Abstract
Purpose
To study choline metabolism in biopsies from non-enhancing Grade 2 (AS2) and Grade 3 (AS3) astrocytomas to determine whether (1) phosphocholine (PC) dominates in AS3, and (2) PC is associated with proliferation or angiogenesis. PC and glycerophosphocholine (GPC) are involved in phospholipid metabolism that accompanies mitosis. PC is the predominant peak in Grade 4 astrocytoma (GBM) while GPC dominates in AS2.
Materials and Methods
We used high resolution magic angle spinning magnetic resonance spectroscopy to compare the concentrations of 10 metabolites in 41 biopsies (16 AS2 and 25 AS3) from 24 tumors. Immunohistochemistry was performed on paired biopsies to determine the cell density, Ki-67 proliferation index, and vascular endothelial growth factor (VEGF) angiogenic marker expression.
Results
AS3 had higher PC than AS2; however, the PC:GPC was less than 1 in all cases irrespective of tumor grade. Within tumors, GPC increased with Ki-67 and PC and tCho increased with cell density. There was no association between any choline compound and VEGF.
Conclusion
These data suggest that PC:GPC less than 1 is not unique to low grade glioma. Further, the PC concentration that is a marker of aggressive glial tumors is not tightly linked to cell proliferation or angiogenesis in non-enhancing astrocytomas.
Keywords: magnetic resonance spectroscopy, HRMAS, astrocytoma, VEGF, Ki-67, non-enhancing
Introduction
Malignant astrocytomas, a subtype of malignant gliomas, are the most common form of brain tumor and present as one of three histologic grades that is the primary determinant of patient outcome. The five-year survival rates for histologic Grades 2, 3, and 4 are 47%, 27%, and 5%, respectively, according to the Surveillance Epidemiology and End Results (SEER) registries from 1995-2006 (1). Grade 2 astrocytomas (AS2) are considered low grade malignant tumors, are distinguished from normal brain by their hypercellularity and nuclear atypia, and typically appear as non-enhancing lesions on gadolinium-enhanced T1-weighted magnetic resonance imaging (MRI) (2). Grade 3 anaplastic astrocytoma (AS3) are considered high grade, are more cellular than low grade astrocytomas, have evidence of mitotic activity, and can appear as either contrast-enhancing or non-enhancing lesions on MRI. Grade 4 astrocytoma, also known as glioblastoma multiforme, or GBM, are the most common high grade glioma, are highly vascular tumors harboring regions of hypoxia and necrosis, and almost always exhibit contrast-enhancement on MRI due to leakage of gadolinium out of angiogenic vessels that have a compromised blood-brain barrier.
Cellular metabolism is also altered in gliomas and studies utilizing magnetic resonance spectroscopy (MRS) have explored the utility of metabolic markers for predicting tumor aggressiveness (3-5). One of the most robust indicators of malignancy is an increase in the choline compounds involved in membrane phospholipid metabolism that is regulated by the cell cycle (6,7); namely, the anabolic compound, phosphocholine (PC), the catabolic compound, glycerophosphocholine (GPC), and free choline (fCho). In vivo and ex vivo MRS studies have shown that high grade gliomas exhibit higher total choline (GPC + PC + fCho) than low grade gliomas (8,9). Further, PC has been shown to be the predominant choline compound in high grade glioma, particularly GBM, while GPC dominates in low grade glioma and normal brain (5,9). The higher PC in GBM has been attributed to higher expression and/or activity of choline kinase (5) which has been shown to be enhanced by hypoxia (10) and has recently gained appeal as a potential target for therapy (11) (5). It is not clear, however, if the predominance of PC is due to the high proliferation, hypoxia, or angiogenesis that are all features of GBM that are not observed in low grade glioma (12,13).
To try and pinpoint the malignant processes that are associated with elevated PC in high grade glioma, we studied the choline metabolism in a cohort of AS2 and AS3 that did not exhibit contrast-enhancement on MRI. Since none of the tumors enhanced on MRI, we expected that they were all at a pre-angiogenic or early angiogenic stage. However, we expected the AS2 and AS3 cohorts to differ in proliferation and cellularity as these are the histopathologic features that distinguish the two grades. To verify our expectations, we measured the expression level of the angiogenic marker vascular endothelial growth factor (VEGF) (14,15), the cell density, and expression level of Ki-67 proliferation antigen in the AS2 and AS3 subgroups.
We chose to restrict our study to the most common subtype of gliomas, astrocytomas, because the metabolism and hypoxic status of oligodendroglial or mixed gliomas may be different from astrocytomas (3,16). The goals of the study were to determine whether non-enhancing AS2 and AS3 differed in terms of (1) cell density, Ki-67 proliferation index, and VEGF expression and (2) MRS metabolite concentrations, with particular focus on the relative levels of choline compounds. We also explored associations between the histologic and metabolic markers of aggressiveness within the entire cohort of non-enhancing astrocytomas.
Methods
Subjects
Twenty-five patients (13 male, 12 female, age mean and SD = 37 +/- 8 years) that were recommended for surgical resection of non-enhancing lesions suspicious for glioma on T1-weighted MRI were recruited for the study. None of the patients had received prior treatment other than surgical resection or biopsy. All patients were consented in accordance with our institutional standards. Patients underwent an MR exam 1-3 days prior to surgery that included a 3D MRS sequence and diffusion tensor imaging (see MRI/MRS). Multiple biopsy targets were identified from the pre-surgery anatomic MRIs and targets were overlaid on the MRIs prior to their transfer to the BrainLab surgical navigation. The biopsy target was a cylinder with a diameter and height of 6 mm to accommodate for the 5 mm accuracy of the surgical planning methodology (17). During surgery, biopsies were collected from one or more of the biopsy target regions deemed safe for surgical removal by the surgeon. A large (20-30 mg) biopsy was retrieved from each target and dissected in the operating room. One half was flash frozen in liquid nitrogen, and the other half was immediately stored in ethanol for subsequent formalin fixation and paraffin embedding. The frozen and ethanol-fixed biopsies were transferred to a laboratory adjacent to the operating room immediately after retrieval and the ethanol-fixed biopsies were transferred to formalin. After biopsies were retrieved from all accessible targets, they were transferred to the Neurosurgery Tissue Bank where the formalin-fixed biopsies were embedded in paraffin. The biopsies were stored at the Neurosurgery Tissue Bank as paraffin blocks (formalin-fixed) or in liquid nitrogen (frozen) until they were requested for immunohistochemical or MRS analysis. One section from each paraffin block was stained with hemotoxylin and eosin and reviewed by the Neuropathologist (J.P.) in the Tissue Bank to confirm that the diagnosis was consistent with the clinical diagnosis. The histopathologic subtype of the image-guided biopsies was astrocytoma in all but one case, which was read as a mixed oligoastrocytoma and turned out to have loss of heterozygosity on chromosomes 1p and 19q which is a hallmark of oligodendroglial tumors. The histologic grades of the image-guided biopsies were all consistent with the clinical diagnosis. Of the 24 remaining patients, ten were classified as AS2 and 14 were classified as AS3. Seven patients had biopsies retrieved from a single location within the tumor and 17 had biopsies from two locations, resulting in a total of 41 biopsies (16 AS2 and 25 AS3).
