Abstract
Microwave assisted acid cleavage was applied directly to intact adenovirus type 5 to achieve denaturation and proteolysis in a single reaction. The speed of the digestion, coupled with the simplicity of MALDI analysis, allowed peptide products to be profiled in less than 5 min. Identification of peptides from a range of proteins by MALDI-TOF confirms that both denaturation and proteolysis were achieved using low concentrations of acetic acid (12.5%) and short incubations (1.5 to 2 min) at high temperatures (140° C). These conditions favor production of peptides that carry Asp on their C-termini. When this cleavage reaction is carried out in highly enriched H2 18O, a single atom of 18O is introduced site-specifically into the carboxyl terminal. The labeling reaction is applied to label reporter peptides from human adenovirus type 5 harvested from HeLa cells. Small peptide products of endogenous processing were also observed.
Keywords: adenovirus, Asp selective cleavage, chemical proteolysis, isotope labeling, MALDI-TOF, endogenous processing
1.0 Introduction
1.1
Adenoviruses are commonly used as vectors in genetic engineering and gene therapy [1] and, as such, have generated continuing interest in new methods for their rapid characterization [for example, 2–4] The adenovirus particle is a large macromolecular assembly (Mr ~ 2×108 Da) composed of a double-stranded DNA genome and multiple copies of at least 13 distinct proteins [3, 5–11]. A viral protease is responsible for processing [12–14]. The small proteome of the adenovirus served earlier as a model system for the development in this laboratory of enzyme-catalyzed 18 O-labeling [15]. In the present report adenovirus type 5 is revisited to demonstrate the capability of Asp-selective rapid microwave-assisted acid cleavage [16–22] concurrently to denature a stable protein complex and to cleave its proteins in a single reaction.
1.2
Much work in proteomics comprises comparative or quantitative analysis, exploiting the capability of mass spectrometry to measure ratios of stable isotope-labeled peptides. Differential stable isotope labels can be introduced into proteins (for example, ICAT [23] and metabolic labeling [24] methods), or into their proteolytic peptide products (C-terminal-18O [25] and iTraQ [26] methods). Desirable attributes of labeling reactions include speed, complete labeling, site specific labeling and the absence of contaminating chemical by-products [27]. The feasibility is examined here of incorporating one atom of O-18 during Asp-selective microwave-supported acid cleavage in highly enriched H2 18O.
1.3
Figure 1 illustrates the dominant mechanism by which acid cleavage is thought to occur [28] in a protein, and the pathway by which it is expected that a single atom of 18O would be incorporated into proteolytic products. 18O-labeling was first optimized on myoglobin and ovalbumin, and then extended to label peptides from human adenovirus type 5.The effect of acid type and concentration on the extent of incorporation of the isotope label has been evaluated. The site of incorporation is confirmed using laser induced dissociation.
Figure 1.
Mechanistic scheme for acid catalyzed cleavage at aspartic acid in a protein (28). Bolded O predicts incorporation of labeled oxygen atoms.
2.0 Material and methods
2.1
Human adenovirus type 5 (ATCC # VR-5) was cultured in HeLa host cells (ATCC # CCL-2) following a published method [15]. HeLa cells were grown in 150 cm2 culture flasks to about 90% confluence, and exposed to virus stock for 1.5 hr with slow rocking. The virus stock was removed and 2% FBS cell maintenance media was added. As soon as cytopathic lysis was observed, at 2.0 to 2.5 days, the media was removed, host cells were detached and all cells were collected by centrifugation at 8000g for 20 min. The cells were washed twice with 0.1 M PBS pH 7.1, resuspended in 10 mM Tris-HCl buffer, pH 7.6, and the crude virus sample was subjected to analysis.
2.2
Acid cleavage was carried out in 12.5% aqueous acetic acid, unless otherwise specified, in 200 ul glass sample tubes in a Benchmate microwave system (CEM Corp., Matthews NC). The temperature was maintained at 140° +− 5° C for 1.5 to 5 min by fluctuation in the microwave power. Peptides were labeled by cleavage in H2 18O, 97.7 atom % 18O normalized (Isotech Inc., Miamisburg OH). Standard proteins were prepared at a concentration of 1 mg/ml in deionized water.
