Abstract
Introduction
The intracellular signaling cysteine proteases, calpains (specifically the ubiquitous calpains 1 and 2), are involved in numerous physiological and pathological phenomena. Several works have notably highlighted the implication of calpains in processes crucial for cancer development and progression, including cell transformation, migration and tumor invasion, apoptosis/survival, as well as angiogenesis. For these reasons, calpains are considered by several authors as potential anti-cancer targets.
Areas covered in this review
This review covers the literature showing how calpains are implicated in cancer formation and development, how these enzymes are deregulated in cancer cells and how these proteases could be targeted by anti-cancer drugs. Studies published in the last 10 years are focused on.
Expert opinion
Targeting calpain activity with specific inhibitors could be a novel approach to limiting the development of primary tumors and the formation of metastases, by inhibiting tumor cell migration and invasion, which allows dissemination as well as tumor neovascularization, which in turn allows for expansion. However, such drugs could interfere with anti-cancer treatments, as ubiquitous calpains play crucial roles in chemotherapy-induced apoptosis. For these reasons, drugs targeting calpains would have to be used selectively to avoid interferences with other treatments and physiological processes. Finally, concerning the other members of calpain family and their potential implication in cancer development, further studies will be required before considering treatments targeting their activity.
Keywords: calpains, cancer, migration, invasion, apoptosis
1- Introduction
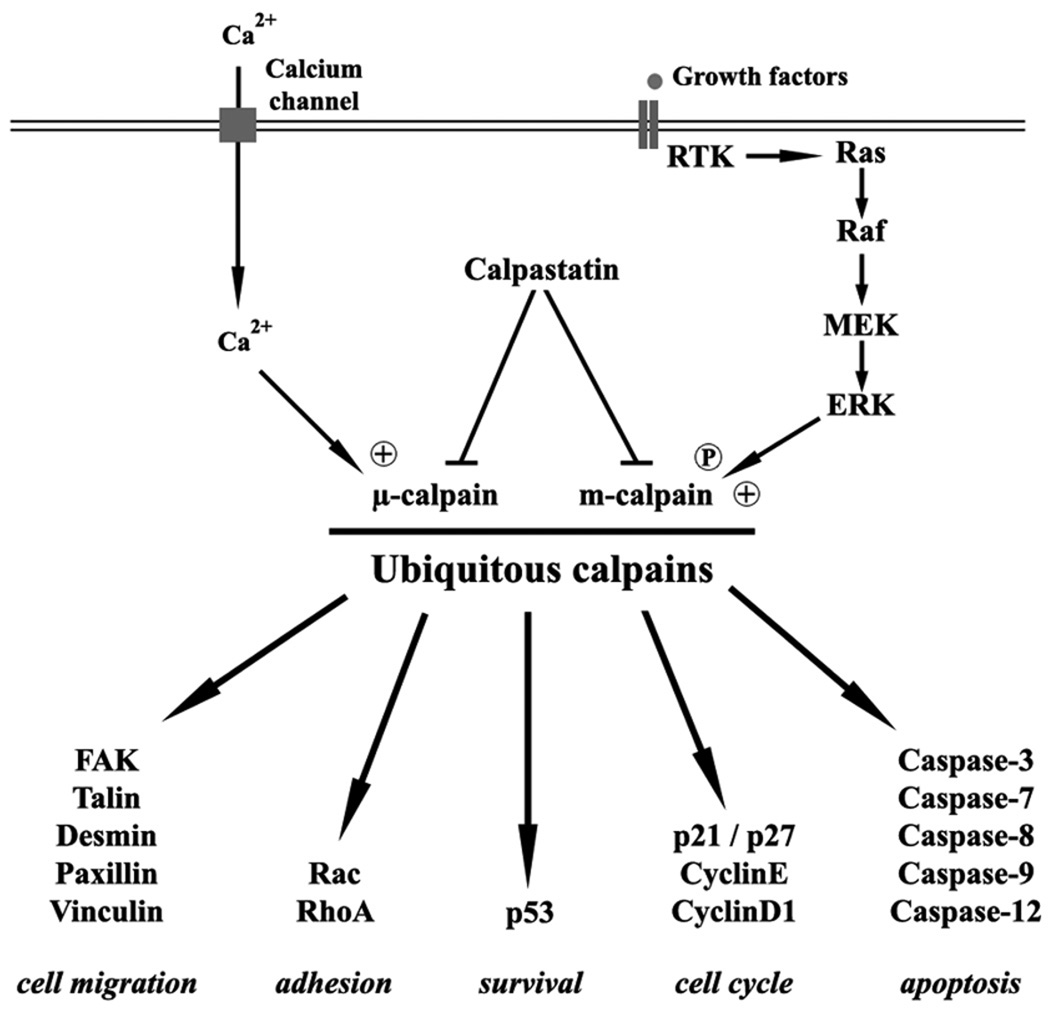
Calpains are intracellular cysteine-proteases described as being calcium-dependent in vitro, though the mechanisms of activation in cells is varied, not always involving calcium. The calpain family is currently constituted by 15 members, classified according to their localization (ubiquitous or tissue-specific) or to the presence or the absence of EF-hands, structures allowing calcium binding (calpains are typical if they encode EF-hands, atypical if they do not) (Table 1, Figure 1) [1]. The calpain system also includes the endogenous inhibitor of calpains 1 and 2, calpastatin, and two regulatory small subunits carrying EF-hands, calpains S1 and S2 [2]. Calpain S1 is known to interact with calpain 1 and calpain 2 to form heterodimers called µ-calpain and m-calpain, respectively (Figure 1). This regulatory subunit is required for the proper folding of the heterodimers as well as in the regulation of their activity. The role(s) played by the S2 subunit and its ability to heterodimerize currently remain unknown. Calpains have a broad spectrum of substrates, from proteins of the cytoskeleton (such as talin, vinculin, …) to transcription factors (p53, c-fos, …), and enzymes (PKC, caspases, RhoA/Rac …) (Figure 2). The proteolysis actuated by calpains is not degradative but rather signaling in that the fragments generated act as dominant-negative or –positive elements (cleavage of RhoA [3], talin [4], EGFR [5], …). Thus, the inhibition of calpains should be considered analogous to abrogation of diverse signaling pathways.
Table 1.
The calpain family
| Calpains | Other names | Gene | Locus (human) | EF-hands | Localization |
|---|---|---|---|---|---|
| Calpain 1 | capn1 | 11q13 | + | ubiquitous | |
| Calpain 2 | capn2 | 1q41 | + | ubiquitous | |
| Calpain 3 | p94 | capn3 | 15q15 | + | muscle, lens, retina |
| Calpain 5 | htra3 | capn5 | 11q14 | − | brain, testis |
| Calpain 6 | CAPNX | capn6 | Xq23 | − | placenta, embryo |
| Calpain 7 | palBH | capn7 | 3p24 | − | ubiquitous |
| Calpain 8 | nCL-2 | capn8 | 1q32-41 | + | stomach |
| Calpain 9 | nCL-4 | capn9 | 1q42-43 | + | digestive tract |
| Calpain 10 | capn10 | 2q37 | − | ubiquitous | |
| Calpain 11 | capn11 | 6p12 | + | testis | |
| Calpain 12 | capn12 | 19q13 | + | hair follicle | |
| Calpain 13 | capn13 | 2p21-22 | + | ubiquitous | |
| Calpain 14 | capn14 | 2p21-22 | − | ? | |
| Calpain 15 | SolH | capn15 | 16p13 | − | ubiquitous |
| Calpain-7-Like | CAPN7L | C6orf103 | 6q24 | − | ? |
| Calpain S1 | Calpain 4 | capns1 | 19q13 | + | ubiquitous |
| Calpain S2 | capns2 | 16q12 | + | NA | |
| Calpastatin | CAST | cast | 5q15 | ubiquitous | |
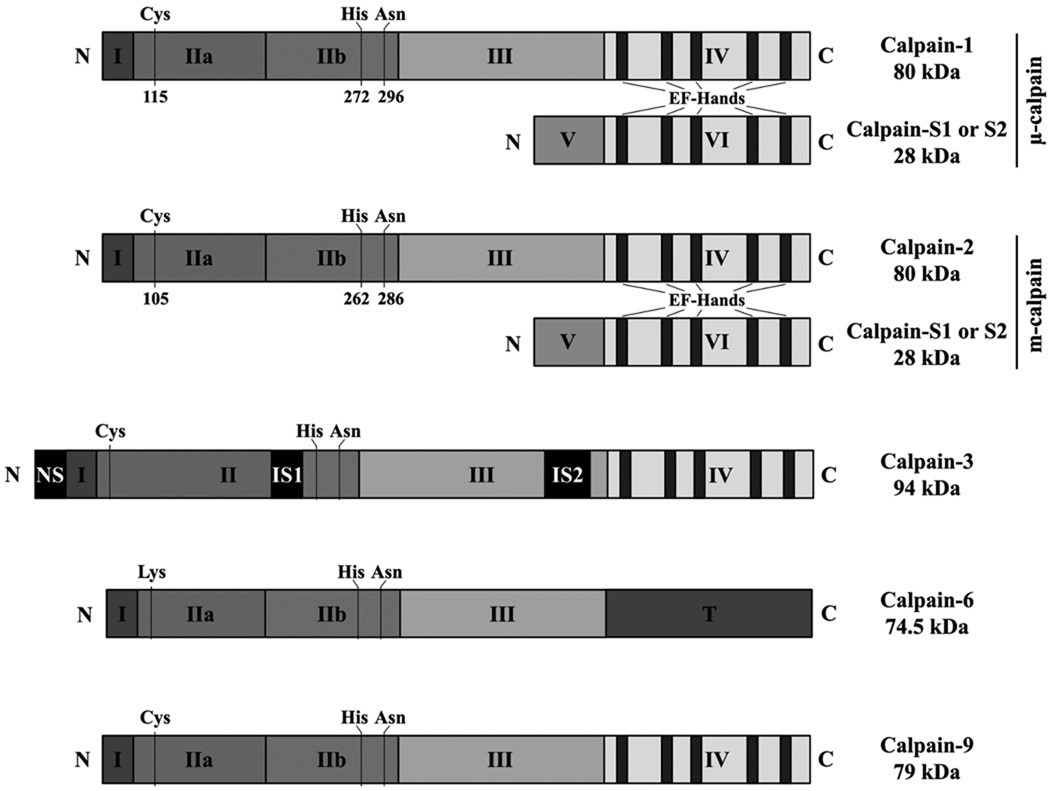
Figure 1.