MRI/MRS
The presurgical MR scans were performed on a 3T GE MRI scanner equipped with an 8-channel phased array head coil. The protocol included a T2-weighted fast spin echo (TR/TE: 3667/107, slice thickness: 1.5 mm, FOV: 260×260×180 mm, matrix: 256×256×120, pixel size: 1.03 mm2), a T1-weighted contrast-enhanced spoiled gradient echo (TR/TE: 425/3.2, slice thickness: 1.5 mm, FOV: 240×240×180, matrix: 256×256×120, pixel size: 0.88 mm2), a six directional diffusion tensor echo-planar imaging (DTI) sequence (TR = 7,000 ms, TE = 63 ms, matrix size = 256 × 256, slice thickness = 3 mm, b = 1000 s/mm2, NEX=4), and a 3D-MRSI sequence (TR/TE: 1200/144, slice thickness: 10mm, FOV: 160×160×160, matrix: 16×16×16, nominal voxel size: 1.0 mm3).
The pre-surgical MRSI data was processed using previously described methods (7,18). Briefly, the data were corrected for each coil signal profile, fourier transformed, phase- and frequency-corrected resulting in a 3D array of spectra containing peaks corresponding to choline compounds, Cre, NAA, and Lip. The peak areas were quantified and the spectral array was co-registered with the presurgical MR images. ADC maps were calculated from the DTI images on a pixel-by-pixel basis using software developed in-house, based on published algorithms (19).
The goal of designating biopsy targets was to improve the likelihood of retrieving tissue from the most aggressive regions of the tumor, which is particularly challenging in tumors that do not exhibit contrast enhancement. Based on a previous study using long-echo in vivo 3D-MRSI (20) that showed that the region in non-enhancing tumors with the highest peaks corresponding to choline-containing compounds (Cho) relative to NAA most often corresponded to the region with highest proliferation and cell density, we attempted to identify at least one target in a region of maximal Cho:NAA ratio. When there was no clear maximum Cho:NAA, the region with minimal ADC was included as a target because of the inverse association demonstrated between ADC and cell density (21).
High-resolution Magic Angle Spinning (HRMAS) MRS
HRMAS MRS is an ex vivo technique that can be used to interrogate the metabolism of intact tissue specimens without the need for chemical extraction, which can disrupt the cell membrane (22). We used a Varian 500MHz spectrometer, equipped with a gHX gradient nanoprobe. Samples were evaluated at 1° C while the tissue was spun at 2250 Hz at the magic angle (theta = 54.7 degrees). The fully relaxed water presaturation sequence parameters were pulse width = 7.8μs, transients = 128, sweep width = 40kHz, and 40,000 points. The ERETIC method was used to provide a constant reference for quantifying the peak sizes in the spectrum (23). Total correlation spectroscopy (TOCSY) was performed to confirm the relative areas of the GPC and PC resonance peaks. The parameters for the TOCSY experiment were: TR = 1.24s, mixing time = 40 ms, NP=4096 and spectral width of 20,000 Hz in F2; Ni=64 and spectral width of 6000 Hz in F1, experiment time = 1 hour.
The absolute concentrations of the following metabolites were quantified from the 1D spectra using the HR-QUEST semi-parametric time-domain fitting algorithm (24). Briefly, the first few timepoints of the free induction decay (FID) were removed prior to analysis to reduce the contribution of macromolecules and lipids to the metabolite spectrum. The remaining macromolecules were estimated using Hankel Lanczos singular value decomposition and the metabolites were parametrically quantified using a basis set created from solutions with known concentrations of metabolites. A considerable advantage of the HR-QUEST algorithm was the ability to adjust the frequency and phase of individual peaks within the basis spectrum to account for differences in the molecular environments of metabolites in solution and tissue. Basis set spectra of 36 resonance peaks representing 15 metabolites were collected in solution and incorporated into the fitting routine. Of the 15 metabolites represented in the basis set, only 10 of them were included in this paper because they were consistently quantified with a Cramer Rao error of 25% or less. The metabolites and their resonance frequencies were (metabolite; ppm) : alanine (Ala; d-1.48, m-3.78), creatine+phosphocreatine (Cre; s-3.02), glycerophosphocholine (GPC; s-3.23), phosphocholine (PC; s-3.22), free choline (fCho; s-3.20), glutamine (Gln; m-2.45, m-3.75), glutamate (Glu; m-2.35, m-3.75), N-Acetylaspartate (NAA; 2.0, 2.49, 2.68, 4.38), taurine (Tau, t-3.26, t-3.43), and myo-inositol (mI; t-4.06, dd-3.55, t-3.62, t-3.28). The chemical shifts and multiplicities were initially assigned according to the values published by Govindaraju et al (25) for rat brain in D20; however, the frequencies reported here show the actual frequencies that we consistently observed in our human tissues under our experimental conditions. Total choline (tCho) was calculated by the summation of PC + GPC + fCho. Of the 10 metabolites quantified, only Ala, Glu, Tau, and NAA had average Cramer Rao bounds that exceeded 10%.