2.3
MALDI spectra were measured on an Axima CFR Plus time-of-flight mass spectrometer (Shimadzu Biotech, Columbia MD) using a 337 nm nitrogen laser. Spectra of peptide samples were acquired in both linear and reflectron modes as averages of 100–200 profile scans, using α-cyanohydroxycinnamic aid (10 mg/ml in 70%/30% (v/v) acetonitrile/deionized water containing 0.1% trifluoroacetic acid) as the matrix. In laser induced dissociation (LID) experiments spectra were acquired in the reflectron mode as averages of 250–500 profiles.
2.4
Nano-electrospray mass spectra were acquired using an LC Packings Ultimate HPLC system (San Francisco CA) interfaced to a PE Sciex API QStar Pulsar I Q-TOF mass spectrometer (Concord ON). Peptide products were desalted using C18 Ziptips (Millipore, Bedford MA) dried and redissolved in 40 ul 0.1% formic acid. Ten uL was loaded onto a 15 cm C18 reverse phase column (I.D. 75µm) (LC Packings) and eluted with a flow rate of 0.20 ul/min under a gradient of 3 to 97 % acetonitrile/0.1 % formic acid (solvent B) in 90 min. Solvent A was 0.1% formic acid.
2.5
Bioinformatic searches were carried out using peptide masses or sequence tags. All peptide [M+H]+ masses between 900 and 2500, obtained using MALDI-TOF mass spectrometry, were searched using the RMIDB website [29]. A tolerance of 1.0 Da was allowed and searches were performed against a custom database comprising peptides formed in silico by Asp-specific cleavage of all adenovirus protein sequences compiled from Swiss-Prot, Genbank, RefSeq and TrEMBL.
Product ion spectra from laser induced dissociation were searched by MASCOT with mass tolerances of ± 1.0 Da and ± 1.5 Da for parent and fragment ions, respectively. Spectra were searched against all NCBlnr entries, using the “formic acid” cleavage option and “other viruses” as the taxonomic search parameter. Nano-electrospray MS/MS product ion spectra were searched with mass tolerances of ± 0.3 and ± 1.0 Da for Parent and fragment ions, respectively. These spectra were searched against the Swiss-Prot database using MASCOT’s “other viruses” taxonomic classification and a “formic acid” option modified in house [19] to accommodate cleavage on both sides of Asp.
3.0 Results and Discussion
3.1
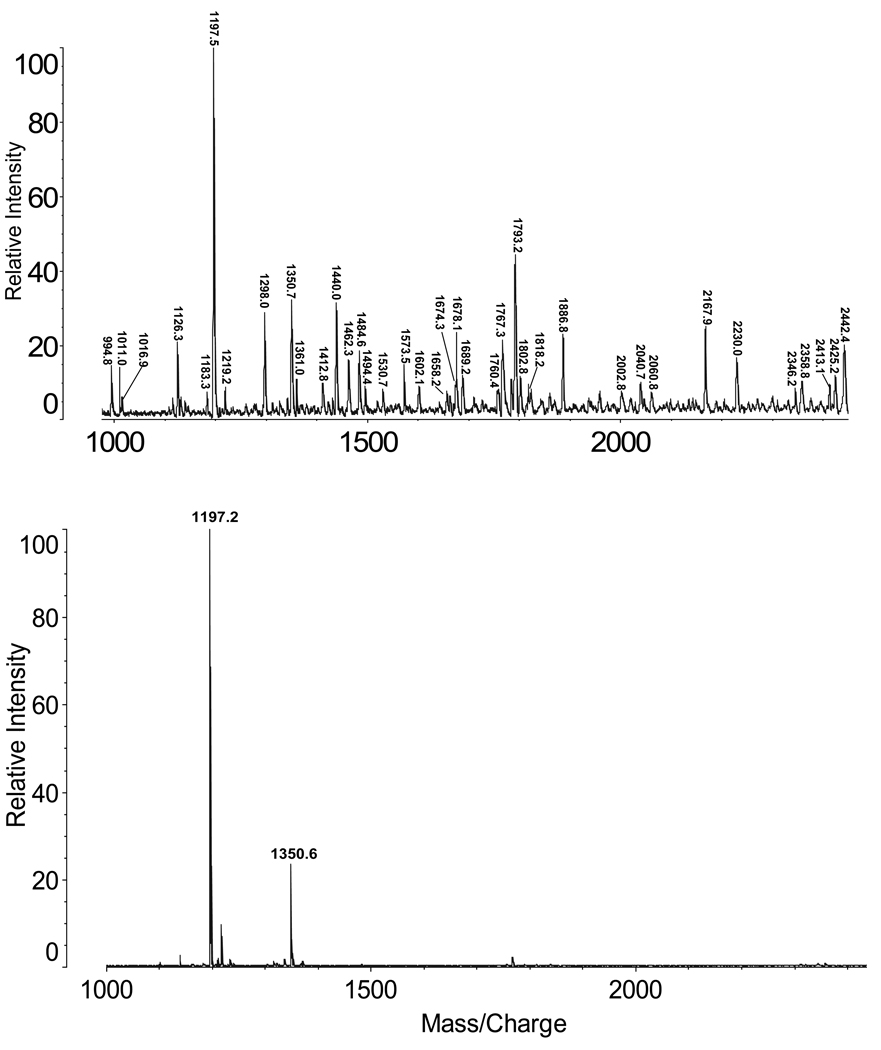