Schematic diagram of the structure of µ- and m-calpain, calpain-3, -6, and -9.
Figure 2.
Schematic representation of ubiquitous calpain regulation and key substrates.
By cleaving these numerous substrates, calpains are involved in a large number of physiological and pathological phenomena, from embryogenesis to cell adhesion, diabetes, and Alzheimer’s disease [1, 6–8] (Figure 2). Key to this discussion, several studies have also linked the calpain system to cancer development and progression. Indeed, the expression and/or activity of several members of the calpain system have been shown to be strongly altered in different types of cancer cells or transformed cells (Table 2). Recent publications have shed light on possible molecular mechanisms; with these focusing mainly on the ubiquitous calpains 1 and 2 (Table 3).
Table 2.
Alterations of calpain system in different types of cancer
| Calpains | Cancer | Modifications | Ref. |
|---|---|---|---|
| Calpain 1 | Breast cancer | Higher activity | [87] |
| Calpain 2 | Colorectal adenocarcinoma | Overexpression calpain 2 | [88] |
| Lung cancer | Phosphorylations and secretion | [65] | |
| Malignant brain tumors | Overexpression, increased activity | [89] | |
| Prostate cancer | Overexpression calpain 2 | [90] | |
| SCC / BCC | Alteration of calpain 1 expression | [91] | |
| Renal carcinoma | Higher expression of calpain 1 | [92] | |
| Rhabdomyosarcoma | Higher activity | [68] | |
| Calpain S1 | Breast cancer | Overexpression, highly proteolyzed | [93] |
| Hepatocellular carcinoma | Overexpression | [94] | |
| LGL Leukemia | Overexpression | [95] | |
| Calpastatin | Endometrial cancer | Higher expression | [96] |
| Rhabdomyosarcoma | Low expression | [68] | |
| Calpain 3 | Melanoma | Downregulation proapototic variants | [81] |
| Urothelial cancer | Expressed in an active form | [82] | |
| Calpain 6 | Uterine cancer | Overexpression | [83] |
| Calpain 9 | Gastric cancer | Downregulation | [85] |
| Calpain 10 | Laryngeal cancer | Lower expression of allele UCNSP-44 | [97] |
Table 3.
Implications of calpain family members in cancer
| Calpains | Cancer | Implications | Ref. |
|---|---|---|---|
| Calpain 1 | Bladder carcinoma | Cell migration, invasion | [55] |
| Calpain 2 | Brain tumors | Cell death | [42] |
| Breast cancer | Invadopodia dynamics, invasion | [29] | |
| Breast cancer | Cyclin E truncation | [13] | |
| Lung cancer | Cell migration, invasion | [65] | |
| Melanoma | Degradation of p27Kip1 | [98] | |
| Meningioma | Degradation merlin, adhesion, contact inh. | [99] | |
| Neuroblastoma | Regulation of NO production | [100] | |
| Osteosarcoma | Invasion, metastasis formation | [70] | |
| Prostate cancer | Cell migration, invasion | [28] | |
| Prostate cancer | Degradation of E-cadherin | [90] | |
| Renal carcinoma | Metastasis formation | [92] | |
| Rhabdomyosarcoma | Cell migration, invasion | [68] | |
| Calpain S1 | Hepatocellular carcinoma | Invasion, metastasis formation | [94] |
| Calpain 3 | Melanoma | Pro-apoptotic role | [81] |
| Urothelial cancer | Proliferation ? | [82] | |
| Calpain 6 | Uterine cancer | Reduction of apoptosis, angiogenesis | [84] |
| Calpain 9 | Breast cancer | Apoptosis, lumen formation | [101] |
| Gastric cancer | Anti-tumor effects | [86] | |
| Calpain 10 | Laryngeal cancer | Lower expression of allele UCNSP-44 | [97] |
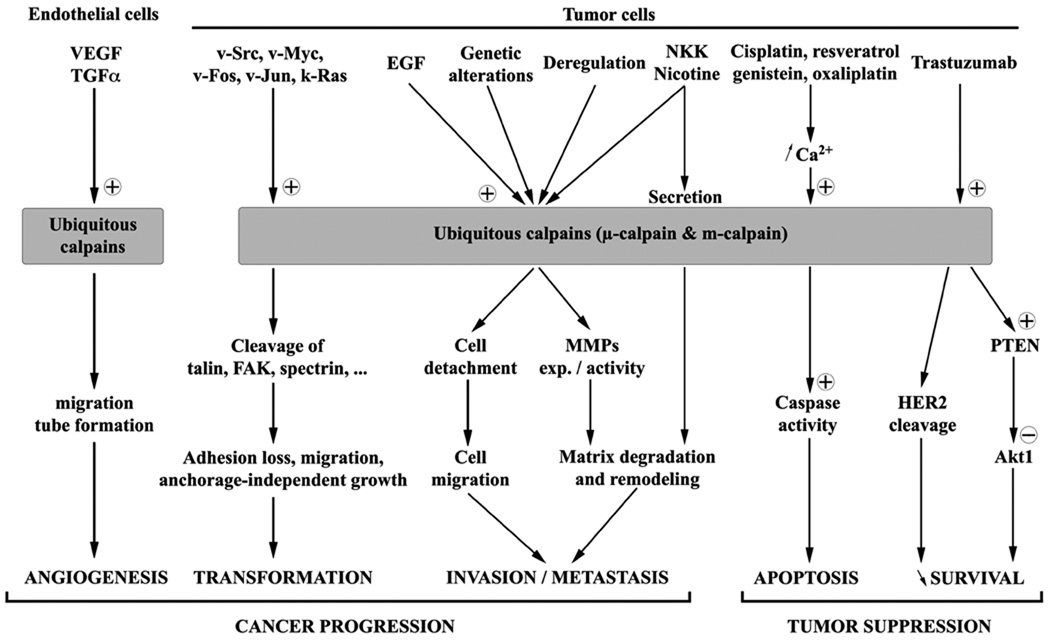
Among the 15 members of the calpain family, the ubiquitous calpains 1 and 2 are the most intensely studied. The heterodimers µ- and m-calpain that they form are known to regulate and even be required for numerous physiological processes. It is important to note that in cells, the contribution of µ-calpain versus m-calpain is often not evident or experimentally distinguished; it is not determined whether this is due to co-regulation or compensation. Several of these processes, such as regulation of cell cycle and apoptosis, adhesion, migration, invasion, and also angiogenesis, are critical for the formation, progression, and growth of cancers (see Figures 2 & 3).
Figure 3.
Schematic representation of the implication of the ubiquitous calpains in the different mechanisms leading to cancer progression or suppression.
The ubiquitous calpains were notably shown to regulate the cell cycle, particularly the transition between the phases G1 and S [9–11]. Indeed several crucial regulators of this phase of the cell cycle are substrates of µ- and m-calpain, including two key cyclins, the cyclin D1 and the cyclin E [12, 13]. In normal cells, the degradation of these two cyclins by the ubiquitous calpains induces the cell cycle arrest. But in cancer cells the opposite occurs; calpains can promote the progression of the cell cycle in transformed cells or tumor cells. Indeed, in these cells calpains were shown to proteolyze two inhibitors of cyclin-dependent kinases (Cdk), p21Cip1 and p27Kip1, thus leading to the activation of the complexes cyclinD-Cdk4 and cyclinE-Cdk2 [14], [15]. Cdk5, a cyclin-dependent kinase implicated in the regulation of neuronal cell cycle was also shown to be a substrate of calpains [16]. By regulating these proteins, ubiquitous calpains are thus strongly involved in the progression of the cell cycle and in cell proliferation.
Ubiquitous calpains are also known to be involved in the regulation of cell death. In fact, calpain was the first protease identified in initiating apoptosis [17]. Several studies have notably highlighted how closely these proteases are linked to caspases. Mu- and m-calpain cleave several members of caspase family, thus activating the caspase-3, -7 and -12 and inactivating the caspase-8 and -9 [18, 19]. By regulating caspases, calpains can thus control indirectly apoptosis. Also, in situations of mass calcium influx, membrane transection or ischemia/reperfusion injury, the ubiquitous calpains are activated and in turn trigger caspase-3 [20]. Ubiquitous calpains were also shown to regulate cell proliferation and apoptosis through the tumor suppressor protein p53 [21]. Indeed µ- and m-calpain can cleave this transcription factor inducing its degradation; the implications of loss of p53 regulation of genomic repair for tumor genetic instability are obvious.
