Abstract
Aims
The present study was designed to examine the mechanism involved in mitochondrial aldehyde dehydrogenase (ALDH2)-induced cardioprotection against ischaemia/reperfusion (I/R) injury with a focus on autophagy.
Methods
Wild-type (WT), ALDH2 overexpression, and knockout (KO) mice (n = 4–6 for each index measured) were subjected to I/R, and myocardial function was assessed using echocardiographic, Langendroff, and edge-detection systems. Western blotting was used to evaluate AMP-dependent protein kinase (AMPK), Akt, autophagy, and the AMPK/Akt upstream signalling LKB1 and PTEN.
Results
ALDH2 overexpression and KO significantly attenuated and accentuated, respectively, infarct size, factional shortening, and recovery of post-ischaemic left ventricular function following I/R as well as hypoxia/reoxygenation-induced cardiomyocyte contractile dysfunction. Autophagy was induced during ischaemia and remained elevated during reperfusion. ALDH2 significantly promoted autophagy during ischaemia, which was accompanied by AMPK activation and mammalian target of rapamycin (mTOR) inhibition. On the contrary, ALDH2 overtly inhibited autophagy during reperfusion accompanied by the activation of Akt and mTOR. Inhibition and induction of autophagy mitigated ALDH2-induced protection against cell death in hypoxia and reoxygenation, respectively. In addition, levels of the endogenous toxic aldehyde 4-hydroxy-2-nonenal (4-HNE) were elevated by ischaemia and reperfusion, which was abrogated by ALDH2. Furthermore, ALDH2 ablated 4-HNE-induced cardiomyocyte dysfunction and protein damage, whereas 4-HNE directly decreased pan and phosphorylated LKB1 and PTEN expression.
Conclusion
Our data suggest a myocardial protective effect of ALDH2 against I/R injury possibly through detoxification of toxic aldehyde and a differential regulation of autophagy through AMPK- and Akt-mTOR signalling during ischaemia and reperfusion, respectively.
Keywords: ALDH2, Myocardial ischaemia/reperfusion, Akt, AMPK, Autophagy, 4-HNE
Introduction
The mitochondrial isoform of aldehyde dehydrogenase (ALDH2) plays a key role in the metabolism of acetaldehyde and other toxic aldehydes.1 Evidence from our laboratory and others has revealed a beneficial role of ALDH2 against alcohol, acetaldehyde and toxic aldehyde-induced reactive oxygen species (ROS) formation, and tissue injury.2–4 More recent seminal finding by Chen et al.5 has indicated a potential role of ALDH2 activation in the cardioprotection against ischaemia injury. Nonetheless, neither the feasibility of ALDH2 activation nor the mechanisms behind ALDH2-offered cardioprotection against ischaemic injury has been defined. Recent observation has indicated that ethanol treatment prior to ischaemia may enhance ALDH2 activity and retard formation of 4-hydroxy-2-nonenal (4-HNE)-protein adducts.6 4-HNE, a specific electrophilic reactive aldehyde, is capable of modifying key enzymes by forming protein adducts to inhibit protein function.7 Kinase function is often disrupted by 4-HNE due to its reactivity with amino acids including cysteine, histidine, and lysine. Interestingly, 4-HNE may be produced as a result of ischaemia/reperfusion (I/R) insult in various tissues including hearts.8 4-HNE imposes severe myocardial toxicity encompassing from disturbed coronary flow9 to impaired cardiac contractility.10,11 Recent findings suggest that elevated HNE content may modify essential cardiac survival signalling molecules including AMP-dependent protein kinase (AMPK)12 and Akt.13 Given the elevated cardiac 4-HNE in ischaemia and reperfusion along with its detrimental role in the heart,8,10,11 it is plausible to speculate a possible action of ALDH2 against I/R injury through the detoxification of the toxic aldehyde.
Autophagy, an evolutionarily conserved process of lysosome-dependent turnover of damaged proteins and organelles, is present in normal hearts and may be upregulated in response to stress and cardiovascular anomalies such as heart failure and ischaemic injury.14 Autophagy is critical to cell survival, the interruption of which may initiate severe ventricular dysfunction and cardiomyopathy.15 Autophagy is believed to exert a beneficial role in I/R as rapamycin, a potent inducer of autophagy, reduces infarct size, and improves functional recovery in ischaemic murine hearts.16,17 Paradoxically, autophagy has been shown to be both the cause and consequence of cardiac cell death. Autophagy is upregulated during myocardial ischaemia and is further enhanced during reperfusion.18,19 Despite the seemingly cardioprotective role of autophagy during ischaemia, the jury is still out with regard to the possible harmful effect of autophagy during reperfusion.14,20 Moreover, the signalling mechanism(s) behind autophagy appear(s) to be rather complicated. Several lines of evidence have indicated a pivotal role of the mammalian target of rapamycin (mTOR) in autophagy such that autophagy may be negatively regulated by mTOR through the phosphorylation of its downstream targets such as p70s6k.21,22 Other evidence suggested that glucose deprivation during I/R triggers autophagy via the activation of AMPK and the inhibition of mTOR in cardiomyocytes.20 In addition, the PI3K-Akt pathway has been indicated to play a critical role in autophagy.23,24 Interestingly, mTOR can be activated by Akt although it is inhibited by AMPK via the phosphorylation of tuberous sclerosis complex 2 (TSC2).25,26 Hence, the AMPK and Akt pair may quarterback a series of post-transcriptional processes such as cardiac hypertrophy and adaptation to stress at the convergence of mTOR. Nevertheless, the precise role of AMPK/Akt/mTOR pathway in the regulation of autophagy in myocardial ischaemia and reperfusion is unclear. Therefore, the aim of this study was to examine (i) whether ALDH2 protects against myocardial I/R injury through detoxification of 4-HNE; (ii) whether autophagy is involved in ALDH2-elicited cardioprotective effect, if any; and (iii) the signalling mechanism(s) behind ALDH2 and/or autophagy-induced myocardial response during I/R with a focus on the AMPK- and Akt-mTOR signalling cascades.
Methods
Experimental animals and ALDH2 activity
All animal procedures were approved by our Institutional Animal Care and Use Committee (University of Wyoming). Production of ALDH2 transgenic mice using the chicken β-actin promoter was described previously.27 ALDH2 knockout (KO) mice were obtained from Dr T. Kawamoto from the University of Occupational and Environmental Health (Kitakyushu, Japan). All mice were housed in a temperature-controlled room under a 12/12 h-light/dark circadian cycle with access to water and food ad libitum. Four-to-six-month-old adult male wild-type (WT), ALDH2, and ALDH KO mice were used. To validate the ALDH2 gene expression, ALDH2 activity was measured in 33 mmol/L sodium pyrophosphate containing 0.8 mmol/L NAD+, 15 µmol/L propionaldehyde, and 0.1 mL protein extract. Propionaldehyde, the substrate of ALDH2, was oxidized in propionic acid, whereas NAD+ was reduced to NADH to estimate ALDH2 activity. NADH was determined by spectrophotometric absorbance at 340 nm. ALDH2 activity was expressed as nmol NADH/min per mg protein.3
Echocardiographic evaluation
Cardiac geometry and function were evaluated using a 2-D guided M-mode echocardiography equipped with a 15-6 MHz linear transducer. Anterior and posterior wall thicknesses and diastolic and systolic left ventricular (LV) dimensions were recorded from M-mode images using the method adopted by the American Society of Echocardiography. Fractional shortening was calculated from end-diastolic diameter (EDD) and end-systolic diameter (ESD) using the equation of (EDD − ESD)/EDD.27
In vivo regional ischaemia and experimental myocardial infarction
Mice were anaesthetized, intubated, and ventilated with oxygen (Rodent Ventilator, Harvard Apparatus, Millis, MA, USA). The core temperature was maintained at 37°C with a heating pad. After left lateral thoracotomy, the left anterior descending artery (LAD) was occluded for 20 min with an 8-0 nylon suture and polyethylene tubing to prevent arterial injury, prior to a 4 h reperfusion. Electrocardiogram confirmed ischaemic repolarization changes (ST-segment elevation) during coronary occlusion. The hearts were then excised and stained to delineate the extent of myocardial necrosis as a percentage of non-perfused ischaemic area at risk.28 Viable tissue in ischaemic region was red-stained by 2,3,5-triphenyltetrazolium, and the non-ischaemic region was blue-stained with Evan's blue. Hearts were fixed and sectioned into 1 mm slices, photographed using a Leica microscope, and analysed using NIH Image software.
