Abstract
We synthesized a galactose derivative, N-octyl-4-epi-β-valienamine (NOEV), for a molecular therapy (chemical chaperone therapy) of a human neurogenetic disease, β-galactosidosis (GM1-gangliosidosis and Morquio B disease). It is a potent inhibitor of lysosomal β-galactosidase in vitro. Addition of NOEV in the culture medium restored mutant enzyme activity in cultured human or murine fibroblasts at low intracellular concentrations, resulting in a marked decrease of intracellular substrate storage. Short-term oral administration of NOEV to a model mouse of juvenile GM1-gangliosidosis, expressing a mutant enzyme protein R201C, resulted in significant enhancement of the enzyme activity in the brain and other tissues. Immunohistochemical stain revealed a decrease in the amount of GM1 and GA1 in neuronal cells in the fronto-temporal cerebral cortex and brainstem. However, mass biochemical analysis did not show the substrate reduction observed histochemically in these limited areas in the brain probably because of the brief duration of this investigation. Chemical chaperone therapy may be useful for certain patients with β-galactosidosis and potentially other lysosomal storage diseases with central nervous system involvement.
Hereditary deficiency of lysosomal acid β-galactosidase (β-galactosidosis) causes two clinically distinct diseases in humans, GM1-gangliosidosis and Morquio B disease (1). The mode of inheritance is autosomal recessive. GM1-gangliosidosis is a generalized neurosomatic disease occurring mainly in early infancy, and rarely in childhood or young adults. Morquio B disease is a rare systemic bone disease without central nervous system involvement.
Glycoconjugates with terminal β-galactose residues accumulate in tissues and urine from patients with these clinical phenotypes. Ganglioside GM1 and its asialo derivative GA1 accumulate in the GM1-gangliosidosis brain. High amounts of oligosaccharides derived from keratan sulfate or glycoproteins are detected in visceral organs and urine from GM1-gangliosidosis and Morquio B disease patients.
At present only symptomatic therapy is available for human β-galactosidosis patients. Allogeneic bone marrow transplantation did not modify subsequent clinical course or cerebral enzyme activity in a Portuguese water dog affected with GM1-gangliosidosis (2). Amniotic tissue transplantation was not effective in a patient with Morquio B disease (3). Enzyme replacement therapy conducted for Gaucher disease and other lysosomal storage diseases is not available at present for β-galactosidosis.
Recently we reported results of a molecular approach (chemical chaperone therapy) for restoration of mutant α-galactosidase in Fabry disease. Galactose and its structural analog, 1-deoxygalactonojirimycin, restored residual enzyme activity in cultured human lymphoblasts from patients with α-galactosidase deficiency (4, 5) and transgenic (Tg) mouse tissues expressing a mutant enzyme causing Fabry disease (5, 6). Some mutant proteins are unstable at neutral pH in the endoplasmic reticulum/Golgi apparatus and are rapidly degraded without appropriate molecular folding (7, 8). Certain exogenous compounds that inhibit enzyme activity in vitro bind to the enzyme intracellularly, resulting in the formation of a complex that stabilizes and transports the catalytically active enzyme to lysosomes. Under the acidic condition in lysosomes, the complex dissociates, and the mutant enzyme remains stabilized and functional.
In this study, we synthesized a compound for possible molecular therapy of brain pathology in β-galactosidosis and confirmed its restorative effect on the model mouse brain after short-term oral administration.
Materials and Methods
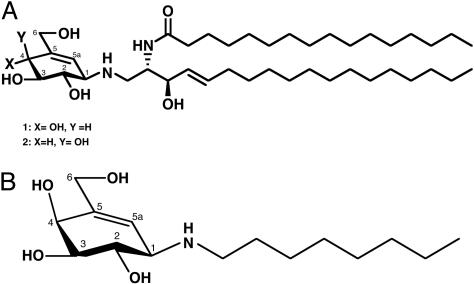
Synthesis of a β-Galactosidase Inhibitor, N-octyl-4-epi-β-valienamine (NOEV). We chemically modified a glucocerebrosidase inhibitor (Fig. 1A; compound 1) (9-11) by replacing the ceramide moiety with simple aliphatic chains (11, 12) and multistep epimerization at C-4 (13). In this study, we chose an N-octyl derivative, N-octyl-4-epi-β-valienamine (Fig. 1B) for experimental studies of chemical chaperone therapy (5) in murine GM1-gangliosidosis. We use the term NOEV as abbreviation of this compound. Its structure was assigned by a combination of COSY, total correlation spectroscopy (TOCSY), and heteronuclear sequential quantum correlation (HSQC) NMR spectroscopy. NMR spectra were recorded with a Varian UNITYINOVA 500 [1H (500 MHz) or13C (125 MHz)] spectrometer. Chemical shifts were expressed in ppm downfiled from the signal for internal Me4Si for solutions in CD3OD. The sample temperature was 23°C, and concentration was 10 mg/ml.