Ubiquitous calpains were shown to control cell adhesion and migration in different types of normal cells (such as fibroblasts, endothelial cells, myoblasts, …). Indeed, in physiological conditions, µ-calpain controls the formation of focal adhesion complexes through the regulation of Rho GTPases, thus allowing cell adhesion [3], while m-calpain controls cell de-adhesion by cleaving several components of these complexes (such as paxillin, talin, vinculin, FAK) [22, 23]. Ubiquitous calpains regulate cell adhesion to the substratum, and thereby cell migration as this process was previously described as a succession of adhesion and de-adhesion steps [24]. Recent publications have shown that the regulation of migration by calpains depends on a compartmentalization of these enzymes: in adherenet cells, µ-calpain is localized at the front of the cells (in the lamellipodia) where it induces focal adhesion complexes, while m-calpain is concentrated at the rear of the cells where it induces cell retraction [25, 26]. However, the distribution of the calpain isoforms in cells of the immune system may be different [27], as motility in these cells qualitatively and quantitatively distinct. By regulating cell migration, calpains can also control tumor dissemination. Several studies have shown that these proteases can regulate the invasiveness of isolated tumor cells by modulating cell migration [28], invadopodia dynamics [29] and MMP (matrix metalloproteinase) expression and activity [30].
These critical implications show how dramatic could be a deregulation of calpain activity. Alterations of the calpain activity balance has been observed in numerous cancer types (Table 2) can reduce apoptosis, increase cell proliferation and stimulate cell migration and invasiveness (Table 3). For these reasons, calpains are considered potential therapeutic targets to treat cancer and to limit its progression. In this review we present how and why these proteases can be targeted as well as the pros and the cons of such an approach.
2- Ubiquitous calpains in carcinoma development – and cell transformation
Oncogenic transformation is the sine qua non for tumorigenesis; this is characterized by a series of major changes in cell morphology and behavior. During this pathological process, oncogenic proteins, the result of genetic insults, induce a loss of cell adhesion, the disruption of actin cytoskeleton, and the deregulation of cell cycle. These events lead to the emergence of malignant cells that have escaped from the usual suppressive mechanisms, and thus present increased migration and proliferation. Several studies, notably those published by Carragher’s group, have highlighted the role played by the two ubiquitous calpains during malignant transformation [31]. It was firstly shown that the balance of calpain system was modified by the oncoprotein v-Src. Indeed, v-Src induces an increase in the expression of calpain 2 and the degradation of its endogenous inhibitor calpastatin, thus leading to a strong increase in calpain activity [32]. This activity is also strongly increased (from 1.5 to 11 fold) by several other oncoproteins (v-Myc, v-Jun, k-Ras and v-Fos) during cell transformation [33]. This enhanced proteolytic activity is responsible for lessened adhesiveness and increased migration observed in these transformed cells, notably through the degradation of cytoskeletal proteins such as talin, paxillin and spectrin, and the regulatory FAK. The increased calpain activity is also responsible for the anchorage-independent growth of the v-Src transformed cells. In the same way, a recent publication has shown that c-Myc stimulates calpain activity through the suppression of calpastatin expression and that the knockdown of calpastatin is enough to promote the transformation of c-Myc deficient cells [34]. These different studies strongly demonstrate that calpains could be major effectors of malignant transformation.
Calpain activity could thus be targeted to prevent the cell transformation induced by oncoproteins. Indeed, a recent study showed that the inhibition of calpain activity using calpastatin overexpression or calpain S1 knockout (to abrogate both ubiquitous calpains) was sufficient to block the cleavage of FAK, focal adhesion disassembly, and morphological changes induced by v-Src [32]. Similarly, it was observed that this calpain inhibition was able to suppress cell cycle progression and proliferation of the transformed cells, as well as their anchorage-independent growth. Very similar results were obtained with a synthetic calpain inhibitor (ALLN). In the same manner, calpain inhibition using different inhibitors was sufficient to repress the effects of the transformation induced by other oncoproteins such as v-Jun, v-Myc, v12k-Ras and v-Fos [33]. Moreover, a recent publication has shown that the simultaneous inhibition of calpains and ERK/MAPK pathway coupled with an activation of p38 MAPK was sufficient to restore ability of v-Src-transformed myoblasts to differentiate [35]. Calpain inhibition was also shown to induce apoptosis of transformed cells. This induction is due to an accumulation of c-Myc, previously identified as a calpain substrate [36]. The activity of the two ubiquitous calpains could thus be targeted and inhibited to block cell transformation by suppressing the adhesion disassembly, the enhanced motility, and the cell cycle progression of the cells transformed by oncoproteins and by inducing their death.
3- Ubiquitous calpains in cell survival/apoptosis
A second critical behavior enabling emergence of carcinomas is the escape from programmed cell death, apoptosis, that is often initiated upon inappropriate proliferative or dedifferentiation stimuli. Apoptosis is a process of programmed cell death critical for the homeostasis of the organism. Caspases are the main effectors of the process. However, caspases are not the only proteases implicated, as the two ubiquitous calpains are also known to be involved in apoptosis. The inhibition of these enzymes with specific inhibitors was notably shown to block cell death induced by dexamethasone, cycloheximide or irradiation [17]. This involvement in apoptosis is due to the role played by µ- and m-calpain in the regulation of caspases, as calpains activate caspase-3, -7 and -12 and inactivate the caspase-8 and -9 [18]. For these reasons calpain activity is crucial for the induction of apoptosis.
Inducing the apoptosis of tumor cells is critical in the treatment of cancer, thus serving a dual function of preventing carcinogenesis and being used to control cancer cells once they arise. Several molecules known to directly or indirectly trigger apoptosis such as cisplatin, 5-fluorouracil, irinotecan, and paclitaxel, are used as chemotherapy drugs. Several recent studies presented below have highlighted the major role played by calpains in the induction of tumor cell apoptosis by some of these therapeutic molecules, particularly when the precipitating event is endoplasmic reticulum (ER) stress.
Calpains were notably shown to be involved in the apoptosis of breast cancer cells (MCF-7) treated with genistein. In a first study published in 2004, genistein was shown to increase the intracellular concentration of calcium leading to the activation of µ-calpain [37]. This calpain cleaves and activates caspase-12 thus triggering the apoptosis of the MCF-7 cells. Such an activation of caspase-12 by calpains during ER stress-induced apoptosis was previously described [19, 38, 39]. A second publication has highlighted the involvement of another caspase in the apoptosis induced by this chemotherapy drug. In the same cell type, genistein was shown to release calcium from the endoplasmic reticulum, allowing calpain and caspase-7 activation, leading to cell death [40]. In hepatocellular carcinomas, genistein was also shown to trigger apoptosis via the activation of calpain and caspase-12, however the calpain isoform implicated in this process was m-calpain, not µ-calpain [41]. Other flavonoids also lead to cancer cell apoptosis (neuroblastoma cells) by increasing the intracellular concentration of calcium and the activation of calpains and caspases [42].
A similar involvement of calpains was observed in the apoptosis of breast cancer cells induced by resveratrol and HL-37 (anthracene derivative) [43, 44]. These two drugs trigger a rise in the levels of intracellular calcium followed by an activation of calpains. However, the caspases activated in these conditions are different depending on the drug and the type of breast cancer cells.
Cisplatin and oxaliplatin, two widely used platinum-based cancer chemotherapies, were also recently shown to induce apoptosis of tumor cells via the activation of ubiquitous calpains. The treatment of lung adenocarcinoma cells with cisplatin induces a rapid and significant increase of calpain activity (within 4 to 6 hours), resulting in the cleavage and the activation of both Bid and caspase-3 [45]. The activation of these two pro-apoptotic proteins leads to the tumor cell death after 8 to 20 hours of treatment. A second publication has completed the study of this phenomenon and µ-calpain was identified as the isoform responsible for the pro-apoptotic effects of cisplatin [46]. Similar results were obtained with oxaliplatin-treated cervical carcinoma cells (HeLa cells) [47]. Indeed, calpain-dependent activations of Bid, caspase-2, caspase-3, caspase-8 and caspase-9 were observed in response to oxaliplatin treatments and are responsible for the tumor cell apoptosis induced by this alkylating agent. Finally, another very recent publication showed that calpain activity is required for the ER-mediated apoptosis of transformed embryonic kidney cells treated with cisplatin [48].
Two other molecules known for their potential anti-cancer properties, aspirin and curcumin, induce tumor cell apoptosis via the pathway described above. Indeed, aspirin and curcumin activate calpain activity leading to the activation of different caspases and to the apoptosis of cervical cancer cells (HeLa) and glioblastoma cells, respectively [49–51].
Likewise, a recent study published in 2010 showed that the effects of trastuzumab on breast cancer cells strongly depend on calpain activity [52]. Indeed, the calpain activity modulates the sensitivity of HER2-positive breast cancer cells to trastuzumab. An increased calpain activity is required for the effects of trastuzumab, and the inhibition of calpain activity promotes the resistance of the tumor cells to this chemotherapy drug. However, contrarily to what was observed for cisplatin, oxaliplatin, genistein, and resveratrol, the ubiquitous calpains are not mediating the effects of trastuzumab by inducing apoptosis via caspases, but by inhibiting signaling pathways responsible for tumor cell survival. In the model described in this publication, the ubiquitous calpains, activated by the trastuzumab, regulate two different pathways. On the one hand, calpains cleave HER2, leading to the inhibition of this oncogene, while, on the other hand, they induce the activation of the tumor suppressor PTEN, thus inhibiting Akt1. By reducing the activity of both HER2 and Akt1, calpains inhibit the cell survival and increase the sensitivity of the tumor cells to the chemotherapy treatment.