Figure 2 presents a MALDI-TOF spectrum of acid cleavage products from the adenovirus sample in the mass range 950 to 2450 (top), and a control spectrum obtained from the adenovirus sample not subjected to acid cleavage (bottom). The top spectrum confirms that many peptides are formed by the acid cleavage reaction. In the bottom spectrum small peptides are detected when no chemical cleavage is employed. Virtually all of the ions detected in the product mixture formed by acid cleavage could be assigned as virus peptides based on their masses (Table 1). Among these, the identities of selected peptides were confirmed by partial sequencing by LID and MS/MS (Table I).
Figure 2.
MALDI-TOF mass spectra of peptides from adenovirus sample harvested at 48 hr. Top: 2 min acid digestion; bottom: no digestion
TABLE 1.
Characterization of Peptides Detected by MALDI TOF MS
| Protein | Observed [M+H]+ | Calculated [M+H]+ | Peptide | Confirmed by MS/MS | MASCOT Expect Value |
|---|---|---|---|---|---|
| Hexon (PII) | 1183.3 | 1183.4 | D.SITQKKFLCD.R | ||
| 1298.0 | 1298.5 | V.DSITQKKFLCD.R | |||
| 1350.7 | 1350.6 | D.RNTELSYQLLL.D | |||
| 1423.7* | 1423.7 | D.PYYTYSGSIPYL.D | ESI CID | 0.036 | |
| 1462.3 | 1462.7 | D.YMNKRVVAPGLVD.C | |||
| 1494.4 | 1493.6 | G.DNLTPKVVLYSED.V | |||
| 1530.7 | 1530.7 | D.GEGYNVAQCNMTKD.W | |||
| 1538.8* | 1538.7 | D.PYYTYSGSIPYLD.G | ESI CID | 1.7e-5 | |
| 1602.1 | 1601.9 | D.RSQRLTLRFIPVD.R | |||
| 1674.3 | 1674.9 | D.RTRYFSMWNQAVD.S | |||
| 1760.4 | 1761.0 | D.VNMVLQSSLGNDLRVD.G | |||
| 1818.2 | 1818.1 | D.RLLTPNEFEIKRSVD.G | |||
| 1886.8 | 1887.2 | D.RSQRLTLRFIPVDRE.D | |||
| 2002.8 | 2002.3 | D.RSQRLTLRFIPVDRED.T | |||
| 2002.8 | 2003.3 | D.GTFYLNHTFKKVAITFD.S | ESI CID | 0.053 | |
| 2040.7 | 2040.2 | D.RTRYFSMWNQAVDSYD.P | ESI CID | 0.011 | |
| 2060.8 | 2061.3 | L.DSIGDRTRYFSMWKQAVD.S | |||
| 2167.9 | 2167.4 | D.NPNTYDYMNKRVVAPGLVD.C | ESI CID | 0.0034 | |
| 2425.2 | 2424.7 | D.RMYSFFRNFQPMSRQVVD.D | |||
| Penton (PIII) | 994.8 | 995.1 | D.VDAYQASLK.D | ||
| 1011.0 | 1011.1 | V.DAYQASLKD.D | |||
| 1011.0 | 1011.2 | D.TRNFRLGF.D | |||
| 1126.3 | 1126.3 | D.TRNFRLGFD.P | |||
| 1573.5 | 1573.8 | D.PQTGIRSWTLLCTP.D | |||
| 1689.2 | 1688.9 | D.PQTGIRSWTLLCTPD.V | ESI CID | 0.0057 | |
| 2044.1* | 2044.1 | D.PVTGLVMPGVYTNEAFHPD.I | ESI CID | 1.5e-6 | |
| 2135.0* | 2134.9 | D.STFTQYRSWYLAYNYGD.P | ESI CID | 0.016 | |
| IVa2 (PIVa2) | 1016.9 | 1017.1 | D.LILEHNYD.V | ||
| 1462.3 | 1461.6 | D.RDAVEQVTELWD.R | |||
| 1658.2 | 1659.0 | D.RLELLGQTLKSMPTA.D | |||
| 2425.2 | 2425.9 | D.LVRENMRVRDMLNEVAPLLR.D | |||