Fig. 1.
Structure of inhibitors for β-glucosidase and β-galactosidase. (A) Synthetic derivatives of glucosylceramide (1) and galactosylceramide (2). (B) Structure of N-octyl-4-epi-β-valienamine (NOEV).
Cell Culture and NOEV Experiments. Human and murine fibroblasts were cultured and used for enzyme inhibition/restoration experiments. Fibroblasts from human patients with GM1-gangliosidosis or Morquio B disease were kindly provided by colleagues in Japan and other countries or purchased from Coriell Institute for Medical Research (Camden, NJ). Mouse fibroblast lines expressing mutant human β-galactosidase were established as reported (14). They were cultured in DMEM with 10% FCS and antibiotics. NOEV was added at various concentrations to the culture medium of fibroblasts from human patients or genetically engineered mice.
Ganglioside Loading of Cultured Mouse Fibroblasts. The mouse fibroblasts expressing wild-type (GP8) or R201C mutant human β-galactosidase were cultured in medium containing bovine ganglioside mixture (0.1 mg/ml; Sigma) for 2 days followed by culture in the presence of NOEV (0.2 μM) for 4 subsequent days. The cells were stained with FITC-labeled cholera toxin B (Sigma) to detect ganglioside GM1 (15).
Gene Mutation Analysis. We analyzed mutant genes as described (14). In short, after extraction of genomic DNA from human fibroblasts, each of 16 exons with flanking sequence was amplified by PCR under the standard conditions. All exons except 1, 4, 7, and 9 were sequenced directly by using ABI PRISM 3100 Genetic Analyzer (Applied Biosystems Japan, Tokyo, Japan). The amplified exons 1, 4, 7, and 9 were subjected to single strand conformation polymorphism (16). Exons with aberrant bands were subcloned into pGEM-T vector (Promega) and sequenced.
Knockout (KO) and KO-Tg Mice. We prepared a C57BL/6-based congenic strain KO mouse with β-galactosidase deficiency as reported (17). Then, we produced a Tg mouse line overexpressing mutant human β-galactosidase with an amino acid substitution R201C that causes juvenile type GM1-gangliosidosis in humans by injecting the DNA fragment containing β-actin promoter (CAG promoter) and human mutant β-galactosidase cDNA (R201C) into C57BL/6 fertilized eggs. Cross-breeding of the KO mice and Tg mice was performed to obtain KO-Tg mice (R201C mice). We used hemizygous Tg mice with the β-galactosidase KO background in this study. Presence of the mutation of human β-galactosidase gene (R201C) in these mice was confirmed by DNA sequencing. We further confirmed that these mice expressed human β-galactosidase but did not express endogenous mouse enzyme by Northern blot analysis and RT-PCR. The genotype of these mice was determined by PCR amplification of tail DNA, using the following primer sets: 5′-GCTGGTTGTTGTGCTGTCTCATCATT-3′ (sense) and 5′-AGTTCCAGGGCACATACGTCTGGAT-3′ (antisense) for detection of human β-galactosidase transgene (PCR product, ≈330 bp); 5′-CTCGCGCCAGCCGAACTGTT-3′ (sense) and 5′-GTTCGAGGCCACACGCGTCA-3′ (antisense) for detection of neor gene (PCR product, 570 bp); and 5′-CATTCCTGCCAAGACAGTAG-3′ (sense) and 5′-ATGGCCTCAGTGTTCAGTGGG-3′ (antisense) for detection of wild-type exon 15 of mouse β-galactosidase gene (PCR product, 242 bp). PCR was performed by using Taq polymerase (EX-Taq, Takara, Ohtsu, Japan) at 30 cycles of reaction at 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The care of experimental animals was carried out in accordance with the guidelines on animal experimentation of the National Institute of Infectious Diseases (Tokyo).
Oral Administration of NOEV to the R201C Mouse. An aqueous solution of NOEV (0.1-1 mM) was given ad libitum orally to the R201C mouse for 1 week. The mouse was anesthetized with ethylether; blood was collected by cardiac puncture, and then brain and other tissues were removed. The brain was immediately divided into two sagittal sections, one for biochemical analysis and the other for pathological analysis. For comparison with ad libitum oral administration of NOEV, we also analyzed mice to which daily doses of 0.5 ml of 1 mM NOEV were administered by gavage.