Altogether, these complex studies show that the activity of ubiquitous calpains is required for the efficacy of several anti-cancer treatments. These molecules used in chemotherapy induce tumor cell apoptosis by activating very similar pathways. They increase the concentration of intracellular calcium followed by the activation of calpains to trigger the final effectors caspases. Trastuzumab is the only treatment to involve calpains in a different manner, as the enzymes are responsible for an inhibition of the survival signals. However, a recent study published in 2010 highlighted an opposite implication of calpains in chemotherapy-induced apoptosis [53]. In this publication, the authors showed that the proteasome inhibitor bortezomib, used to treat myeloma by increasing the stability of IκBα, failed to stabilize the inhibitor of NF-κB in lung cancer cells. On the opposite, bortezomib induces the calpain-dependent degradation of IκBα by activating the ubiquitous calpains. Contrarily to what was observed for the other chemotherapy treatments, the inhibition of calpains strongly enhances the efficiency of bortezomib to induce the apoptosis of lung cancer cells.
Even if these studies show a different implication of the ubiquitous calpains in cancer cell survival and/or apoptosis, they all prove that these enzymes could be potential targets to treat cancer. Except for bortezomib, a stimulation of calpain activity could be interesting to improve the sensitivity of the tumor cells and the efficiency of the chemotherapy treatments. On the opposite, an inhibition of calpain activity could be useful to improve the sensitivity of lung cancer cells to the proteasome inhibitor bortezomib. It is important to note that this inhibition would be a mistake for these other treatments, as it would decrease their efficiency by increasing the resistance of the cells to the drugs as it was observed for trastuzumab. It is possible that while calpain inhibition may decrease apoptosis, the cells may be redirected to other modes of death. Still the sum of the published data suggest an interesting approach would be to target calpains to improve chemotherapy efficiency, even if the treatment (activating or inhibiting the enzymes) will strongly depends on the type of cancer and the chemotherapy drugs used.
4- Ubiquitous calpains in cancer dissemination – migration and invasion
Tumor dissemination is the most ominous development for patients, as this negates cures in most all solid tumors. While dissemination can take two distinct forms with differential underlying behaviors and controls, localized invasion into adnexia and distant metastasis, both require growth factor-induced cell migration [54]. It was notably shown that cell motility is crucial as it is rate-limiting for carcinoma cell invasion [55]. The involvement of µ- and m-calpain in tumor invasion was extensively studied during the last decade. Recent studies have clearly shown that calpain activity is required for tumor invasion and that the ubiquitous calpains are implicated in the aforementioned processes.
As previously stated, the involvement of the two ubiquitous calpains in cell migration has been established, both in physiological and pathological conditions. Calpain activity is required for both the formation of focal adhesion complexes at the front of the migrating cells (µ-calpain) and the disassembly of these complexes at the rear of the cells (m-calpain) [22]. Cell motility is limited by the ability of the cell to retract; the role of m-calpain is thus crucial for cell motility [56]. In physiological conditions, m-calpain regulates the motility of fibroblasts [57], endothelial cells [58, 59], keratinocytes [60] as well as myoblasts [61, 62], and this regulation is under the control of pro-migratory growth factors such as EGF, PDGF, and VEGF [26, 58, 63]. The fact that EGF activates m-calpain is critical as EGFR is overexpressed in a wide variety of cancers ([54], for a review about EGFR and cancer see [64]). This involvement of calpains in cell migration was also observed for tumor cells from several types of cancer, from lung cancer to prostate cancer and rhabdomyosarcoma.
In lung cancer, two studies have shown that the migration of the tumor cells induced by nicotine and NNK (formed by the nitrosation of nicotine) was dependent on the activation of both µ- and m-calpain [65, 66]. These two cigarette smoke components were shown to increase the phosphorylation of the two ubiquitous calpains, leading to their activation, an increased migration and an enhanced invasiveness. However, the signaling pathways responsible for these effects seem to be different for the two components of cigarette smoke. While the phosphorylation of calpains induced by NNK was shown to be mediated by ERK/MAPK pathway [65], like it was observed in physiological conditions, PKCiota would be responsible for the effects of nicotine [66]. Another study has highlighted the involvement of m-calpain in the migration and invasion of lung cancer cells. According to this study, fibronectin stimulates the migration and the invasion capacities of these tumor cells by activating FAK and the downstream ERK/MAPK pathway, leading to an increase of m-calpain and MMP-9 expression [67].
In the same manner, m-calpain was shown to play a crucial role in the migration and invasion of prostate carcinoma cells [28]. First, it was shown that the migration and the invasion of the carcinoma cells are EGFR- and calpain-dependent. In these cells, EGF stimulates both cell migration and invasion by activating m-calpain, and these effects can be blocked in vitro as well as in vivo by blocking calpain activity or expression. These data are consistent with those obtained previously showing that EGF induces fibroblast migration by activating m-calpain via ERK/MAPK pathway. Interestingly, and in contrast to the data obtained for lung cancer, the expression of calpain 2 remains only slightly elevated if at all in invasive prostate tumor cells.
A similar involvement of µ- and m-calpain in tumor cell migration and invasion was also observed with rhabdomyosarcoma (RMS) cells [68]. However, the major alterations of the calpain system observed for these cells are very different from those observed in lung and prostate cancer cells. A recent study shows that both the expression, the regulation and the activity of the two ubiquitous calpains are modified. The expression of both µ- and m-calpain is downregulated in RMS cells in comparison to normal muscle cells. However, the expression of the endogenous inhibitor of µ- and m-calpain, calpastatin, is dramatically reduced in the RMS cells (by 75–85% according to the type of RMS). This drastic reduction leads to an abnormally high calpain activity. This over-activation of the two ubiquitous calpains triggers a disorganization of the actin cytoskeleton and a strong reduction of the capacity of the RMS cells to adhere. This increased calpain activity is also responsible for the high motility and the strong invasiveness of the RMS when compared to normal myoblasts. These three examples of calpain levels moving in different directions but with the net result being higher calpain signaling, demonstrate that expressome or simple proteome analyses are not fully explanatory but rather one needs to determine the activation status of key molecules.
Even if migration is rate-limiting for the invasiveness of tumor cells, the degradative remodeling of the extracellular matrix is also crucial for tumor invasion (for a review see [69]). Indeed, the tumor cells need to degrade the components of the extracellular matrix in order to be able to invade the surrounding tissues and to reach the vascular conduits for dissemination. Another family of proteases, the matrix metalloproteinases (MMP), was shown to be a main actor in matrix degradation and remodeling. The involvement of µ- and m-calpain in the degradation in the extracellular matrix is not as well described as for tumor cell migration, however a couple of recent publications highlight the direct and indirect roles of these two calpains. Two studies published in 2003 and 2009 show that the ubiquitous calpains are able to modulate MMP expression and secretion. The data of the first publication show that the calpain/calpastatin system regulates the RNA expression of MMP-2 and MMP-9 as well as the secretion of these two MMP, and therefore the invasiveness of the leukemic cells used in this study [30]. The results presented in the second publication are similar, showing that µ- and m-calpain regulate the invasiveness of osteosarcoma cells by controlling the secretion of MMP-2 [70]. These data support an indirect implication of calpains in the degradation of the extracellular matrix by tumor cells, however a study published in 2004 highlights a more direct involvement. Indeed, this study shows that µ- and m-calpains can be secreted by lung cancer cells treated with components of the cigarette smoke [65]. The possibility that the intracellular proteases calpains could be secreted is controversial, even if it was previously observed in physiological conditions with normal myoblasts [71]. However, the secretion of both µ- and m-calpain observed in this study could explain, at least in part, the invasiveness of these cells. Indeed, the high concentration of calcium observed outside of the cells would induce a strong activation of the secreted enzymes and would thus allow the degradation of the components of the extracellular matrix.
Taken together, these studies highlight the strong involvement of the ubiquitous calpains in tumor invasion, by regulating both the motility of the tumor cells and their capacities to remodel the extracellular matrix. Even if the alterations of the calpain system observed in these publications are very different according to the types of cancer, they all support the same conclusion: calpain activity is required for tumor invasion, and a strong activation of calpains increases invasiveness. For this reason, calpain activity could be targeted by inhibitors to reduce the invasiveness of the tumor cells and thus block their dissemination. Indeed all these publications have shown that inhibition of calpains reduces the invasiveness of the tumor cells. For lung cancer cells, the addition of calpain inhibitors, such as calpeptin, blocks calpain activation and reduces the invasiveness of the cancer cells by blocking their migration [65, 66]. The treatment of the same cells with C2-ceramide, an activator of the phosphatase PP2A, limits tumor invasion by inducing the dephosphorylation of calpains and thus their inactivation [72]. Likewise, the inhibition of m-calpain using synthetic inhibitors (leupeptin, calpain inhibitor I) or antisense reduces the invasiveness of prostate carcinoma cells [28]. Very similar results were obtained with rhabdomyosarcoma treated with calpeptin [68]. Indeed the invasiveness of these cells is dramatically reduced in the presence of this calpain inhibitor to a level close to the one observed with normal myoblasts. Very interestingly inhibition of calpains can also reduce the expression of MMPs. Indeed, the treatment of leukemic cells with CP1B, a specific inhibitor of calpains derived from calpastatin, decreases the expression as well as the secretion of MMP-2 and MMP-9, thus reducing matrix degradation and tumor invasion [30].