Immunohistochemistry
The proliferative activity, cell density, and VEGF expression in the paraffin-embedded biopsies was assessed with immunohistochemistry (IHC). The tissue blocks were cut into 5 micron sections, deparaffinized in xylenes, and rehydrated in graded dilutions of ethanol. After microwave antigen retrieval, endogenous peroxidases were blocked with 3% hydrogen peroxide. Sections were then incubated with either anti-Ki-67 (Ventana Medical Systems, Tucson, AZ) or anti-VEGF (Thermo Scientific, Fremont, CA), and antibody binding was subsequently detected with 3,3′-diaminobenzidine (DAB Detection Kit, Ventana, Tucson, AZ). The sections were then counterstained with hematoxylin and visualized under a brightfield microscope. The proliferation index was calculated on a single slide from each biopsy by counting at least 1000 cells and dividing the number of Ki-67-stained cells by the total number of cells counted. Central regions of the tissue sample with the highest proliferative activity were consistently used for the Ki-67 assessment and care was taken to avoid the tissue edges. Expression of VEGF was evaluated using a scoring system that consisted of three levels. The first and lowest level of expression included samples that showed no staining or very light staining in the extracellular matrix. The second level showed moderate to dark extracellular staining. The third level showed both intracellular and extracellular staining, often with evidence of perivascular staining. Because the clearest distinction in staining pattern was between score 3 and the other specimens, the scores were re-grouped as VEGF-negative (scores 1 and 2) and VEGF-positive (score 3). The cell density for all sections was calculated as the total number of cells in the microscopic fields of view used for the Ki-67 measurement divided by the number of FOVs and given in units of cells per mm2.
Data Analysis
Comparisons of ex vivo HRMAS metabolites and continuous IHC data across all biopsies were performed using a linear mixed model with “Patient” as the random effect to control for the multiple observations (biopsies) that were obtained from each subject (patient). Within-patient correlations between the choline measures, Ki-67, and cell density were performed by first ranking the values for each parameter by “Patient” and performing a univariate analysis with each ranked choline measure as the independent variable and the ranked histologic measure as the dependent variable. Comparisons of binary VEGF levels in AS2 and AS3 were performed using cross tabulation and a Gamma test was used to determine the strength of the association. Spearman rank correlation and the same mixed model described above were used to investigate associations between the metabolic, proliferative and cell density data. For statistical tests, a significance level of p ≤ 0.05 was used.
Results
Histologic and metabolic differences between non-enhancing grades 2 and 3 astrocytomas
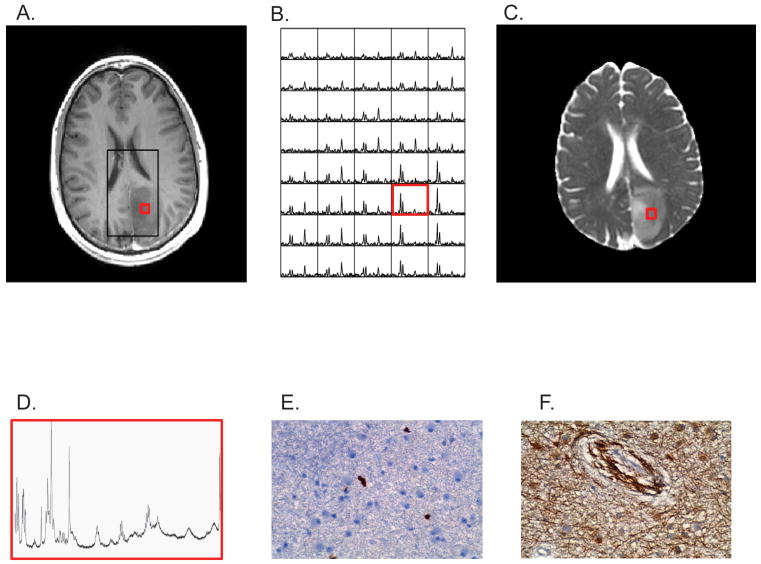
Figure 1 shows (A) a contrast-enhanced T1-weighted MR image, (B) the in vivo 3D MR spectra, and (C) the ADC image map of a patient with an AS3 that were obtained prior to surgery and used to choose the target location for biopsy (red box). The ex vivo MR spectra obtained from a flash-frozen biopsy from the target region and the Ki-67 and VEGF stained sections from the paraffin-embedded specimen obtained from the same target location are shown in Figures 1D-1F.
Figure 1.
Non-enhancing Grade 3 astrocytoma (A) contrast-enhanced T1-weighted MRI showing PRESS box (black) where MRS data was acquired and biopsy location (red). (B) 3D-MR spectra and (C) apparent diffusion coefficient image map were that were used to select the biopsy location prior to surgery. Post-surgical (F) HRMAS MR spectrum, immunohistochemical (D) Ki-67 and (E) VEGF labeling of biopsied tissue sample.
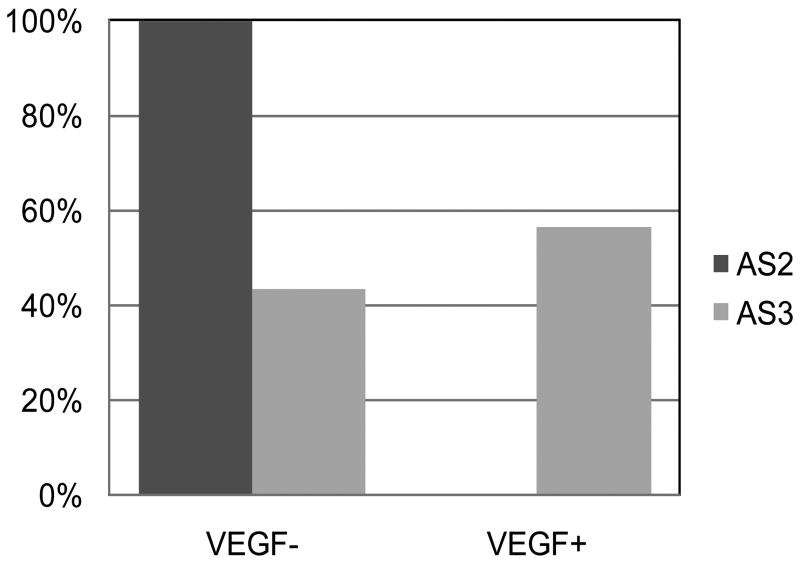
The AS3 biopsies had higher Ki-67 labeling and a trend toward higher cell density than the AS2 biopsies (Table 1). Figure 2 shows the distribution of VEGF-positive samples among the AS2 and AS3 biopsies. All of the VEGF-positive biopsies were from AS3 tumors. In all, 56.5% of the AS3 tumors were clearly positive for VEGF and 43.5% were VEGF-negative. The association between VEGF-positivity and histologic grade was so strong that the variables could not be considered as independent (crosstabs gamma = 1.0).