Mouse heart perfusion
Mouse hearts were retrogradely perfused with a Krebs–Henseleit buffer containing 7 mmol/L glucose, 0.4 mmol/L oleate, 1% BSA, and a low fasting concentration of insulin (10 µU/mL). Hearts were perfused at a constant flow of 4 mL/min (equal to an aortic pressure of 80 cmH2O) at baseline for 60 min. A fluid-filled latex balloon connected to a solid-state pressure transducer was inserted into the left ventricle through a left atriotomy to measure pressure. Left ventricular developed pressure (LVDP), the first derivative of LVDP (±dP/dt), and heart rate were recorded using a digital acquisition system at a balloon volume which resulted in a baseline LV end-diastolic pressure of 5 mmHg.29
Cardiomyocyte isolation and mechanics
Mouse cardiomyocytes were isolated using Librase as described.27 Myocyte yield was ∼75%, which was not affected by ALDH2 overexpression or KO. Only rod-shaped cells with clear edges were selected for mechanical study. Mechanical properties of myocytes were assessed using an IonOptix™ soft-edge system.27 Cell shortening and relengthening were assessed using the following indices: peak shortening (PS), time-to-PS (TPS), time-to-90% relengthening (TR90), and maximal velocities of shortening/relengthening (±dL/dt). For in vitro study, cardiomyocytes were incubated with 20 µmol/L 4-HNE for 50 min.13
Cell viability MTT assay
The assay is based on the transformation of the tetrazolium salt MTT by active mitochondria to an insoluble formazan salt. Cardiomyocytes from WT and ALDH2 mice were treated with the autophagy inhibitor 3-methyladenine (3-MA, 10 mmol/L), the autophagy inducer rapamycin (100 nmol/L), or vehicle for 5 min at 37°C before a 20 min exposure to hypoxia (95% nitrogen/5% CO2) followed with or without a 30 min reoxygenation (room air/5% CO2). The cells were plated in microtitre plate at a density of 3 × 105 cells/mL. MTT was added to each well with a final concentration of 0.5 mg/mL, and the plates were incubated for another 2 h at 37°C. Formazan was quantified spectroscopically at 560 nm using a SpectraMax® 190 spectrophotometer.30
Western blotting analysis
Membrane proteins were separated on SDS–polyacrylamide gels and were transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% milk and incubated overnight with anti-ALDH2, anti-p-AMPK (Thr172), anti-AMPKα, anti-p-Akt (Thr308), anti-Akt, anti-p-mTOR (Ser2448), anti-mTOR, anti-p-p70S6K (Thr389), anti-p70S6K, anti-LC3, anti-p-LKB1 (Ser428), anti-LKB1, anti-p-PTEN (Ser380), and anti-PTEN antibodies (Cell Signaling, Beverly, MA, USA, 1:1000). HNE-protein adducts were determined as described.6,8 A specific antibody against the reductively stabilized HNE amino acid adducts was used (Calbiochem, Gibbstown, NJ, USA; 1:1000). No single protein was selected for HNE analysis. Rather, the density in each lane was assessed as a whole for the integrated density (all different protein adducts formed) within that lane.8 The antigens were detected by the luminescence method.27
Carbonyl formation
Samples were resuspended in 10 mmol/L 2,4-dinitrophenylhydrazine solution for 30 min at room temperature before 20% trichloroacetic acid was added. Samples were centrifuged and the precipitate was resuspended in 6 mol/L guanidine solution. Maximum absorbance (360–390 nm) was read against appropriate blanks and carbonyl content was calculated using the formula: absorption at 360 nm × 45.45 nmol/protein content (mg).27
Statistical analysis
A total of 11–12 mice were used for each of the WT, ALDH2 overexpression, and KO groups although only 4–6 mice per group were studied for each parameter measured. Data were mean ± SEM. Statistical significance (P < 0.05, two-side) was estimated by one-way analysis of variance (ANOVA) (two-way ANOVA for mouse heart perfusion study) followed by Tukey's test for post hoc analysis (GraphPad4.0, GraphPad Software, La Jolla, CA, USA).
Results
General feature and cardiac phenotype of wild-type, ALDH2 overexpression, and ALDH2 knockout mice
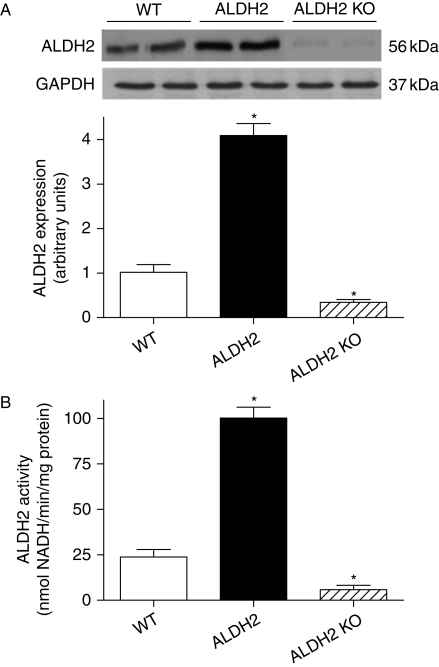
Neither overexpression nor KO of ALDH2 affected body and organ weights or the organ-to-body weight ratio. Echocardiographic assessment revealed comparable heart rate, wall thickness, EDD, ESD, normalized LV mass, and fractional shortening among the WT, ALDH2 overexpression, and KO mice (Supplementary material online, Table S1). Both protein expression and enzymatic activity were significantly increased and decreased, respectively, in ALDH2 overexpression and KO mouse hearts (Figure 1).
Figure 1.
Validation of ALDH2 transgenic overexpression and knockout (KO). Myocardial ALDH2 protein expression and enzymatic activity were evaluated using western blot analysis and spectrophotometry in wild-type (WT), ALDH2 overexpression, and ALDH2 knockout mice. (A) ALDH2 expression with representative gel blots of ALDH2 and GAPDH (loading control); (B) ALDH2 enzymatic activity; mean ± SEM, n = 5–6 per group, *P < 0.05 vs. WT group.