Histochemistry and Immunohistochemistry of Mouse Tissues. Brain tissue was immersed in 4% phosphate-buffered paraformaldehyde overnight and frozen for embedding in OTC (optimal cutting temperature) compound (Sakura Finetechnical Co., Tokyo, Japan). Sections of 8-μm thickness were stained with hematoxylin and eosin. The frozen slices of brain and liver were reacted with 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal, Wako, Osaka), 50 mM ferrocyanide, 50 mM ferricyanide, 2 mM MgCl2, and 0.1 M sodium acetate (pH 4.5), in 20m M NaCl, for 12 h at 37°C. Serial sections were also immunostained for GM1 and GA1. Details of the procedures were described in a previous report (18).
For immunohistochemistry, monoclonal anti-GM1 antibody (clone GMB16) and monoclonal anti-GA1 antibody (clone AG-1) were purchased from Seikagaku Corporation (Tokyo). Brain tissue sections were permeabilized with 0.25% Triton X-100 in PBS for 15 min at room temperature, blocked with 1% BSA in PBS for 1 h at room temperature, and incubated with the first antibody at 4°C overnight. The anti-GM1 had been diluted 1:50 and anti-GA1 1:25 with 0.1% BSA in PBS before use. The sections were washed with 1% BSA in PBS three times for 5 min each at room temperature. Then, they were incubated with FITC-conjugated anti-mouse IgM (1:50 dilution with 0.1% BSA in PBS, washed with PBS 3 times for 5 min each, and mounted on a slide glass (19).
Biochemical Analysis of Mouse Tissues. The left half of the whole brain was used for enzyme assay and lipid analysis. Activities of lysosomal enzymes were assayed by using 4-methylumbelliferyl derivatives (Sigma) as described (20). Protein was determined with the DC Assay Kit (Bio-Rad) or BCA Protein Assay Kit (Pierce).
Total lipids were extracted with chloroform:methanol (2/1, vol/vol) and applied to a chromatography column of DEAE-Sepahadex A-25 (acetate form). Neutral glycosphingolipids were eluted with chloroform:methanol:water (30/60/8, vol/vol/vol), and then gangliosides were eluted with chloroform:methanol:0.8 M sodium acetate (30/60/8, vol/vol/vol) (21). Gangliosides were saponified with 0.5 ml of 0.2 M KOH/methanol, desalted with Sep-Pak C18 reverse-phase cartridge (22) (Waters), and then acetylated with pyridine-acetic anhydride (3:1, vol/vol). Acetylated glycosphingolipids were purified by Sep-Pak Florisil cartridge (Waters) chromatography by using acetone-dichloroethane (1:1, vol/vol) as an elution solvent according to Saito and Hakomori (23). For deacetylation, the acetylated glycosphingolipids were saponified as described in the ganglioside purification procedure. Individual gangliosides and glycosphingolipids were separated by high performance TLC on a plate precoated with superfine silica gel 60 (Merck, Darmstadt, Germany), with chloroform:methanol:0.2% CaCl2 (60/35/8, vol/vol/vol) for development and visualized with orcinol reagent for qualitative and quantitative analysis (24).
Results
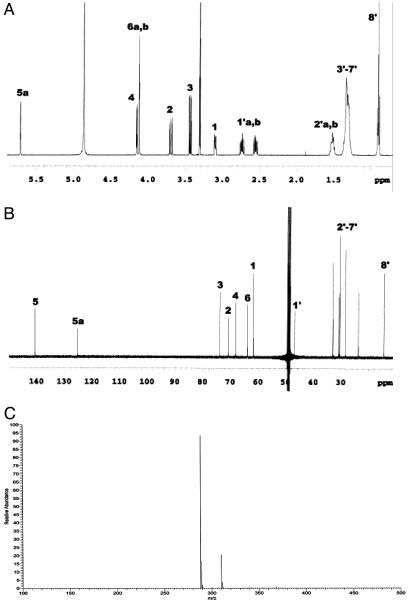
Characterization of NOEV. 1H- and 13C-NMR data of NOEV are shown in Fig. 2 A and B. The evidence for β linkage was attributed to the doublet signal for H-1 at δ 3.101 (J1,2 = 8.1 Hz, J1,5a = 2.2 Hz) (Fig. 2 A). A positive-ion electrospray ionization (ESI) mass spectrum of NOEV produced peaks at m/z 288.04 and 310.04, corresponding respectively to [M + H]+ and [M + Na]+ (Fig. 2C). NOEV had Rf 0.33 and 0.24 (CHCl3:MeOH:H2O, 60:35:8); [α]D +2.64° (C = 1.03, MeOH) NOEV was found to be stable at room temperature and a strong inhibitor of human β-galactosidase in vitro. It is freely soluble in methanol or dimethyl sulfoxide, and it is soluble in water up to 3-5 mM at room temperature. The molecular weight is 287.40. The IC50 is 0.2 μM toward human β-galactosidase. This compound did not inhibit galactosyltransferase activity. Accordingly, substrate deprivation did not occur with NOEV.