Table 1. Comparison of histologic features of non-enhancing Grades 2 and 3 astrocytoma.
| AS2 (mean +/- SD) |
AS3 (mean +/- SD) |
p | |
|---|---|---|---|
| Proliferation Index | 1.3 +/- 1.5 | 3.0 +/- 1.5 | 0.03* |
| Cell Density (cells/mm2) | 1672 +/- 826 | 2236 +/- 993 | 0.08 |
statistically significant at p ≤ 0.05
Figure 2.
Distribution of VEGF immunopositive tissue samples among non-enhancing Grades 2 (AS2) and Grades 3 (AS3) glioma. None of the Grade 2 samples expressed VEGF while approximately half (56%) of the Grade 3 samples were VEGF positive.
The ex vivo metabolite concentrations in AS2 and AS3 are shown in Table 2 and example spectra are shown in Figure 3. Although the AS3 biopsies had higher concentrations of PC, tCho and Tau as compared with AS2, only the difference in PC was significant once we controlled for multiple biopsies from each patient. We repeated the comparison while controlling for Ki-67 and found that the difference in PC (p = 0.01) remained, suggesting that higher PC in AS3 was independent of their higher proliferation rate.
Table 2. HRMAS MRS metabolite concentrations (mmol/kg) in non-enhancing astrocytoma.
| AS2 (mean +/- SD) |
AS3 (mean +/- SD) |
p | |
|---|---|---|---|
| Alanine | 0.5 +/- 0.5 | 0.7 +/- 0.4 | 0.42 |
| Cre+PCre | 3.5 +/- 1.5 | 4.3 +/- 2.2 | 0.28 |
| fCho | 0.2 +/- 0.2 | 0.3 +/- 0.2 | 0.31 |
| Gln | 1.7 +/- 1.7 | 2.1 +/- 1.6 | 0.61 |
| Glu | 2.1 +/- 1.0 | 3.0 +/- 1.9 | 0.22 |
| GPC | 1.7 +/- 0.9 | 2.0 +/- 0.9 | 0.39 |
| mI | 7.3 +/- 4.0 | 9.5 +/- 5.0 | 0.25 |
| NAA | 0.2 +/- 0.3 | 0.2 +/- 0.2 | 0.80 |
| PC | 0.3 +/- 0.2 | 0.6 +/- 0.3 | <0.01* |
| PC:GPC | 0.3 +/- 0.4 | 0.3 +/- 0.2 | 0.96 |
| Tau | 1.3 +/- 0.7 | 1.8 +/- 1.1 | 0.14 |
| tCho | 2.3 +/- 0.9 | 2.9 +/- 1.1 | 0.13 |
statistically significant at p ≤ 0.05
Figure 3.
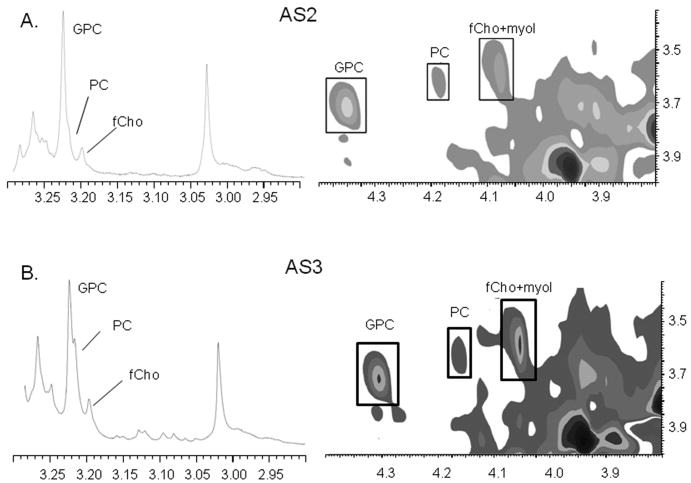
Example 1D and 2D TOCSY spectra from (A) AS2 and (B) AS3 showing GPC as the dominant choline compound.
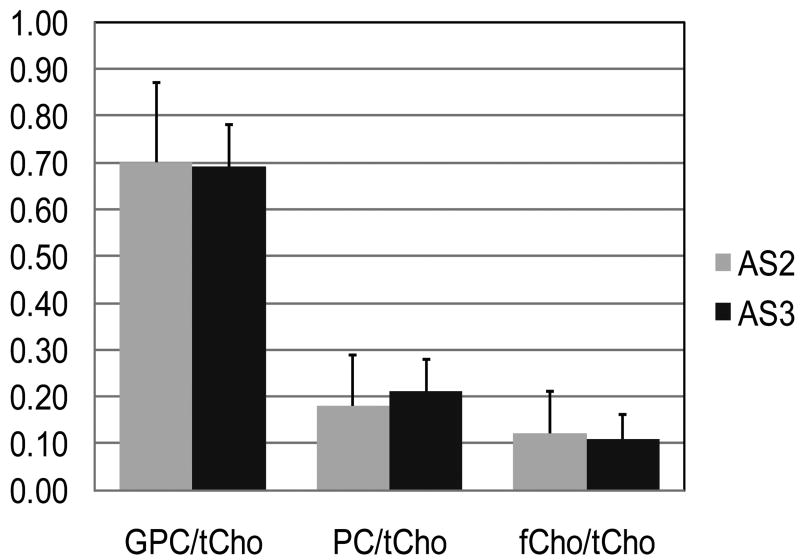
A striking and unexpected feature of the spectra from all of the tumors in this study was that GPC was the most prominent peak and had the highest concentration of all the choline compounds, irrespective of histologic grade. There was not a single case in which PC was higher than GPC in the one-dimensional HRMAS MRS analysis. Figure 3 shows 1D and 2D TOCSY spectra from (A) an AS2 and (B) an AS3 showing GPC as the predominant peak in both cases. Table 2 shows that the PC:GPC ratio did not differ between the two grades. To directly compare our results with other groups that have shown PC provides the greatest contribution to tCho in high grade gliomas, we compared the PC:tCho, GPC:tCho, and fCho:tCho ratios from the AS2 and AS3 groups and found that the GPC:tCho ratio was greater than 65% for both (Figure 4). There was no difference in any of the three ratios between the AS2 and AS3 biopsies.
