Abstract
Purpose
Our previous studies have shown that benzyl isothiocyante (BITC) suppresses pancreatic cancer growth by inducing apoptosis but the molecular mechanism was unclear. In the present study we hypothesized the involvement of PI3K/AKT/FOXO pathway in BITC induced apoptosis.
Experimental design
Mice were implanted BxPC-3 tumor xenografts and orally gavaged with 12μmol BITC. Plasma and tumor BITC concentration was estimated by LC/MS/MS. BxPC-3 and PanC-1 cells were used to elucidate PI3K/AKT/FOXO pathway. EMSA, DNA binding activity, immunofluorescence and gene transfection were used to delineate the mechanism.
Results
BITC-treated mice showed 43% less tumor growth as compared to control mice and correlated well with the therapeutic concentrations of 6.5μM BITC achieved in plasma and 7.5μmol/g BITC in tumor tissue. Western blot analyses and immunohistochemistry revealed that tumors from BITC-treated mice demonstrated reduced phosphorylation of PI3K, AKT, PDK1, mTOR, FOXO1, FOXO3a and increased apoptosis. Complementing our in vivo results, we made similar observations in a dose and time-dependent manner in BITC-treated BxPC-3 and Panc-1 cells. Binding of FOXO1 with 14-3-3 proteins was also reduced drastically by BITC treatment indicating nuclear retention of FOXO1 and this observation was further confirmed with EMSA, immunofluorescence, DNA binding and up regulation of FOXO-responsive proteins Bim, p27 and p21 in BxPC-3 cells. Overexpression of AKT by transient transfection significantly blocked the modulation of FOXO proteins and protected the cells from BITC mediated apoptosis and growth suppression.
Conclusions
Our results provide convincing evidence on the involvement of PI3K/AKT/FOXO pathway in BITC mediated pancreatic tumor growth suppression.
Keywords: Benzyl isothiocyanate, AKT, FOXO, Pancreatic cancer, chemoprevention
Introduction
The American Cancer Society reported that 42,470 Americans were diagnosed with pancreatic cancer in the year 2009 out of which 35,420 died, making pancreatic cancer the fourth leading cause of cancer-related deaths (1). The high mortality rate is due to resistance to chemotherapy, radiotherapy, and the poor prognosis of the disease and 40–50% patients rapidly progress to metastatic disease (2).
Phosphatidylinositol-3-kinase (PI3K)/AKT is a potent survival pathway that may mediate resistance to the apoptotic effects of chemotherapy drugs and radiation therapy in a variety of cancer types including pancreatic cancer (3). Although, majority of pancreatic cancer cell lines examined to date harbor constitutively activated AKT, the mechanism behind AKT activation in pancreatic cancer remains elusive (4). A recent study has shown that 59% of pancreatic adenocarcinomas demonstrated hyperactivation of AKT (5). The mammalian FOXO family of transcription factors, consisting of FOXO1, FOXO3a and FOXO4, function downstream of PI3K signaling pathway, are important regulators of cell death as well as promote cell survival and resistance (6). In fact, a number of anticancer drugs, such as doxorubicin and paclitaxel have been shown to induce apoptosis through oxidative stress, which enhances FOXO3a activity by stimulating its nuclear translocation (7) and thus causing overexpression of FOXO responsive genes such as Bim, p27 and p21 (8). Regulation of FOXO factors by AKT pathway is receiving increasing attention in cancer research.
Currently, there is no effective treatment for pancreatic cancer since conventional chemotherapy and radiation treatment have shown very limited success in improving patient survival. Therefore, novel treatment strategies are urgently needed. Case controlled epidemiological studies continue to support the notion that consumption of cruciferous vegetables reduces the risk of pancreatic cancer (9–11). Benzyl isothiocyanate (BITC) is present in cruciferous vegetables such as garden cress (12–14). In our previous studies, we demonstrated that BITC suppress the proliferation of human pancreatic cancer cells by causing DNA damage resulting in G2/M cell cycle arrest and apoptosis (15). Our results also revealed that BITC was not toxic to normal pancreatic epithelial cells (16).
In the present study, our results demonstrate that orally feeding BITC significantly suppress the growth of BxPC-3 pancreatic tumor xengraft. The tumor growth inhibition by BITC was associated with inhibition of activation and expression levels of PI3K/AKT/FOXO proteins as analyzed by immunohistochemistry and western blotting. We further confirmed our in vivo observations in BxPC-3 and PanC-1 cells.
Materials and Methods
Cell culture
Human pancreatic cancer cell lines BxPC-3 and Panc-1 were procured from ATCC and normal human pancreatic duct epithelial cell line, HPDE-6, was a generous gift from Dr Ming-Sound Tsao (University of Toronto, Canada). All cell lines were maintained as described by us previously (16).
Tumor Therapy Model
Tumor therapy experiment was performed as described by us previously (16) with minor modifications. The use of athymic nude mice and their treatment was approved by the Institutional Animal Care and Use Committee (IACUC), Texas Tech University Health Sciences Center, and all the experiments were carried out in strict compliance with their regulations. Exponentially growing BxPC-3 (1×106) cells were injected subcutaneously into the left and right flanks of ten mice. When the tumors reached a size of about 70mm3, mice were randomly segregated into two groups and test group of mice received 12μmol BITC in PBS by oral gavage every day for 46 days, whereas control mice received vehicle alone. Tumor volume and animal weights were taken as described us previously (16).
Western Blot Analysis
BxPC-3, Panc-1 and HPDE-6 cells were treated with varying concentrations of BITC (0, 5, 10, and 20μM) for 24h. For time-dependent experiment, cells were treated with 10μM BITC for 0, 2, 4, 8, 16 and 24h. The nuclear portion from control and treated cells or tumors was isolated using a commercially available kit from Pierce (Pierce, Rockford, IL), according to manufacturer’s instruction. Forty microgram protein was subjected to SDS-PAGE and western blot was carried out as described us previously (17).
Immunohistochemistry
Tumors excised from control and BITC-treated animals were fixed in formalin and 4μm sections were used for immunohistochemistry for p-PI3K (Tyr458), p-AKT (Ser-473) and p-FOXO3a (Ser-253) as described by us previously (16).