Fig. 2.
Spectral data of NOEV. Mass spectrum data of NOEV. (A)1H-NMR. δ 0.900 (t, 3 H, J8′7′ = 6.8 Hz, H-8′), 1.290-1.370 (m, 10 H, H-3′, 4′, 5′, 6′, and 7′), 1.490-1.540 (m, 2 H, H-2′), 2.555 (ddd, 1 H, Jgem = 11.2 Hz, J1′b,2′ = 7.4 Hz, H-1′b), 2.739 (ddd, 1 H, Jgem = 11.2 Hz, J1′a,2′ = 7.4 Hz, H-1′a), 3.101 (dd, 1 H, J1,2 = 8.1 Hz, J1,5a = 2.2 Hz, H-1), 3.435 (dd, 1 H, J3,2 = 10.0 Hz, J3,4 = 4.2 Hz, H-3), 3.696 (dd, 1 H, J2,1 = 8.1 Hz, J2,3 = 10.0 Hz, H-2), 4.121 (t, 2 H, J6a,6b = 1.7 Hz, H-6a and 6b), 4.154 (d, 1H, J4,3 = 4.2 Hz, H-4), 5.718 (d, 1H, J5a,1 = 2.2 Hz, H-5a). (B)13C-NMR, δ 14.466 (C-8′), 23.766, 28.480, 30.448, 30.666, 30.983, 33.050 (C-2′≈7′), 46.925 (C-1′), 61.848 (C-1), 63.958 (C-6), 68.192 (C-4), 70.862 (C-2), 73.929 (C-3), 125.326 (C-5a), 140.716 (C-5). (C) Electrospray ionization mass. [M + H]+, [M + Na]+: m/z 288.04, 310.04.
Effect of NOEV on β-Galactosidase Activity in Cultured Cells. Among the mouse fibroblasts expressing mutant human β-galactosidase R201C and R201H cell strains (14), catalytic activity was markedly increased after exposure to NOEV at 0.2-2 μM in the culture medium for 4 days (Table 1). Lesser augmentation of β-galactosidase activity occurred in fibroblasts with R457Q, W273L, and Y83H mutations. Almost the same or more restorative effect was achieved at 2,500-fold lower concentration than that with 1-deoxygalactonojirimycin or N-butyl-deoxygalactonojirimycin.
Table 1. Effect of NOEV on mouse fibroblasts expressing mutant human β-galactosidase.
| Addition
|
|||||
|---|---|---|---|---|---|
| Phenotype | Cell line | None | DGJ (fold) | NB-DGJ (fold) | NOEV (fold) |
| Normal | GP8 | 67.6 | 112.9 (1.7) | 94.5 (1.4) | 78.9 (1.2) |
| Juvenile GM1 | R201C | 22.9 | 131.9 (5.8) | 113.5 (5.0) | 116.3 (5.1) |
| Adult GM1 | R201H | 19.1 | 34.2 (1.8) | 52.7 (2.8) | 86.0 (4.5) |
| R457Q | 5.9 | 31.1 (5.3) | 35.6 (6.1) | 14.2 (2.4) | |
| Morquio B | W273L | 8.1 | 11.3 (1.4) | 11.0 (1.4) | 17.7 (2.2) |
| Y83H | 10.1 | 17.1 (1.7) | 12.4 (1.2) | 20.2 (2.0) | |
Enzyme activity is expressed as nmol/mg of protein per h. Increase after addition is expressed in parentheses as ratio of values with and without inhibitors. GM1, GM1-gangliosidosis; Cell line, cells expressing wild (GP8) or mutant (R201C, R201H, R457Q, W273L, Y83H) β-galactosidase causing various phenotypes of human β-galactosidosis; DGJ, 1-deoxygalactonojirimycin (0.5 mM); NB-DGJ, N-butyl-deoxygalactonojirimycin (0.5 mM); NOEV, N-octyl-4-epi-β-valienamine (0.2 μM).
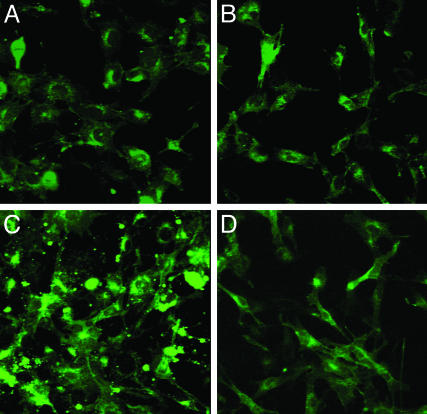
After adding the mixture of gangliosides to the culture medium, intracellular GM1 increased remarkably in R201C cells but only slightly in GP8 cells (Fig. 3). Incubation with NOEV significantly reduced GM1 storage in R201C cells.