Figure 4.
Ratio of choline compounds to total choline in non-enhancing Grades 2 and 3 astrocytoma. There was no significant difference in any of the ratios. The GPC/tCho ratio was the highest in all samples. GPC = glycerophosphocholine, PC = phosphocholine, fCho = free Choline
Associations between IHC and metabolic markers in non-enhancing astrocytoma
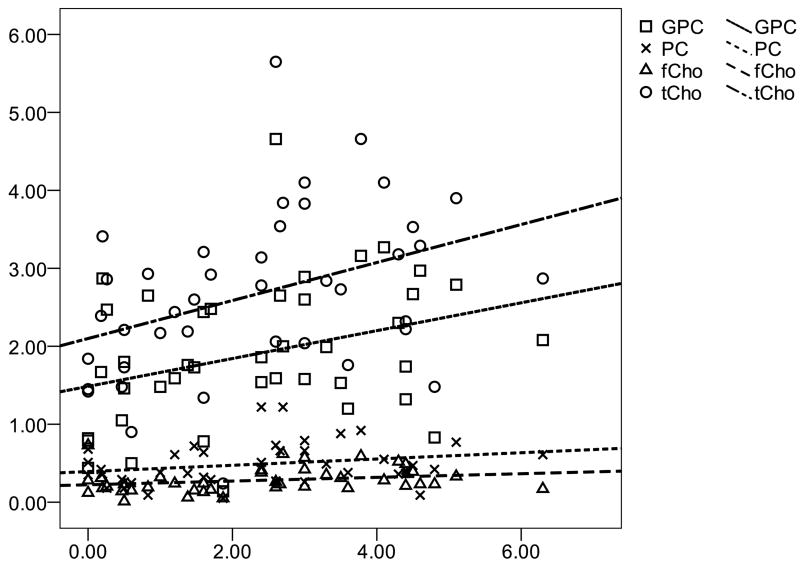
To determine whether the tumor metabolic properties were associated with tumor growth, we looked for correlations between the metabolic levels, Ki-67 index, and cell density across all biopsies. Figure 5 shows the results of a Spearman rank correlation indicating positive associations between Ki-67 index and GPC (p=0.02), fCho (p=0.03) and tCho (p=0.005). A similar trend was found between Ki-67 and PC but it did not reach statistical significance (p=0.07). We repeated the analysis using a mixed effects model to control for multiple observations from each patient and found that only the association between Ki-67 and tCho (p=0.03) remained significant. No other metabolite was associated with Ki-67 index or with cell density using either the Spearman rank or mixed effects method. We then looked for within-patient correlations between the choline measures, Ki-67, and cell density. Table 3 shows that significant within-patient associations were observed between Ki-67 and GPC, cell density and PC, and cell density and tCho.
Figure 5.
Associations between choline compounds and Ki-67 proliferation index in non-enhancing astrocytoma. Spearman rank correlation showed that tCho (r = 0.432, p = 0.005), fCho (r = 0.332, p = 0.034) and GPC (r = 0.397, p = 0.015) were correlated with proliferation while PC (r = 0.286, p = 0.070) had a similar trend but did not reach statistical significance.
Table 3. Within-patient correlations between choline and histologic measures.
| Ki-67 r (p) |
Cell Density r (p) |
|
|---|---|---|
| PC | -0.01 (0.97) | 0.63 (< 0.01*) |
| GPC | 0.31 (0.05*) | 0.30 (0.07) |
| fCho | 0.21 (0.20) | 0.30 (0.07) |
| tCho | 0.21 (0.20) | 0.56 (<0.01*) |
statistically significant at p ≤ 0.05
To determine whether the tumor metabolic properties were associated with VEGF expression, we compared the metabolic levels in the VEGF-positive and VEGF-negative specimens. The concentrations for all of the choline compounds as well as any metabolite that differed between the two groups are shown in Table 4. Tau and mI were higher in the VEGF-positive group; however, there was no difference in the any of the choline measures. We questioned whether the differences were primarily due to the difference in histologic grade rather than VEGF expression, particularly in light of the fact that the two variables were strongly associated. We therefore performed the comparison on the subset of AS3 tumors only and found that only mI was still significantly higher in the VEGF-positive specimens. There was no difference in the proliferation rate or cell density of the VEGF-positive and VEGF-negative specimens (data not shown).