| 2442.4 | 2442.8 | Y.DVSDPRNIFAQAAARGPIAIIMD.E | |||
| Fiber (PIV) | 1183.3 | 1183.3 | D.PEYWNFRNG.D | MALDI LID | 0.023 |
| 1298.0 | 1298.3 | D.PEYWNFRNGD.L | ESI CID | 9.7e-5 | |
| 1412.8 | 1413.4 | L.DPEYWNFRNGD.L | |||
| 1674.3 | 1673.9 | D.SQGNMQLNVAGGLRID.S | |||
| Hexon-associated (PIX) | 1767.3 | 1767.0 | D.LRQQVSALKASSPPNAV.- | ESI CID | 2.8e-5 |
| Late 100 kDa. | 1498.8* | 1498.7 | D.SLTAPSEFATTASTD.A | ESI CID | 1.9e-5 |
| 1802.8 | 1802.0 | D.LWTAFNERSVAAHLAD.I | |||
| 1858.1* | 1858.0 | D.IASLNEVPKIFEGLGRD.E | ESI CID | 0.00048 | |
| 2709.5* | 2709.3 | D.AANAPTTFPVEAPPLEEEEVIIEQD.F | ESI CID | 2.3e-5 | |
| Minor Capsid (PVI) | 1350.7 | 1350.7 | GVQSLKRRRCF (Endogenous) | MALDI LID | |
| 1655.0* | 1655.9 | D.LANQAVQNKINSKLD.P | ESI CID | 0.0037 | |
| Major Core (PVII) | 1197.5 | 1197.5 | GLRFPSKMFGG (Endogenous) | MALDI LID | |
| 2442.4 | 2442.7 | D.AIDAVVEEARNYTPTPPPVSTVD.A | |||
| Minor Core (PV) | 1919.1* | 1919.0 | D.AAVQAVAAAASKTSTEVQTD.P | ESI CID | 2.7e-5 |
indicates that peptide was only found in ESI mass spectra. D is shown at the carboxy terminus.
3.2
Table I indicates that 37 peptides are identified in the MALDI spectrum (Figure 2) from 8 proteins on the basis of molecular masses and LID sequence tags. Another 9 peptides are listed from ESI MS/MS experiments in order to strengthen the protein identifications and the argument for denaturation. A ninth protein, the minor core protein (PV) was detected only by ESI. Hexon (PII) and penton (PIII) proteins are well represented by peptides. The hexon protein is the second most abundant (720 copies) in each virus, while the penton protein III has only 60 copies per virus. It is of special interest that peptides are identified from the fiber protein (PIV), which participates in attachment to host cells [30]. It is readily lost from viral particles and is often not encountered in protein analysis [5]. It may be retained in the samples studied here because they have not been subjected to final cleanup by gradient centrifugation. The late 100 kDa protein is expressed later in the cycle of infection. In cases where LID and CID experiments are reported, Asp is confirmed to be located at the carboxy terminal of peptide products as previously reported [16–22]. Obtaining high protein sequence coverage was not an objective in this experiment, however it is anticipated that additional peptides would be characterized by repeated injections in HPLC-MSMS experiments.