Fig. 3.
Effect of NOEV after ganglioside loading on GM1 storage in cultured mouse fibroblasts expressing human β-galactosidase gene. The cells expressing mutant (R201C) or wild-type (GP8) human β-galactosidase were loaded with ganglioside mixture, and then cultured with additional 0.2 μM NOEV (4 days). Shown is staining with FITC-labeled cholera toxin B. (A and C) R201C cells. (B and D) GP8 cells. (A and B) After NOEV treatment. (C and D) Before NOEV treatment. GM1 storage was reduced to the control (GP8) level after NOEV treatment. There was no change in GP8 cells.
In experiments with human fibroblasts, all of the cells derived from juvenile GM1-gangliosidosis patients and 30% of infantile GM1-gangliosidosis patients expressed an increase of β-galactosidase activity after NOEV treatment (data not shown). Most of them were compound heterozygotes with various mutations, particularly of R201C or R457Q.
KO-Tg Mouse Expressing Human R201C-Mutant β-Galactosidase. The R201C mice, expressing the human R201C-mutant β-galactosidase but lacking the endogenous mouse β-galactosidase, had very low β-galactosidase activity in the brain (≈4% of the wild-type activity). They exhibited an apparently normal clinical course for the first 6 months after birth that was followed by slowly progressive neurological deterioration, such as tremor and gait disturbance during the next 9 months. Death ensued around 15 months of age due to malnutrition and emaciation. Neuropathological examination revealed vacuolated or ballooned neurons, but they were less abundant than those in the KO mouse brain described in our previous reports (17, 18). Intracytoplasmic storage materials were present in pyramidal neurons and brainstem motor neurons, but not in neurons in the other areas of the brain. There was no storage in glial cells.
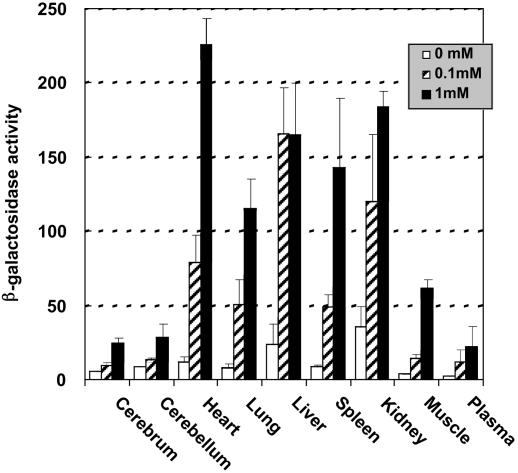
Oral Administration of NOEV Restores β-Galactosidase Activity in R201C Mouse Tissues. Aqueous solutions of NOEV (0.1-1 mM) were given ad libitum for 1 week to R201C mice. In this short-term experiment, all of the tissues that were examined showed a remarkable increase of β-galactosidase activity with 1 mM NOEV and a lesser degree with 0.1 mM NOEV (Fig. 4). The increase was relatively high in the heart, lungs, spleen, and muscle as compared with cerebrum and cerebellum. The augmented enzyme activity was sufficient for substrate degradation in these tissues (see below). With 1 mM NOEV, the amount of daily intake was estimated to be 1.4 mg, based on the observation that the daily consumption of water was ≈5 ml per mouse. All of the tissues of mice that received a single daily dose of 0.5 ml of 1 mM NOEV by gavage showed the same increase of β-galactosidase activity that occurred in mice with ad libitum oral daily intake of 5 ml of 0.1 mM NOEV (data not shown).
Fig. 4.
β-Galactosidase activity in mouse tissues after NOEV treatment. Results are shown for oral administration of NOEV water solution (0.1-1 mM) ad libitum for 1 week to R201C mice (3 months old). β-Galactosidase activity is expressed as nmol/mg of protein per 30 min (mean ± SD; n = 3).
We used X-Gal staining for evaluation of β-galactosidase expression in individual cells. X-Gal was mainly positive in neurons in the cerebral cortex, thalamus, caudo-putamen, brainstem nuclei, and Purkinje cells, as well as glial cells in cerebral and cerebellar white matter and hepatic cells (Fig. 5). The intensity of staining correlated well with enzyme activity in vitro.
Fig. 5.