Estimation of BITC in Plasma and Tumors
BITC was analyzed by LC/MS/MS as described previously (18). The LC/MS/MS system consisted of a 1200 LC/MS (Quad 42) mass spectrometer (Varian) equipped with a heated nebulizer interface, a Varian Prostar Model 210 pump, and an autosampler. HPLC separation was carried out on a C18 (particle size 5 μm; 150×2.0 mm) column (Varian) and the mobile phase consisted of acetonitrile/5mM formic acid (30:70, v/v). The flow rate was 0.2mL/min and the injection volume was 10μL. The mass spectrometer operated in a positive ionization mode and the mass spectrometer setting was optimized for benzylthiourea and phenylthiourea (IS) to give optimum ion yield. Multiple reaction monitoring (MRM) of MS/MS was used for specific detection of the derivatives of PEITC as an internal standard (IS) and BITC by measuring the characteristic ion transitions for PEITC m/z 181 (parent ion) to m/z 105 (product ion) and BITC 167.0 (parent ion) to m/z 91 (product ion), respectively. BITC standards (0.001–50μM) were prepared in acetonitrile and 50 μL of each concentration were added to the plasma sample such that the final concentration of BITC in plasma ranged from 0.00008-4.13μM. Samples were extracted with n-hexane twice and ammonia (2M in 2-Methanol) was added for derivatization. The mixture was incubated for 6h at room temperature and dried under N2 stream and reconstituted with 200μL of acetonitrile/H2O (60:40, v/v). The reconstituted sample was transferred into a 100-μL autosampler insert for analysis by LC/MS/MS.
AKT Kinase Assay
BxPC-3 cells were treated with various concentrations of BITC and cells were collected. Cell lysates were analyzed for AKT kinase activity using a kit (Assay Designs, MI) according to manufacturer’s instructions.
Immunoprecipitation
Immunoprecipitation was performed as described by us previously (19). Briefly, 1×106 BxPC-3 cells were plated in 100mm dish and treated with different concentrations of BITC for 24h. Whole cell lysates were prepared using RIPA buffer and immunoprecipitated with FOXO1 antibody. Immune-complexes were resolved on SDS-PAGE and immunoblotted for 14-3-3 binding motif. Same blot was stripped and reprobed for acetylated lysine.
FOXO1 DNA Binding Assay
BxPC-3 cells were treated with different concentrations of BITC for 24h and nuclear fraction was isolated. Equal protein (15μg) from control and BITC-treated cells was subjected to DNA binding assay using a kit (Universal EZ-TFA Kit, Millipore, MA) according to manufacturer’s instructions.
Immunofluorescence
BxPC-3 cells treated with 10μM BITC for 24h. Treated and untreated cells were immunostained with anti-FOXO1 antibody as described by Andrew et al (20).
Electrophoretic Mobility Shift Assay (EMSA)
Cells treated with 10μM BITC for 24h were collected and nuclear fraction was isolated. Nuclear FOXO1 protein was captured using biotin labeled FOXO1 binding site oligomer 5’-CAAAACAACAAAACAACAAAACAA-3’ and subjected to DNA binding activity using the commercially available kit from Panomics (Santa Clara, CA).
AKT Transient Transfection
BxPC-3 cells were transiently transfected with plasmid containing wild type AKT (a generous gift from Dr Daniel Altschuler, University of Pittsburgh) using lipofectamin LTX (Invitrogen). Briefly 3×105 cells were transfected with 0.5μg of the AKT plasmid diluted in Opti-MEM serum-free medium to which lipofectamine reagent was added before the mixture was added to cells. Cells were incubated with plasmid-lipofectamine mixture for 5h and then media was replaced with fresh growth medium and incubated for another 24h. Transfected cells were treated with 10μM BITC for 24h and analyzed for cytotoxicity or apoptosis. Whole cell lysates (40μg) was subjected to western blot analysis and actin was used as loading control.
Annexin-FITC Apoptosis Assay
Cells were treated with various concentrations of BITC for 24h and apoptosis was evaluated using Annexin V-FITC kit (Calbiochem, CA) by flow-cytometer (Accuri C6, MI) according to manufacturer’s instructions.
Statistical Analysis
All the statistical analysis was performed using Prism 5.0 (GraphPad Software Inc., San Diego, CA). Results were expressed as Means ± SD or S.E.M. of at least three independent experiments. Data was analyzed by Student’s t-test or one way ANOVA followed by Bonferroni’s post hoc analysis for multiple comparisons. Differences were considered statistically significant at p<0.05.
Results
BITC suppresses tumor growth in nude mice
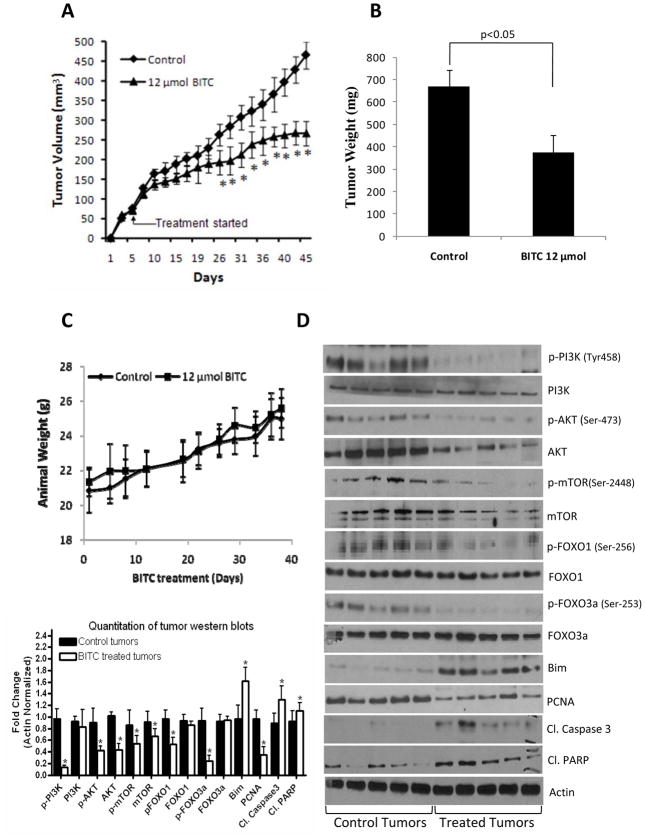
To test the possibility that BITC treatment would suppress pancreatic tumor growth, BxPC-3 tumors-bearing mice were fed 12μmol BITC every day and tumor growth was periodically recorded. Our results show that oral gavage of 12μmol BITC significantly reduced the growth of the tumors starting day 10 of treatment and continued till the end of the experiment (Fig. 1A). At day 46 of the treatment, tumor volume in the treated group was reduced by 43% as compared to control groups [465.8±30.8mm3 vs 266.7±35.4mm3; (n=20)] (Fig. 1A). Similarly, weight of the tumors dissected from treated mice was about 45% less than the weight of the tumors from control mice (Fig. 1B). The weight of the mice did not changed significantly, indicating no apparent systemic toxicity in BITC-treated mice (Fig. 1C).
Figure 1. BITC inhibits the growth of in vivo xenograft by inhibiting the PI3K/AKT/FOXO pathway.