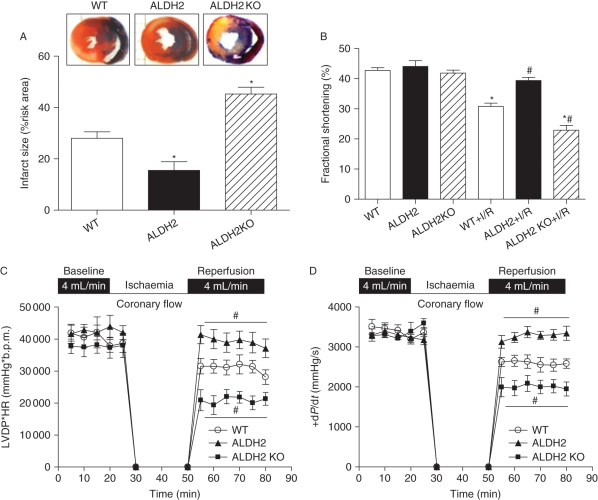
Effect of ALDH2 overexpression and knockout on myocardial ischaemia/reperfusion injury and post-ischaemic left ventricular function
To evaluate the effect of ALDH2 on myocardial I/R injury, myocardial infarct size was examined in WT, ALDH2 overexpression, and KO mouse hearts following in vivo regional I/R (20 min coronary artery ligation, followed by a 4 h reperfusion). Our data revealed that the infarct size following I/R was significantly reduced and enlarged, respectively, in ALDH2 and KO hearts (Figure 2A). Echocardiographic results depicted significantly suppressed fractional shortening in WT mice following I/R, the effect of which was ablated and accentuated by ALDH2 overexpression and KO, respectively (Figure 2B). Consistently, the post-ischaemic LV contractile function following in vitro ischaemia (20 min) and reperfusion (30 min) was greatly improved and exacerbated by ALDH2 overexpression and KO, respectively, as assessed by the heart rate–LV pressure product (Figure 2C) or maximal LV pressure development (+dP/dt) (Figure 2D) using the Langendorff perfusion system.
Figure 2.
Effect of ALDH2 overexpression and knockout (KO) on ischaemia/reperfusion (I/R)-induced myocardial injury. Effect of ALDH2 overexpression and KO on myocardial infarct size, fraction shortening, and post-ischaemic left ventricular function was evaluated following ischaemia (20 min)/reperfusion (4 h for panels A and B and 30 min for C and D). (A) Myocardial infarct size with representative tissue sectioning; (B) echocardiographic fraction shortening; (C) left ventricular developed pressure (LVDP)-heart rate (HR) product; and (D) maximal velocity of pressure development (+dP/dt). Mean ± SEM, n = 5–6 hearts per group, *P < 0.05 vs. wild-type (WT) group, #P < 0.05 vs. WT + I/R group.
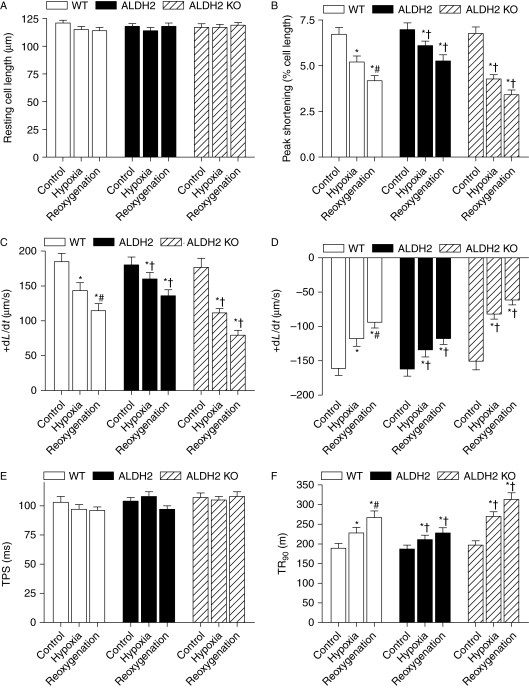
Effect of ALDH2 on hypoxia/reoxygenation-induced cardiomyocyte contractile dysfunction
We went on to examine the effect of ALDH2 overexpression and KO on hypoxia and reoxygenation (H/R)-elicited cardiomyocyte contractile response. Hypoxia (20 min) alone triggered an overt contractile dysfunction manifested as depressed PS and maximal velocity of shortening/relengthening (±dL/dt), as well as prolonged TR90 in WT murine cardiomyocytes. In line with their effects on myocardial I/R injury, ALDH2 overexpression significantly alleviated, whereas ALDH2 KO accentuated hypoxia-induced cardiomyocyte contractile dysfunction. Our data also revealed a further decline in cardiomyocyte contractile function following post-hypoxic reoxygenation (30 min). Similar to the effect under hypoxic condition, ALDH2 overexpression and KO significantly attenuated and exacerbated the H/R-elicited cardiomyocyte mechanical defects (Figure 3).
Figure 3.
Cardiomyocyte contractile properties following hypoxia/reoxygenation. Effect of ALDH2 overexpression and knockout (KO) on hypoxia/reoxygenation-induced cardiomyocyte dysfunction was evaluated. Cardiomyocytes were subjected to hypoxia (95% nitrogen/5% CO2) for 20 min followed with or without a 30 min reoxygenation (room air/5% CO2). (A) Resting cell length; (B) peak shortening (PS, normalized to cell length); (C) maximal velocity of shortening (+dL/dt); (D) maximal velocity of relengthening (−dL/dt); (E) time-to-PS (TPS) and (F) time-to-90% relengthening (TR90). Mean ± SEM, n = 68–74 cells from four mice per group, *P < 0.05 vs. respective control group, #P < 0.05 vs. wild-type (WT) hypoxia group, †P < 0.05 vs. respective WT hypoxia or reoxygenation group.
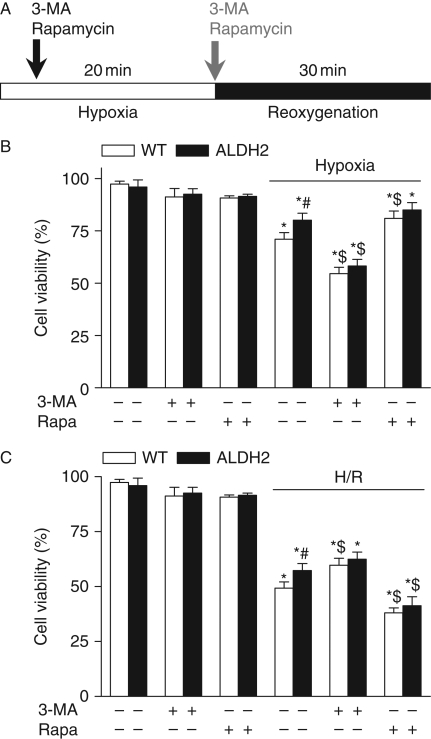
Differential autophagy effect in cell survival under hypoxia or hypoxia/reoxygenation
Autophagy has been shown to possess distinct roles during ischaemia and reperfusion.31 To evaluate whether autophagy plays a role in ALDH2-induced myocardial response, cardiomyocytes from WT and ALDH2 overexpression mice were subjected to a 20 min hypoxia followed with or without a 30 min reoxygenation (H/R) in the presence or absence of the autophagy inhibitor 3-MA or the autophagy inducer rapamycin. Hypoxia significantly decreased the cell viability, the effect of which was attenuated by ALDH2. 3-MA significantly enhanced hypoxia-induced cardiomyocyte death while mitigating the protective role of ALDH2. On the other hand, rapamycin effectively rescued hypoxia-induced cell death in a manner reminiscent to ALDH2 (Figure 4A). Our data further revealed that H/R greatly lessened cell survival although with a less pronounced effect in the ALDH2 group. Interestingly, 3-MA significantly attenuated H/R-elicited cell death in a manner similar to ALDH2, whereas rapamycin greatly accentuated H/R-induced cell death in both WT and ALDH2 groups (Figure 4B). These data suggest a likely paradoxical role of autophagy in ALDH2-induced cardioprotection under hypoxia and H/R.