Targeting the activity of the two ubiquitous calpains with specific inhibitors could thus be a realistic way to block the invasiveness of tumor cells by inhibiting their migration and the matrix degradation. However this kind of treatment has limits, as tumor cells use two modes of locomotion through the matrix, the mesenchymal migration, calpain- and integrin-dependent, and the amoeboid migration, which is not depending on integrins, calpains, and matrix degradation. In the case of amoeboid migration and invasion, treatments targeting calpain activity would have no effect on invasiveness. Moreover, a recent publication has shown that some tumor cells treated with protease inhibitors undergo a mesenchymal to amoeboid transition, thus becoming resistant to the treatment [73]. This phenomenon could be a mode by which tumor cells avoid calpain-targeted inhibition of invasion.
5- Ubiquitous calpains in tumor growth - angiogenesis
For tumors to grow beyond microscopic size, either in situ or in an ectopic metastatic site, the tumor mass must establish a viable blood supply via angiogenesis. Tumor neovascularization may also be critical for dissemination as the cancer-associated neovessels are leaky allowing for easy intravasation. The ability of cancer cells to induce the formation of new blood vessels is crucial as these vessels are required to provide oxygen and nutrient supply to the tumor. Moreover, these blood vessels allow the dissemination of the tumor cells in the organism and thus the formation of metastasis. Previous studies have shown that cancer cells induce angiogenesis by secreting large amounts of angiogenic factors including VEGF and TGFα/HB-EGF, thus stimulating the migration and the proliferation of the surrounding endothelial cells (see the reviews [74] and [75]).
For all these reasons, angiogenesis has emerged as a favorite target in efforts to block cancer progression. Indeed, inhibiting angiogenesis would prevent tumor neovascularization, thus blocking the oxygen and nutrient supply of the cancer cells, leading to their necrosis. VEGF is one of the potential therapeutic targets to inhibit angiogenesis and humanized monoclonal antibodies against VEGF-A (Bevacizumab, Ranibizumab) are in use in a variety of cancers as an adjuvant to cancer cell-directed therapies. However, a recent study has shown that Bevacizumab does not prolong disease-free survival of patients with colorectal cancer despite prolonging progression-free survival in a subset of such patients [76]. Moreover, there are concerns that these treatments could interfere with physiological processes requiring the signaling by VEGF. Thus, other targets that could limit angiogenesis are being sought.
Calpains are strongly involved in angiogenesis, notably by regulating the migration and survival of endothelial cells [58]. Like it was previously observed with fibroblasts, calpains are required for the growth factor-induced migration of microvascular endothelial cells. These observations highlight particularly the role played by m-calpain during angiogenesis. Indeed, VEGF stimulates the endothelial cell migration by inducing an increase of m-calpain expression and activity to enable tail retraction [59]. Inhibition of calpains with synthetic inhibitors or by over-expression of calpastatin blocks the effects of VEGF on endothelial cell migration. Calpain inhibition could thus be used to block tumor neovascularization. However this inhibition could be problematic and have side effects. Indeed, while m-calpain activity is needed for productive endothelial cell migration, µ-calpain activation in the cells leads to apoptosis and the regression of blood vessels [77]. Because of this dual action of the ubiquitous calpains, nonspecific inhibition of these enzymes, targeting both µ- and m-calpain, would block the endothelial cell migration but also blunt the regression of existing blood vessels.
This could be circumvented by a physiological effector network. A recent study has reported that the ligands of the receptor CXCR3, expressed during wound healing to control excess vascularization, inhibits VEGF-induced migration of endothelial cells by blocking the activation of m-calpain [58]. Like for fibroblasts, this inhibition is due to the phosphorylation of the enzyme by the protein kinase A (PKA) [78]. This family of chemokines was also shown to inhibit the effects of EGF on cell migration [26]. Moreover, these chemokines do not inhibit µ-calpain; on the opposite, they were shown to activate this enzyme thus inducing endothelial cell apoptosis and vessel regression. However, these treatments could also be problematic. Indeed, two isoforms of the receptor CXCR3 were identified. While CXCR3B is responsible for the angiostatic effects of IP-10 on endothelial cells, inhibiting cell migration, proliferation and inducing apoptosis, CXCR3A induces cell proliferation and migration [79]. Treatments with ligands of CXCR3 would thus block the formation of new blood vessels and induce the regression of existing vessels, but it could also stimulate the proliferation and the motility of the tumor cells.
Taken together, these data clearly show that the ubiquitous calpains could be interesting targets to limit tumor neovascularization, but the treatments would have to be isoform-specific, and possibly cell-specific, inhibiting m-calpain and activating µ-calpain in endothelial cells to block angiogenesis and induce the involution of the existing vessels, while avoiding stimulation of cancer cells.
6- Involvement of calpain-3, -6, and -9 in cancer
Even if µ- and m-calpain are the best described and many of their roles in cancer development and progression is now well known, several other calpains have been implicated in this pathological process in specific cancers. Results obtained for three tissue-specific calpains, the calpain-3, -6, and -9, are particularly interesting and suggest that these proteases could be targeted to treat their respective cancers, like calpain-1 and -2.
The tissue-specific calpain-3, also known as p94, is mainly expressed in the skeletal muscle [1] (Figure 1). This protease is noted as causative for the muscle dystrophy LGMD2A (limb girdle muscular dystrophy type 2A) [80]. However, two recent publications highlight the potential implication of this calpain in the development and progression of cancer. In the first study, published in 2009, the authors have identified two new splicing variants of calpain 3 expressed in melanoma cells [81]. They have also observed that the expression and the autoproteolytic cleavage of these unstable variants are increased in pre-apoptotic cells treated with cisplatin. Moreover the expression of these variants is downregulated in cells from aggressive melanoma with increased invasiveness and a high resistance to chemotherapy. These observations suggest that calpain-3 could play a role in the chemotherapy-induced apoptosis of melanoma cells, similar to µ- and m-calpain. However, the second publication supports another hypothesis. Indeed, the authors have observed that calpain-3 is expressed in an active form in urothelial tumor cells, and that this over activation could be responsible for the increased proliferation of the tumor cells through the overexpression of E2F3 [82]. Even though these data are contradictory, they support the possible involvement of calpain-3 in cancer and identify this protease as a potential anti-cancer target.
The involvement of calpain-6 in cancer was also recently suggested. This tissue-specific calpain, also known as calpamodulin or calpain-like protease X-linked, is encoded by a gene located on the X chromosome and is notably expressed in the fetus during embryogenesis as well as in the placenta [1] (Figure 1). Recent publications have shown that calpain-6 is highly expressed in cancer cells, notably in human cervical cancer cells, while its expression is extremely low in normal cells, being representative of an onco-fetal protein [83]. Moreover, this expression increases during the progression of uterine cervical neoplasia. Calpain-6 was reported to protect the cancer cells from the cisplatin-induced apoptosis by inhibiting caspase-3 activity [84]. Moreover, the expression of calpain-6 in endothelial cells enhances the effects of VEGF on migration and invasion, thus promoting angiogenesis. Thus, this calpain could be an interesting target to treat uterine cancer with minimal side effects, as it is by and large not expressed in normal tissues. However, contrarily to the other members of calpain family, calpain-6 lacks the cysteine of the catalytic triad and there is currently no evidence that this protein possesses any proteolytic activity. For this reason it is premature to suggest the generation of inhibitors to calpain-6 and to speculate as to the type of treatments that could be used to target this calpain.
Similarly to calpain-3 and -6, calpain-9 (also called nCL-4, Figure 1) was shown to be a potential anti-cancer target. However, contrarily to calpain-6, this tissue-specific protease expressed in the normal cells of the digestive tract; appears to suppress tumorigenesis. A first publication has shown that the expression of the gene encoding calpain-9 is depleted in several gastric cancer cell lines [85]. These observations were complemented by a second study showing that the inactivation of this gene in fibroblasts promoted the anchorage-independent growth of these cells and the formation of tumors in nude mice [86]. Because of the tumorigenic effects of the suppression of its expression, calpain-9 activation appears to be a potential approach to treat gastric cancer. However further studies are required, notably to observe the effects of an overexpression of calpain 9 on the development and progression of gastric cancers.
These data concerning calpain-3, calpain-6 and calpain-9 highlight the fact that µ- and m-calpain are not the only potential targets to treat cancer in calpain family. However, further work is required to better understand their role in the tumorigenesis. It is notably impossible to target calpain-6 activity to limit uterine cancer development without indentifying or developing an inhibitor of this protease. Moreover, the exact role of calpain-3 has to be clarified, as the current data are contradictory. However, calpain isoform-specific inhibitors may be beneficial to avoid side effects of inhibition of the ubiquitous calpains.
7- Conclusions
Calpains are known to be directly or indirectly implicated in a very large number of phenomena with cancer being one of them. Even if the members of calpain family are not directly responsible for the formation and the development of cancer, these enzymes are important effectors of the different processes leading to the formation of the tumor, its growth and the formation of metastasis. Importantly for patients, calpains are also linked to chemotherapeutic efficacy. As shown in Tables 2 and 3, there are numerous alterations and deregulations of the calpain system in different cancer types; the expression and/or the activity of these enzymes are very often modified leading to transformation and increases in proliferation, motility, and invasiveness, and providing resistance of the tumor cells to chemotherapies (see Figure 3).
Among the different members of the calpain family, the ubiquitous calpains µ- and m-calpain are clearly the calpains the most widely implicated in the development and progression of cancer. Indeed, the studies published during the last decade clearly show how involved the ubiquitous calpains are in the transformation, the migration, and the invasiveness of the cancer cells, as well as for the neovascularization of the tumor. They are also implicated in the apoptosis of the tumor cells. Thus, these signaling proteases are potentially viable targets for cancer control and treatment.