Athymic nude mice were kept on antioxidant free diet for one week before starting the experiment. Exponentially growing one million BxPC-3 cells were injected into both right and left flanks of each animal in PBS:Matrigel suspension. When tumors reached 70mm3 mice were randomly divided into two groups with ten mice in each group. Treated group received 12μmol BITC by oral gavage everyday while control group received vehicle alone. Tumors were measured three times a week. Effect of BITC on tumor volume (A), tumor weight (B) and mice body weight (C) was evaluated. The mechanism of tumor growth inhibition by BITC was determined in the tumor lysates from control and BITC-treated mice (D). Tumors were homogenized, lysed and 40μg protein was resolved on SDS-PAGE and probed for p-PI3K (Tyr-458), PI3K, p-AKT (Ser-473), AKT, p-mTOR (Ser-2448), mTOR, p-FOXO1 (Ser-256), FOXO1, p-FOXO3a (Ser-253), FOXO3a, Bim, PCNA, cleaved Caspase-3 and cleaved PARP. The blots were stripped and reprobed for actin to ensure equal protein loading. Lower (left) panel shows the quantitation of tumor western bolts. Values in (A and B) are means ± SEM of 20 samples and values in (C and D) are means ± SD of 10 samples.* P<0.05 statistically significant when compared with control.
Tumor growth inhibition was associated with inhibition of PI3K/AKT/FOXO pathway
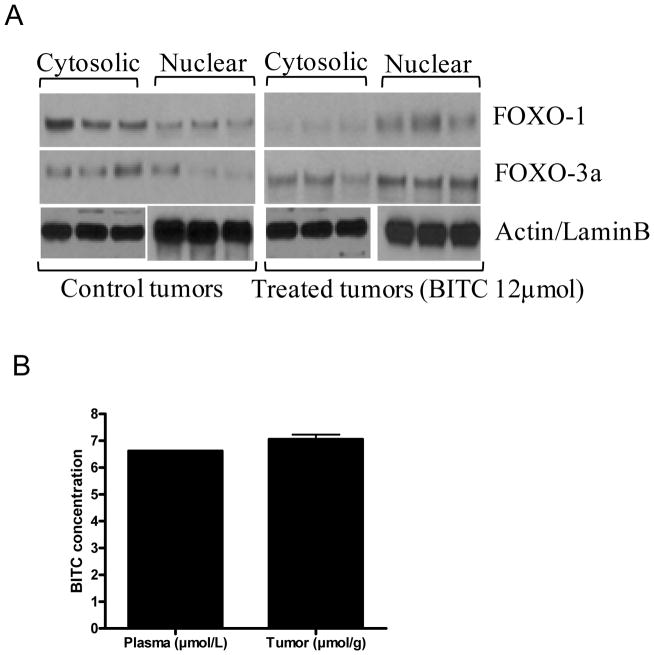
PI3K/AKT is constitutively activated in majority of pancreatic tumors (5). We hypothesized that the pancreatic tumor growth inhibition by BITC in our model was due to inhibition of PI3K/AKT pathway. To test our hypothesis, constitutive levels of PI3K and AKT were examined in the tumor lysates by western blotting. As shown in Fig. 1D, phosphorylation of PI3K at Tyr-458 and AKT at Ser-473 was drastically suppressed by BITC treatment. The expression level of AKT but not PI3K was also reduced in the tumors of BITC-treatment mice (Fig. 1D). Next we investigated the downstream molecules of AKT pathway such as mTOR, FOXO1 and FOXO3a, which are known to play critical role in tumorigenesis. Our results show that phosphorylated and protein levels of mTOR and phosphorylated levels, but not protein levels of FOXO1 and FOXO3a were decreased in the tumors of BITC-treated mice (Fig. 1D). On the other hand, the levels of FOXO regulated pro-apoptotic protein Bim was substantially increased in the tumors of BITC-treated mice as compared to control animals (Fig. 1D) indicating that PI3K/AKT/FOXO pathway plays a critical role in BITC mediated pancreatic tumor suppression. In addition, we observed cleaved products of caspase-3 and PARP in the tumors from BITC-treated mice indicating apoptosis (Fig. 1D). PCNA levels were decreased in the tumors from BITC-treated mice indicating reduced mitosis (Fig 1D). To further confirm the mechanism by which BITC feeding reduced tumor growth, tumors from control and BITC-treated mice were evaluated by immunohistochemistry (Suppl. Fig.1). As analyzed by TUNEL assay, substantially increased numbers of apoptotic bodies were observed in the tumor sections obtained from BITC-treated mice as compared with control mice, whereas reduced staining for PCNA was noticed in the similar sections suggesting that the suppression of tumor growth in BITC-treated mice was due to reduced mitosis and increased apoptosis. Furthermore, as compared to controls, BITC treatment substantially reduced the staining of p-PI3K (Tyr-458), p-AKT (Ser-473) and p-FOXO3a (Ser-253) in the tumor sections (Suppl. Fig. 1). To determine the nuclear localization, FOXO proteins were determined in the nuclear and cytosolic fractions of the tumors from control and BITC-treated mice. As shown in Fig 2A, in control tumors, FOXO1 expression was reduced in the nucleus as compared to cytosol, whereas in the tumors from BITC-treated mice, FOXO1 was retained in the nucleus. Similar observations were made for FOXO3a (Fig 2A).These observations further confirm that BITC suppress tumor growth by inducing apoptosis which is associated with the inhibition of PI3K/AKT/FOXO pathway.
Figure 2. BITC mediates nuclear retention of FOXO proteins in the tumors of BITC-treated mice.
Nuclear retention of FOXO proteins (A). Nuclear and cytosolic proteins from control and BITC 10μmol treated tumors were probed for FOXO proteins. Same membrane was stripped and reprobed for actin or lamin B to ensure equal protein loading. Concentration of BITC in the plasma and tumor tissues by LC/MS/MS (B). BITC concentration was evaluated in the plasma of the mice after 1h of oral gavage of 12μmol BITC, whereas cumulative concentration of BITC was determined in the tumors of mice after 46 days of BITC administration by LC/MS/MS. Bar represents the quantification of BITC in 10 samples (Mean ± SD).
BITC concentration in plasma and tumor
In order to see whether therapeutic concentration of BITC in the plasma and tumor was achieved by oral gavage, we evaluated BITC in the plasma and tumors of BITC-fed mice by LC/MS/MS. The mean BITC concentration in plasma after one hour of BITC (12μmol) oral gavage was 6.5±0.1μM (n=10), whereas accumulated BITC concentration in the tumors after 46 days was 7.5±0.3μmol/g (n=10) (Fig. 2B). These results indicate that the therapeutic concentration of BITC could be achieved in vivo by oral feeding and that the tumor growth suppression correlated with the concentration of BITC in plasma and tumor. It is important to mention that we only evaluated BITC and not the metabolites of BITC. The detailed study of the bioavailability and pharmacokinetics of BITC in mice is currently underway in our laboratory.