Figure 4.
Cell viability of cardiomyocytes following hypoxia–reoxygenation. Cell viability was examined using MTT assay in cardiomyocytes from wild-type (WT) and ALDH2 overexpression mice subjected to a 20 min hypoxia or a 20 min hypoxia followed by a 30 min reoxygenation (H/R) in the presence or absence of the autophagy inhibitor 3-MA (10 mmol/L) or the autophagy inducer rapamycin (Rapa, 100 nmol/L). (A) Experimental protocol; (B) cell viability under hypoxia; and (C) cell viability under H/R. Mean ± SEM, n = 5, *P < 0.05 vs. WT without inhibitor treatment, #P < 0.05 vs. respective WT hypoxia or H/R group, $P < 0.05 vs. respective WT hypoxia or H/R in the absence of 3-MA or rapamycin.
Role of AMP-dependent protein kinase and Akt activation in ALDH2-elicited response in ischaemia and ischaemia/reperfusion
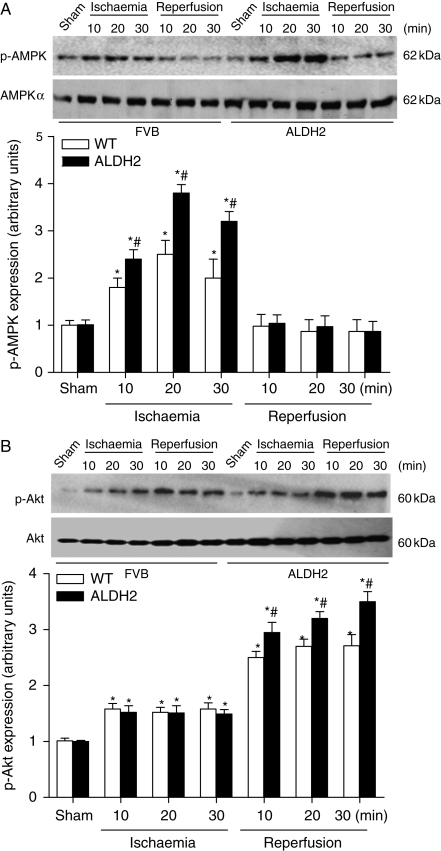
To examine the potential mechanism(s) behind ALDH2-elicited cardioprotection against ischaemia or I/R, key cardiac surviving factors AMPK and Akt were evaluated. Our data revealed that ischaemia markedly increased AMPK phosphorylation in WT mice, the effect of which was markedly augmented by ALDH2 overexpression. Furthermore, during the reperfusion phase after a 20 min ischaemia, AMPK phosphorylation was rapidly declined to basal levels in both WT and ALDH2 groups (Figure 5A). Following ischaemia, Akt activation was comparable between WT and ALDH2 groups. However, during the reperfusion phase, Akt phosphorylation was markedly elevated in both WT and ALDH2 overexpression groups, with a further increase in ALDH2 mice (Figure 5B).
Figure 5.
Phosphorylation of AMP-dependent protein kinase (AMPK) and Akt during ischaemia and reperfusion. Time course of in vivo ischaemia (10, 20, and 30 min) or reperfusion (10, 20, and 30 min) preceded by a 20 min ischaemia on phosphorylation of AMPK (A) and Akt (B) was evaluated in murine myocardium from wild-type (WT) and ALDH2 overexpression mice. Inset: Representative gel blots depicting total and phosphorylation of AMPK or Akt. Mean ± SEM, n = 4 hearts, *P < 0.05 vs. respective sham group, #P < 0.05 vs. respective WT ischaemia or reperfusion group.
Signalling mechanisms involved in autophagy during ischaemia and ischaemia/reperfusion
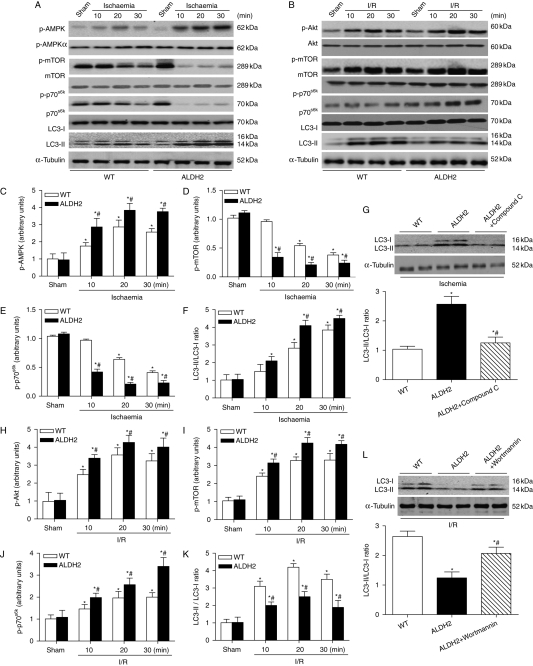
Mammalian target of rapamycin is a critical mediator of autophagy under the positive control of Akt,22 whereas the activation of AMPK has been shown to phosphorylate TSC2 to inhibit mTOR.25 Activation of AMPK, Akt, and mTOR was assessed along with autophagy under ischaemia and I/R. Our result depicted a significant concurrent increase in LC3-II/LC3-I ratio (autophagy) and p-AMPK under ischaemia in WT and ALDH2 overexpression mice, with a more pronounced effect in the ALDH2 group. The rise in autophagy and p-AMPK coincided with a decline in the phosphorylation of mTOR (Ser2481) and Thr389 phosphorylation of p70s6k, a downstream target of mTOR, the effect of which was more pronounced in ALDH2 mice (Figure 6A, C–F). In addition, compound C (10 mmol/L), an AMPK inhibitor, markedly attenuated the ALDH2-induced increase in LC3-II/LC3-I ratio in response to hypoxia (Figure 6G). Our further experiment revealed a significant increase in autophagy (LC3-II/LC3-I ratio) and p-Akt during I/R in WT and ALDH2 groups. Interestingly, ALDH2 significantly increased I/R-induced Akt activation while dampening I/R-induced rise in autophagy. Changes in autophagy and p-Akt coincided with the increase in the phosphorylation of mTOR and p70s6k during I/R, with a much greater response in ALDH2 mice (Figure 6B, H–K). Wortmannin (100 nmol/L), an Akt inhibitor, markedly restored the ALDH2-induced drop in LC3-II/LC3-I ratio following reoxygenation (Figure 6L). These findings suggest a likely disparate role of autophagy in the ALDH2-offered cardioprotection against ischaemia and I/R involving AMPK-mTOR and Akt-mTOR signalling, respectively.
Figure 6.