One issue in targeting calpains relates to the various steps in tumor development and treatment. Concerning cell transformation, recent studies show that several oncoproteins such as Src induce a strong activation of the ubiquitous calpains, allowing for transformation of the cells [33]. Therefore, inhibition of µ- and m-calpain activity would block the effects of the oncoproteins. Similarly, several studies show that calpain activity is necessary for the migration and the invasion of the tumor cells [28, 65, 68]. In several types of cancer, the tumor cells present an abnormally high activity of µ- and/or m-calpains, and this activity allow the cells to migrate faster, and also to invade the surrounding tissue. Indeed, calpains are able to regulate the expression and the activity of the MMPs, thus controlling the degradative remodeling of the extracellular matrix [30]. Interestingly, one study performed with lung cancer cells also showed that µ- and m-calpain can be secreted by these cells to directly alter the matrix to facilitate their invasion [65]. Most of these publications also show that inhibiting calpain activity using synthetic inhibitors, calpastatin, or molecular downregulation of ubiquitous calpain expression reduces the motility and/or the invasiveness of the tumor cells. Lastly, calpain activity is required for endothelial cell motility that provides for tumor neovascularization. Indeed, growth factors secreted by cancer cells such as VEGF and TGFα/HB-EGF, stimulate endothelial cell migration and tube formation that require the activation of ubiquitous calpains; inhibition of these enzymes reduces the migration of the endothelial cells and thus blocks angiogenesis [58]. In these situations, blockade of calpains would provide benefit in limiting carcinogenesis or progression. For these reasons, specific inhibitors of ubiquitous calpain activity could be used to prevent cancer progression by inhibiting migration and invasion of the tumor cells needed for dissemination as well as blocking the tumor neovascularization necessary for growth to clinically relevant size.
However, the situation is not so simple. With the exception of bortezomib, calpain activity was also shown to be required for the apoptosis of the tumor cells induced by several chemotherapy drugs, such as cisplatin, genistein or trastuzumab [37, 45, 52]. Most of these drugs activate the ubiquitous calpains by inducing an increase of the concentration of intracellular calcium, thus leading to the activation of caspases and to cell death. In these conditions, an inhibition of calpain activity inhibits apoptosis and thus reduces the efficacy of the drugs used in chemotherapy.
To summarize the data concerning µ- and m-calpains, the activity of these enzymes is required for both the development and the progression of the cancer (by allowing cell transformation, the migration and the invasion of tumor cells, and the neovascularization of the tumor) and the suppression of the tumor by chemotherapy (see Figure 3). Ubiquitous calpains can thus clearly be considered as excellent targets to treat cancer, however their involvement in such opposite processes is problematic, notably to determine the type of treatment to use. Indeed, inhibiting calpain activity would be an efficient way to block the development of a tumor by blocking the transformation and the proliferation of the cells, as well as the vascularization of the tumor. It would stop the growth of the tumor and the lack of nutrients and oxygen would induce the necrosis of the tumor. More importantly, it would prevent the formation of metastasis, notably in the cases of lung and prostate cancers, by inhibiting the migration and the invasion of the tumor cells. However, an inhibition of the activity of the ubiquitous calpains would also block the activation of these enzymes induced by the chemotherapy drugs, thus preventing the activation of caspases and the initiation of apoptosis. It would strongly limit the effects of chemotherapy by increasing the resistance of the tumor cells to death. Targeting ubiquitous calpains to treat cancer would thus have to be timed and not used during chemotherapeutic regimens. Rather, one could envision a situation wherein calpain inhibition was used as a more chronic maintenance therapy, while during chemotherapy calpain agonists would be adjuvant to promote killing and cause collapse of tumor neovasculature.
Still, such therapeutic approaches must be cognizant of potential side effects. The processes in which the ubiquitous calpains are implicated in cancers are the same as they function in physiology and homeostasis. For instance, inhibition of calpain activity would be detrimental to wound repair, as it would prevent cellular replacement and the re-establishment of normal vascularity. Thus, if there were a surgical intervention, the major part of healing would need to be completed prior to initiating anti-calpain therapy.
Finally, the other calpain family members implicated in specific cancers, calpains -3, -6, and -9, offer opportunities for inhibition without concerns about timing and side effects, as long as the inhibitors show isoform selectivity. These tissue-specific calpains could become interesting anti-cancer targets. However, these promising data and the involvement of these calpains in cancer development require further studies first to confirm involvement and more importantly define mechanistic roles. One key development, however, is the lack of such isoform-specific inhibitors, a challenge made even more daunting by the close sequence similarities of the family members.
8- Expert opinion
Calpains and more particularly µ- and m-calpain, can definitively be considered as potential anti-cancer targets, as these enzymes are involved in several steps of the development and progression of a wide variety of common cancers (Figure 3). Treating tumor cells with inhibitors of the ubiquitous calpains could be a useful and efficient way to retard the development and dissemination of the cancer cells. Of course, one would expect cancer cells to eventually develop resistance to such inhibition; one example would be a switch from mesenchymal to amoeboid invasion. Still, such global inhibition could interfere with chemotherapy drugs used to induce the apoptosis of the tumor cells, such as cisplatin or trastuzumab. Even avoiding the post-surgical wound repair window to minimize side-effects and the acute chemotherapy regimen windows so as to not blunt efficacy, would not be a panacea, as the calpains are involved in normal homeostasis. Thus, the treatment would have to be subtotal and thus used primarily as a maintenance therapy to slow progression and prolong survival, turning cancer into a chronic condition. This is still theoretical, as agents to inhibit calpains in humans have not been approved, and thus these and especially isoform-specific inhibitors will require further development.
Article highlights
Calpain activity is increased by oncoproteins and required for cell transformation
Ubiquitous calpains control tumor cell migration and invasiveness
Ubiquitous calpains regulate MMP expression and activity
Calpains play a critical role in tumor neovascularization by regulating endothelial cell migration
Chemotherapy-induced apoptosis requires calpain-dependent activation of caspases
Acknowledgments
Declaration of interest
This work has been supported by grants from the Veterans Administration (USA) and National Institutes of Health (USA).
References
- 1. Goll DE, Thompson VF, Li H, et al. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. ** A very complete review of the calpain system and its complexity
- 2.Schád E, Farkas A, Jékely G, et al. A novel human small subunit of calpains. Biochem J. 2002;362:383–388. doi: 10.1042/0264-6021:3620383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulkarni S, Goll DE, Fox JEB. Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J Biol Chem. 2002;277:24435–24441. doi: 10.1074/jbc.M203457200. [DOI] [PubMed] [Google Scholar]
- 4.Huang C, Rajfur Z, Yousefi N, et al. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat Cell Biol. 2009;11:624–630. doi: 10.1038/ncb1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregoriou M, Willis AC, Pearson MA, et al. The calpain cleavage sites in the epidermal growth factor receptor kinase domain. Eur J Biochem. 1994;223:455–464. doi: 10.1111/j.1432-1033.1994.tb19013.x. [DOI] [PubMed] [Google Scholar]
- 6.Franco SJ, Rodgers MA, Perrin BJ, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 7.Horikawa Y, Oda N, Cox NJ, et al. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163–175. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 8.Saito K, Elce JS, Hamos JE, et al. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci USA. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellgren RL. Evidence for participation of a calpain-like cysteine protease in cell cycle progression through late G1 phase. Biochem Biophys Res Commun. 1997;236:555–558. doi: 10.1006/bbrc.1997.7003. [DOI] [PubMed] [Google Scholar]
- 10.Jánossy J, Ubezio P, Apáti A, et al. Calpain as a multi-site regulator of cell cycle. Biochem Pharmacol. 2004;67:1513–1521. doi: 10.1016/j.bcp.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Joy J, Nalabothula N, Ghosh M, et al. Identification of calpain cleavage sites in the G1 cyclin-dependent kinase inhibitor p19(INK4d) Biol Chem. 2006;387:329–335. doi: 10.1515/BC.2006.044. [DOI] [PubMed] [Google Scholar]
- 12.Choi YH, Lee SJ, Nguyen P, et al. Regulation of cyclin D1 by calpain protease. J Biol Chem. 1997;272:28479–28484. doi: 10.1074/jbc.272.45.28479. [DOI] [PubMed] [Google Scholar]