BITC down regulates PI3K/AKT pathway in BxPC-3 and PanC-1 but not in HPDE-6 cells
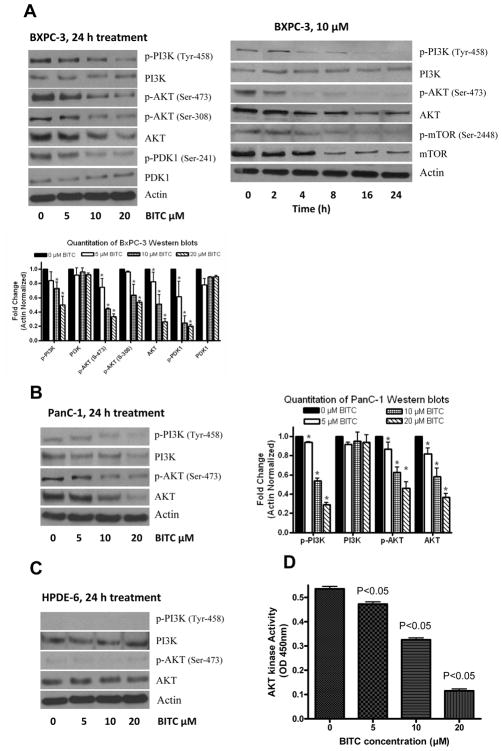
To model and elucidate the molecular observations made in vivo, we treated BxPC-3 and PanC-1 cells with varying concentrations of BITC for 24h. As shown in Fig. 3A, BITC treatment significantly suppressed the phosphorylation of PI3K (Tyr-458) in BxPC-3 cells in a concentration-dependant manner. In a time-dependant study, 10μM BITC suppressed the phosphorylation of PI3K as early as 4h after treatment and continued till 24h (Fig. 3A). The protein levels of PI3K remained unchanged even after 24h of treatment, indicating that BITC specifically target the activation of PI3K. Furthermore, BITC treatment significantly reduced the protein levels as well as phosphorylation of AKT at Ser-473 and Ser-308 in both BxPC-3 and PanC-1 cells (Fig. 3A–B). We then investigated the effect of BITC on PDK1, the upstream regulator of AKT. Our results indicate that BITC treatment significantly suppressed the phosphorylation of PDK1 at Ser-241 (Fig. 3B). Interestingly, phosphorylation of PI3K or AKT was not detected in normal human pancreatic ductal epithelial (HPDE-6) cells (Fig. 3C), which is consistent with the general understanding that PI3K or AKT are activated in transformed cells (21). Moreover, BITC treatment did not altered the protein levels of PI3K or AKT in HPDE-6 cells indicating the specificity of BITC towards cancer cells.
Figure 3. BITC treatment causes dose and time dependant inhibition of PI3K/AKT pathway in BxPC-3 and PanC-1 cells.
Effect of BITC on PI3K/AKT pathway in BxPC-3 (A), PanC-1 (B) and normal HPDE-6 (C) cells. Cells were treated with varying concentrations of BITC for 24h or 10μM BITC for various time intervals and immunoblotted for p-AKT (Ser-473), AKT, p-FOXO1 (Ser-256), FOXO1, p-FOXO3a (Ser-253), FOXO3a, Bim, cleaved caspase-3 and cleaved PARP. The same blot was stripped and reprobed for actin to ensure equal protein loading. Bar diagram shows the quantitation of respective western blots. BITC inhibits AKT kinase activity (D). AKT kinase activity was performed using nonradioactive kit according to the manufacturer’s instructions. Each bar represents Mean±SD of at least three independent experiments.
AKT kinase activity
Since we observed a decrease in AKT phosphorylation and expression by BITC treatment, we wanted to see if this corresponds to a decrease in the functionality of AKT, by measuring the kinase activity. As shown in Fig. 3D, a significant reduction in the kinase activity of AKT was observed in BxPC-3 cells. For example, treatment of cells with 10–20μM BITC for 24h resulted in the inhibition of about 45–75% of AKT kinase activity as compared to control cells.
BITC regulates downstream molecules of AKT
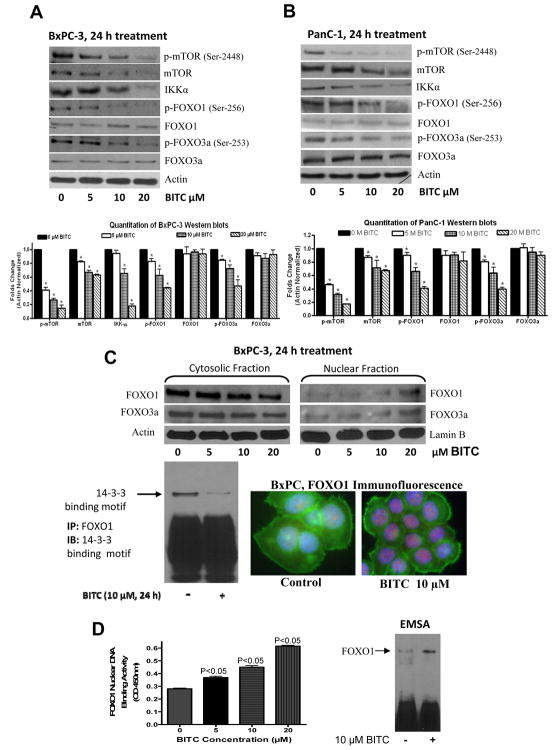
AKT regulates various cellar processes by directly acting on the downstream molecules such as mTOR, FOXO and IKK. First, we examined the effect of BITC on mTOR and IKKα. Our results reveal that BITC treatment substantially suppressed the protein levels and phosphorylation of mTOR (Ser-2448) in both BxPC-3 and PanC-1 cells (Fig. 4A–B). The protein level of IKKα was also reduced by BITC treatment in both the cell lines (Fig. 4A–B). Next, we evaluated the effect of BITC on FOXO transcriptional factors, which are downstream to AKT. Our results demonstrate that BITC treatment for 24h significantly reduced the phosphorylation of FOXO1 (Ser-256) and FOXO3a (Ser-253) in BxPC-3 and PanC-1 cells (Fig. 4A–B). The reduced phosphorylated levels of FOXOs were not due to decreased protein levels because protein levels of FOXO1 and FOXO3a did not changed by BITC treatment (Fig. 4A–B).
Figure 4. BITC down regulates cytosolic mTOR, IKK-α, FOXO and sequester FOXO 1 and FOXO3a in nucleus.