Autophagy signalling pathway. Effect of ALDH2 overexpression on ischaemia or ischaemia/reperfusion-induced phosphorylation of AMP-dependent protein kinase (AMPK), Akt, mammalian target of rapamycin (mTOR), and p70s6k as well as autophagy (LC3-I/II) was examined in myocardium from wild-type (WT) and ALDH2 overexpression mice. (A, C–F) Hearts were subjected to in vivo ischaemia (10, 20, and 30 min). (A) Representative gel bolts depicting respective protein expression using specific antibodies; (C) phosphorylated AMPK (p-AMPK); (D) phosphorylated mTOR (p-mTOR); (E) phosphorylated p70s6k (p-p70s6k); and (F) LC3-II-to-LC3-I ratio. Mean ± SEM, n = 5, *P < 0.05 vs. sham, #P < 0.05 vs. respective WT ischaemia group. (G) Cardiomyocytes were subjected to 20 min hypoxia (95% nitrogen/5% CO2) in vitro with or without the pre-treatment of the AMP-dependent protein kinase (AMPK) inhibitor compound C (10 mmol/L) for 5 min. (B, H–K) Hearts were subjected to in vivo ischaemia (20 min) followed by reperfusion (10, 20, and 30 min). (B) Representative gel bolts depicting respective protein expression using specific antibodies; (H) phosphorylated Akt (p-Akt); (I) phosphorylated mammalian target of rapamycin (mTOR) (p-mTOR); (J) phosphorylated p70s6k (p-p70s6k); and (K) LC3-II-to-LC3-I ratio. n = 5, *P < 0.05 vs. sham, #P < 0.05 vs. respective WT ischaemia/reperfusion (I/R) group. (L) Cardiomyocytes were subjected to 20 min hypoxia and 30 min reoxygenation in vitro with or without the pre-treatment of the Akt inhibitor wortmannin (100 nmol/L) for 5 min. Insets: Representative gel blots depicting autophagy proteins LC3-I and LC3-II. For panels G and L, mean ± SEM, n = 5, *P < 0.05 vs. WT group, #P < 0.05 vs. ALDH2 group.
Effect of ALDH2 on ischaemia and ischaemia/reperfusion-induced 4-HNE-protein adduct content in the hearts
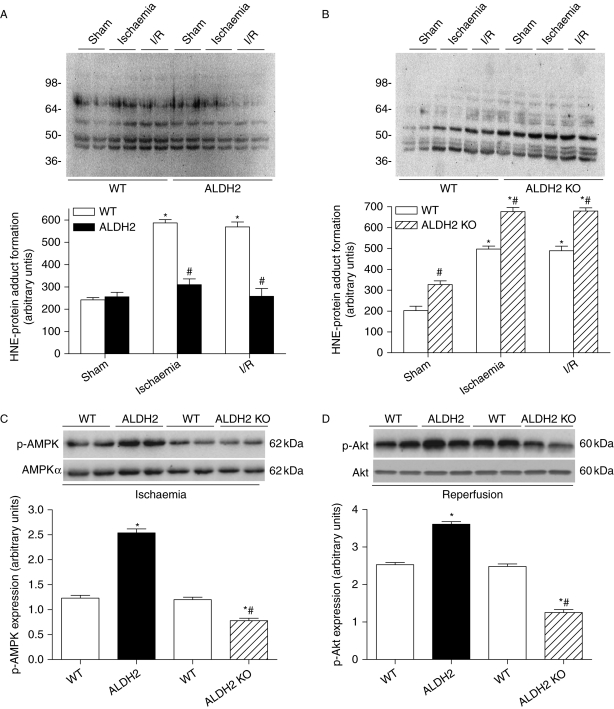
Considering the key role of 4-HNE in ischaemic cardiac injury,8 we examined the effect of ALDH2 on in vivo ischaemia and I/R-induced 4-HNE-protein adduct content in the heart. Although the levels of 4-HNE-protein adduct were low in sham condition, cardiac 4-HNE-protein adduct formation was significantly increased in response to ischaemia and remained elevated during reperfusion. Although ALDH2 overexpression itself exhibited little effect in the sham group, it significantly depressed ischaemia and I/R-induced rise in 4-HNE-protein adduct content (Figure 7A). To the contrary, ALDH2 KO further accentuated the rise in 4-HNE-protein adduct content under ischaemia and I/R conditions. In addition, ALDH2 KO itself enhanced the HNE-protein adduct formation. Further evaluation of AMPK and Akt phosphorylation revealed that both p-AMPK and p-Akt were augmented and attenuated, respectively, by ALDH2 overexpression and KO under ischaemia and I/R (Figure 7C and D).
Figure 7.
4-HNE-protein adducts formation and differential phosphorylation of AMP-dependent protein kinase (AMPK)/Akt. (A) HNE-protein adducts formation evaluated with immunoblotting in hearts from wild-type (WT) and ALDH2 overexpression mice subjected to ischaemia or ischaemia/reperfusion (I/R). Inset: Representative gel blots depicting HNE-protein adducts formation. Molecular protein standards are shown on the left. The density of staining in each lane was assessed as a whole to generate a single value of the integrated density of all HNE-protein adducts formed within that lane; (B) HNE-protein adducts formation in hearts from WT and ALDH2 KO mice using immunoblotting; (C) In vivo regional ischaemia (20 min)-stimulated differential phosphorylation of AMPK in WT, ALDH2 overexpression, and ALDH2 KO mouse hearts. Inset: Representative gel blots depicting total and phosphorylation AMPK; and (D) in vivo regional ischaemia (20 min)/reperfusion (30 min)-stimulated differential phosphorylation of Akt in WT, ALDH2 overexpression, and ALDH2 KO mouse hearts. Inset: Representative gel blots depicting total and phosphorylation Akt. Mean ± SEM, n = 6–8, *P < 0.05 vs. sham or WT group under basal condition, #P < 0.05 vs. corresponding WT or ALDH2 group under ischaemia or I/R condition.
4-HNE-induced mechanical and cell signalling response
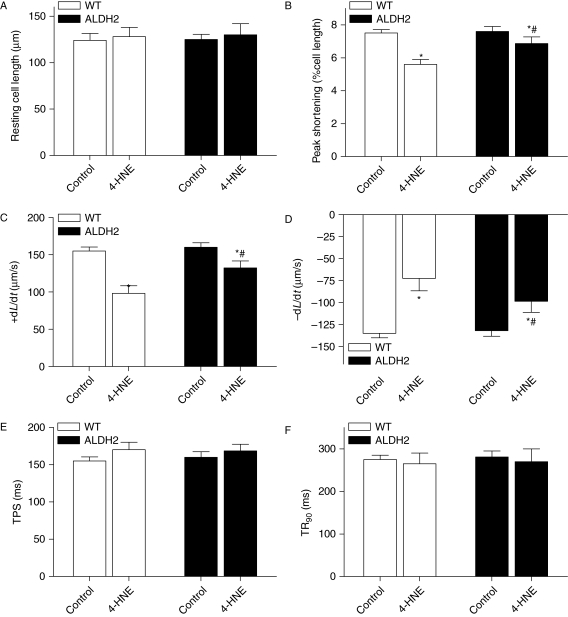
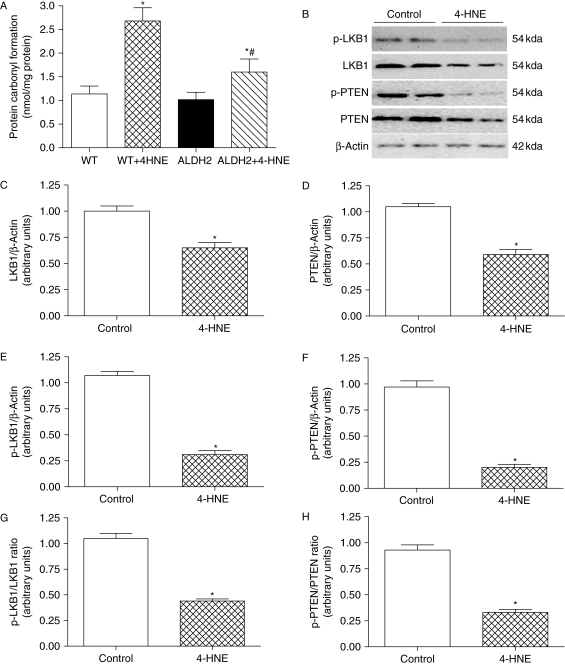
Our data depicted a cardioprotective effect of ALDH2 possibly via the detoxification of toxic aldehyde. To further consolidate the role of 4-HNE in ALDH2-elicited cardioprotection against ischaemia–reperfusion, the 4-HNE-exerted mechanical response was evaluated in cardiomyocytes from WT and ALDH2 overexpression mice. Treatment of 4-HNE (20 µmol/L) for 50 min13,32 significantly compromised cardiomyocyte mechanical function manifested as reduced PS and ±dL/dt with normal TPS and TR90 in the WT group, the effect of which was significantly attenuated by ALDH2 overexpression (Figure 8). Evaluation of protein carbonyl formation, an indicative of 4-NHE-elicited protein oxidation and damage, revealed enhanced protein carbonyl formation in response to 4-HNE exposure which was significantly attenuated by ALDH2 overexpression (Figure 9A).