- 13.Wang XD, Rosales JL, Magliocco A, et al. Cyclin E in breast tumors is cleaved into its low molecular weight forms by calpain. Oncogene. 2003;22:769–774. doi: 10.1038/sj.onc.1206166. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Knutson E, Kurosky A, et al. Degradation of p21cip1 in cells productively infected with human cytomegalovirus. J Virol. 2001;75:3613–3625. doi: 10.1128/JVI.75.8.3613-3625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delmas C, Aragou N, Poussard S, et al. MAP kinase-dependent degradation of p27Kip1 by calpains in choroidal melanoma cells. Requirement of p27Kip1 nuclear export. J Biol Chem. 2003;278:12443–12451. doi: 10.1074/jbc.M209523200. [DOI] [PubMed] [Google Scholar]
- 16.Smith PD, Mount MP, Shree R, et al. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2006;26:440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squìer MK, Miller AC, Malkinson AM, et al. Calpain activation in apoptosis. J Cell Physiol. 1994;159:229–237. doi: 10.1002/jcp.1041590206. [DOI] [PubMed] [Google Scholar]
- 18.Chua BT, Guo K, Li P. Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J Biol Chem. 2000;275:5131–5135. doi: 10.1074/jbc.275.7.5131. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- 21.Kubbutat MH, Vousden KH. Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol Cell Biol. 1997;17:460–468. doi: 10.1128/mcb.17.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. ** An interesting review about the regulation of cell migration by calpains
- 23.Bhatt A, Kaverina I, Otey C, et al. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J Cell Sci. 2002;115:3415–3425. doi: 10.1242/jcs.115.17.3415. [DOI] [PubMed] [Google Scholar]
- 24.Sheetz MP, Felsenfeld D, Galbraith CG, et al. Cell migration as a five-step cycle. Biochem Soc Symp. 1999;65:233–243. [PubMed] [Google Scholar]
- 25.Shao H, Chou J, Baty CJ, et al. Spatial localization of m-calpain to the plasma membrane by phosphoinositide biphosphate binding during epidermal growth factor receptor-mediated activation. Mol Cell Biol. 2006;26:5481–5496. doi: 10.1128/MCB.02243-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leloup L, Shao H, Bae YH, et al. m-Calpain activation is regulated by its membrane localization and by its binding to phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2010;285:33549–33566. doi: 10.1074/jbc.M110.123604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franco S, Perrin B, Huttenlocher A. Isoform specific function of calpain 2 in regulating membrane protrusion. Exp Cell Res. 2004;299:179–187. doi: 10.1016/j.yexcr.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 28. Mamoune A, Luo J, Lauffenburger DA, et al. Calpain-2 as a target for limiting prostate cancer invasion. Cancer Res. 2003;63:4632–4640. ** A detailed study of the regulation of tumor cell migration and invasion by calpains
- 29.Cortesio CL, Chan KT, Perrin BJ, et al. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J Cell Biol. 2008;180:957–971. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popp O, Heidinger M, Ruiz-Heinrich L, et al. The calpastatin-derived calpain inhibitor CP1B reduces mRNA expression of matrix metalloproteinase-2 and -9 and invasion by leukemic THP-1 cells. Biol Chem. 2003;384:951–958. doi: 10.1515/BC.2003.107. [DOI] [PubMed] [Google Scholar]
- 31.Carragher NO, Frame MC. Calpain: a role in cell transformation and migration. Int J Biochem Cell Biol. 2002;34:1539–1543. doi: 10.1016/s1357-2725(02)00069-9. [DOI] [PubMed] [Google Scholar]
- 32.Carragher NO, Westhoff MA, Riley D, et al. v-Src-induced modulation of the calpain-calpastatin proteolytic system regulates transformation. Mol Cell Biol. 2002;22:257–269. doi: 10.1128/MCB.22.1.257-269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carragher NO, Fonseca BD, Frame MC. Calpain activity is generally elevated during transformation but has oncogene-specific biological functions. Neoplasia. 2004;6:53–73. doi: 10.1016/s1476-5586(04)80053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niapour M, Yu Y, Berger SA. Regulation of calpain activity by c-Myc through calpastatin and promotion of transformation in c-Myc-negative cells by calpastatin suppression. J Biol Chem. 2008;283:21371–21381. doi: 10.1074/jbc.M801462200. [DOI] [PubMed] [Google Scholar]
- 35.Ciuffini L, Castellani L, Salvati E, et al. Delineating v-Src downstream effector pathways in transformed myoblasts. Oncogene. 2008;27:528–539. doi: 10.1038/sj.onc.1210665. [DOI] [PubMed] [Google Scholar]
- 36.Small GW, Chou T, Dang CV, et al. Evidence for involvement of calpain in c-Myc proteolysis in vivo. Arch Biochem Biophys. 2002;400:151–161. doi: 10.1016/S0003-9861(02)00005-X. [DOI] [PubMed] [Google Scholar]
- 37.Sergeev IN. Genistein induces Ca2+ -mediated, calpain/caspase-12-dependent apoptosis in breast cancer cells. Biochem Biophys Res Commun. 2004;321:462–467. doi: 10.1016/j.bbrc.2004.06.173. [DOI] [PubMed] [Google Scholar]
- 38.Martinez JA, Zhang Z, Svetlov SI, et al. Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells. Apoptosis. 2010;15:1480–1493. doi: 10.1007/s10495-010-0526-4. [DOI] [PubMed] [Google Scholar]
- 39.Tan Y, Dourdin N, Wu C, et al. Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J Biol Chem. 2006;281:16016–16024. doi: 10.1074/jbc.M601299200. [DOI] [PubMed] [Google Scholar]
- 40.Shim H, Park J, Paik H, et al. Genistein-induced apoptosis of human breast cancer MCF-7 cells involves calpain-caspase and apoptosis signaling kinase 1-p38 mitogen-activated protein kinase activation cascades. Anticancer Drugs. 2007;18:649–657. doi: 10.1097/CAD.0b013e3280825573. [DOI] [PubMed] [Google Scholar]
- 41.Yeh T, Chiang P, Li T, et al. Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem Pharmacol. 2007;73:782–792. doi: 10.1016/j.bcp.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 42.Das A, Banik NL, Ray SK. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer. 2010;116:164–176. doi: 10.1002/cncr.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sareen D, Darjatmoko SR, Albert DM, et al. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol Pharmacol. 2007;72:1466–1475. doi: 10.1124/mol.107.039040. [DOI] [PubMed] [Google Scholar]
- 44.Xie S, Zhang Z, Hu G, et al. HL-37, a novel anthracene derivative, induces Ca(2+)-mediated apoptosis in human breast cancer cells. Toxicology. 2008;254:68–74. doi: 10.1016/j.tox.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Xing D, Chen WR, et al. Calpain-mediated pathway dominates cisplatin-induced apoptosis in human lung adenocarcinoma cells as determined by real-time single cell analysis. Int J Cancer. 2008;122:2210–2222. doi: 10.1002/ijc.23378. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Xing D, Chen WR. Micro-calpain regulates caspase-dependent and apoptosis inducing factor-mediated caspase-independent apoptotic pathways in cisplatin-induced apoptosis. Int J Cancer. 2009;125:2757–2766. doi: 10.1002/ijc.24626. [DOI] [PubMed] [Google Scholar]
- 47.Anguissola S, Köhler B, O'Byrne R, et al. Bid and calpains cooperate to trigger oxaliplatin-induced apoptosis of cervical carcinoma HeLa cells. Mol Pharmacol. 2009;76:998–1010. doi: 10.1124/mol.109.058156. [DOI] [PubMed] [Google Scholar]
- 48.Wu Z, Chao CC. Knockdown of NAPA using short-hairpin RNA sensitizes cancer cells to cisplatin: implications to overcome chemoresistance. Biochem Pharmacol. 2010;80:827–837. doi: 10.1016/j.bcp.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 49.Lee SK, Park MS, Nam MJ. Aspirin Has Antitumor Effects via Expression of Calpain Gene in Cervical Cancer Cells. J Oncol. 2008;2008:285374. doi: 10.1155/2008/285374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karmakar S, Banik NL, Patel SJ, et al. Curcumin activated both receptor-mediated and mitochondria-mediated proteolytic pathways for apoptosis in human glioblastoma T98G cells. Neurosci Lett. 2006;407:53–58. doi: 10.1016/j.neulet.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Karmakar S, Banik NL, Ray SK. Curcumin suppressed anti-apoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells. Neurochem Res. 2007;32:2103–2113. doi: 10.1007/s11064-007-9376-z. [DOI] [PubMed] [Google Scholar]
- 52. Kulkarni S, Reddy KB, Esteva FJ, et al. Calpain regulates sensitivity to trastuzumab and survival in HER2-positive breast cancer. Oncogene. 2010;29:1339–1350. doi: 10.1038/onc.2009.422. ** An interesting study highlighting the importance of calpains for chemotherapy treatments
- 53.Li C, Chen S, Yue P, et al. Proteasome inhibitor PS-341 (bortezomib) induces calpain-dependent IkappaB(alpha) degradation. J Biol Chem. 2010;285:16096–16104. doi: 10.1074/jbc.M109.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells A, Kassis J, Solava J, et al. Growth factor-induced cell motility in tumor invasion. Acta Oncol. 2002;41:124–130. doi: 10.1080/028418602753669481. [DOI] [PubMed] [Google Scholar]
- 55.Kassis J, Radinsky R, Wells A. Motility is rate-limiting for invasion of bladder carcinoma cell lines. Int J Biochem Cell Biol. 2002;34:762–775. doi: 10.1016/s1357-2725(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 56.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dourdin N, Bhatt AK, Dutt P, et al. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem. 2001;276:48382–48388. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- 58.Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. 2006;98:617–625. doi: 10.1161/01.RES.0000209968.66606.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su Y, Cui Z, Li Z, et al. Calpain-2 regulation of VEGF-mediated angiogenesis. FASEB J. 2006;20:1443–1451. doi: 10.1096/fj.05-5354com. [DOI] [PubMed] [Google Scholar]
