, et al. Energy intake and monounsaturated fat in relation to bone mineral density among women and men in Greece. Prev Med 1997;26:395–400 [DOI] [PubMed] [Google Scholar]
- 53.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(suppl):1220S–8S; discussion 1229S–31S [DOI] [PubMed] [Google Scholar]
- 54.Willett WC. Nutritional epidemiology. 2nd ed New York, NY: Oxford University Press, 1998 [Google Scholar]
- 55.Kris-Etherton PM, Harris WS, Appel LJ. AHA Scientific Statement. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747–57 [DOI] [PubMed] [Google Scholar]
- 56.De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr 2000;71(suppl):213S–23S [DOI] [PubMed] [Google Scholar]
- 57.Tasaki Y, Takamori R, Koshihara Y. Prostaglandin D2 metabolite stimulates collagen synthesis by human osteoblasts during calcification. Prostaglandins 1991;41:303–13 [DOI] [PubMed] [Google Scholar]
- 58.Koshihara Y, Takamori R, Nomura K, Sugiura S, Kurozumi S. Enhancement of in vitro mineralization in human osteoblasts by a novel prostaglandin A1 derivative TEI-3313. J Pharmacol Exp Ther 1991;258:1120–6 [PubMed] [Google Scholar]
- 59.Weaver KL, Ivester P, Chilton JA, Wilson MD, Pandey P, Chilton FH. The content of favorable and unfavorable polyunsaturated fatty acids found in commonly eaten fish. J Am Diet Assoc 2008;108:1178–85 [DOI] [PubMed] [Google Scholar]
- 60.National Osteoporosis Foundation How the foods you eat affect your bones. Available from: http://www.nof.org/aboutosteoporosis/prevention/foodandbones (cited 27 November 2010)
- 61.Blanaru JL, Kohut JR, Fitzpatrick-Wong SC, Weiler HA. Dose response of bone mass to dietary arachidonic acid in piglets fed cow milk-based formula. Am J Clin Nutr 2004;79:139–47 [DOI] [PubMed] [Google Scholar]
- 62.Weiler H, Fitzpatrick-Wong S, Schellenberg J, et al. Maternal and cord blood long-chain polyunsaturated fatty acids are predictive of bone mass at birth in healthy term-born infants. Pediatr Res 2005;58:1254–8 [DOI] [PubMed] [Google Scholar]
- 63.Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab 2007;32:619–34 [DOI] [PubMed] [Google Scholar]
- 64.Dichtl W, Ares MP, Jonson AN, et al. Linoleic acid-stimulated vascular adhesion molecule-1 expression in endothelial cells depends on nuclear factor-kappaB activation. Metabolism 2002;51:327–33 [DOI] [PubMed] [Google Scholar]
- 65.Park HJ, Lee YW, Hennig B, Toborek M. Linoleic acid-induced VCAM-1 expression in human microvascular endothelial cells is mediated by the NF-kappa B-dependent pathway. Nutr Cancer 2001;41:126–34 [DOI] [PubMed] [Google Scholar]
- 66.Weiss LA, Barrett-Connor E, von Muhlen D. Ratio of n−6 to n−3 fatty acids and bone mineral density in older adults: the Rancho Bernardo Study. Am J Clin Nutr 2005;81:934–8 [DOI] [PubMed] [Google Scholar]
- 67.Macdonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr 2004;79:155–65 [DOI] [PubMed] [Google Scholar]
- 68.Griffin BA. How relevant is the ratio of dietary n−6 to n−3 polyunsaturated fatty acids to cardiovascular disease risk? Evidence from the OPTILIP study. Curr Opin Lipidol 2008;19:57–62 [DOI] [PubMed] [Google Scholar]