Figure 8.
4-HNE-induced cardiomyocyte contractile dysfunction. Cardiomyocytes from wild-type (WT) and ALDH2 overexpression mice were incubated with 4-HNE (20 µmol/L) or vehicle for 50 min before cardiomyocyte mechanical function was assessed. (A) Resting cell length; (B) peak shortening (PS, normalized to cell length); (C) maximal velocity of shortening (+dL/dt); (D) maximal velocity of relengthening (−dL/dt); (E) time-to-PS (TPS); and (F) time-to-90% relengthening (TR90). Mean ± SEM, n = 150–200 cells from three mice per group, *P < 0.05 vs. respective control, #P < 0.05 vs. 4-HNE group from WT mice.
Figure 9.
Effect of 4-HNE on carbonyl formation, phosphorylation of LKB1 and PTEN. Cardiomyocytes from wild-type (WT) or ALDH2 overexpression mice were incubated with 4-HNE (20 µmol/L) or vehicle for 50 min prior to the assessment of carbonyl, total, and phosphorylated LKB1 and PTEN. (A) Carbonyl formation; (B) representative gel blots of total and phosphorylated LKB1 and PTEN (β-actin was used as loading control); (C and D) total LKB1 and PTEN expression; (E and F) phosphorylated LKB1 and PTEN; (G and H) phosphorylated-to-pan protein ratio of LKB1 and PTEN. Mean ± SEM, n = 5, *P < 0.05 vs. WT or control group, #P < 0.05 vs. WT + 4-NHE group.
4-HNE may inhibit the activity of AMPK12 and Akt,13 and is capable of modifying the LKB1/AMPK/mTOR pathway.12 LKB1 is also known to inactivate phosphatase and tensin homolog deleted on chromosome 10 (PTEN) through phosphorylation.33 Given the important roles of LKB1 and PTEN in the regulation of AMPK and Akt,34,35 the effect of 4-HNE on LKB1 and PTEN (pan and phosphorylated) was evaluated in cardiomyocytes from WT mice. Our data revealed that 4-HNE significantly decreased the levels of both pan and phosphorylated (absolute and ratio) LKB1 and PTEN (Figure 9), suggesting a role of LKB1 and PTEN signalling in 4-HNE-induced inhibition of AMPK and Akt activity.
Discussion
The ALDH2 enzyme was recently demonstrated to retard ischaemic injury in the hearts.5 Findings from this study provided compelling evidence, for the first time, that ALDH2 protects against myocardial I/R injury through a paradoxical regulation of autophagy during ischaemia and reperfusion phases. Our study further depicted a possible role of AMPK activation and mTOR inhibition in autophagy induction en route to the beneficial effect of ALDH2 during ischaemia. To the contrary, data from our study favoured activation of Akt and mTOR in reduced autophagy en route to the beneficial action of ALDH2 during reperfusion when AMPK is no longer active. The concerted action between AMPK and Akt at the converging point of mTOR seems to play a pivotal role in cell survival and myocardial function. Moreover, our results depicted that ALDH2 significantly inhibited the I/R-induced cytotoxic 4-HNE formation, protein damage, and 4-HNE-induced cardiomyocyte dysfunction while 4-HNE compromised the LKB1 and PTEN signalling. These regulatory mechanisms of ALDH2 on AMPK and Akt as well as the upstream (LKB1 and PTEN) signalling may be dependent on the detoxification of 4-HNE (Supplementary material online, Scheme 1). Taken together, these findings should shed some light towards a better understanding of a protective role of ALDH2 against myocardial ischaemia–reperfusion injury.
Our earlier studies have demonstrated that ALDH2 alleviates acetaldehyde and alcohol-induced myocardial injury.3,27 Nonetheless, little information is available with regard to the effect of ALDH2 on ischaemia and reperfusion in hearts. Data from our present study have provided compelling evidence that ALDH2 is capable of counteracting I/R injury and post-I/R or -ischaemic LV contractile dysfunction, at both whole-heart and cardiomyocyte levels. This is also substantiated by the accentuated I/R or H/R-myocardial injury under ALDH2 KO condition. These data convincingly support the notion that an increase in ALDH2 expression and/or activity may serve as a novel therapeutic remedy in the management against ischaemic heart diseases. Ethanol treatment prior to ischaemia has been shown to enhance ALDH2 activity,6 representing a possible protective mechanism of ALDH2.