3.3
Digestion of the intact virus with immobilized trypsin on the MALDI sample plate produced few peptides (data not shown) because the complex was not denatured. Tryptic digestion of adenovirus proteins is typically preceded by separate denaturation with detergents, reduction and alkylation steps [e.g.,3, 5–7] and takes much longer than the 5 min required for microwave assisted acid cleavage and MALDI-TOF profiling.
3.4
The smaller of the two endogenous peptides (m/z 1197.5) detected in Figure 2 (bottom) was identified by LID sequencing as GLRFPSKMFGG. This sequence is part of the major core protein VII [13,14], and the peptide has also been detected by others using HPLC-based analyses [5,6]. Cleavage of this small peptide by the adenovirus protease is reported to be required before incorporation of the major capsid protein VII into the mature viron [14]. The other peptide detected in the control spectrum, at m/z 1350.6, may also be a product of endogenous cleavage. The human adenovirus minor core protein VI is known to undergo processing by adenovirus protease at position 239 [13,14] to produce the peptide GVQSLKRRRCF, which has a calculated average [MH]+ mass of 1350.6. This sequence is also supported by an LID experiment. Adenovirus protease activity is required for the production of infectious virus particles and the ability to detect these peptide products rapidly may be useful to researchers evaluating protease inhibitors to prevent viral infections. In our studies the two peptides were not detectable in samples harvested at 2 and 4 hr post-infection. They were clearly detected in samples harvested at 24 hr.
3.5
Using myoglobin and ovalbumin as model proteins, formic acid (6.0 % and12.5%) and acetic acid (6.0 % and 12.5 %) were evaluated for efficient incorporation of the isotope label from H2 18O. These concentrations were already known to provide extensive cleavage (16–19). Reaction times of 15 sec. 30 sec, 90 sec, 3 min, 5 min, 15 min and 30 min were evaluated at the constant temperature of 140°C. Non-specific or side-chain incorporation of isotopes was observed when formic acid was used, and at extended reaction times in acetic acid. Based on the extent of proteolysis and high incorporation of a single isotope, 12.5 % acetic acid and 90 sec were chosen as standard labeling conditions for the rest of the study. Partial mass spectra are shown in Figure 3 of a peptide, STRTQINKVVRFD, produced from ovalbumin by cleavage at those conditions in H2 16O (top) and in H2 18O (bottom). A single atom of 18O is found to be incorporated >90% in the latter digestion.
Figure 3.
Partial MALDI-TOF mass spectra of the peptide STRTQINKVVRFD produced by acid digestion of ovalbumin in H2 16O (top) and H2 18O (bottom).
3.6
In order to confirm that the label is incorporated at the carboxy-terminal (see mechanism in Figure 1), tandem mass spectrometry experiments were performed. Figure 4 shows a fragment ion spectrum of a peptide AQGAMTKALELFRND from myoglobin cleaved in heavy water. The label is shown to be localized on the carboxy-terminal Asp moiety, based on the 2 Da mass shift of the y ions observed, and the absence of any mass increase in b ions formed. The original protein carboxyl terminal of myoglobin, observed in the peptide (D)IAAKYKELGRQG, remained unlabeled, and no evidence of random labeling was observed among other myoglobin peptides examined under these conditions.
Figure 4.
Laser induced dissociation spectrum of the peptide AQGAMTKALELFRND produced by acid digestion of myoglobin. Inset is a list of predicted b and y ions.
Peptide products that retain Asp at the C-terminus are preferentially formed at digestion times <2 min [16–22]. Depending on the incubation time, some of these peptides undergo a second hydrolysis to lose C-terminal Asp. Loss of C-terminal Asp in this step was observed to proceed as a second reaction with nonspecific incorporation of either 18O or 16O, which originated within the peptide.