- 60.Satish L, Blair HC, Glading A, et al. Interferon-inducible protein 9 (CXCL11)-induced cell motility in keratinocytes requires calcium flux-dependent activation of mu-calpain. Mol Cell Biol. 2005;25:1922–1941. doi: 10.1128/MCB.25.5.1922-1941.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dedieu S, Poussard S, Mazères G, et al. Myoblast migration is regulated by calpain through its involvement in cell attachment and cytoskeletal organization. Exp Cell Res. 2004;292:187–200. doi: 10.1016/j.yexcr.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 62.Leloup L, Mazères G, Daury L, et al. Involvement of calpains in growth factor-mediated migration. Int J Biochem Cell Biol. 2006;38:2049–2063. doi: 10.1016/j.biocel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 63.Glading A, Chang P, Lauffenburger DA, et al. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- 64.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37 Suppl 4:S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 65. Xu L, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induces phosphorylation of mu- and m-calpain in association with increased secretion, cell migration, and invasion. J Biol Chem. 2004;279:53683–53690. doi: 10.1074/jbc.M409889200. * A nice study showing how calpain activation and secretion can regulate lung cancer cell migration and invasion
- 66.Xu L, Deng X. Protein kinase Ciota promotes nicotine-induced migration and invasion of cancer cells via phosphorylation of micro- and m-calpains. J Biol Chem. 2006;281:4457–4466. doi: 10.1074/jbc.M510721200. [DOI] [PubMed] [Google Scholar]
- 67.Meng XN, Jin Y, Yu Y, et al. Characterisation of fibronectin-mediated FAK signalling pathways in lung cancer cell migration and invasion. Br J Cancer. 2009;101:327–334. doi: 10.1038/sj.bjc.6605154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roumes H, Leloup L, Dargelos E, et al. Calpains: markers of tumor aggressiveness? Exp Cell Res. 2010;316:1587–1599. doi: 10.1016/j.yexcr.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 69.Tryggvason K, Höyhtyä M, Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987;907:191–217. doi: 10.1016/0304-419x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- 70.Fan D, Dai J, Tang J, et al. Silencing of calpain expression reduces the metastatic potential of human osteosarcoma cells. Cell Biol Int. 2009;33:1263–1267. doi: 10.1016/j.cellbi.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 71.Brustis JJ, Elamrani N, Balcerzak D, et al. Rat myoblast fusion requires exteriorized m-calpain activity. Eur J Cell Biol. 1994;64:320–327. [PubMed] [Google Scholar]
- 72.Xu L, Deng X. Suppression of cancer cell migration and invasion by protein phosphatase 2A through dephosphorylation of mu- and m-calpains. J Biol Chem. 2006;281:35567–35575. doi: 10.1074/jbc.M607702200. [DOI] [PubMed] [Google Scholar]
- 73.Carragher NO, Walker SM, Scott Carragher LA, et al. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene. 2006;25:5726–5740. doi: 10.1038/sj.onc.1209582. [DOI] [PubMed] [Google Scholar]
- 74.Martiny-Baron G, Marmé D. VEGF-mediated tumour angiogenesis: a new target for cancer therapy. Curr Opin Biotechnol. 1995;6:675–680. doi: 10.1016/0958-1669(95)80111-1. [DOI] [PubMed] [Google Scholar]
- 75.Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333:F179–F199. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 76.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III Trial Assessing Bevacizumab in Stages II and III Carcinoma of the Colon: Results of NSABP Protocol C-08. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bodnar RJ, Yates CC, Rodgers ME, et al. IP-10 induces dissociation of newly formed blood vessels. J Cell Sci. 2009;122:2064–2077. doi: 10.1242/jcs.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shiraha H, Glading A, Chou J, et al. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Biol. 2002;22:2716–2727. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lasagni L, Francalanci M, Annunziato F, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ono Y, Shimada H, Sorimachi H, et al. Functional defects of a muscle-specific calpain, p94, caused by mutations associated with limb-girdle muscular dystrophy type 2A. J Biol Chem. 1998;273:17073–17078. doi: 10.1074/jbc.273.27.17073. [DOI] [PubMed] [Google Scholar]
- 81.Moretti D, Del Bello B, Cosci E, et al. Novel variants of muscle calpain 3 identified in human melanoma cells: cisplatin-induced changes in vitro and differential expression in melanocytic lesions. Carcinogenesis. 2009;30:960–967. doi: 10.1093/carcin/bgp098. [DOI] [PubMed] [Google Scholar]
- 82.Roperto S, De Tullio R, Raso C, et al. Calpain3 is expressed in a proteolitically active form in papillomavirus-associated urothelial tumors of the urinary bladder in cattle. PLoS ONE. 2010;5:e10299. doi: 10.1371/journal.pone.0010299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee S, Kim B, Choi Y, et al. Increased expression of calpain 6 during the progression of uterine cervical neoplasia: immunohistochemical analysis. Oncol Rep. 2008;19:859–863. [PubMed] [Google Scholar]
- 84.Rho SB, Byun H, Park S, et al. Calpain 6 supports tumorigenesis by inhibiting apoptosis and facilitating angiogenesis. Cancer Lett. 2008;271:306–313. doi: 10.1016/j.canlet.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 85.Yoshikawa Y, Mukai H, Hino F, et al. Isolation of two novel genes, down-regulated in gastric cancer. Jpn J Cancer Res. 2000;91:459–463. doi: 10.1111/j.1349-7006.2000.tb00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu K, Li L, Cohen SN. Antisense RNA-mediated deficiency of the calpain protease, nCL-4, in NIH3T3 cells is associated with neoplastic transformation and tumorigenesis. J Biol Chem. 2000;275:31093–31098. doi: 10.1074/jbc.M005451200. [DOI] [PubMed] [Google Scholar]
- 87.Shiba E, Kambayashi JI, Sakon M, et al. Ca²+;-Dependent Neutral Protease (Calpain) Activity in Breast Cancer Tissue and Estrogen Receptor Status. Breast Cancer. 1996;3:13–17. doi: 10.1007/BF02966957. [DOI] [PubMed] [Google Scholar]
- 88.Lakshmikuttyamma A, Selvakumar P, Kanthan R, et al. Overexpression of m-calpain in human colorectal adenocarcinomas. Cancer Epidemiol Biomarkers Prev. 2004;13:1604–1609. [PubMed] [Google Scholar]
- 89.Das A, Banik NL, Patel SJ, et al. Dexamethasone protected human glioblastoma U87MG cells from temozolomide induced apoptosis by maintaining Bax:Bcl-2 ratio and preventing proteolytic activities. Mol Cancer. 2004;3:36. doi: 10.1186/1476-4598-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rios-Doria J, Day KC, Kuefer R, et al. The role of calpain in the proteolytic cleavage of E-cadherin in prostate and mammary epithelial cells. J Biol Chem. 2003;278:1372–1379. doi: 10.1074/jbc.M208772200. [DOI] [PubMed] [Google Scholar]
- 91.Reichrath J, Welter C, Mitschele T, et al. Different expression patterns of calpain isozymes 1 and 2 (CAPN1 and 2) in squamous cell carcinomas (SCC) and basal cell carcinomas (BCC) of human skin. J Pathol. 2003;199:509–516. doi: 10.1002/path.1308. [DOI] [PubMed] [Google Scholar]
- 92.Braun C, Engel M, Seifert M, et al. Expression of calpain I messenger RNA in human renal cell carcinoma: correlation with lymph node metastasis and histological type. Int J Cancer. 1999;84:6–9. doi: 10.1002/(sici)1097-0215(19990219)84:1<6::aid-ijc2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 93.Daniel KG, Anderson JS, Zhong Q, et al. Association of mitochondrial calpain activation with increased expression and autolysis of calpain small subunit in an early stage of apoptosis. Int J Mol Med. 2003;12:247–252. [PubMed] [Google Scholar]
- 94.Bai D, Dai Z, Zhou J, et al. Capn4 overexpression underlies tumor invasion and metastasis after liver transplantation for hepatocellular carcinoma. Hepatology. 2009;49:460–470. doi: 10.1002/hep.22638. [DOI] [PubMed] [Google Scholar]
- 95.Kothapalli R, Bailey RD, Kusmartseva I, et al. Constitutive expression of cytotoxic proteases and down-regulation of protease inhibitors in LGL leukemia. Int J Oncol. 2003;22:33–39. [PubMed] [Google Scholar]
- 96.Salehin D, Fromberg I, Haugk C, et al. Immunhistochemical analysis for expression of calpain 1, calpain 2 and calpastatin in endometrial cancer. Anticancer Res. 2010;30:2837–2843. [PubMed] [Google Scholar]
- 97.Esteban F, Royo JL, González-Moles MA, et al. CAPN10 alleles modify laryngeal cancer risk in the Spanish population. Eur J Surg Oncol. 2008;34:94–99. doi: 10.1016/j.ejso.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 98.Akashiba H, Matsuki N, Nishiyama N. Calpain activation is required for glutamate-induced p27 down-regulation in cultured cortical neurons. J Neurochem. 2006;99:733–744. doi: 10.1111/j.1471-4159.2006.04100.x. [DOI] [PubMed] [Google Scholar]
- 99.Kaneko T, Yamashima T, Tohma Y, et al. Calpain-dependent proteolysis of merlin occurs by oxidative stress in meningiomas: a novel hypothesis of tumorigenesis. Cancer. 2001;92:2662–2672. doi: 10.1002/1097-0142(20011115)92:10<2662::aid-cncr1620>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 100.Averna M, Stifanese R, De Tullio R, et al. Calpain-mediated activation of NO synthase in human neuroblastoma SK-N-BE cells. J Neurochem. 2009;110:412–421. doi: 10.1111/j.1471-4159.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 101.Chen C, Nguyen T, Shively JE. Role of calpain-9 and PKC-delta in the apoptotic mechanism of lumen formation in CEACAM1 transfected breast epithelial cells. Exp Cell Res. 2010;316:638–648. doi: 10.1016/j.yexcr.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]