Table 4. Association between VEGF and metabolite levels in non-enhancing astrocytoma.
| VEGF+ (mean +/- SD) |
VEGF- (mean +/- SD) |
p | p (AS3 only) | |
|---|---|---|---|---|
| PC | 0.6 +/- 0.3 | 0.5 +/- 0.3 | 0.18 | -- |
| GPC | 2.2 +/- 1.1 | 1.8 +/- 0.8 | 0.27 | -- |
| fCho | 0.3 +/- 0.1 | 0.3 +/- 0.2 | 0.75 | -- |
| tCho | 3.1 +/- 1.3 | 2.6 +/- 0.9 | 0.18 | -- |
| PC:GPC | 0.3 +/- 0.2 | 0.3 +/ 0.3 | 0.82 | -- |
| Tau | 2.1 +/- 1.3 | 1.4 +/- 0.8 | 0.05 | 0.17 |
| mI | 12.0 +/- 4.9 | 7.5 +/- 3.7 | 0.01* | 0.05* |
statistically significant at p ≤ 0.05
Discussion
We studied the metabolic, proliferative, and angiogenic features of non-enhancing Grades 2 and 3 astrocytic gliomas to (1) confirm that the relative PC and GPC levels were consistent with previous studies of choline metabolism in gliomas, and (2) to determine whether there was any association between the metabolic and histologic markers. We compared the levels of 10 different metabolites and paid particular attention to the contributions of PC and GPC to the tCho levels in each group to determine whether histologic grade was the primary determinant of the relative PC to GPC concentrations. We also looked for associations between the levels of choline compounds, Ki-67 labeling, and VEGF staining. This was important for understanding the time course of alterations in choline metabolism with respect to changes in cellular proliferation and initiation of angiogenesis within astrocytomas.
Of the 10 metabolic measures that were compared, only PC was significantly higher in AS3 than AS2. This finding is consistent with a previous report by Sabatier (9), who reported higher levels of PC in high grade glioma as compared with low grade glioma. There were also trends toward higher tCho and Tau in the AS3 although they did not reach statistical significance. In vivo MRS studies have shown that high grade – in particular Grade 3 – gliomas typically have higher resonance peaks at 3.2 ppm corresponding to choline compounds (8,26). The broad resonance peaks of in vivo MRS, however, often contain resonances from multiple metabolites, such as the GPC (3.22 ppm), PC (3.21 ppm), and fCho (3.20 ppm) peaks that we summed as tCho. It is possible that the Tau triplet at 3.24 ppm may also contribute to the in vivo choline-compound peak but this postulate needs to be confirmed. The fact that Tau had one of the highest Cramer Rao errors in our study, however, lessens the impact of the trend toward higher levels in the high grade tumors.
Despite the higher PC levels in AS3, GPC was the predominant choline compound in all 41 tissue biopsies that we studied with HRMAS. In fact, there was no difference in the PC:GPC ratio nor the ratios of each choline compound to tCho between the AS2 and AS3 samples. The GPC predominance is consistent with a recent study by Wright et al (27) which reported mean PC = 0.45 and GPC = 0.77 mM in a cohort of five Grade 3 gliomas. However, these findings are in contrast to reports by Righi and Sabatier (5,9) that stated the predominant choline compound in low grade glioma is GPC, while PC dominates in high grade glioma. One possible reason for the different findings may be the difference in the histopathological classification of the tumors in the “high grade” cohorts of the three studies. Righi et al (5) included eight GBM in their cohort of 14 high grade astroctyomas, which are distinguished from AS2 and AS3 by the presence of microvascular hyperplasia and pseudopalisading necrosis that contains regions of hypoxia and expresses high levels of the hypoxic marker hypoxia-inducible factor-1 alpha (HIF-1alpha) (12,15). Sabatier et al (9) included oligodendroglial tumors in their high grade cohort which also have been shown to express HIF-1alpha (16). Of the five astrocytoma (two Grade 2 and three Grade 3) studied by Sabatier et al, GPC was the predominant peak in three of them (9). Thus, the dominance of PC that has been observed in cohorts of high grade glioma that include GBM and/or oligodendroglioma may not be a feature of non-enhancing Grade 3 astrocytoma.
The three choline compounds that were measured – PC, GPC, and fCho – all play a role in membrane phospholipid metabolism that accompanies the cell cycle (28). Cancer-associated alterations in choline metabolism have been best characterized in breast cancer (29,30) where PC has been shown to increase in a stepwise fashion during malignant transformation (31) via increased phosphorylation by choline kinase (32,33). Concomitant decreases in GPC have also been shown to accompany malignant transformation of breast cancer cells (33). Moreover, GPC is the predominant choline compound in normal mammary epithelial cells, and a “GPC to PC switch” to a PC-dominant metabolic phenotype occurs when the cells were immortalized (31). Interestingly, despite the role that phospholipid metabolism plays in generating sufficient membrane for new cells created during mitosis, no established correlation has been observed between any of the choline compounds and cell proliferation in breast cancer. However, a recent study showed that hypoxia increases choline kinase expression in prostate cancer cells (10). Such a mechanism may also play a role in glioma and may explain why PC did not predominate in our AS3 tumors which do not harbor the high hypoxic fractions seen in GBM.
We demonstrated a positive correlation between GPC and Ki-67 proliferation index across all biopsies and among biopsies within the same tumor. TCho,fCho, and PC also increased with proliferation across all biopsies; however, the association with PC did not reach statistical significance. The lack of a clear association between PC and proliferation is further evidence that choline kinase expression and/or activity may not be directly associated with proliferation in gliomas. Indeed, we found that the higher PC in AS3 tumors was not due to the higher proliferation in those tumors. Cell density, on the hand, was positively associated with both PC and tCho, while fCho and GPC exhibited similar trends with cell density that did not reach statistical significance. Thus, when comparing different regions of the same tumor, the density of tumor cells within a sub-region of non-enhancing astrocytoma appears to influence the concentration of all of the choline compounds, while the proliferation in a given sub-region primarily impacts GPC concentrations.