3.7
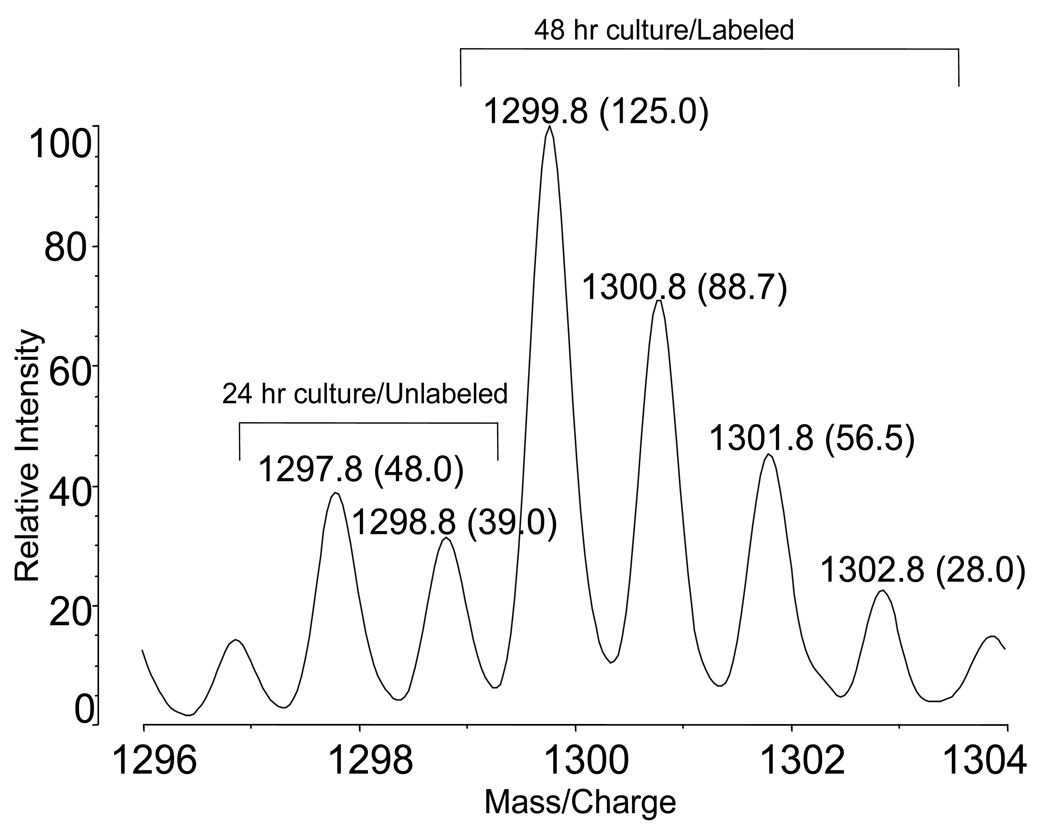
This rapid cleavage and isotope labeling technique was used to follow the growth of human adenovirus type 5 in HeLa cells. Figure 5 shows isotope envelopes for the peptide PEYWNFRNGD from the adenovirus fiber protein. Adenovirus was harvested from four flasks 24 hour after infection and digested in 12.5 % acetic acid in H2 16O for 90 sec. The virus was harvested from four culture flasks at 48 hr and digested in H2 18O. Aliquots of peptide products from the two samples were combined 1:1 by volume to produce the mass spectrum shown. After subtraction of contributions by 13C isotope species, the 16O/18O ratio indicates the presence of approximately twice as much virus in the 48 hr incubation compared to the 24 hr incubation, and indicates that the growth rate has not yet begun to level off.
Figure 5.
Partial MALDI-TOF mass spectrum of the peptide PEYWNFRNGD in solutions of peptide products combined 1:1 by volume from labeled adenovirus (48 hr harvest) and unlabeled adenovirus (24 hr harvest). Ion intensities are shown in parentheses.
4.0 Conclusions
In some circumstances it may be advantageous to characterize viruses rapidly and with a minimum of processing. In the present paper the capability of microwave assisted acid cleavage is demonstrated to denature human adenovirus and simultaneously to produce peptides without the addition of buffers and chaotropic agents. This proteolytic reaction can also incorporate a single atom of 18O quantitatively and site-specifically into peptides that end in Asp. It is compatible with various automated proteomic workflows [17,19,29].
Acknowledgements
The research was supported in part by a grant from the NIH, GM 021248. Dr. Laine was supported by a fellowship from the Helsingin Sanomat 100th Anniversary Foundation. We thank Prof. Nathan Edwards for advice on the use of RMIDB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Catherine Fenselau, Email: Fenselau@umd.edu.
Olli Laine, Email: olli.laine@suomi24.fi.
Stephen Swatkoski, Email: sswatko1@jhmi.edu.