Enhanced myocardial autophagy is present in a wide array of cardiovascular diseases,36 although the role of autophagy in the regulation of cardiovascular function has not been clearly defined. Data from our study provided direct evidence that ALDH2 overexpression moderates upregulated autophagy in the ischaemia phase and lessened autophagy in the reperfusion phase. A combination of these actions appears to be responsible for the ultimate cardioprotective effect of ALDH2 against I/R injury. Decker et al.37 found that a 40 min ischaemia may result in an increased number of autophagosomes, which was further elevated during reperfusion in Langendorff-perfused rabbit hearts. Along the same line, myocardial adaptation to ischaemia by repeated brief episodes of I/R prior to lethal I/R was found to upregulate the autophagosomal membrane-specific proteins LC3-II and Beclin-1.38 These in vivo and in vitro observations suggest that autophagy is an essential mediator in cell survival in response to I/R and H/R. In our hand, induction of autophagy with rapamycin promotes cardiomyocyte survival during hypoxia, reminiscent of the cardioprotection of ALDH2. However, inhibition of autophagy using 3-MA improves cell survival under reoxygenation. Interestingly, 3-MA and rapamycin mitigate ALDH2-offered cytoprotection under hypoxia and H/R, respectively, indicating a paradox role of autophagy under ischaemia and I/R. An ample of evidence supported the pro-survival effect of autophagy during I/R.19 Consistently, agents capable of inducing autophagy have been found to be cardioprotective, such as rapamycin39 and statins.40 Paradoxically, autophagy has also been associated with cell death during I/R. Using the beclin1 heterozygous KO (beclin 1+/−) mice, Matsui et al.20 found reduced autophagy during reperfusion, accompanied by significantly lessened infarct size and apoptosis. Inhibition of autophagy with the downregulation of Beclin 1 or 3-MA was reported to retard cardiomyocyte cell death following simulated I/R,41 consistent with our finding. Thus, although ischaemia-induced autophagy in cardiomyocytes may be generally protective, it may be switched into a detrimental role during the reperfusion phase.14,31
Up-to-date, a number of signalling molecules are speculated to serve as the upstream initiators of autophagy during I/R. Several lines of evidence have depicted a critical role for mTOR in the regulation of autophagy. Mammalian target of rapamycin as an important negative regulator of autophagy is regulated by several kinases including AMPK and Akt via TSC1/2. In the heart, both AMPK and Akt are deemed key regulators of myocardial function.42,43 Nonetheless, potential cross-talk between the two under physiological or pathophysiological state is unclear. It was shown that mTOR is activated by Akt, whereas it is inhibited by AMPK via the phosphorylation of TSC2.25,26 The convergence of the two at the level of mTOR may serve as a critical avenue for the cross-talk between Akt and AMPK during pathophysiological adaptation. Data from our study revealed that AMPK and Akt possess apparently different active windows during I/R. In ischaemia, ALDH2 activates AMPK to inhibit mTOR, favouring autophagy. When AMPK is no longer active during reperfusion, Akt phosphorylation is kicked on to activate mTOR, resulting in an Akt-dependent inhibition of autophagy. These observations suggest that ALDH2 may upregulate autophagy in ischaemia while inhibiting autophagy during reperfusion. Such dual regulatory autophagy paradox may underscore the homeostatic machinery for ALDH2-elicited cardiac benefits against I/R injury. It is worth mentioning that other mechanisms should not be ruled out at this time. Reactive oxygen species may mediate autophagosome formation through oxidative modification.44 In addition, ALDH2 is capable of activating nitrates inside mitochondria to exert cytoprotective effect.2
Our data demonstrated a key role of 4-HNE detoxification in ALDH2-elicited responses in cardiac function, the activity of AMPK and Akt. There is a plethora of evidence indicating a detrimental role of the lipid peroxidation product in cardiac injury in ischaemia and reperfusion. 4-HNE was reported to inhibit glycolytic enzyme activity during ischaemia and reperfusion.45 4-HNE, via adduct formation with COX subunits, interrupts mitochondrial function during reperfusion.46 Our data provided direct evidence that in vivo ischaemia and reperfusion significantly increased 4-HNE-protein adduct content, which was restored to near-basal levels in murine hearts with ALDH2 overexpression. Our further results revealed that ALDH2 attenuated 4-HNE exposure-induced cardiomyocyte contractile dysfunction. Consistently, cardiac 4-HNE-protein adduct content was accentuated in response to I/R in the ALDH2 KO group. These findings are in line with the report that elevated ALDH2 enzymatic activity may retard the formation of 4-HNE-protein adducts.6 Moreover, ischaemia-induced AMPK activation was overtly enhanced by ALDH2 overexpression, whereas it was decreased in KO hearts. Likewise, reperfusion-induced Akt activation was enhanced by ALDH2 overexpression although it was attenuated in KO hearts. Given the correlative findings among HNE content, 4-HNE-induced cardiomyocyte mechanical defect, and ischaemia–reperfusion injury in WT and ALDH2 overexpression mice, our findings suggest a role of cytotoxic aldehyde detoxification in ALDH2-offered beneficial effects against interrupted AMPK and Akt signalling as well as compromised myocardial dysfunction under I/R. HNE has been shown to inhibit AMPK12 and Akt13 activity, consistent with our findings of downregulated pan and phosphorylated LKB1 with 4-NHE exposure in murine cardiomyocytes. Our results demonstrated that 4-HNE led to a significant reduction in pan and phosphorylated PTEN in cardiomyocytes. PTEN is the main phosphatase serving as a negative regulator of the PI3K/Akt and is inactivated through phosphorylation.35 LKB1 may also phosphorylate PTEN.33,34 Detoxification of 4-HNE with ALDH2 should remove the suppression of 4-HNE on LKB1/PTEN-mediated AMPK and Akt signalling, thus preserving the critical kinase activity in I/R. It is worth mentioning that 4-HNE may impair cardiac contractile function through other cell signalling cascades such as HNE metabolism and MAP kinase activation.11
In summary, our data have demonstrated an essential role of ALDH2 in cardioprotection against myocardial I/R injury, possibly through an AMPK-dependent induction of autophagy during ischaemia and a paradoxical Akt-dependent reduction in autophagy during reperfusion. Our evidence favours a role of detoxification of toxic aldehyde in ALDH2-elicited regulation of AMPK and Akt pathway, possibly via LKB1 and PTEN signalling. These data suggest not only the therapeutic potential of ALDH2 against I/R injury but also a pivotal role of the AMPK-Akt-mTOR signalling cascade in autophagy regulation in the maintenance of cardiomyocyte survival homeostasis. It should be mentioned that one major limitation of our study is the relatively low sample size per group, although more large-scale studies are in need to fully unveil the important cardioprotective property and the underlying clinical implication of ALDH2. Further studies are warranted to examine the association between ALDH2 gene and cardiovascular risk in human.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by NIAAA 1R01 AA013412 (J.R.), NCRR P20RR016474 (J.R.), and AHA Postdoctoral Fellowship 09POST2250477 (H.M.).
Conflict of interest: none declared.
Acknowledgements
The authors wish to thank Dr Paul N. Epstein from the University of Louisville (Louisville, KY, USA) for the production of the ALDH2 transgenic line. The ALDH2 KO mice were kindly provided by Drs Kyoko Kitagawsa (Hamamatsu University School of Medicine, Shizuoka, Japan), Keiichi I. Nakayama (Kyushu University, Fukuoka, Japan) and Toshihiro Kawamoto (University of Occupational and Environmental Health, Kitakyshu, Japan).