Although none of the tumors in our study cohort exhibited frank contrast-enhancement on MRI, we questioned whether they were at different stages of angiogenesis and whether the expression of the angiogenic marker, vascular endothelial growth factor (VEGF), was associated with the choline metabolism within the tissue samples. Contrast-enhancement reflects the leakage of contrast material across the blood-brain barrier and into the interstitium, and is therefore thought to be less pronounced in tumors that do not have angiogenic neovasulature which is characteristically leaky. However, studies have shown a range of expression levels of VEGF within cohorts of non-enhancing (34) and low grade astrocytoma (35). Further, the VEGF levels were shown to be associated with astrocytoma histologic grade within the non-enhancing cohort (34) and future malignant transformation and survival within the low grade cohort (35). In the current study, none of the AS2 tumors expressed VEGF, while approximately half of the AS3 were VEGF-positive. When we grouped the tumors according to their VEGF status, there was no difference in the levels of any of the choline compounds. The only metabolite that differed between the VEGF-positive and VEGF-negative tumors was mI. VEGF-positive tumors exhibited higher mI even when only AS3 tumors were considered. These findings contradicted in vivo studies by Castillo et al (36) that reported a negative association between mI:Cre ratios and glioma grade in their study of previously-treated patients. In the current study, we found no difference in the mI or mI:Cre (data not shown) in the AS2 and AS3, suggesting that treatment effects may have influenced either the mI or Cre levels in the study by Castillo et al. Indeed, a study by Hattingen et al suggested that the mI levels in gliomas were associated with the presence of astrogliosis and reported no difference between the mI levels in low and high grade glioma (37). To our knowledge, this is the first report of a possible association between VEGF and mI and we can only speculate as to the biological basis of the association. MI is a precursor to inositol phosphates that are regulated by VEGF (38), however, more studies are needed to determine whether such a signaling pathway underlies the association between mI and VEGF observed in the astrocytomas in this study.
A limitation of this study was the limited amount of tissue for immunohistochemical analysis which prevented a direct measurement of hypoxia in the biopsy specimens using a marker such as HIF-1alpha. The exclusion of contrast-enhancing Grade 3 astrocytoma from this study also prevented us from explicitly determining whether our results are unique to non-enhancing tumors or extend to the overall population of Grade 3 astrocytomas.
In conclusion, our results showed that GPC was the predominant peak in non-enhancing astrocytic glioma, irrespective of histologic grade, which is in contrast to the predominant PC levels that have been observed in GBM and high grade breast cancer. GPC was associated with proliferation within tumors, PC and tCho were associated with cell density within tumors and no choline compound was associated with VEGF immunopositivity. Taken together, these data suggest that choline kinase activity, which is the primary source of the increased PC seen in aggressive breast cancer and hypoxic glial tumors, does not appear to be tightly linked to cell proliferation or angiogenesis in non-enhancing astrocytomas. These results add to the growing body of information regarding the role of phospholipid metabolism in the malignant progression of glial tumors.
Acknowledgments
UCSF NMR Laboratory, UCSF Surbeck Laboratory for Advanced Imaging, UCSF Neurosurgery Tissue Bank, Jan Wooten. The authors would also like to acknowledge Dr. Andrew Zektzer, in memoriam.
This work funded by NIH R01- CA116041
References
- 1.CBTRUS . 2009-2010 CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in Eighteen States in 2002-2006. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2009. [Google Scholar]
- 2.Kleihues P, Burger PC, Scheithauer BW, Zülch KJ. Histological typing of tumours of the central nervous system. Berlin ; New York: Springer-Verlag; 1993. p. 112. [Google Scholar]
- 3.Erb G, Elbayed K, Piotto M, et al. Toward improved grading of malignancy in oligodendrogliomas using metabolomics. Magn Reson Med. 2008;59(5):959–965. doi: 10.1002/mrm.21486. [DOI] [PubMed] [Google Scholar]
- 4.Opstad KS, Bell BA, Griffiths JR, Howe FA. Taurine: a potential marker of apoptosis in gliomas. Br J Cancer. 2009;100(5):789–794. doi: 10.1038/sj.bjc.6604933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Righi V, Roda JM, Paz J, et al. 1H HR-MAS and genomic analysis of human tumor biopsies discriminate between high and low grade astrocytomas. NMR Biomed. 2009;22(6):629–637. doi: 10.1002/nbm.1377. [DOI] [PubMed] [Google Scholar]
- 6.Negendank WG, Sauter R, Brown TR, et al. Proton magnetic resonance spectroscopy in patients with glial tumors: a multicenter study. Journal of Neurosurgery. 1996;84(3):449–458. doi: 10.3171/jns.1996.84.3.0449. [DOI] [PubMed] [Google Scholar]
- 7.Nelson SJ. Analysis of Volume MRI and MR Spectroscopic Imaging Data for the Evaluation of Patients with Brain Tumors. Magnetic Resonance in Medicine. 2001;46:228–239. doi: 10.1002/mrm.1183. [DOI] [PubMed] [Google Scholar]
- 8.Howe FA, Barton SJ, Cudlip SA, et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2003;49(2):223–232. doi: 10.1002/mrm.10367. [DOI] [PubMed] [Google Scholar]
- 9.Sabatier J, Gilard V, Malet-Martino M, et al. Characterization of choline compounds with in vitro 1H magnetic resonance spectroscopy for the discrimination of primary brain tumors. Invest Radiol. 1999;34(3):230–235. doi: 10.1097/00004424-199903000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Glunde K, Shah T, Winnard PT, Jr, et al. Hypoxia regulates choline kinase expression through hypoxia-inducible factor-1 alpha signaling in a human prostate cancer model. Cancer Res. 2008;68(1):172–180. doi: 10.1158/0008-5472.CAN-07-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glunde K, Serkova NJ. Therapeutic targets and biomarkers identified in cancer choline phospholipid metabolism. Pharmacogenomics. 2006;7(7):1109–1123. doi: 10.2217/14622416.7.7.1109. [DOI] [PubMed] [Google Scholar]