References
- 1.Walther W, Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000;60:249–271. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- 2.Lehmberg E, Traina JA, Chakel JA, Chang RJ, Parkman M, McCaman MT, Murakami PK, Lahidji V, Nelson JW, Hancock WS, Nestaas E, Pungor EJ. Reversed-phase high-performance liquid chromatographic assay for the adenovirus type 5 proteome. J.Chomatogr. B. 1999;732:411–423. doi: 10.1016/s0378-4347(99)00316-3. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Vellekakmp G, Chen G, Mirza UA, Wylie D, Twarowska B, Tang JT, Porter FW, Wang S, Nagabhusan TL, Pramanik BN. Proteomic study of recombinant adenovirus 5 encoding human p53 by matrix-assisted laser desorption/ionization mass spectrometry in combination with database search. Int. J.Mass Spectrom. 2003;226:55–69. [Google Scholar]
- 4.Blyn LB, Hall TA, Libby B, Ranken R, Sampath R, Rudnick K, Moradi E, Desai A, Metzgar D, Russell KL, Freed NE, Balansay M, Broderick MP, Osuna MA, Hofstadler SA, Ecker DJ. Rapid detection and molecular serotyping of adenovirus by use of PCR followed by electrospray ionization mass spectrometry. J.Clin. Microbiol. 2008;46:644–651. doi: 10.1128/JCM.00801-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanche F, Monegier B, Faucher D, Duchesne M, Audhuy F, Barbot A, Bouvier S, Daude G, Dubois H, Guillemin T, Maton L. Polypeptide composition of an adenovirus type 5 used in cancer gene therapy. J. Chromatog. A. 2001;921:39–48. doi: 10.1016/s0021-9673(01)00896-2. [DOI] [PubMed] [Google Scholar]
- 6.Roitsch C, Achstetter T, Benchaibi M, Bonfils E, Cauet G, Gloeckler R, L’hote H, Keppi E, Nguyen M, Spehner D, Van Dorsselaer A, Malatme D. Characterization and quality control of recombinant adenovirus vectors for gene therapy. J.Chromatog. B. 2001;752:263–280. doi: 10.1016/s0378-4347(00)00557-0. [DOI] [PubMed] [Google Scholar]
- 7.Chelius D, Huhmer AR, Shieh CH, Hehmberg E, Traina JA, Slattery TK, Pungor E. Analysis of the adenovirus type 5 proteome by liquid chromatography and tandem mass spectrometry methods. J.Prot.Res. 2002;1:501–513. doi: 10.1021/pr025528c. [DOI] [PubMed] [Google Scholar]
- 8.Chroboczek J, Bieber F, Jacrot B. The sequence of the genome of adenovirus type 5 and its comparison with the genome of adenovirus type 2. Virol. 1992;186:280–285. doi: 10.1016/0042-6822(92)90082-z. [DOI] [PubMed] [Google Scholar]
- 9.Shenk T. Adenoviridiae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, Chanock RM, Melnick JL, Monath TP, Roizman B, Straus SE, editors. Fundamental virology. 3rd edn. Philadelphia: Lippincott-Raven; 1996. pp. 2111–2148. [Google Scholar]
- 10.Stewart PL, Burnett RM. Adenovirus structure by X-ray crystallography and electron microscopy. Curr. Top. Microbiol Immunol. 1995;199:25–38. doi: 10.1007/978-3-642-79496-4_2. [DOI] [PubMed] [Google Scholar]
- 11.van Oostrum J, Burnett RM. Molecular Composition of the adenovirus type 2 virion. J.Virol. 1985;56:439–448. doi: 10.1128/jvi.56.2.439-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson CW. The proteinase polypeptide of adenovirus type 2 virons. Virol. 1990;177:259–272. doi: 10.1016/0042-6822(90)90479-b. [DOI] [PubMed] [Google Scholar]
- 13.Webster A, Leith IR, Hay RT. Activation of adenovirus-coded protease and processing of preterminal protein. J. Virology. 1994;68:7292–7300. doi: 10.1128/jvi.68.11.7292-7300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diouri M, Keyvani-Amineh H, Geoghegan KF, Weber JM. Cleavage efficiency by adenovirus protease is site-dependent. J. Biolog. Chem. 1996;271:32511–32514. doi: 10.1074/jbc.271.51.32511. [DOI] [PubMed] [Google Scholar]