The effect of BITC on mTOR, IKK-α, FOXO1 and FOXO3a was determined in BxPC-3 (A) and PanC-1 (B) cells. Cells were treated with different concentrations of BITC for 24h, lysed and 40μg cell lysate was resolved by SDS-PAGE. Membranes were stripped and reprobed for βactin to ensure equal protein loading. The experiment was repeated three times with similar observations. Bar diagram shows the quatitation of respective western blots. Effect of BITC on nuclear retention of FOXO (C). To track the subcellular localization of FOXO proteins, nuclear and cytosolic proteins from control and BITC-treated BxPC-3 cells was probed for FOXO1 and FOXO3a. Same membrane was stripped and reprobed for actin or lamin B to ensure equal protein loading. In another experiment, whole cell lysate from control and BITC-treated BxPC-3 cells was incubated with FOXO1 antibody overnight, resolved on SDS-PAGE and immunoblotted for 14-3-3 binding motif. To further confirm nuclear retention of FOXO1, BxPC-3 cells were immunostained with FOXO1 (red) and actin (green) antibodies and visualized under microscope (Olympus Inc.). FOXO1 DNA binding activity was evaluated by EMSA and Universal DNA binding assay (D). Values are Mean±SD of three individual experiments.
BITC regulates nuclear shuttling of FOXO proteins
Once phosphorylated by AKT, FOXO1 binds to 14-3-3 chaperone proteins, which serve as escort proteins for FOXO1 to move out of the nucleus (22) leading to transcriptional down regulation of Bim, p21 and p27 (8). Hence, we determined the interaction of FOXO proteins with 14-3-3 chaperones. As expected, BITC treatment drastically decreased 14-3-3 binding sites on FOXO1 proteins indicating its retention in the nucleus (Fig. 4C). To confirm nuclear localization of FOXO proteins, we immunoblotted nuclear and cytosolic proteins and our results show that both FOXO1 and FOXO3a protein levels steadily increased in nuclear fraction and decreased in cytosolic fraction of BITC-treated BxPC-3 cells (Fig. 4C). Further, our immunofluorescence studies show intensified staining of FOXO1 in the nucleus of BITC-treated cells as compared to control cells, confirming the nuclear accumulation of FOXO1 proteins (Fig. 4C). Next we sought to determine the DNA binding activity of FOXO1 by EMSA to see if the retention of FOXO in the nucleus causes increased DNA binding resulting in the transcription of responsive genes Bim and p27. Indeed, EMSA results shows that BITC treatment significantly increased the DNA binding ability of FOXO1 protein (Fig. 4D). The DNA binding activity of FOXO1 was further confirmed by Universal-DNA binding Assay. Our results shows that BITC treatment increased the DNA binding activity of FOXO1 in the nuclear fraction of the cells in a concentration-dependant manner (Fig. 4D)
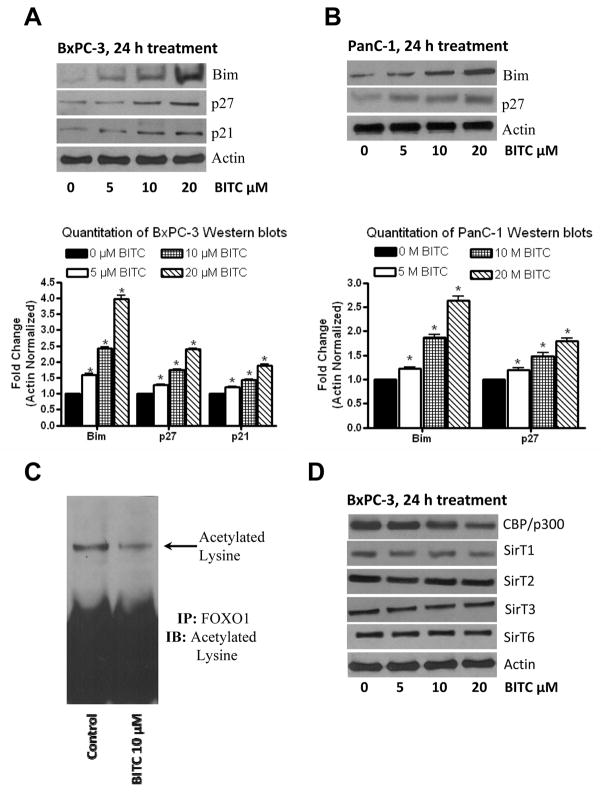
Nuclear translocation of FOXO proteins are expected to up-regulate the transcription of FOXO-responsive genes such as Bim, p21 and p27 (23). Our results clearly show that BITC treatment substantially enhanced the expression of Bim and p27 in BxPC-3 and PanC-1 cells (Fig. 5A–B), indicating that BITC induces nuclear localization of FOXO transcription factor.
Figure 5. BITC induces the expression of Bim and p27.
Effect of BITC on Bim and p27 was evaluated in BxPC-3 (A) or PanC-1 (B) cells. Cells were treated with BITC for 24h and 40μg protein was resolved by SDS-PAGE. The membranes were probed for Bim, p27 and p21. Same membrane was stripped and reprobed for β-actin to ensure equal protein loading. The experiment was repeated and similar results were obtained. Bar diagrams shows the quantitation of respective western blots. Deacetylation of lysine by BITC was determined in BxPC-3 cells (C). FOXO1 was immunoprecipitated from control and BITC-treated cells and immunoblotted against acetylated lysine. BITC-treated cells were examined for the expression of CBP/p300, SirT1, SirT2, SirT3 and SirT6 (D). The experiments were repeated three times and similar results were obtained.
BITC regulates FOXO transcriptional activity by acetylation
Another level of regulation of FOXO transcription is by acetylation or deacetylation of critical lysine residues on FOXO by CBP (CREB binding protein) and SirTs, respectively (24). Hence, we evaluated the acetylated levels of lysine. FOXO1 proteins from control and BITC-treated BxPC-3 cells were immunoprecipitated with FOXO1 antibody and immunoblotted for acetylated lysine. Our results show that acetylated lysine levels on FOXO1 were significantly decreased by BITC treatment (Fig. 5C). We also observed that CBP/p300 protein levels were decreased by BITC treatment (Fig. 5D). Interestingly, none of the SirT levels were affected by BITC treatment (Fig. 5D).