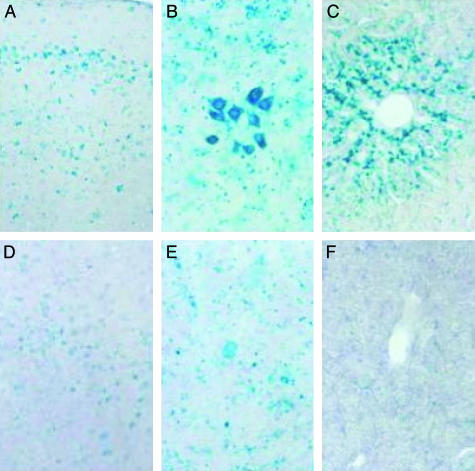
X-Gal stain of mouse tissues. The R201C mouse, 3 months old, was given orally a water solution of 1 mM NOEV ad libitum for 1 week. The mouse tissues after NOEV treatment are more intensely stained than those without treatment. (A and D) Fronto-temporal cerebral cortex. (B and E) Trigeminal nucleus of brainstem. (C and F) Liver. (A, B, and C) After NOEV treatment. (D, E, and F) Before NOEV treatment. All tissues showed a remarkable increase of X-Gal stain.
Substrate Storage in the Mouse Brain After NOEV Treatment. Immunostaining of the brain tissue revealed a clear decrease of both GM1 and GA1 in neuronal cells of the fronto-temporal cerebral cortex and brainstem after oral administration of NOEV to the R201C mouse for 1 week (Fig. 6). We did not, however, detect a reduction of GM1 or GA1 on mass biochemical analysis, presumably because of the short duration of these experiments and localized accumulation of these sphingolipids in neurons in specific regions of the brain.
Fig. 6.
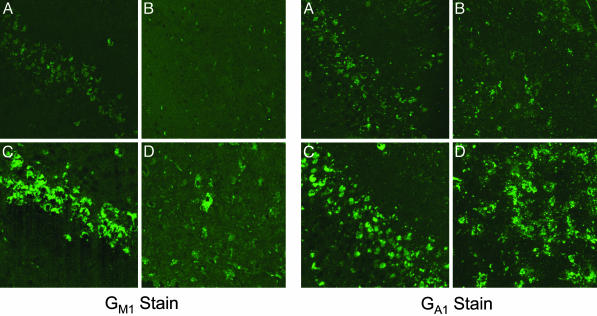
Immunostaining of GM1 and GA1 in the mouse brain. The R201C mouse, 3 months old, was given orally a water solution of 1 mM NOEV ad libitum for 1 week, and the fronto-temporal cortex and brainstem were stained as described in Materials and Methods. (A and C) Fronto-temporal cerebral cortex. (B and D) Trigeminal nucleus of brainstem. (A and B) After NOEV treatment. (C and D) Before NOEV treatment. A remarkable decrease of both GM1 and GA1 is observed in the brain after the NOEV treatment.
Discussion
Low molecular weight compounds for chemical chaperone therapy act as inhibitors at high concentrations in vitro and as in situ activators at low concentrations. We first demonstrated this apparent paradoxical phenomenon in Fabry disease (4-6) and then in β-galactosidosis (this study) and Gaucher disease (β-glucosidase deficiency) (unpublished data). Intracellular NOEV concentration in the cell and animal experiments in this study must have been much lower than IC50 of NOEV in vitro. In fact, the concentration of NOEV in the tissue culture medium was approximately the IC50 in our cell culture experiments.
In a molecular analysis of GM1-gangliosidosis, Zhang et al. (25) reported essentially the same data as ours concerning the mutant enzyme in an infantile patient. The mutation R148S resulted in a major conformational change of the protein molecule with normal catalytic activity. In that case, the mutant protein failed to reach the lysosome and did not express catalytic activity. The report supports the principle of our approach to the molecular rescue of mutant proteins in patients with GM1-gangliosidosis.
Substrate deprivation is another possibility for the treatment of lysosomal storage diseases. N-butyl-deoxynojirimycin, an inhibitor of ceramideglucosyltransferase, produced clinical and morphological effects in the Tay-Sachs mouse (26), Sandhoff mouse (27), and Fabry mouse (28), and in patients with Gaucher disease (29). For chemical chaperone therapy, only a low level of the material is needed for restoration of the catalytic activity of mutant enzymes compared with that for inhibition of biosynthetic reactions. It is hoped that the administration of such low levels of useful inhibitors would occur without clinical problems.