- 15.Yao X, Freas A, Ramirez J, Demirev PA, Fenselau C. Proteolyic 18O labeling for comparative proteomics: model studies with two serotoypes of adenovirus. Anal.Chem. 2001;73:2836–2842. doi: 10.1021/ac001404c. [DOI] [PubMed] [Google Scholar]
- 16.Swatkoski S, Russell S, Edwards N, Fenselau C. Rapid chemical digestion of small acid-soluble spore proteins for Rapid Analysis of Bacillus Spores. Anal. Chem. 2006;78:181–188. doi: 10.1021/ac051521d. [DOI] [PubMed] [Google Scholar]
- 17.Swatkoski S, Gutierrez P, Ginter J, Petrov A, Dinman JD, Edwards N, Fenselau C. Integration of residue-specific acid cleavage into proteomic workflows. J. Proteome Res. 2007;6:4525–4527. doi: 10.1021/pr0704682. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Shefcheck K, Callahan J, Fenselau C. Extension of microwave-accelerated residue-specific acid cleavage to proteins with carbohydrate side chains and disulfide linkages. Internat.J. Mass Spectrom. 2008;278:109–113. doi: 10.1016/j.ijms.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swatkoski S, Gutierrez P, Wynne C, Petrov A, Dinman JD, Edwards N, Fenselau C. Evaluation of microwave-accelerated residue-specific acid cleavage for proteomic applications. J. Proteome Res. 2008;7:579–586. doi: 10.1021/pr070502c. [DOI] [PubMed] [Google Scholar]
- 20.Hauser NJ. Online Microwave D-cleavage LC-ESI-MS/MS of intact proteins: Site - specific cleavages at aspartic acid residues and disulfide bonds. J. Proteome Res. 2008;7:1012–1026. doi: 10.1021/pr700596e. [DOI] [PubMed] [Google Scholar]
- 21.Hauser NJ, Han HL, McLuckey SA, Basile F. Electron transfer dissociation of peptides generated by microwave D-cleavage digestion of proteins. J.Prot. Res. 2008;7:1867–1872. doi: 10.1021/pr700671z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua L, Low TY, Sze SK. Microwave-assisted specific chemical digestion for rapid protein identification. Proteomics. 2006;6:586–591. doi: 10.1002/pmic.200500304. [DOI] [PubMed] [Google Scholar]
- 23.Tao WA, Aebersold R. Advances in quantitative proteomics via stable isotope tagging and mass spectrometry. Curr. Opin. Biotechnol. 2003;14:110–118. doi: 10.1016/s0958-1669(02)00018-6. [DOI] [PubMed] [Google Scholar]
- 24.Ong SE, Kratchmarova I, Mann M. Properties of 13C-substituted arginine in stable isotope labeling by amino acids in cell culture. J. Proteome Res. 2003;2:173–181. doi: 10.1021/pr0255708. [DOI] [PubMed] [Google Scholar]
- 25.Yao X, Afonso C, Fenselau C. Dissection of Proteolytic 18O Labeling: Endoprotease-Catalyzed 16O-to-18O Exchange of Truncated Peptide Substrates. J. Proteome Res. 2003;2:147–152. doi: 10.1021/pr025572s. [DOI] [PubMed] [Google Scholar]
- 26.Ross PI, Huang YN, Marchese JN, Williamson V, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed Protein Quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Molec. Cell. Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Fenselau C, Yao X. 18O2-Labeling in Quantitative proteomic Strategies: A Status Report. J. Proteome Res. 2009;8:2140–2143. doi: 10.1021/pr8009879. [DOI] [PubMed] [Google Scholar]
- 28.Li AQ, Sowder RC, Henderson LE, Moore SP, Garfinkel DJ, Fisher RJ. Chemical Cleavage at Aspartyl Residues for Protein Identification. Anal.Chem. 2001;73:5395–5402. doi: 10.1021/ac010619z. [DOI] [PubMed] [Google Scholar]
- 29. www.RMIDB.org 01-20-10.
- 30.Philipson L. Structure and assembly of adenoviruses, Current Top. Microbiol. Immun. 1983;109:1–52. doi: 10.1007/978-3-642-69460-8_1. [DOI] [PubMed] [Google Scholar]