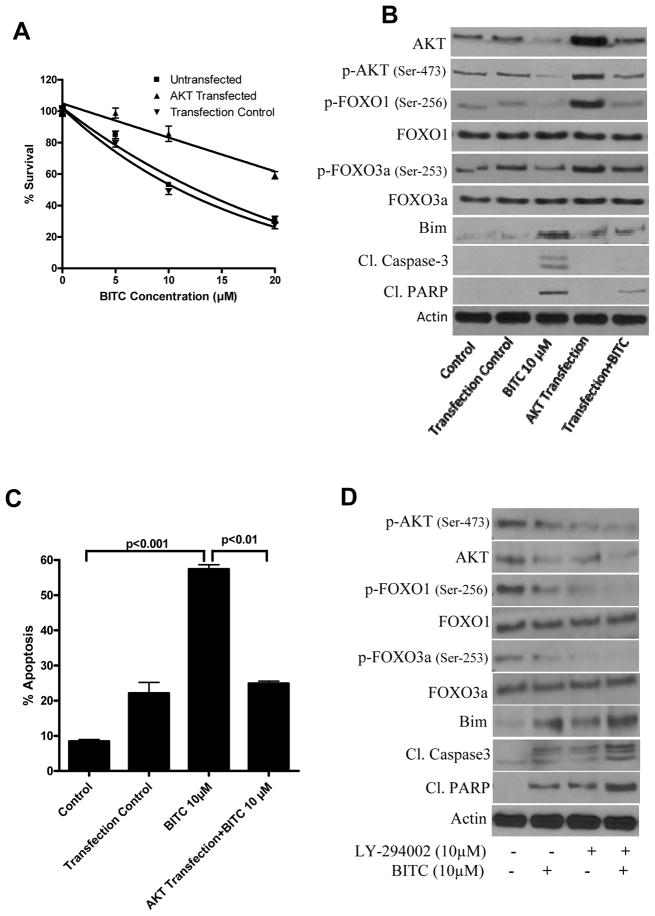
AKT overexpression abrogates BITC mediated nuclear localization of FOXO and apoptosis
To confirm the role of AKT signaling in BITC mediated tumor growth suppression, BxPC-3 cells were transfected with AKT plasmid and the effect of BITC on FOXO/Bim pathway was evaluated. We were able to achieve about 4.8 fold enhanced expression of AKT over constitutive level in non-transfected cells. Our results show that AKT overexpression significantly increased the viability of BITC-treated BxPC-3 cells as compared to BITC-treated non-transfected cells (Fig. 6A). The percent survival of wild type BxPC-3 cells by10μM BITC treatment was 53.2±2.6% whereas in AKT transfected BxPC-3 cells survival was 85.6±8.6% indicating a 32.4% survival advantage (Fig. 6A). We next examined the effect of BITC on FOXO and Bim levels in AKT transfected cells. Our results show that the decline in FOXO1 and FOXO3a phosphorylation by BITC treatment was blocked by AKT overexpression (Fig. 6B). We also observed that BITC-induced Bim protein expression was significantly reduced in cells overexpressing AKT (Fig. 6B). We then determined apoptosis in control and AKT transfected BxPC-3 cells after BITC treatment. Western blot analysis shows significantly reduced cleavage of caspase-3 and PARP in AKT-transfected BxPC-3 cells as compared to BITC-treated non-transfected cells (Fig. 6B). We confirmed apoptosis by AnnexinV-FITC by flow-cytometery. Our results show that 10μM BITC induced about 57.4% apoptosis whereas, in AKT transfected cells, apoptosis was reduced to 25% indicating 32.4% decrease in apoptosis (Fig. 6C). To further confirm AKT as a target of BITC, we pretreated BxPC-3 cells with LY-294002 (10μM, 1h) followed by BITC (10μM, 24h). Our results show that LY-294002, which is a known inhibitor of PI3K, together with BITC substantially decreased the phosphorylation of AKT and FOXO and increased apoptosis as compared to BITC alone treated BxPC-3 cells (Fig 6D). Taken together, these results establish the critical role of AKT inhibition in BITC-induced apoptosis through FOXO-Bim in our model.
Figure 6. AKT overexpression abrogates BITC-induced apoptosis in BxPC-3 cells.
Cells were transfected with 0.5μg of AKT plasmid for 24h using Lipofectamine-LTX and treated with different concentrations of BITC for another 24h and analyzed by cell survival assay (A). Results were confirmed by three independent experiments. Control and BITC-treated AKT transfected cells were analyzed for p-AKT (Ser-473), AKT, p-FOXO1 (Ser-256), FOXO1, p-FOXO3a (Ser-253), FOXO3a, Bim, cleaved caspase-3 and cleaved PARP (B). Same blots were stripped and reprobed for β-actin. Cells from above mentioned treatment were also analyzed for apoptosis using annexinV by Accuri flow-cytometer (C). Each experiment was performed in triplicate. Results are expressed as Means±SD. (D) Effect of BITC with LY-294002. BxPC-3 cells were treated with LY-294002 and then with BITC (10μM, 24h). Cell lysates were analyzed by western blot.
Discussion
We have shown previously that BITC suppresses the growth of pancreatic cancer by inducing apoptosis, however the exact mechanism of BITC mediated induction of apoptosis was not clear. Our current results demonstrate that orally feeding 12μmol BITC to athymic nude mice significantly suppressed the growth of pancreatic tumor xenograft. Interestingly, 6.4 μM BITC was observed in plasma after 24h of oral gavage of 12μmol BITC and a cumulative concentration of 7.5μmol BITC/g tumor tissue was observed indicating that the therapeutic concentration of BITC can be achieved in vivo and is directly linked to the tumor growth suppression in the present study. Furthermore, tumor growth suppression by BITC treatment was associated with increased apoptosis in the tumor cells which in turn was linked with the inhibition of PI3K, AKT and FOXO activation. Pro-apoptotic protein Bim, which is negatively regulated by AKT through FOXO proteins (25) was up-regulated in the tumors of the BITC-treated mice. Inhibition of activated levels of PI3K, AKT and FOXO were confirmed in BxPC-3 and PanC-1 pancreatic cancer cells by western blotting, EMSA, kinase activity and immunoflorescence. Overexpression of AKT by transient transfection substantially blocked the growth suppressive and apoptosis-inducing effects of BITC. To the best of our knowledge, our results for the first time demonstrate the involvement of PI3K/AKT/FOXO in BITC-mediated pancreatic tumor suppression.
Constitutive activation of AKT has been reported in various cancer types including breast, colon, ovarian, prostate and pancreatic cancers (26). A study conducted by West et al. has shown that over 55% of the cancers have hyper-activation of AKT, making it as an attractive molecular target (27). Furthermore, most of the cancers acquire drug resistance due to the activation of PI3K/AKT pathway (28). For instance, paclitaxel (29), doxorubicin (30) in breast cancer and gemcitabine in pancreatic cancer (31) acquires drug resistance due to hyper activation of PI3K/AKT survival pathway. Our results show that BITC treatment substantially suppressed the phosphorylation of AKT at both Ser-473 and Ser-308 in a dose and time dependant manner in BxPC-3 and PanC-1 cells. In agreement with these results, tumors from BITC-treated mice also demonstrated drastic suppression of AKT phosphorylation at Ser-473. Under normal physiological condition, AKT is under the control of PI3K catalyzed products IPP2 and PIP3 and these products bind to the PH domain of AKT causing translocation of AKT to plasma membrane (32). The full activation of AKT requires phosphorylation of AKT at Ser-308 and Ser-473 (33). Phosphorylation of AKT at Ser-308 is catalyzed by PDK1 (34) which is again regulated PI3K, but the second kinase PDK2 that is responsible for phosphorylation at Ser-473is not yet fully understood (35). BITC treatment drastically reduced the phosphorylated but not protein levels of PI3K in both BxPC-3 and PanC-1, and BITC-treated tumors. Phosphorylated PI3K levels on the other hand were undetectable in normal pancreatic HPDE-6 cells, which is in agreement with previous investigations showing that PI3K is constitutively activated mostly in cancer cells (36). Our results indicate that BITC suppress the growth of pancreatic cancer cells by targeting constitutively activated-PI3K without affecting the protein levels. However, a recent study has shown that BITC reduces protein levels of PI3K in HT-29 cells, indicating that BITC acts differentially in different cancer cells (37).