- 12.Dreyfuss JM, Johnson MD, Park PJ. Meta-analysis of glioblastoma multiforme versus anaplastic astrocytoma identifies robust gene markers. Mol Cancer. 2009;8:71. doi: 10.1186/1476-4598-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haapasalo JA, Nordfors KM, Hilvo M, et al. Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin Cancer Res. 2006;12(2):473–477. doi: 10.1158/1078-0432.CCR-05-0848. [DOI] [PubMed] [Google Scholar]
- 14.Murat A, Migliavacca E, Hussain SF, et al. Modulation of angiogenic and inflammatory response in glioblastoma by hypoxia. PLoS One. 2009;4(6):e5947. doi: 10.1371/journal.pone.0005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rong Y, Durden DL, Van Meir EG, Brat DJ. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65(6):529–539. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Birner P, Gatterbauer B, Oberhuber G, et al. Expression of hypoxia-inducible factor-1 alpha in oligodendrogliomas: its impact on prognosis and on neoangiogenesis. Cancer. 2001;92(1):165–171. doi: 10.1002/1097-0142(20010701)92:1<165::aid-cncr1305>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Keles GE, Lamborn KR, Berger MS. Coregistration accuracy and detection of brain shift using intraoperative sononavigation during resection of hemispheric tumors. Neurosurgery. 2003;53(3):556–562. doi: 10.1227/01.neu.0000080949.44837.4c. discussion 562-554. [DOI] [PubMed] [Google Scholar]
- 18.Star-Lack J, Spielman D, Adalsteinsson E, Kurhanewicz J, Terris DJ, Vigneron DB. In vivo lactate editing with simultaneous detection of choline, creatine, NAA, and lipid singlets at 1.5 T using PRESS excitation with applications to the study of brain and head and neck tumors. J Magn Reson. 1998;133(2):243–254. doi: 10.1006/jmre.1998.1458. [DOI] [PubMed] [Google Scholar]
- 19.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 20.McKnight TR, Lamborn KR, Love TD, et al. Correlation of magnetic resonance spectroscopic and growth characteristics within Grades II and III gliomas. J Neurosurg. 2007;106(4):660–666. doi: 10.3171/jns.2007.106.4.660. [DOI] [PubMed] [Google Scholar]
- 21.Gupta RK, Cloughesy TF, Sinha U, et al. Relationships between choline magnetic resonance spectroscopy, apparent diffusion coefficient and quantitative histopathology in human glioma. J Neurooncol. 2000;50(3):215–226. doi: 10.1023/a:1006431120031. [DOI] [PubMed] [Google Scholar]
- 22.Cheng LL, Chang IW, Louis DN, Gonzalez RG. Correlation of high-resolution magic angle spinning proton magnetic resonance spectroscopy with histopathology of intact human brain tumor specimens. Cancer Res. 1998;58(9):1825–1832. [PubMed] [Google Scholar]
- 23.Albers MJ, Butler TN, Rahwa I, et al. Evaluation of the ERETIC method as an improved quantitative reference for 1H HR-MAS spectroscopy of prostate tissue. Magn Reson Med. 2009;61(3):525–532. doi: 10.1002/mrm.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratiney H, Albers MJ, Rabeson H, Kurhanewicz J. Semi-parametric time-domain quantification of HR-MAS data from prostate tissue. NMR Biomed. doi: 10.1002/nbm.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Toyooka M, Kimura H, Uematsu H, Kawamura Y, Takeuchi H, Itoh H. Tissue characterization of glioma by proton magnetic resonance spectroscopy and perfusion-weighted magnetic resonance imaging: glioma grading and histological correlation. Clin Imaging. 2008;32(4):251–258. doi: 10.1016/j.clinimag.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Wright AJ, Fellows GA, Griffiths JR, Wilson M, Bell BA, Howe FA. Ex-vivo HRMAS of adult brain tumours: metabolite quantification and assignment of tumour biomarkers. Mol Cancer. 9:66. doi: 10.1186/1476-4598-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackowski S. Cell cycle regulation of membrane phospholipid metabolism. J Biol Chem. 1996;271(34):20219–20222. doi: 10.1074/jbc.271.34.20219. [DOI] [PubMed] [Google Scholar]
- 29.Katz-Brull R, Seger D, Rivenson-Segal D, Rushkin E, Degani H. Metabolic markers of breast cancer: enhanced choline metabolism and reduced choline-ether-phospholipid synthesis. Cancer Res. 2002;62(7):1966–1970. [PubMed] [Google Scholar]
- 30.Ting YL, Sherr D, Degani H. Variations in energy and phospholipid metabolism in normal and cancer human mammary epithelial cells. Anticancer Res. 1996;16(3B):1381–1388. [PubMed] [Google Scholar]
- 31.Aboagye EO, Mori N, Bhujwalla ZM. Effect of malignant transformation on lactate levels of human mammary epithelial cells. Adv Enzyme Regul. 2001;41:251–260. doi: 10.1016/s0065-2571(00)00019-4. [DOI] [PubMed] [Google Scholar]
- 32.Glunde K, Bhujwalla ZM. Choline kinase alpha in cancer prognosis and treatment. Lancet Oncol. 2007;8(10):855–857. doi: 10.1016/S1470-2045(07)70289-9. [DOI] [PubMed] [Google Scholar]
- 33.Glunde K, Jie C, Bhujwalla ZM. Molecular causes of the aberrant choline phospholipid metabolism in breast cancer. Cancer Res. 2004;64(12):4270–4276. doi: 10.1158/0008-5472.CAN-03-3829. [DOI] [PubMed] [Google Scholar]
- 34.Maia AC, Jr, Malheiros SM, da Rocha AJ, et al. MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. AJNR Am J Neuroradiol. 2005;26(4):777–783. [PMC free article] [PubMed] [Google Scholar]
- 35.Abdulrauf SI, Edvardsen K, Ho KL, Yang XY, Rock JP, Rosenblum ML. Vascular endothelial growth factor expression and vascular density as prognostic markers of survival in patients with low-grade astrocytoma. J Neurosurg. 1998;88(3):513–520. doi: 10.3171/jns.1998.88.3.0513. [DOI] [PubMed] [Google Scholar]
- 36.Castillo M, Smith JK, Kwock L. Correlation of myo-inositol levels and grading of cerebral astrocytomas. AJNR Am J Neuroradiol. 2000;21(9):1645–1649. [PMC free article] [PubMed] [Google Scholar]
- 37.Hattingen E, Raab P, Franz K, Zanella FE, Lanfermann H, Pilatus U. Myo-inositol: a marker of reactive astrogliosis in glial tumors? NMR Biomed. 2008;21(3):233–241. doi: 10.1002/nbm.1186. [DOI] [PubMed] [Google Scholar]
- 38.Downes CP, Macphee CH. myo-inositol metabolites as cellular signals. Eur J Biochem. 1990;193(1):1–18. doi: 10.1111/j.1432-1033.1990.tb19297.x. [DOI] [PubMed] [Google Scholar]