Chemical chaperone therapy has two major advantages over enzyme replacement therapy, oral administration and accessibility to the brain. NOEV administration in this study was for a short-term, and we did not anticipate a decrease in overall substrate storage in the brain of experimental animals in the late-onset type disease. The R201C mutation causes a mild clinical phenotype of GM1-gangliosidosis, and substrate storage is not full-blown in the early stage of life either in mice or humans. Intracytoplasmic ballooning was observed only in pyramidal cells and brainstem motor neurons in the R201C mouse brain. Because of the brief duration of the investigation and potential limitation of high glycosphingolipid turnover to neuronal cells in the fronto-temporal cerebral cortex and brainstem, we did not see a reduction of GM1 or GA1 on mass biochemical analysis. More time may be needed to demonstrate a mass substrate reduction in the brain of the model mouse that represents juvenile GM1-gangliosidosis with a protracted clinical course.
We also need long-term experiments to establish an optimal dose for prevention or reduction of substrate storage in these mice. Possible adverse or toxic effects should be carefully evaluated before starting human clinical experiments. We are aware that this molecular approach is not justified for all patients with a single lysosomal enzyme deficiency disorder. Biosynthesis of a catalytically active enzyme is prerequisite in chemical chaperone therapy. Our initial survey indicates that at least 30% of β-galactosidosis (mainly GM1-gangliosidosis) patients express unstable but catalytically active protein and respond to NOEV treatment in cultured fibroblasts. Patients of this type may be reasonable candidates for chemical chaperone therapy. This strategy is in principle applicable to all lysosomal storage diseases if specific compounds can be found for each enzyme that is involved. Special drug design technology may be needed to screen appropriate inhibitors. Bioinformatics analysis may reveal a new aspect of molecular pathology in lysosomal storage diseases (30, 31) and be a useful adjunct for the development of effective chemical chaperone therapy.
Acknowledgments
We thank the following investigators for supplying human fibroblasts in this study: Nils U. Bosshard (University Children's Hospital, Zurich), Koji Inui (Osaka University, Osaka), Jana Ledvinova (Charles University, Prague), Gert Matthijs (Universitaire Ziekenhuizen, Leuven), Toshihiro Oura (Tohoku University, Sendai), Alan Percy (The Children's Hospital of Alabama), Konrad Sandhoff and Gerhild van Echten-Deckert (Kekule-Institut für Organische Chemie und Biochemie, Bonn), George H. Thomas (Kennedy Krieger Institute, Baltimore), David A. Wenger (Thomas Jefferson University, Philadelphia), and Marie-Therese Zabot (Hôpital Debrousse, Lyon). This research was supported by Ministry of Education, Culture, Science, Sports, and Technology of Japan Grants 13680918 and 14207106, and Ministry of Health, Labour, and Welfare of Japan Grant 14221201.
Abbreviations: NOEV, N-octyl-4-epi-β-valienamine; KO, knockout; Tg, transgenic; X-Gal, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside.
References
- 1.Suzuki, Y., Oshima, A. & Nanba, E. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S., Valle, D., Childs, B. & Vogelstein, B. (McGraw-Hill, New York), 8th Ed., pp. 3775-3809.
- 2.O'Brien, J. S., Storb, R., Raff, R. F., Harding, J., Appelbaum, F., Morimoto, S., Kishimoto, Y., Graham, T., Ahern Rindell, A. & O'Brien, S. L. (1990) Clin. Genet. 38, 274-280. [DOI] [PubMed] [Google Scholar]
- 3.Tylki-Szymanska, A., Maciejko, D., Kidawa, M., Jablonska-Budaj, U. & Czartoryska, B. (1985) J. Inherit. Metab. Dis. 8, 101-104. [DOI] [PubMed] [Google Scholar]
- 4.Okumiya, T., Ishii, S., Takenaka, T., Kase, R., Kamei, S., Sakuraba, H. & Suzuki, Y. (1995) Biochem. Biophys. Res. Commun. 214, 1219-1224. [DOI] [PubMed] [Google Scholar]
- 5.Fan, J. Q., Ishii, S., Asano, N. & Suzuki, Y. (1999) Nat. Med. 5, 112-115. [DOI] [PubMed] [Google Scholar]
- 6.Ishii, S., Kase, R., Sakuraba, H., Taya, C., Yonekawa, H., Okumiya, T., Matsuda, Y., Mannen, K., Takeshita, M. & Suzuki, Y. (1998) Glycoconj. J. 15, 591-594. [DOI] [PubMed] [Google Scholar]
- 7.Okumiya, T., Ishii, S., Kase, R., Kamei, S., Sakuraba, H. & Suzuki, Y. (1995) Hum. Genet. 95, 557-561. [DOI] [PubMed] [Google Scholar]
- 8.Ishii, S., Kase, R., Okumiya, T., Sakuraba, H. & Suzuki, Y. (1996) Biochem. Biophys. Res. Commun. 220, 812-815. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa, S., Tsunoda, H. & Inokuchi, J.-i. (1994) J. Chem. Soc. Chem. Commun. 1317-1318.