Previous investigations have indicated that pancreatic cancer growth can be modulated by PTEN regulating agents such as TGF-beta (38) and PPAR-gamma PTEN (39). Our results show that BITC did not alter the protein or phosphorylated levels of PTEN in either BxPC-3 or PanC-1 cells (data not shown). However, activated levels but not protein levels of PDK1 were down regulated by BITC treatment. In conclusion, BITC modulates AKT in pancreatic cancer cells by inhibiting the upstream molecules PI3K and PDK1.
AKT indirectly regulates apoptosis by inhibiting the transcription of Bim through phosphorylation of FOXO transcription factors and directly inhibits the apoptosis by regulating CREB or IKK (40). In addition, AKT also regulates protein synthesis through mTOR pathway during oxidative stress (41). Our previous results have demonstrated that BITC causes oxidative stress in pancreatic cancer cells by ROS generation (17). Our current results reveal that both activated and protein levels of mTOR were decreased by BITC treatment. These results are in agreement with other investigators who reported that phenethyl isothiocyanates, a close analogue of BITC suppresses mTOR pathway in prostate cancer (42). Our results also reveal that BITC treatment decrease the expression of IKKα in BxPC-3 and PanC-1cells. These results provide the evidence that BITC induces apoptosis by inhibiting AKT regulated proteins.
The major mechanism by which AKT regulates FOXO transcription factors is by subcellular localization and phosphorylation of FOXO (43). Upon phosphorylation by AKT, FOXO proteins specifically binds to 14-3-3 chaperones leading to conformational change that causes masking of nuclear localization signal (NLS) and exposing the nuclear export signal (NES). This results in moving FOXO from the nucleus into cytosol and prevents the transactivation of responsive genes such as Bim and p27 (44). We hypothesized that inhibition of AKT by BITC treatment would lead to nuclear sequestration of FOXO proteins and increased transcription of responsive genes such as Bim and p27. In agreement with our hypothesis, our results showed that phosphorylated levels of FOXO1 and FOXO3a were decreased with BITC treatment resulting in the nuclear retention of these proteins. These observations were further supported by our results showing that binding of FOXO1 with 14-3-3 binding sites were decreased by BITC treatment. Furthermore, FOXO responsive proteins p27 and Bim were up-regulated providing evidence that BITC treatment causes localization of FOXO proteins in the nucleus thus leading the cells to apoptosis.
Another plausible mechanism by which FOXO proteins are regulated is through acetylation and deacetylation by CBP (CREB binding protein) and SirT protein respectively at conserved lysine residues in the DNA binding site of FOXO proteins, resulting in the reduced DNA binding ability of FOXO proteins (45). CBP proteins such as p300 levels were reduced with BITC treatment suggesting reduced acetylation of FOXO proteins. Inhibition of histone deacetylases such as HDAC1/3 by BITC was recently observed by us in pancreatic cancer cells (19). These observations were further confirmed with reduced acetylation at lysine in BITC-treated cells in the present study. Increased DNA binding capacity of FOXO1 was observed in response to BITC treatment as compared to control cells in the nucleus resulting in the transcription of FOXO1 responsive proteins p27 and Bim. Overexpression of AKT severely abrogated the apoptosis-inducing and growth suppressive effects of BITC.
The pharmacokinetics of BITC in humans is not yet reported. However, consumption of 100g watercress by human volunteers resulted in about 928 ± 250 nM PEITC in the plasma (19). Phenethyl isothiocyanate (PEITC) is a close analog of BITC. In another study, consumption of a single dose of hydrolyzed extract of 3-day old broccoli sprouts (containing about 200μmol total isothiocyanates) resulted in a peak concentration of 0.94–2.27μM isothiocyanates in the plasma, serum and erythrocytes within 1h of broccoli consumption in humans (46). In a recent study, oral administration of 10μmol/kg PEITC in rats resulted in about 9.2 ± 0.6 μM PEITC in the plasma after 0.44h of treatment (47). In agreement with these observations, our current studies reveal that oral administration of 12μmol BITC to athymic nude mice after 1h resulted in about 6.5 ± 0.1 μM BITC in the plasma and about 7.5 ± 0.3 μmol/g cumulative concentration of BITC in the tumors after 46 days of treatment. These results indicate that the therapeutic concentration of BITC can be achieved. Nonetheless, detailed pharmacokinetic studies on BITC are required and the focus of our future studies.
In conclusion, our in vitro and in vivo results demonstrate that BITC suppress the growth of the pancreatic tumor in strong association with an inhibition and targeting of PI3K/AKT, and probably in major part, their downstream FOXO pathway.
Supplementary Material
Acknowledgments
This investigation was supported in part by R01 grants CA106953 and CA129038 (to S.K.S.) awarded by the National Cancer Institute. The funds from Texas Tech University Health Sciences Center, School of Pharmacy (to S.K.S.) are also acknowledged. The authors would like to thank Dr. Ming-Sound Tsao, University of Toronta, Canada for providing the HPDE-6 cells and Dr. Daniel Altschuler, University of Pittsburgh for providing AKT plasmid.
Footnotes
Grant Support: Supported in part by R01 grants CA106953 and CA129038 (to S.K.S) awarded by the National Cancer Institute. The funds from Texas Tech University Health Sciences Center, School of Pharmacy (to S.K.S.) are also acknowledged.