References
- 1.Ren J. Acetaldehyde and alcoholic cardiomyopathy: lessons from the ADH and ALDH2 transgenic models. Novartis Found Symp. 2007;285:69–76. doi: 10.1002/9780470511848.ch5. Discussion 76–69, 198–199. doi:10.1002/9780470511848.ch5. [DOI] [PubMed] [Google Scholar]
- 2.Daiber A, Wenzel P, Oelze M, Munzel T. New insights into bioactivation of organic nitrates, nitrate tolerance and cross-tolerance. Clin Res Cardiol. 2008;97:12–20. doi: 10.1007/s00392-007-0588-7. doi:10.1007/s00392-007-0588-7. [DOI] [PubMed] [Google Scholar]
- 3.Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, Ren J. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006;40:283–294. doi: 10.1016/j.yjmcc.2005.11.006. doi:10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Li SY, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol. 2008;44:992–1001. doi: 10.1016/j.yjmcc.2008.02.276. doi:10.1016/j.yjmcc.2008.02.276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science (New York, NY) 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churchill EN, Disatnik MH, Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46:278–284. doi: 10.1016/j.yjmcc.2008.09.713. doi:10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renner A, Sagstetter MR, Harms H, Lange V, Gotz ME, Elert O. Formation of 4-hydroxy-2-nonenal protein adducts in the ischemic rat heart after transplantation. J Heart Lung Transplant. 2005;24:730–736. doi: 10.1016/j.healun.2004.02.021. doi:10.1016/j.healun.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Eaton P, Li JM, Hearse DJ, Shattock MJ. Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat heart. Am J Physiol. 1999;276:H935–H943. doi: 10.1152/ajpheart.1999.276.3.H935. [DOI] [PubMed] [Google Scholar]
- 9.van der Kraaij AM, de Jonge HR, Esterbauer H, de Vente J, Steinbusch HW, Koster JF. Cumene hydroperoxide, an agent inducing lipid peroxidation, and 4-hydroxy-2,3-nonenal, a peroxidation product, cause coronary vasodilatation in perfused rat hearts by a cyclic nucleotide independent mechanism. Cardiovasc Res. 1990;24:144–150. doi: 10.1093/cvr/24.2.144. doi:10.1093/cvr/24.2.144. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa T, Esterbauer H, Sies H. Role of cardiac glutathione transferase and of the glutathione S-conjugate export system in biotransformation of 4-hydroxynonenal in the heart. J Biol Chem. 1986;261:1576–1581. [PubMed] [Google Scholar]
- 11.Aberle NS, II, Picklo MJ, Sr, Amarnath V, Ren J. Inhibition of cardiac myocyte contraction by 4-hydroxy-trans-2-nonenal. Cardiovasc Toxicol. 2004;4:21–28. doi: 10.1385/ct:4:1:21. doi:10.1385/CT:4:1:21. [DOI] [PubMed] [Google Scholar]
- 12.Dolinsky VW, Chan AY, Robillard Frayne I, Light PE, Des Rosiers C, Dyck JR. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. doi:10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Akhand AA, Takeda K, Kawamoto Y, Itoigawa M, Kato M, Suzuki H, Ishikawa N, Nakashima I. Protein phosphatase 2A-linked and -unlinked caspase-dependent pathways for downregulation of Akt kinase triggered by 4-hydroxynonenal. Cell Death Differ. 2003;10:772–781. doi: 10.1038/sj.cdd.4401238. doi:10.1038/sj.cdd.4401238. [DOI] [PubMed] [Google Scholar]
- 14.Matsui Y, Kyoi S, Takagi H, Hsu CP, Hariharan N, Ago T, Vatner SF, Sadoshima J. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4:409–415. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. doi:10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 16.Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia–reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41:256–264. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Qian J, Ren X, Wang X, Zhang P, Jones WK, Molkentin JD, Fan GC, Kranias EG. Blockade of Hsp20 phosphorylation exacerbates cardiac ischemia/reperfusion injury by suppressed autophagy and increased cell death. Circ Res. 2009;105:1223–1231. doi: 10.1161/CIRCRESAHA.109.200378. doi:10.1161/CIRCRESAHA.109.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Meyer GR, Martinet W. Autophagy in the cardiovascular system. Biochim Biophys Acta. 2008;1793:1485–1495. doi: 10.1016/j.bbamcr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. doi:10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 20.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. doi:10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 21.Goswami SK, Das DK. Autophagy in the myocardium: dying for survival? Exp Clin Cardiol. 2006;11:183–188. [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009;16:46–56. doi: 10.1038/cdd.2008.110. doi:10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- 23.Gu Y, Wang C, Cohen A. Effect of IGF-1 on the balance between autophagy of dysfunctional mitochondria and apoptosis. FEBS Lett. 2004;577:357–360. doi: 10.1016/j.febslet.2004.10.040. doi:10.1016/j.febslet.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 24.Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–35246. doi: 10.1074/jbc.C100319200. doi:10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- 25.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. doi:10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 26.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. doi:10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 27.Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119:1941–1949. doi: 10.1161/CIRCULATIONAHA.108.823799. doi:10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. doi:10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 29.Russell RR, III, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q, Yang X, Sreejayan N, Ren J. Insulin-like growth factor I deficiency prolongs survival and antagonizes paraquat-induced cardiomyocyte dysfunction: role of oxidative stress. Rejuvenation Res. 2007;10:501–512. doi: 10.1089/rej.2007.0552. doi:10.1089/rej.2007.0552. [DOI] [PubMed] [Google Scholar]
- 31.Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104:150–158. doi: 10.1161/CIRCRESAHA.108.187427. doi:10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usatyuk PV, Parinandi NL, Natarajan V. Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J Biol Chem. 2006;281:35554–35566. doi: 10.1074/jbc.M607305200. doi:10.1074/jbc.M607305200. [DOI] [PubMed] [Google Scholar]
- 33.Mehenni H, Lin-Marq N, Buchet-Poyau K, Reymond A, Collart MA, Picard D, Antonarakis SE. LKB1 interacts with and phosphorylates PTEN: a functional link between two proteins involved in cancer predisposing syndromes. Hum Mol Genet. 2005;14:2209–2219. doi: 10.1093/hmg/ddi225. doi:10.1093/hmg/ddi225. [DOI] [PubMed] [Google Scholar]
- 34.Mocanu MM, Yellon DM. PTEN, the Achilles' heel of myocardial ischaemia/reperfusion injury? Br J Pharmacol. 2007;150:833–838. doi: 10.1038/sj.bjp.0707155. doi:10.1038/sj.bjp.0707155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. doi:10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Halapas A, Armakolas A, Koutsilieris M. Autophagy: a target for therapeutic interventions in myocardial pathophysiology. Expert Opin Ther Targets. 2008;12:1509–1522. doi: 10.1517/14728220802555554. doi:10.1517/14728220802555554. [DOI] [PubMed] [Google Scholar]
- 37.Decher RS, Poole AR, Dingle JT, Wildenthal K. Influence of methylprednisolone of the sequential redistribution of cathepsin D and other lysosomal enzymes during myocardial ischemia in rabbits. J Clin Invest. 1978;62:797–804. doi: 10.1172/JCI109191. doi:10.1172/JCI109191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurusamy N, Lekli I, Gorbunov NV, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2009;13:373–387. doi: 10.1111/j.1582-4934.2008.00495.x. doi:10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takemura G, Maruyama R, Goto K, Kanamori H, Tsujimoto A, Minatoguchi S, Fujiwara H. Fate of isolated adult cardiomyocytes undergoing starvation-induced autophagic degeneration. Autophagy. 2009;5:90–92. doi: 10.4161/auto.5.1.7206. doi:10.4161/auto.5.1.7206. [DOI] [PubMed] [Google Scholar]
- 40.Araki M, Motojima K. Hydrophobic statins induce autophagy in cultured human rhabdomyosarcoma cells. Biochem Biophys Res Commun. 2008;367:462–467. doi: 10.1016/j.bbrc.2007.12.166. doi:10.1016/j.bbrc.2007.12.166. [DOI] [PubMed] [Google Scholar]
- 41.Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS, Stephanou A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. doi:10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 42.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100:474–488. doi: 10.1161/01.RES.0000258446.23525.37. doi:10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 43.Clerk A, Cole SM, Cullingford TE, Harrison JG, Jormakka M, Valks DM. Regulation of cardiac myocyte cell death. Pharmacol Ther. 2003;97:223–261. doi: 10.1016/s0163-7258(02)00339-x. doi:10.1016/S0163-7258(02)00339-X. [DOI] [PubMed] [Google Scholar]
- 44.Hickson-Bick DL, Jones C, Buja LM. Stimulation of mitochondrial biogenesis and autophagy by lipopolysaccharide in the neonatal rat cardiomyocyte protects against programmed cell death. J Mol Cell Cardiol. 2008;44:411–418. doi: 10.1016/j.yjmcc.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Lucas DT, Szweda LI. Declines in mitochondrial respiration during cardiac reperfusion: age-dependent inactivation of alpha-ketoglutarate dehydrogenase. Proc Natl Acad Sci USA. 1999;96:6689–6693. doi: 10.1073/pnas.96.12.6689. doi:10.1073/pnas.96.12.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Henderson GI, Freeman GL. Role of 4-hydroxynonenal in modification of cytochrome c oxidase in ischemia/reperfused rat heart. J Mol Cell Cardiol. 2001;33:1919–1927. doi: 10.1006/jmcc.2001.1454. doi:10.1006/jmcc.2001.1454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.