- 10.Tsunoda, H., Inokuchi, J.-i., Yamagishi, K. & Ogawa, S. (1995) Liebigs Ann. 279-284.
- 11.Ogawa, S., Ashiura, M., Uchida, C., Watanabe, S., Yamazaki, C., Yamagishi, K. & Inokuchi, J.-i. (1996) Bioorg. Med. Chem. Lett. 6, 929-932. [Google Scholar]
- 12.Ogawa, S., Kobayashi, Y., Kabayama, K., Jimbo, M. & Inokuchi, J.-i. (1998) Bioorg. Med. Chem. 6, 1955-1962. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa, S., Matsunaga, Y. K. & Suzuki, Y. (2002) Bioorg. Med. Chem. 10, 1967-1972. [DOI] [PubMed] [Google Scholar]
- 14.Tominaga, L., Ogawa, Y., Taniguchi, M., Ohno, K., Matuda, J., Oshima, A., Suzuki, Y. & Nanba, E. (2001) Brain Dev. 23, 284-287. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto, Y., Ninomiya, H., Ohsaki, Y., Higaki, K., Davies, J. P., Ioannou, Y. A. & Ohno, K. (2001) Proc. Natl. Acad. Sci. USA 98, 12391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang, H., Nanba, E., Yamamoto, T., Ninomiya, H., Ohno, K., Mizuguchi, M. & Takeshita, K. (1999) J. Hum. Genet. 44, 391-396. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda, J., Suzuki, O., Oshima, A., Ogura, A., Noguchi, Y., Yamamoto, Y., Asano, T., Takimoto, K., Sukegawa, K., Suzuki, Y., et al. (1997) Glycoconj. J. 14, 729-736. [DOI] [PubMed] [Google Scholar]
- 18.Itoh, M., Matsuda, J., Suzuki, O., Ogura, A., Oshima, A., Tai, T., Suzuki, Y. & Takashima, S. (2001) Brain Dev. 23, 379-384. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi M., Shinoda Y., Ninomiya H., Vanier M. T. & Ohno K. (2001) Brain Dev. 23, 414-421. [DOI] [PubMed] [Google Scholar]
- 20.Sakuraba, H., Aoyagi, T. & Suzuki, Y. (1982) Clin. Chim. Acta 125, 275-282. [DOI] [PubMed] [Google Scholar]
- 21.Yu, R. K. & Ledeen, R. W. (1972) J. Lipid Res. 13, 680-686. [PubMed] [Google Scholar]
- 22.Williams, M. A. & McCluer, R. H. (1980) J. Neurochem. 35, 266-269. [DOI] [PubMed] [Google Scholar]
- 23.Saito, T. & Hakomori, S. I. (1971) J. Lipid Res. 12, 257-259. [PubMed] [Google Scholar]
- 24.Sewell, A. C. (1979) Clin. Chim. Acta 92, 411-414. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, S., Bagshaw, R., Hilson, W., Oho, Y., Hinek, A., Clarke, J. T. & Callahan, J. W. (2000) Biochem. J. 348, 621-632. [PMC free article] [PubMed] [Google Scholar]
- 26.Platt, F. M., Neises, G. R., Reinkensmeier, G., Townsend, M. J., Perry, V. H., Proia, R. L., Winchester, B., Dwek, R. A. & Butters, T. D. (1997) Science 276, 428-431. [DOI] [PubMed] [Google Scholar]
- 27.Jeyakumar, M., Butters, T. D., Cortina-Borja, M., Hunnam, V., Proia, R. L., Rerry, V. H., Dwek, R. A. & Platt, F. M. (1999) Proc. Natl. Acad. Sci. USA 96, 6388-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Platt, F. M., Jeyakumar, M., Andersson, U., Heare, T., Dwek, R. A. & Butters, T. D. (2003) Philos. Trans. R. Soc. London B. Biol. Sci. 358, 947-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox, T., Lachmann, R., Hollak, C., Aerts, J., van Weely, S., Hrebicek, M., Platt, F., Butters, T., Dwek, R., Moyses, C., et al. (2000) Lancet 355, 1481-1485. [DOI] [PubMed] [Google Scholar]
- 30.Durand, P., Fabrega, S., Henrissat, B., Mornon, J.-P. & Lehn, P. (2000) Hum. Mol. Genet. 9, 967-977. [DOI] [PubMed] [Google Scholar]
- 31.Fabrega, S., Durand, P., Codogno, P., Bauvy, C., Delomenie, C., Henrissat, B., Martin, B. M., McKinney, C., Ginns, E. I., Mornon, J. P., et al. (2000) Glycobiology 10, 1217-1224. [DOI] [PubMed] [Google Scholar]