References
- 1.Li J, Wientjes MG, Au JL. Pancreatic cancer: pathobiology, treatment options, and drug delivery. AAPS J. 2010;12:223–32. doi: 10.1208/s12248-010-9181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borja-Cacho D, Jensen EH, Saluja AK, Buchsbaum DJ, Vickers SM. Molecular targeted therapies for pancreatic cancer. Am J Surg. 2008;196:430–41. doi: 10.1016/j.amjsurg.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahy BN, Schlieman M, Virudachalam S, Bold RJ. AKT inhibition is associated with chemosensitisation in the pancreatic cancer cell line MIA-PaCa-2. Br J Cancer. 2003;89:391–7. doi: 10.1038/sj.bjc.6601037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3'-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002;1:989–97. [PubMed] [Google Scholar]
- 5.Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89:2110–5. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes AR, Brosens JJ, Lam EW. Resist or die: FOXO transcription factors determine the cellular response to chemotherapy. Cell Cycle. 2008;7:3133–6. doi: 10.4161/cc.7.20.6920. [DOI] [PubMed] [Google Scholar]
- 7.van Gorp AG, Pomeranz KM, Birkenkamp KU, Hui RC, Lam EW, Coffer PJ. Chronic protein kinase B (PKB/c-akt) activation leads to apoptosis induced by oxidative stress-mediated Foxo3a transcriptional up-regulation. Cancer Res. 2006;66:10760–9. doi: 10.1158/0008-5472.CAN-06-1111. [DOI] [PubMed] [Google Scholar]
- 8.Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–29. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 9.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 10.Bueno de Mesquita HB, Maisonneuve P, Runia S, Moerman CJ. Intake of foods and nutrients and cancer of the exocrine pancreas: a population-based case-control study in The Netherlands. Int J Cancer. 1991;48:540–9. doi: 10.1002/ijc.2910480411. [DOI] [PubMed] [Google Scholar]
- 11.Ji BT, Chow WH, Gridley G, McLaughlin JK, Dai Q, Wacholder S, et al. Dietary factors and the risk of pancreatic cancer: a case-control study in Shanghai China. Cancer Epidemiol Biomarkers Prev. 1995;4:885–93. [PubMed] [Google Scholar]
- 12.Hecht SS. Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism. J Nutr. 1999;129:768S–74S. doi: 10.1093/jn/129.3.768S. [DOI] [PubMed] [Google Scholar]
- 13.Stoner GD, Morse MA. Isothiocyanates and plant polyphenols as inhibitors of lung and esophageal cancer. Cancer Lett. 1997;114:113–9. doi: 10.1016/s0304-3835(97)04639-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R, Loganathan S, Humphreys I, Srivastava SK. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. J Nutr. 2006;136:2728–34. doi: 10.1093/jn/136.11.2728. [DOI] [PubMed] [Google Scholar]
- 16.Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J Natl Cancer Inst. 2009;101:176–93. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahu RP, Zhang R, Batra S, Shi Y, Srivastava SK. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis. 2009;30:1744–53. doi: 10.1093/carcin/bgp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji Y, Morris ME. Determination of phenethyl isothiocyanate in human plasma and urine by ammonia derivatization and liquid chromatography-tandem mass spectrometry. Anal Biochem. 2003;323:39–47. doi: 10.1016/j.ab.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Batra S, Sahu RP, Kandala PK, Srivastava SK. Benzyl Isothiocyanate-Mediated Inhibition of Histone Deacetylase Leads to NF-{kappa}B Turnoff in Human Pancreatic Carcinoma Cells. Mol Cancer Ther. 2010;9:1596–608. doi: 10.1158/1535-7163.MCT-09-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–20. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, et al. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene. 2001;20:6073–83. doi: 10.1038/sj.onc.1204736. [DOI] [PubMed] [Google Scholar]
- 22.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 23.Reagan-Shaw S, Ahmad N. The role of Forkhead-box Class O (FoxO) transcription factors in cancer: a target for the management of cancer. Toxicol Appl Pharmacol. 2007;224:360–8. doi: 10.1016/j.taap.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 24.van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem Sci. 2005;30:81–6. doi: 10.1016/j.tibs.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Kuiperij HB, van der Horst A, Raaijmakers J, Weijzen S, Medema RH, Bos JL, et al. Activation of FoxO transcription factors contributes to the antiproliferative effect of cAMP. Oncogene. 2005;24:2087–95. doi: 10.1038/sj.onc.1208450. [DOI] [PubMed] [Google Scholar]
- 26.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 27.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5:234–48. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 28.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–7. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 29.Gehrmann M, Schmidt M, Brase JC, Roos P, Hengstler JG. Prediction of paclitaxel resistance in breast cancer: is CYP1B1*3 a new factor of influence? Pharmacogenomics. 2008;9:969–74. doi: 10.2217/14622416.9.7.969. [DOI] [PubMed] [Google Scholar]
- 30.Smith L, Watson MB, O'Kane SL, Drew PJ, Lind MJ, Cawkwell L. The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol Cancer Ther. 2006;5:2115–20. doi: 10.1158/1535-7163.MCT-06-0190. [DOI] [PubMed] [Google Scholar]
- 31.Sayaka Mori-Iwamoto YK, Ryozawa Shomei, Taba Kumiko, Fujimoto Masanori, Okita Kiwamu, Nakamura Kazuyuki, Sakaida Isao. A proteomic profiling of gemcitabine resistance in pancreatic cancer cell lines. Molecular Medicine Reports. 2008;1:6. [PubMed] [Google Scholar]
- 32.Melinda M, Mortenson JMG, Schlieman Michael G, Bold Richard J. AKT: A novel target in pancreatic cancer therapy. Cancer Therapy. 2004;2:12. [Google Scholar]
- 33.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346(Pt 3):561–76. [PMC free article] [PubMed] [Google Scholar]
- 34.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–45. [PubMed] [Google Scholar]
- 35.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–76. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 36.Sahu RP, Batra S, Srivastava SK. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br J Cancer. 2009;100:1425–33. doi: 10.1038/sj.bjc.6605039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS, Wu SH, et al. Benzyl isothiocyanate (BITC) inhibits migration and invasion of human colon cancer HT29 cells by inhibiting matrix metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC and MAPK signaling pathway. J Agric Food Chem. 2010;58:2935–42. doi: 10.1021/jf9036694. [DOI] [PubMed] [Google Scholar]
- 38.Chow JY, Ban M, Wu HL, Nguyen F, Huang M, Chung H, et al. TGF-beta downregulates PTEN via activation of NF-kappaB in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol. 2010;298:G275–82. doi: 10.1152/ajpgi.00344.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teresi RE, Waite KA. PPARgamma, PTEN, and the Fight against Cancer. PPAR Res. 2008;2008:932632. doi: 10.1155/2008/932632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 41.Park S, Chapuis N, Tamburini J, Bardet V, Cornillet-Lefebvre P, Willems L, et al. Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia. Haematologica. 2010;95:819–28. doi: 10.3324/haematol.2009.013797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bommareddy A, Hahm ER, Xiao D, Powolny AA, Fisher AL, Jiang Y, et al. Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells. Cancer Res. 2009;69:3704–12. doi: 10.1158/0008-5472.CAN-08-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–88. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 44.Dijkers PF, Birkenkamp KU, Lam EW, Thomas NS, Lammers JW, Koenderman L, et al. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J Cell Biol. 2002;156:531–42. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–95. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.